In 1882, Giulio Bizzozero initially described the hemostatic properties of platelets[1] and, for approximately 100 years, any alternative attributes ascribed to these anucleated cells have been treated with a degree of skepticism. For instance, while most platelet research has centered around their thrombotic properties, more recently, their role in inflammation has been clearly established and, now is firmly accepted. Conversely, while platelets have been clearly identified to have a transcriptomic profile, research understanding their ability to support the process of translation is still limited. It is with this degree of measured skepticism, one must examine the study by Malaver et al[2] in the current issue of the Journal of Thrombosis and Haemostasis. The authors propose that inhibition of platelet NFκB leads to modulation of platelet function. NFκB is a transcription factor which controls the gene expression of inflammatory mediators. Under normal conditions, NFκB is retained in the cytosol and, in response to stimuli, the free NFκB complex translocates to the nucleus where it initiates gene transcription. As the platelet is an anuclueated cell without genomic DNA, the idea that NFκB could regulate platelet function is fascinating.
The study by Malaver et al [2] raises the question; can platelets have nongenomic function due to a pathway that is only known as a genomic regulator? While there is limited data on genomic function, platelets are known to have a clear transcriptomic profile[3] and, specifically, are known to contain the transcript for NFκB (unpublished data). However, whether the transcript contained in the platelet is a reflection of the megakaryocyte, a specific phenotype, or merely remnant RNA is not yet clear. A limited number of studies have convincingly shown that, in platelets, there is new synthesis of proteins from previously transcribed RNAs in response to activating signals.[4] In addition, select nuclear receptors have been shown to have “nongenomic” function in platelets. One example is estrogen which is well known to regulate specific kinase pathways in other cell types[5] and, in platelets, mediates function via various pathways including increased GPVI receptor expression after oral estrogen replacement (GPVI).[6] Notably and unlike NFκB, these studies specifically examine receptors which are already known to have effects (i.e. stimulation of kinase pathways) that do not require genomic regulation.[5]
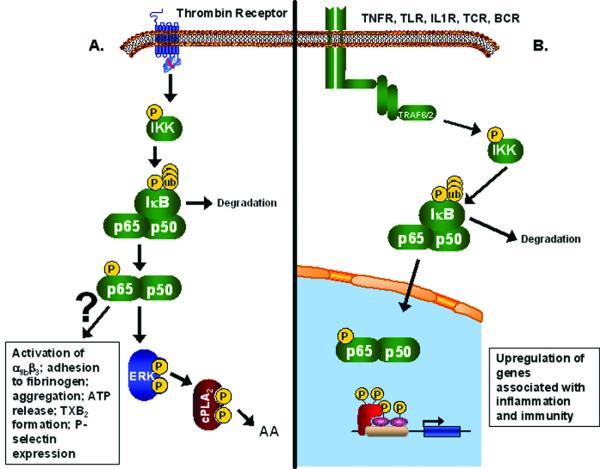
The NFκB family of transcription factors is comprised of 5 members: p50, p52, p65, RelB, and c-Rel. NFκB complexes are mainly found sequestered in the cytoplasm in an inactive state, bound to the inhibitory proteins, IκBs. In this complex, IκBs mask the nuclear localization sequence on the NFκB. The combinations of NFκB dimers and IκBs indicate the diverse function of this signaling pathway. The NFκB pathway begins with the activation of IKK (Figure 1B). Numerous receptors, mainly associated with inflammation and immunity, are involved in IKK activation, including tumor necrosis factor receptors (TNFRs), toll-like receptors (TLRs), interleukin-1 receptor (IL1R), T cell receptors (TCR), and B cell receptors (BCR). However, other cellular stresses can trigger the activation of this pathway including DNA damage by UV and ionizing radiation, and exposure to free radicals and reactive oxygen species. These receptors activate proteases and adaptors that will both activate IKK and other signaling pathways, such as JNK, p38, ERK, and caspases. Upon the phosphorylation of 2 serine residues on its β subunit, IKK will phosphorylate IκB, which leads to a conformational change and release of NFκB. The phosphorylated IκB is recognized by SCRF ubiquitin ligase machinery, which polyubiquinates lysine residues on IκBs, leading to its degradation. NFκB translocates into the nucleus and will activate the transcription of various genes mainly involved in inflammation and immunity. Activation into a transcription factor occurs through post-translational modifications, including phosphorylation and acetylation, of NFκB.[7, 8] Dysfunction in these pathways has been associated with numerous pathologies, including cancer, diabetes, cardiovascular disease, and inflammatory disorders.
