Abstract
Background
Kinematic and kinetic measurements used in laboratory settings can quantify upper extremity movement impairment following stroke, but their relationship to clinical methods of evaluating movement impairment is unclear.
Objective
To test whether the Arm Coordination Training 3D device (ACT3D) could provide a repeatable quantitative measurement of range of motion during upper extremity reaching along a range of functional levels of loads on the arm and correlate with clinical assessments of arm impairment.
Methods
Work area during reaching along clockwise and counterclockwise hand paths was measured under 9 limb-loading conditions ranging from no load to twice the weight of the upper extremity in 11 individuals with chronic hemiparetic stroke on 2 separate occasions. Participants were given a battery of clinical assessments that included the Fugl-Meyer Motor Assessment, Chedoke McMaster Stroke Assessment, Reaching Performance Scale, Modified Ashworth Scale, and the Stroke Impact Scale, by a physical therapist who did not know the results of the kinematic studies.
Results
A reproducible test-retest reduction in work area was found when participants were required to support up to and beyond the weight of their limb. Work area was correlated with most upper extremity clinical assessments, suggesting criterion validity.
Conclusions
Reaching work area during various loading conditions is a robust measurement that quantifies the effect of abnormal joint torque coupling and provides useful data that can be applied in the clinical setting.
Keywords: Stroke, Arm, Kinematics, Rehabilitation evaluation, Haptic, Robotics
Substantial gains have been made in the description of upper extremity discoordination following hemiparetic stroke through the laboratory-based implementation of quantitative kinetic and kinematic analyses. These efforts have established that under isometric conditions, abnormal muscle coactivation occurs between shoulder abductors and elbow flexors when individuals with stroke attempt to abduct at the paretic shoulder.1 Furthermore, abnormal muscle coactivation results in synergistic joint torque coupling of shoulder abduction with shoulder external rotation and extension and most strongly with elbow flexion,2-4 reflecting the observations of “flexion synergy” noted in early studies.5,6 In other simultaneous lines of inquiry, we have demonstrated the effects of abnormal joint torque coupling on dynamic reaching with the affected arm. As individuals with stroke actively support their arm against gravity, they are limited primarily in the ability to extend at the elbow when reaching outward. However, when the weight of the limb is externally supported, reaching range of motion remains mostly preserved.7 Our most recent work in rehabilitation robotics has been successful in quantifying with great specificity the effect of active shoulder abduction due to limb loading on reaching kinematics using the Arm Coordination Training 3-Dimensional Device (ACT3D).8 This work has identified a monotonic relationship between the amount of limb support actively performed by the individual and the amount of total available reaching range of motion measured in terms of maximum hand-path area (work area). The reaching direction most impacting the total work area involves elbow extension in the ipsilateral half of the reaching work area and is attributed to abnormal coactivation of elbow flexors and inhibition of elbow extensors during volitional activation of the shoulder abductors.4
Although both kinematic and kinetic analyses are widely used tools in rehabilitation research dedicated to elucidating upper extremity control following stroke, only a few efforts have been made to evaluate the relationship between laboratory-based quantitative measurements with existing clinical assessments of movement and activity limitation in individuals with stroke. Endpoint reaching trajectories and interjoint coordination variables measured during passively supported planar reaching have been correlated with clinical impairment scores from the arm motor portion of the Fugl-Meyer Motor Assessment and the Ashworth spasticity scale.9 The same investigator, in a study more comparable to the present one, identified a relationship between temporal interjoint coordination of the subcomponents of an unsupported active reaching task with arm motor impairment and spasticity scores.10 We sought to expand upon the kinematic analysis of unsupported reaching by analyzing the work area encompassed under various abductor-loading conditions8 and to identify relationships between work area and clinical scores to demonstrate the validity and clinical usefulness of the kinematic analysis. In clinical assessment, the validity of a measurement is established by documenting that inferences made about the magnitude of an impairment are based on a relevant observed behavior or response.11 Specifically, criterion-related validity can be practically tested by comparing the relationship of a new measurement to a known “gold standard.” Many other studies using kinematic and kinetic analyses have made inferences about the magnitude of stroke-related movement impairments but did not include comparisons with known clinical evaluation tools, thus limiting their immediate clinical application.7,12-14
In the present study, we took the first step in investigating the relationship of our laboratory-based measurements with known qualitative clinical assessments in an effort to provide evidence for its criterion validity and therefore applicability to clinical practice. Because there is no stand-alone gold standard of upper extremity impairment in stroke, we chose a battery of clinical assessments with established psychometric properties as comparison measures. Traditional standardized clinical assessments for the upper extremity such as the Fugl-Meyer Motor Assessment,15 Chedoke-McMaster Stroke Assessment,16 Reaching Performance Scale,17 Modified Ashworth Scale,18 and Stroke Impact Scale19 offer various strengths and are suitable as criterion measures. The importance to clinical practice of investigating the criterion validity of the work area measure is that work area would naturally translate from a laboratory tool into a viable clinical tool that could augment traditional qualitative evaluation of hemiparetic arm movement. Work area provides a discrete value specifying the impact of abductor activation on reaching range of motion. Furthermore, principal joint range of motion limitations contributing to work area losses can be easily distilled and targeted in impairment-based interventions.
