Abstract
Objectives
We examined the effects of early life exposure to poor nutrition and infectious diseases on adult heart disease and diabetes using season of birth as an indicator to help disentangle the effects on health of early life exposure from effects associated with other childhood conditions.
Methods
Using data from 60- to 74-year-old Puerto Ricans who lived in rural areas during childhood (n = 1,457), we estimated the effects of seasonal exposure during late gestation on the probability of diabetes and heart disease, controlling for adult obesity and other childhood conditions (health, socioeconomic status, knee height).
Results
We found (a) strong associations between exposure and heart disease; (b) weaker associations between exposure and diabetes, although significant negative interaction effects between exposure and having a family member with diabetes suggest the possibility of either strong gene–environment or early adult–environment interactions; (c) virtually no attenuation of effects of self-reported childhood health with controls for exposure.
Discussion
Timing of birth may reveal conditions experienced perinatally that affect adult heart disease and diabetes. The results suggest that examination of the effects of season of birth on these chronic conditions among older Puerto Rican adults and among older adults from similar populations deserves deeper scrutiny.
Keywords: Early life exposure, Heart disease, Diabetes, Older adult health, Puerto Rico
HEART disease and diabetes are two major chronic conditions facing countries throughout the world. Obesity, a risk factor for both conditions, is also on the rise (Murray & Lopez, 1996; World Health Organization, 2000). Although lifestyle, and in some cases genetics, may play a role in the prevalence of such diseases, the hypothesis that nutrition in utero (especially late gestation) and early infancy influences adult chronic diseases such as diabetes and heart disease has also been proposed as an important determinant of older adult health (Barker, 1998). Yet although research continues to accumulate supporting this hypothesis (Gluckman & Hanson, 2006), there has been relatively little empirical research in the Latin American and Caribbean region examining the topic.
The aim of our article is to build on our previous research (Palloni, McEniry, Dávila, & García Gurucharri, 2005) and to examine the degree to which the Barker hypothesis has merit in helping to understand the determinants of heart disease and diabetes among older adults in a Latin American and Caribbean context. In particular, we examine the degree to which seasonality of birth, a potential useful indicator of early life conditions (Doblhammer, 2004), is predictive of adult heart disease and diabetes in Puerto Rico.
There are two important points worthy of mention. First, by examining prenatal and early infant exposure of older adults in Puerto Rico, we contribute to the literature on early conditions (Barker, 1998; Gluckman & Hanson, 2006) and also the literature on the seasonal effects of nutrition on health (Costa, 2005; Doblhammer, 2004; Gavrilov & Gavrilova, 2005) in the Latin American and Caribbean region. The studies on seasonal nutrition provide evidence in support of the Barker hypothesis and are thus very much related to the purpose of our article. Our focus on heart disease and diabetes contributes to this literature, which has focused largely on mortality, with the exception of the work of Doblhammer.
Puerto Rico presents an intriguing case study. During the 1920s and 1930s and early 1940s, a large proportion of Puerto Ricans were unemployed or underemployed during extended periods of the year, and many of them lived in rural areas where conditions were precarious (Clark, 1930). Most families living in rural areas were poor, were landless, did not own their own homes, and were scattered and isolated, with no strong social organization. Because most rural families did not have a garden plot nor did they own livestock, wages were the most important means to purchase food and have a proper diet. Agricultural employment was highly cyclical and thoroughly dominated by the sugar cane industry. In 1929, about 52% of the total acres planted for the international market for sugar cane, tobacco, and coffee (main cash crops in Puerto Rico) were devoted to production of sugar cane, as compared with 36% devoted to coffee and 12% to tobacco. However, sugar cane produced almost 99% of the total tons in production of these three crops (U.S. Census Bureau, 1932). Because of the dominance of the sugar cane industry, employment was highest during the first 6 months of the year and lowest during the later part of the year. The cutting or harvest season for sugar cane was intense and occurred in the first half of the year (January–June). This period was followed by a slack season lasting most of the second half of the year. There were limited opportunities for alternative sources of employment that could have supplemented income. In addition to these conditions, the hurricane season peaked between August and October, during the economically leaner part of the year, bringing with it a long period of hot and humid weather. This augmented exposure to infectious diseases such as dysentery, diarrhea, malaria, and dengue fever (Rigau Pérez, 2000). Thus, the effects of poor nutrition due to reduced purchasing power were complicated by increased exposure to infectious diseases.