Figure. The NFκB Pathway.
(A) Depiction of the novel function of the NFκB pathway in platelets based on the findings from Malayer, et al., which shows NFκB involved in the activation of this anucleated cell by thrombin. (B) Depiction of the traditional NFκB pathway in nucleated cells.
The function of NFκB has been studied exclusively in nucleated cells. The question at hand is whether or not this transcription factor is functionally present in anucleated platelets. In a large epidemiological cohort looking at the relationship of platelet RNA levels and cardiovascular disease, NFκB1 RNA was consistently present in platelets (unpublished). Previous work by Liu, et al [9], has shown that platelets express 3 members of the NFκB pathway - IκB, NFκB p65, and IKK. PMA or TRAP treatment led to the phosphorylation of IKK on serine 32 and phosphorylation and degradation of IκBα; however, the NFκB phosphorylation level remained unchanged upon activation[9]. In this issue of the Journal of Thrombosis and Haemostasis, Malayer, et al [2] present additional data further confirming the presence of NFκB in platelets with a summary of their data as compared to known NFκB pathways shown in the summary Figure. Through immunofluorescent microscopy and western blots, the presence of NFκB p65 is shown in platelets, which is not attributed to any leukocyte contamination [2]. In addition, they also identify the presence of IκBα in platelets through western blots [2]. Taken together with past data, it seems possible that NFκB and other members of its pathway are present in platelets; however, the question remains if this pathway is functioning in a novel way, unrelated to transcriptional regulation. Platelets pretreated with NFκB pathway inhibitors, BAY 11-7082 and Ro 106-9920, responded to stimulation with arachadonic acid but not other agonists, including thrombin, epinephrine, collagen, and ADP[2]. These inhibitors prevented multiple platelet activation mechanisms – platelet-platelet interactions, platelet adhesion to fibrinogen, integrin αIIbβ3 activation, P-selectin release, ATP release, and TXB2 formation [2]. Although these inhibitors did not effect arachadonic acid-induced aggregation, it was determined that they blocked the phosphorylation of ERK, which regulates the phosphorylation of cPLA2, the main enzyme responsible for the release of arachadonic acid [2] (data summarized in Figure 1A). Although there is no doubt that these inhibitors are affecting platelet function, the question remains whether or not these inhibitors are effecting more that just the NFκB pathway, such as caspases. In addition, it would have been more conclusive to show that the NFκB pathway is involved in platelet aggregation by using a known activator of this pathway. It has been established that platelets express functional IL1R and TLRs, specifically TLR4 and TLR2 [10], receptors known to activate the NFκB pathway. This paper also concludes that NFκB pathway is involved in the regulation of the MAPK-ERK pathway. With the addition of either pharmacological inhibitor, there is noted loss of ERK phosphorylation as shown by western blot when the platelets are treated with thrombin [2]. Traditionally, ERK phosphorylation has led to the regulation of the NFκB pathway, in particular through phosphorylation of IκBα bound to NFκB p65 [11]. Regulation of ERK activation has only been shown to occur through the phosphorylation and degradation of NFκB p105 [12].
Strong evidence for the functionality of the findings presented by Malaver et al[2] should be from the in vivo findings in which mice have the NFκB inhibitor BAY 11-7082 administered intraperitoneally and, after 24 hours and a repeat dose, bleeding time and platelet aggregation measured. However, in this study,[2] no differences were detected by bleeding time but aggregation was decreased in animals treated with BAY 11-7082. Unfortunately, these in vivo pharmacological studies raise more questions than they answer. The NFκB inhibitor is not specific for platelets; a point highlighted by the discrepant findings looking at platelet function vs. bleeding time which reflects both platelet and endothelial effects. Additionally, inhibition of NFκB throughout the vasculature could influence transcription in nucleated cells including mediators of platelet function such as CD40/CD40L[13], and megakaryocytes, in which NFκB has been shown to have a role the maturation of these cells prior to platelet production [14]. Perhaps, if platelets that had been incubated with an NFκB inhibitor were reinfused, and bleeding times immediately measured, changes in function could be assumed to be platelet specific with a greater degree of certainty. Ideally, the use of conditional knockout mice models would be of greater benefit. However, in the case of platelets, conditionally removing components of the NFκB pathway, will also affect megakaryocytes, leading to problems with platelet development. Infusion of platelets from a conditional knockout mouse into a normal mouse would be a method that would best determine if NFκB has a functional role in platelets.