The quantitative measurement of kinematics as a function of active limb support provided by the ACT3D may provide additional information to the clinician to identify appropriate interventions to target this specific movement impairment. Values for reaching work area under specific limb loading conditions would provide optimized starting points for multijoint strength training and identify specific regions of the work area that should be targeted with functional movement retraining. We hypothesize that kinematic variables from the ACT3D are related to existing standardized clinical assessments of arm movement following stroke (criterion validity) and can be acquired repeatedly with little variation (test-retest reliability) and suggest that additional information valuable to a clinician can be gained such that arm movement impairment is better characterized and interventions better initiated and guided by the rehabilitation specialist.
METHODS
Participants
Eleven individuals (ranging 51-78 years in age) with chronic hemiparesis, 2 to 12 years postinfarct, were recruited for this study (Table 1). All participants were recruited from a departmental research database under search criteria for score on the arm motor portion of the Fugl-Meyer Motor Assessment. Inclusion criteria for the study was a score within the range of 10 and 50 out of a possible 66, to exclude individuals with near complete paralysis or individuals who lacked impairment. All participants were screened for inclusion in the study by the first author. Potential participants were excluded if they had difficulty with prolonged sitting (self-report), recent changes in the medical management of hyper-tension (self-report), any acute or chronic painful condition in the upper limbs or spine, or greater than minimal sensory loss in the affected upper limb as determined by a tactile localization and awareness of movement task.20 In addition, all individuals with stroke had to be able to lift their arm volitionally and extend the elbow slightly. Passive range of motion of the affected upper limb was measured to verify at least 90 degrees of shoulder flexion, abduction, neutral internal/ external rotation, and full extension of the elbow in order to participate in the study. Subluxation was not an exclusionary factor. However, pain at the end of the range of passive motion was used as a medical screening to verify the presence of an inflammatory condition at the shoulder, elbow, wrist, and fingers. All participants provided informed consent in accordance with the Declaration of Helsinki prior to participation in this study, which was approved by the Institutional Review Board of Northwestern University.
Table 1.
General Demographics of Participant Pool
| Participant | Age | Gender | Affected | Dominant | Lesion Location | FMAt | CMSa |
|---|---|---|---|---|---|---|---|
| 1 | 51 | F | L | R | * | 16 | 2 |
| 2 | 55 | M | L | R | IC,P,CL | 10 | 3 |
| 3 | 59 | M | R | R | P,GP,IC,SupTH | 14 | 2 |
| 4 | 64 | M | L | R | CN,IC,LatTH | 37 | 5 |
| 5 | 50 | F | R | L | PostIC,SupTH,BG | 18 | 3 |
| 6 | 71 | F | L | R | BG,CN,P | 34 | 3 |
| 7 | 60 | M | L | R | FL/PL,BG,LatTH,IC,CR | 25 | 3 |
| 8 | 54 | M | L | R | IC,TH | 17 | 3 |
| 9 | 61 | M | L | R | CR | 43 | 4 |
| 10 | 60 | M | L | R | TH,IC,GP | 24 | 3 |
| 11 | 78 | M | L | R | IC,TH | 35 | 4 |
FMAt = total arm score of the Fugl-Meyer Motor Assessment; CMSa = arm score of the Chedoke-McMaster Stroke Assessment; BG = basal ganglia; CL = claustrum; CN = caudate nucleus; CR = corona radiata; FL/PL = cortical and subcortical frontal and parietal lobe; GP = globus pallidus; IC = internal capsule; P = putamen; TH = thalamus.
Right side lesion, MRI data unavailable, participant not MRI compatible.