The second important note is the use of season of birth as an indicator of early life conditions (early growth and development) and as a way to disentangle the effects of substandard nutrition in utero or shortly after birth from the effects of associated childhood conditions (such as poverty) that could influence adult health status. The measurement of early childhood conditions in population surveys of older adults relies on retrospective questions regarding childhood health or socioeconomic status (SES) and on anthropometric measures (birth weight, height, knee height, leg length). Birth weight is an indicator of early growth (Barker, 1998), although researchers have criticized it as being contaminated by the influence of other life course factors (Huxley, Neil, & Collins, 2002; Joseph & Kramer, 1996), and it is only rarely available in population surveys of older adults. Height is a marker of cumulated nutritional status throughout childhood and adolescence, whereas leg length and knee height are sensitive markers of nutritional status during early childhood (Davey Smith et al., 2001; Gunnell, Davey Smith, Holly, & Frankel, 1998; Leitch, 1951; Wadsworth, Hardy, Paul, Marshall, & Cole, 2002). However, leg length and knee height are seldom included in population surveys, and it is difficult to use them to distinguish different time periods of growth and development (i.e., in utero, very early infancy, early childhood). Self-rating of childhood health by adult respondents has been associated with indicators of early growth (Haas, 2007), but it is not yet clear which dimensions of health are being captured or the extent to which adult health status self-perception influences self-rating of childhood health. Thus, measures of early childhood conditions used in population surveys are, for the most part, either weak markers of exposure to poor nutrition in utero and/or early infancy, or their effects are confounded with those of other life course factors and individual socioeconomic background.
Even if one had suitable measures of early growth and development, the validity of inferences would be threatened to the extent that background conditions that influence early growth and development also have independent effects on later health and mortality. Thus, failing to control properly for such background conditions will inflate the association between a valid indicator of early growth and adult health and mortality.
A way out of this quandary is to utilize an indicator of early growth and development that is unrelated to background conditions and that more clearly relates to in utero or very early infancy conditions. In theory, season of birth is such an indicator. There is agreement that it is largely independent of social class of origin (Doblhammer, 2004). Marked seasonality in the supply of food can lead to a similar seasonal pattern in maternal nutrition. In decades past, food supply (quantity, variety, freshness) varied sharply according to season. Differences in access to high-quality food could potentially influence intrauterine growth depending on the month of gestation. Being born during or right after a harvest, when nutritional supplies are more plentiful, is associated with longer life (Bengtsson & Lindstrom, 2003; Costa, 2005; Doblhammer, 2004; Gavrilov & Gavrilova, 2005). The period of late gestation (third trimester of pregnancy) is particularly important in terms of later adult heart disease (Barker, 1998; Gardiner, 2007) and diabetes (Ravelli et al., 1998). Under these circumstances, season of birth may be a good indicator of maternal nutritional status and of the extent to which the unborn child is exposed to higher risk of poor nutrition in utero.
In addition to nutritional status, season of birth may also capture increased risks of infectious and parasitic diseases affecting mother and fetus alike. These risks may work together with the effects of nutritional deficiencies. Climate conditions may become favorable to the reproduction of vectors, thus augmenting exposure to infectious and parasitic diseases. To the extent that maternal nutritional status is hampered by contraction of infectious or parasitic diseases, normal intrauterine growth will be impaired. By the same token, as increased exposure translates into increased incidence of infectious and parasitic diseases, we expect deterioration in the quantity and quality of nutrition received by babies from breastfeeding, the main source of nutrition in early life (John, Menken, & Chowdhury, 1987). In fact, being born at the beginning of seasons with more moderate temperatures is beneficial at least with regard to risk of infectious diseases in early infancy (Doblhammer, 2004; Kevan, 1979).