Most importantly, the conclusions from this study are entirely based on the use of two pharmacological inhibitors known to inhibit at specific targets in the NFκB pathway. BAY 11-7082, which has an IC50 of 10 μM [15], inhibits cytokine-induced IκBα phosphorylation. Ro 106-9920 inhibits TNF-α and LPS induced IκBα ubiquitination, has an IC50 of 3 μM [16]. Concentrations used in this study are well above these values. Therefore, the question remains if these concentrations are affecting other pathways or do they have cytotoxic effects. It has been reported that BAY 11-7082 can activate caspase 3, independent of the NFκB pathway[17] at a lower concentration than that used in this study. In addition, contrary to the data shown in this study, BAY 11-7082 was shown to not have an effect on the phosphorylation of ERK[18], albeit at a lower concentration; however, it has been shown to increase the phosphorylation of p38 and JNK1 [15]. Finally, Ro 106-9920 is also not specific for NFκB pathway inhibition at higher concentrations. At 10 μM, this inhibitor will reduce the activity by 50% or more of CGRP (which has been shown to inhibit platelet aggregation and tissue factor release [19]), EGFR kinase, iNOS, and 5-lipoxygenase, while increasing the activity by 50% or more of P2Y1 and COX1(both functional in platelets; P2Y regulates activation and COX1 is involved in prostaglandin production)[16]. Those enzymes inhibited 30% or more include, BZD central and phospholipase C (PLC; involved in platelet shape change) [16]. Not all of these enzymes function in platelets, but nevertheless this shows that the two inhibitors at 10 μM are able to augment various cellular pathways and reactions. In conclusion, with the use of such high concentrations of NFκB pharmacological inhibitors, there can only be concern that these results are an artifact of the treatment and do not identify a novel function of NFκB in platelets.
Thus, in 2009, we can comfortably agree that platelets are more than anucleated cells which mediate hemostasis and induce thrombosis. Clearly, a wealth of basic and clinical studies have shown their importance in inflammation, non-thrombotic vascular homeostasis, and immunity. Emerging studies are defining the relevance of the platelet transcriptome. Therefore, with the optimistic view of the expanding role of platelets in the vasculature, the study by Malaver, et al [2] should be initially viewed. These findings show that NFκB is intact in the platelet and undergoes similar regulation as compared to other inflammatory cells. However, there are significant limitations including a paucity of mechanistic explanation, a purely pharmacologic approach, and a lack of convincing in vivo data; suggesting that further studies are needed to definitely state that NFκB, through nongenomic and unknown pathways, mediates platelet function.
References
- 1.Bizzozero G. Ueber einen neuen Forrnbestandteil des Blutes und dessen Rolle bei der Thrombose und Blutgerinnung. Archiv fur pathologische; Anatomie und Physiologie und fur klinische Medicin. 1881;90:261–332. [Google Scholar]
- 2.Malaver E, Romaniuk MA, D'Atri LP, Pozner RG, Negrotto S, Benzadon R, Schattner M. NF-kB inhibxitors impair platelet activation responses. Journal of Thrombosis and Haemostasis. 2009 doi: 10.1111/j.1538-7836.2009.03492.x. [DOI] [PubMed] [Google Scholar]
- 3.Freedman JE. Transcriptomics: new targets for diagnosing and treating cardiovascular disease. Cardiovasc Ther. 2008;26:179–81. doi: 10.1111/j.1755-5922.2008.00055.x. [DOI] [PubMed] [Google Scholar]
- 4.Weyrich AS, Denis MM, Schwertz H, Tolley ND, Foulks J, Spencer E, Kraiss LW, Albertine KH, McIntyre TM, Zimmerman GA. mTOR-dependent synthesis of Bcl-3 controls the retraction of fibrin clots by activated human platelets. Blood. 2007;109:1975–83. doi: 10.1182/blood-2006-08-042192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang L, Li X, Zhao L, Zhang G, Wang J, Wei L. Nongenomic effect of estrogen on the MAPK signaling pathway and calcium influx in endometrial carcinoma cells. J Cell Biochem. 2009;106:553–62. doi: 10.1002/jcb.22017. [DOI] [PubMed] [Google Scholar]