Protocol: Laboratory Measurements
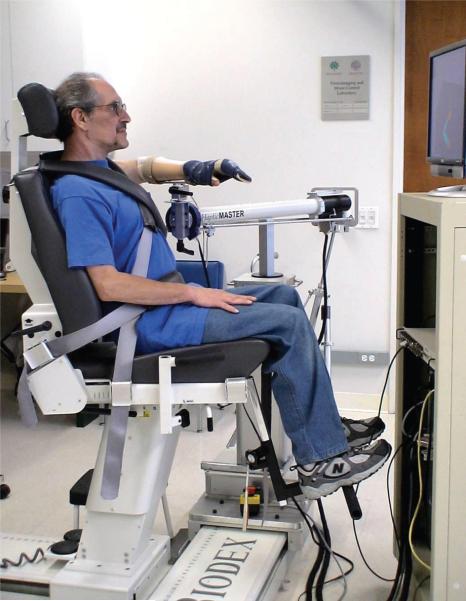
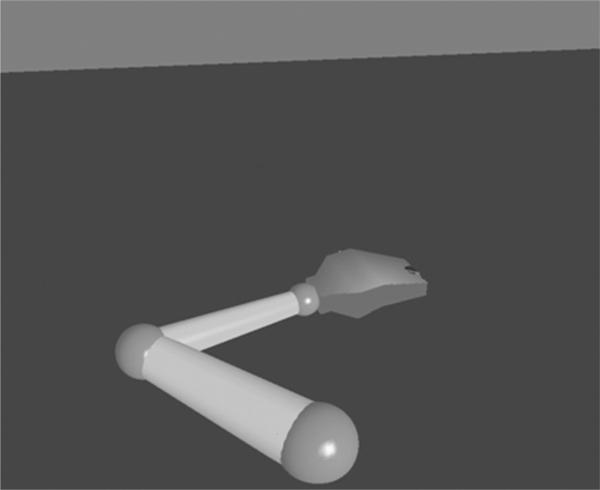
Quantitative kinematic measurements were performed for each participant in the laboratory on 2 sessions separated by 1 week, using the protocol described previously.8 Participants sat in a Biodex chair with their affected arm resting in a forearm-hand orthosis attached to the ACT3D (Figure 1; see reference 8 for a detailed description of the device). The orthosis maintained the wrist and hand in a neutral position, and the participant's trunk was immobilized by a set of straps attached to the chair. The trunk was immobilized to prevent compensatory trunk movements, therefore maximizing elbow and shoulder joint excursions and optimizing interjoint coordination21 to capture the greatest reaching magnitude. At the same time, the shoulder girdle was immobilized maintaining the shoulder joint center of rotation fixed for online inverse dynamics calculations required for the visual display (Figure 2). The shoulder was positioned at 90 degrees of abduction when the tested arm was resting on the haptically rendered table. Participants were manually placed in an initial position of 40 degrees of shoulder flexion or horizontal adduction and 90 degrees of elbow flexion using a goniometer. The ACT3D software was used to calculate the position of the shoulder, and a graphic representation of the arm was illustrated on a computer screen in front of the participant (Figure 2). Participants were asked to move their arm in a circular motion producing the largest hand path possible while it was fully supported by and gliding on the haptic table. Participants were asked to perform the reaching work area task slowly, limiting their joint rotation to approximately 5 degrees per second to minimize the effects of stretch reflex hyperactivity or spasticity. Participants performed the task in a clockwise and in a counterclockwise direction, the order of which was randomized, in order to capture the full reaching work area upon superimposition of the area obtained for each of 2 directions. For each direction, a 15-second recording time was sufficient to capture approximately 3 full hand path circles. Rest was given between each direction to eliminate fatigue, and verbal feedback was given to encourage the participant to achieve the maximum movement excursion while moving slowly. The chair was then elevated by approximately 1 inch, and participants were required to actively support their arm just above the haptic table resulting in 90 degrees of shoulder abduction. The same work area task as above was repeated while the ACT3D provided forces along its vertical axis to alter the amount of active limb support the participant was required to generate. A total of 9 support levels were randomized for testing. They ranged from 0% to 200% of active limb weight support, in increments of 25% of limb weight. Kinematic data obtained by the ACT3D was collected for all trials and saved for future analysis.
Figure 1.
Participant seated in the Arm Coordination Training 3D device (ACT3D) system. Straps secure his trunk, and the arm is attached via a lightweight splint. He is looking at the computer monitor for visual feedback.
Figure 2.
Graphic representation of the participant's arm illustrated on the visual display.
Clinical Assessments
The clinical assessment battery for all study participants was performed in a private room by the same physical therapist. This physical therapist was blinded to the laboratory-based kinematic measurements. The clinical assessment battery included the Fugl-Meyer Motor Assessment, Chedoke-McMaster Stroke Assessment, Reaching Performance Scale, Modified Ashworth Scale, and the Stroke Impact Scale. Data obtained from the clinical assessments were recorded on paper score sheets, transferred to an Excel spreadsheet, and saved for future analysis.
Analysis
The total work area for each level of limb support was calculated offline using customized software in the Matlab environment (Mathworks, Inc, Natick, MA). Work area was defined as the total area in square meters contained within the perimeter of the superimposed clockwise and counterclockwise hand paths. Areas for all participants were normalized to the area they were able to achieve on the table to account for differences in limb length. For example, to calculate normalized work area at the 0% active limb support level, the work area in m2 at the 0% level was divided by the work area in m2 that was achieved while supported on the haptic table. This procedure was performed for all support levels of each participant. Statistical analyses were performed using Data Desk (Ithaca, NY). All data was tested for normality of distribution using the Shapiro-Wilkes Test.
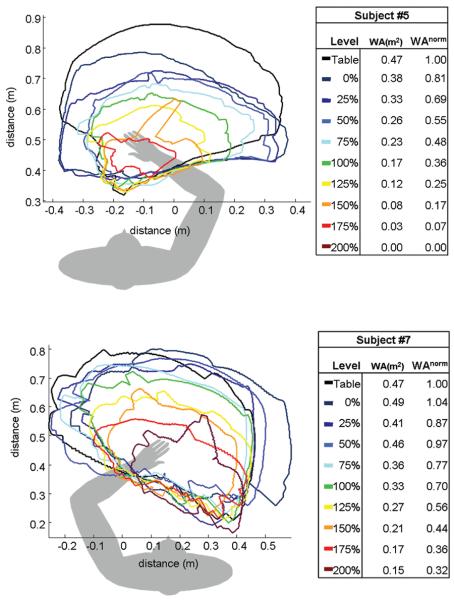
Representative data from 2 of the moderately impaired participants (participants 5 and 7) is illustrated in Figure 3. The 2 exemplars were chosen because they represented the middle of the study sample and, most important, scored the same on the arm Chedoke-McMaster Stroke Assessment and similarly on the arm portion of the Fugl-Meyer Motor Assessment. Despite similar scores on the clinical assessments, it can be seen from the hand path tracings that work area was impacted by abductor activation differently in the 2 participants, illustrating that additional information may be gained with the quantitative kinematic assessment. For example, work area was affected to a greater degree by abductor activation in participant 5 until no active reaching occurred at the 200% level.
Figure 3.
Hand path tracings for each of 9 different required limb support levels for participants 5 (top) and 7 (bottom). Work area (WA) and normalized work area (WAnorm) have been calculated and are reported for each level.
A single-factor ANOVA was used to test for the effect of limb support level (n = 9) on normalized work area for session 1. If a significant effect was found, post hoc testing was done using Scheffe's test. An effect or difference was considered significant if the P value was less than .05. A repeated-measures ANOVA was used to test the effect of session (repeat) at all limb support levels on normalized work area. Interaction effect of repeat and level was also tested. An effect was considered significant if the P value was less than .05.
A Spearman rank correlation coefficient was used to determine the relationship between normalized work area at each of the 9 support levels (0%-200%) from session 1 and each of the following clinical assessments: the Fugl-Meyer Motor Assessment score for the shoulder/ elbow subcomponent (FMAs) and total arm score (FMAt), the Chedoke-McMaster Stroke Assessment score for the arm (CMSa) and hand (CMSh), the Reaching Performance Scale score for the close target (RPSc) and far target (RPSf), the 9 domains of the Stroke Impact Scale (SIS1-9), and the Modified Ashworth Scale score for the elbow flexors (MASf) and extensors (MASe). A coefficient was considered significant if its P value was less than .05.
RESULTS
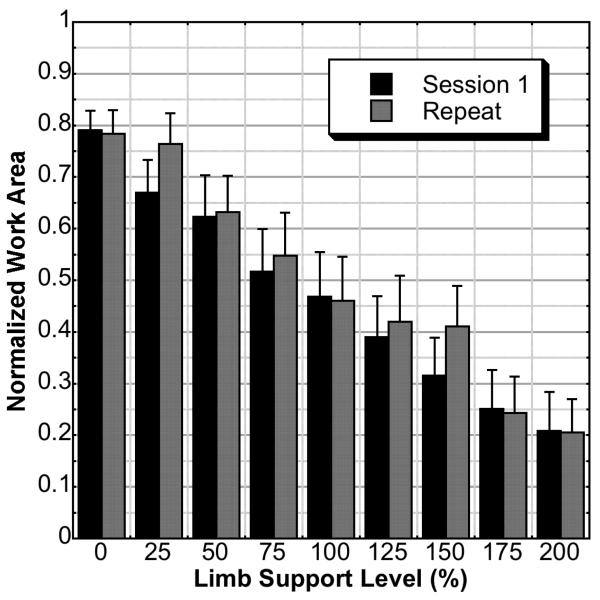
The single-factor ANOVA for session 1 indicated a significant effect of support level (P ≤ .0001). Post hoc testing indicated that there was a significant difference between levels separated by 2 intervals. For example, the 0% active support level was significantly greater than the 75% active support level, and so forth. The repeated-measures ANOVA indicated that there was no effect of repeat on normalized work area (P = .1202). Additionally, there was no secondary interaction between session and limb support level (P = .6703). Mean normalized work area and standard errors are illustrated in Figure 4.
Figure 4.
Normalized work area including standard error bars for each of 9 different required limb support levels of the first and repeated sessions. There was no effect of session (P = .1202) and no interaction effect between session and level (P = .6703).