- 6.Leng XH, Zhang W, Nieswandt B, Bray PF. Effects of estrogen replacement therapies on mouse platelet function and glycoprotein VI levels. Circ Res. 2005;97:415–7. doi: 10.1161/01.RES.0000181025.43762.cf. [DOI] [PubMed] [Google Scholar]
- 7.Xiao W. Advances in NF-kappaB signaling transduction and transcription. Cell Mol Immunol. 2004;1:425–35. [PubMed] [Google Scholar]
- 8.Hayden MS, Ghosh S. Signaling to NF-kappaB. Genes Dev. 2004;18:2195–224. doi: 10.1101/gad.1228704. [DOI] [PubMed] [Google Scholar]
- 9.Liu F, Morris S, Epps J, Carroll R. Demonstration of an activation regulated NF-kappaB/I-kappaBalpha complex in human platelets. Thromb Res. 2002;106:199–203. doi: 10.1016/s0049-3848(02)00130-5. [DOI] [PubMed] [Google Scholar]
- 10.Blair P, Rex S, Vitseva O, Beaulieu L, Tanriverdi K, Chakrabarti S, Hayashi C, Genco CA, Iafrati M, Freedman JE. Stimulation of Toll-like receptor 2 in human platelets induces a thromboinflammatory response through activation of phosphoinositide 3-kinase. Circ Res. 2009;104:346–54. doi: 10.1161/CIRCRESAHA.108.185785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu S, Bayat H, Hou X, Jiang B. Ribosomal S6 kinase-1 modulates interleukin-1beta-induced persistent activation of NF-kappaB through phosphorylation of IkappaBbeta. Am J Physiol Cell Physiol. 2006;291:C1336–45. doi: 10.1152/ajpcell.00552.2005. [DOI] [PubMed] [Google Scholar]
- 12.Parameswaran N, Pao CS, Leonhard KS, Kang DS, Kratz M, Ley SC, Benovic JL. Arrestin-2 and G protein-coupled receptor kinase 5 interact with NFkappaB1 p105 and negatively regulate lipopolysaccharide-stimulated ERK1/2 activation in macrophages. J Biol Chem. 2006;281:34159–70. doi: 10.1074/jbc.M605376200. [DOI] [PubMed] [Google Scholar]
- 13.Chakrabarti S, Blair P, Freedman JE. CD40-40L signaling in vascular inflammation. J Biol Chem. 2007;282:18307–17. doi: 10.1074/jbc.M700211200. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Y, Sun S, Wang Z, Thompson A, Kaluzhny Y, Zimmet J, Ravid K. Signaling by the Mpl receptor involves IKK and NF-kappaB. J Cell Biochem. 2002;85:523–35. doi: 10.1002/jcb.10141. [DOI] [PubMed] [Google Scholar]
- 15.Pierce JW, Schoenleber R, Jesmok G, Best J, Moore SA, Collins T, Gerritsen ME. Novel inhibitors of cytokine-induced IkappaBalpha phosphorylation and endothelial cell adhesion molecule expression show anti-inflammatory effects in vivo. J Biol Chem. 1997;272:21096–103. doi: 10.1074/jbc.272.34.21096. [DOI] [PubMed] [Google Scholar]
- 16.Swinney DC, Xu YZ, Scarafia LE, Lee I, Mak AY, Gan QF, Ramesha CS, Mulkins MA, Dunn J, So OY, Biegel T, Dinh M, Volkel P, Barnett J, Dalrymple SA, Lee S, Huber M. A small molecule ubiquitination inhibitor blocks NF-kappa B-dependent cytokine expression in cells and rats. J Biol Chem. 2002;277:23573–81. doi: 10.1074/jbc.M200842200. [DOI] [PubMed] [Google Scholar]
- 17.White DE, Burchill SA. BAY 11-7082 induces cell death through NF-kappaB-independent mechanisms in the Ewing's sarcoma family of tumours. Cancer Lett. 2008;268:212–24. doi: 10.1016/j.canlet.2008.03.045. [DOI] [PubMed] [Google Scholar]
- 18.Lee SJ, Long M, Adler AJ, Mittler RS, Vella AT. The IKK-neutralizing compound Bay11 kills supereffector CD8 T cells by altering caspase-dependent activation-induced cell death. J Leukoc Biol. 2009;85:175–85. doi: 10.1189/jlb.0408248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li D, Peng J, Xin HY, Luo D, Zhang YS, Zhou Z, Jiang DJ, Deng HW, Li YJ. Calcitonin gene-related peptide-mediated antihypertensive and anti-platelet effects by rutaecarpine in spontaneously hypertensive rats. Peptides. 2008;29:1781–8. doi: 10.1016/j.peptides.2008.06.010. [DOI] [PubMed] [Google Scholar]