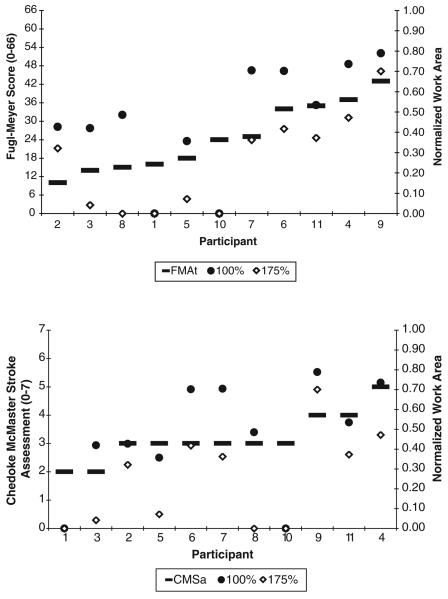
The Spearman rank correlation test indicated a positive and significant relationship between the work area and each clinical assessment with the exception of the CMSh, domains 2-6 and 8-9 on the SIS, and the MAS (see Table 2). The FMAt and CMSa are ordered by value and plotted against the work area achieved at the 100% level of loading and at the 175% level for all participants to illustrate both the correlational trend and the additional information available from the quantitative kinematic measurement (Figure 5). The 100% and 175% levels were specifically highlighted because of their functional relevance in that they reflect reaching at limb weight (100% level) and reaching while transporting an object (175% level). Participants with very similar or identical scores on both the FMAt and CMSa have a variable range of work area measurements. For example, when supporting the full weight of the arm (100% support level), participants scoring a 3 on the CMSa produced work areas between 0% and 70% of passively supported work area. Although FMAt scores in these same participants covered a larger bandwidth than the CMSa, the work area scores continued to provide more descriptive measurement of the effect of abductor activation on reaching range of motion.
Table 2.
Spearman Rank Correlation Coefficients (rho) for Each of the Clinical Assessments That Had Significant P Values
| Level | FMAs | FMAt | CMSa | RPSc | RPSf | SIS1 | SIS7 |
|---|---|---|---|---|---|---|---|
| 0% | 0.32 | 0.36 | 0.44** | 0.32 | 0.29 | 0.14 | 0.13 |
| 25% | 0.71* | 0.74* | 0.76* | 0.66* | 0.63* | 0.62* | 0.62* |
| 50% | 0.60* | 0.62* | 0.56* | 0.68* | 0.64* | 0.31 | 0.51** |
| 75% | 0.74* | 0.76* | 0.74* | 0.57* | 0.52** | 0.56* | 0.67* |
| 100% | 0.68* | 0.69* | 0.72* | 0.64* | 0.59* | 0.37 | 0.61* |
| 125% | 0.59* | 0.59* | 0.64* | 0.77* | 0.71* | 0.36 | 0.46** |
| 150% | 0.69* | 0.69* | 0.68* | 0.79* | 0.72* | 0.44** | 0.66* |
| 175% | 0.76* | 0.76* | 0.74* | 0.80* | 0.72* | 0.47** | 0.71* |
| 200% | 0.50** | 0.49** | 0.38 | 0.79* | 0.75* | 0.17 | 0.54* |
FMAs = shoulder/elbow score of the Fugl-Meyer Motor Assessment; FMAt = total arm score of the Fugl-Meyer Motor Assessment; CMSa = arm score of the Chedoke-McMaster Stroke Assessment; RPSc = Reaching Performance Scale score for the close target; RPSc = Reaching Performance Scale score for the far target; SIS1 = first domain of the Stroke Impact Scale; SIS7 = seventh domain of the Stroke Impact Scale.
P ≤ .05.
P ≤ .10.
Figure 5.
Fugl-Meyer Motor Assessment (top) and ChedokeMcMaster Stroke Assessment score for the arm (bottom) scores are ordered progressively (left y-axis) and superimposed on normalized work area values at the 100% and 175% levels (right y-axis) for each participant. The correlation between clinical scale and quantitative kinematic measurement is evident; however, additional information is gained and can mostly be appreciated in participants with similar clinical scale scores.
DISCUSSION
This study demonstrates that a quantitative kinematic variable representative of reaching impairment (work area) and obtained from a haptic laboratory-based device, the ACT3D, could be repeated with little variability. Our initial kinematic studies showed that abnormal torque coupling dramatically reduced reaching range of motion when an individual is required to lift the limb against gravity and reach outward.7 This impairment was not related to proximal weakness or abnormalities in elbow flexor/extensor imbalances.22 We attributed the impairment to abnormal muscle coactivation of elbow flexors1 and inhibition of elbow extensors4 with activation of abductors and the resultant joint torque coupling of abduction with elbow flexion.2 More recent work with the ACT3D has shown a linear relationship between abduction level and work area specifically identifying limited elbow extension as a primary component of range of motion reductions.8 The current study built upon this work by not only reproducing equivalent findings but also demonstrating the repeatability of this kinematic measurement.
The ACT3D measurement was related to clinical assessments of stroke-induced upper extremity impairments and self-reported activity limitations and produced information that may augment the clinical evaluation. In general, work area was related to the total score and shoulder/elbow subscore of the FMA, the arm score of the CMS, the close and far target scores of the RPS, and domains 1 (perceived strength) and 7 (perceived hand-specific activities of daily living) of the SIS. Others have shown a relationship between kinematic variables of reaching with the FMA9,10,23,24 and CMS.25 Although Kamper and colleagues25 suggested that active range of motion should be targeted in rehabilitation, it was not established how kinematic measurements and limb-loading variables could be applied to augment clinical evaluation. Others have reported that clinically related measures of joint individuation were more closely related to kinematic variables of reaching in the chronic stroke population,26 whereas composite upper extremity strength scores were more related to kinematic variables of reaching in the acute stroke population.27 Subsequently, it was suggested that rehabilitation should be directed toward these impairments in the respective populations, but again it was not indicated how the discussed kinematic analyses could assist a clinician in evaluating reaching in patients with stroke. In addition to positive correlations with clinical assessments, the quantitative measurement of work area at various abduction levels using the ACT3D proved to offer additional information that can be directly applied in the evaluation of reaching impairments in the clinical setting. For example, Figure 5 illustrates that several individuals, although scoring in a narrow bandwidth of FMA or CMS scores, had variable work areas at the 100% and 175% levels. This exemplifies a critical limitation of qualitative clinical assessments that are based on ordinal or nominal scales of measurement. A score of 3 on the CMSa indicates that individuals can fully extend their elbow while reaching downward to the knee (minimal abductor activation). A score of 4 indicates that individuals can fully extend their elbow while reaching outward to an elevation angle of 90 degrees. From previous work,8 it has been demonstrated that elbow extension is impacted progressively by abductor activation. Constrained by a nominal scale of measurement, the CMSa is unable to fully describe the reaching impairment that exists within and between scores of 3 and 4. This is also the reason why correlations become the strongest at higher levels of limb support (Table 2). Because the ordinal- and nominal-based scales do not have the resolution to detect small increments of impairment, the correlations become larger when the work area is more profoundly impacted at higher abductor activation levels. Although the work area measurement offers greater resolution, the 100% and 175% levels may be especially useful in the clinical setting, because values obtained can quantitatively define movement impairment at different functional thresholds. The 100% active support level represents the reaching range of motion available during a functional movement such as reaching to retrieve an object, whereas the 175% active support level represents reaching with additional loading to the arm such as when transporting an object toward or away from the body. Work area as a function of limb loading therefore provides a quantitative kinematic measurement with functionally relevant mechanical thresholds that can be utilized on a single-participant basis such as in the example of participants 5 and 7. Despite similar qualitative clinical scores of these participants, work area measurement elucidates the detrimental impact of abductor activation. The rate of work area reduction is greater in participant 5 with work areas reducing to 0 m2 at the 200% level, whereas participant 7 has 32% of remaining work area at the same level. Furthermore, it can be observed in the illustration that work area is reduced primarily by elbow extension in participant 7 and more equally by shoulder flexion and elbow extension in participant 5. A clinician may utilize work area values in the same way to quantitatively identify the impact of functional thresholds of abductor activation on reaching work area while also qualitatively interpreting the hand path tracings to identify primary directions or joint contributions to overall work area reductions.
In addition to augmenting clinical evaluation, data obtained from kinematic analyses such as work area combined with limb loading can be readily applied in the development, initiation, progression, and evaluation of a therapeutic intervention. Quantitative kinematic and kinetic variables such as temporal coordination,10 joint individuation,26 composite strength,27 and work area8 directly measure impairment and produce descriptive values that identify areas that should be targeted with rehabilitation. For example, kinematic variables distilled from the work area measurement can be used to determine starting levels of limb loading during therapeutic reaching practice. The active support level at which full passive reaching range of motion into the ipsilateral workspace is reduced by 50% can serve as a starting point for active support level required during forward reaching practice. In this application, kinematic variables may better guide the initiation and progression of an impairment-based intervention than the qualitative ordinal or nominal values obtained from existing clinical assessments.
Not surprisingly, we found that some clinical assessments (CMSh, MASf, MASe, and SIS 2-6, 8-9) were unrelated to the work area measurement. As expected, work area was not correlated with the hand score from the CMS and was likely due to the immobilization of the wrist/hand during work area measurement removing it as a potential contributor to work area. Similarly, work area was not correlated with the MAS scores for either the flexors or extensors. Kinematic variables have been shown to be related to spasticity scores previously in reaching tasks where the elbow joint angular velocity was near 50 degrees/sec9 and 100 degrees/sec10 (data taken from figures) and in reaching tasks where the reach was performed as fast as possible.24 In fact, in the current study, the effect of spasticity was minimized by limiting movement speed to <5 degrees/sec, explaining lack of correlation while also demonstrating that hyperactive stretch reflex of the elbow flexors and abnormal elbow flexor coactivation with shoulder abductors are independent impairments that may occur at various severities following stroke. Others have reported no relationship between spasticity and kinematic variables during a “fast as possible” reaching task but attributed the lack of correlation to a small sample size and large homogeneity in clinical scores.23 The Stroke Impact Scale also had several domains that were unrelated to work area. We suggest that these domains of the SIS were unrelated to work area because they covered domains dissociated from the upper extremity such as memory/thinking (domain 2), mood/ emotions (domain 3), communication/understanding (domain 4), mobility/gait (domain 6), and social activities (domain 8). Although we expected the lack of relationship between these domains and work area, it was important to include them in the analysis to thoroughly test the validity of the work area measurement. Overall, work area was found to be related to all upper extremity–associated clinical assessments of movement both of the impairment-type and activity limitation-type.
IMPLICATIONS
As complex laboratory-based methods of kinematic and kinetic measurements evolve and provide new knowledge regarding stroke-induced impairments, efforts to demonstrate immediate clinical applications of basic science methods will facilitate the advancement of stroke rehabilitation principles. The first steps can be achieved by determining the relationship between quantitative measurements and existing clinical assessments, such as in this study. However, these findings may not generalize to all individuals with stroke. All individuals in this study sustained cortical/subcortical lesions involving the sensory motor cortex. We did not include individuals with cerebellar or brainstem lesions who may not present with abductor-induced reductions in work area. Furthermore, the spread of impairment severity in the participants in this study was slightly limited, with mildly impaired individuals being underrepresented. Results from our recent work8 that utilized some individuals with mild impairment found the same linear reduction in work area as a function of abductor activation, so presumably, the same relationship with clinical assessments would be expected. Finally, suggestions for immediate application of technology such as that presented in this study including its clinical relevance and benefits should be addressed. This is a difficult task because the average clinical setting does not have laboratory-caliber measurement equipment or budgetary allocations for high-priced diagnostic equipment for rehabilitation clinicians. However, this work provides evidence justifying the investment by health care institutions in commercially available kinematic and kinetic measurement equipment. Basic scientists will need to continue working toward the development of commercially viable products that can be used to implement their “clinically applicable” procedures so that basic science principles can be fully translated to clinical practice in neurorehabilitation.
ACKNOWLEDGMENTS
This work was supported in part by the National Institute of Disability and Rehabilitation Research (H133G030143; J. Dewald), National Institutes of Health (2R01HD039343-06A1 & 2R42HD049923-02; J. Dewald/ M. Ellis), American Heart Association Greater Midwest Affiliate Postdoctoral Fellowship (0520110Z; M. Ellis), and National Science Foundation Graduate Research Fellowship (T. Sukal). The authors would like to thank Dr Ana Maria Acosta, PhD, for her constructive critique of this manuscript.
REFERENCES
- 1.Dewald JP, Pope PS, Given JD, et al. Abnormal muscle coactivation patterns during isometric torque generation at the elbow and shoulder in hemiparetic subjects. Brain. 1995;118(Pt 2):495–510. doi: 10.1093/brain/118.2.495. [DOI] [PubMed] [Google Scholar]
- 2.Dewald JP, Beer RF. Abnormal joint torque patterns in the paretic upper limb of subjects with hemiparesis. Muscle Nerve. 2001;24:273–283. doi: 10.1002/1097-4598(200102)24:2<273::aid-mus130>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 3.Beer RF, Given JD, Dewald JP. Task-dependent weakness at the elbow in patients with hemiparesis. Arch Phys Med Rehabil. 1999;80:766–772. doi: 10.1016/s0003-9993(99)90225-3. [DOI] [PubMed] [Google Scholar]
- 4.Ellis MD, Acosta AM, Yao J, et al. Position-dependent torque coupling and associated muscle activation in the hemiparetic upper extremity. Exp Brain Res. 2007;176:594–602. doi: 10.1007/s00221-006-0637-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brunnstrom S. Motor testing procedures in hemiplegia: based on sequential recovery stages. Phys Ther. 1966;46:357–375. doi: 10.1093/ptj/46.4.357. [DOI] [PubMed] [Google Scholar]
- 6.Twitchell TE. The restoration of motor function following hemiplegia in man. Brain. 1951;74:443–480. doi: 10.1093/brain/74.4.443. [DOI] [PubMed] [Google Scholar]
- 7.Beer RF, Dewald JP, Dawson ML, et al. Target-dependent differences between free and constrained arm movements in chronic hemiparesis. Exp Brain Res. 2004;156:458–470. doi: 10.1007/s00221-003-1807-8. [DOI] [PubMed] [Google Scholar]
- 8.Sukal TM, Ellis MD, Dewald JP. Shoulder abduction-induced reductions in reaching work area following hemiparetic stroke: neuroscientific implications. Exp Brain Res. 2007;183:215–223. doi: 10.1007/s00221-007-1029-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levin MF. Interjoint coordination during pointing movements is disrupted in spastic hemiparesis. Brain. 1996;119(Pt 1):281–293. doi: 10.1093/brain/119.1.281. [DOI] [PubMed] [Google Scholar]
- 10.Cirstea MC, Mitnitski AB, Feldman AG, et al. Interjoint coordination dynamics during reaching in stroke. Exp Brain Res. 2003;151:289–300. doi: 10.1007/s00221-003-1438-0. [DOI] [PubMed] [Google Scholar]
- 11.Portney LG, Watkins MP. Foundations of Clinical Research: Applications to Practice. Prentice Hall; Upper Saddle River, NJ: 2000. [Google Scholar]
- 12.Beer RF, Dewald JP, Rymer WZ. Deficits in the coordination of multijoint arm movements in patients with hemiparesis: evidence for disturbed control of limb dynamics. Exp Brain Res. 2000;131:305–319. doi: 10.1007/s002219900275. [DOI] [PubMed] [Google Scholar]
- 13.Haaland KY, Prestopnik JL, Knight RT, et al. Hemispheric asymmetries for kinematic and positional aspects of reaching. Brain. 2004;127:1145–1158. doi: 10.1093/brain/awh133. [DOI] [PubMed] [Google Scholar]
- 14.Lang CE, Beebe J. Relating movement control at 9 upper extremity segments to loss of hand function in people with chronic hemiparesis. Neurorehabil Neural Repair. 2007;21:279–291. doi: 10.1177/1545968306296964. [DOI] [PubMed] [Google Scholar]
- 15.Fugl-Meyer AR, Jaasko L, Leyman I, et al. The post-stroke hemiplegic patient. 1. a method for evaluation of physical performance. Scand J Rehabil Med. 1975;7:13–31. [PubMed] [Google Scholar]
- 16.Gowland C, Stratford P, Ward M, et al. Measuring physical impairment and disability with the Chedoke-McMaster Stroke Assessment. Stroke. 1993;24:58–63. doi: 10.1161/01.str.24.1.58. [DOI] [PubMed] [Google Scholar]
- 17.Levin MF, Desrosiers J, Beauchemin D, et al. Development and validation of a scale for rating motor compensations used for reaching in patients with hemiparesis: The Reaching Performance Scale. Phys Ther. 2004;84:8–22. [PubMed] [Google Scholar]
- 18.Bohannon RW, Smith MB. Interrater reliability of a modified Ashworth scale of muscle spasticity. Phys Ther. 1987;67:206–207. doi: 10.1093/ptj/67.2.206. [DOI] [PubMed] [Google Scholar]
- 19.Duncan PW, Wallace D, Lai SM, et al. The stroke impact scale version 2.0. Evaluation of reliability, validity, and sensitivity to change. Stroke. 1999;30:2131–2140. doi: 10.1161/01.str.30.10.2131. [DOI] [PubMed] [Google Scholar]
- 20.O'Sullivan SB, Schmitz TJ. Physical Rehabilitation: Assessment and Treatment. F. A. Davis; Philadelphia: 2001. [Google Scholar]
- 21.Michaelsen SM, Luta A, Roby-Brami A, et al. Effect of trunk restraint on the recovery of reaching movements in hemiparetic patients. Stroke. 2001;32:1875–1883. doi: 10.1161/01.str.32.8.1875. [DOI] [PubMed] [Google Scholar]
- 22.Beer RF, Ellis MD, Holubar BG, et al. Impact of gravity loading on post-stroke reaching and its relationship to weakness. Muscle Nerve. 2007;36:242–250. doi: 10.1002/mus.20817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Archambault P, Pigeon P, Feldman AG, et al. Recruitment and sequencing of different degrees of freedom during pointing movements involving the trunk in healthy and hemiparetic subjects. Exp Brain Res. 1999;126:55–67. doi: 10.1007/s002210050716. [DOI] [PubMed] [Google Scholar]
- 24.Cirstea MC, Levin MF. Compensatory strategies for reaching in stroke. Brain. 2000;123(Pt 5):940–953. doi: 10.1093/brain/123.5.940. [DOI] [PubMed] [Google Scholar]
- 25.Kamper DG, McKenna-Cole AN, Kahn LE, et al. Alterations in reaching after stroke and their relation to movement direction and impairment severity. Arch Phys Med Rehabil. 2002;83:702–707. doi: 10.1053/apmr.2002.32446. [DOI] [PubMed] [Google Scholar]
- 26.Zackowski KM, Dromerick AW, Sahrmann SA, et al. How do strength, sensation, spasticity and joint individuation relate to the reaching deficits of people with chronic hemiparesis? Brain. 2004;127:1035–1046. doi: 10.1093/brain/awh116. [DOI] [PubMed] [Google Scholar]
- 27.Wagner JM, Lang CE, Sahrmann SA, et al. Relationships between sensorimotor impairments and reaching deficits in acute hemiparesis. Neurorehabil Neural Repair. 2006;20:406–416. doi: 10.1177/1545968306286957. [DOI] [PubMed] [Google Scholar]