Suppose that the Barker hypothesis that nutrition in utero and early infancy influences adult chronic diseases, particularly diabetes and heart disease, has merit. Suppose also that season of birth is a good proxy for supply of nutrients and for conditions that alter prospects for “normal” intrauterine and infant growth patterns and is indeed independent of background factors such as SES. Further suppose that we have a good measure of childhood health conditions. Finally, assume that the empirical evidence discussed above regarding weather and seasonality of employment accurately reflect the past history of Puerto Rico. We then seek to identify two regularities. First, it follows that the probability of adult chronic conditions such as diabetes and heart disease should be higher among those who lived in the countryside as a child and who were born during lean seasons (prior to the beginning of the harvest) and lower for those born during seasons of abundance (close to the end of the harvest season). In particular, the probability of diabetes and heart disease should be highest among those born toward the end of the lean season when exposure to poor conditions completely overlapped with late gestation. It should be lowest among those born toward the close of the harvest season. The second regularity is as follows. We know that early childhood health can have important consequences on adult health (Davey Smith & Lynch, 2004; Elo & Preston, 1992; Kuh & Ben-Shlomo, 2004; Lundberg, 1991; O'Rand & Hamil-Luker, 2005). Thus, consider the case that measures of early childhood health status (such as self-reported child health status and knee height) do in fact mediate the effects of early nutrition and growth. Then, if season of birth is a good proxy for early nutritional status and if the latter is indeed an influential factor for adult health conditions, we expect that the effects of childhood health should be attenuated when we control for season of birth.
Figure 1 illustrates a weighted employment index across the year for the sugar, tobacco, and coffee industries in Puerto Rico from the 1920s (Clark, 1930). The strong cyclical nature of these employment numbers reflects the seasonality of the predominant sugar cane industry, in which periods of higher employment occurred during the sugar cane harvest (January–June) and were followed by periods of below average employment (July–December). Superimposed on the figure are our conjectures regarding the timing of exposure to poor nutrition and infectious diseases during late gestation and the association with heart disease and diabetes if the Barker hypothesis has merit.
Figure 1.
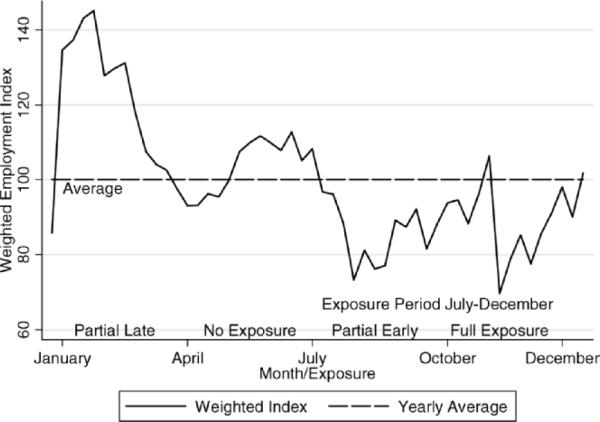
Seasonal variation in plantation employment and hypothesized exposure during late gestation. Data are for sugar, tobacco, and coffee plantations, 1924–1926. Source: Clark (1930).
METHODS
Data
The Puerto Rican Elderly: Health Conditions (PREHCO; 2007) project was designed to gather quality baseline data on issues related to the health of older adult Puerto Ricans. It is a cross-sectional survey of the noninstitutionalized population aged 60 and older (targets) and their surviving spouses. The sample is a multistage, stratified sample of older adults residing in Puerto Rico, with oversamples of regions heavily populated by people of African descent and of individuals older than 80. We used the 2000 U.S. Census of Population and Housing as the sampling frame for the study.
The data were gathered through face-to-face interviews with targets (including those with cognitive limitations who required the presence of a proxy to provide information) and with their surviving spouses, regardless of age. A total of 4,291 interviews with targets were conducted between May 2002 and May 2003, and second-wave data were collected during 2006–2007. Only 4.7% of respondents refused to participate, and 1.4% were not at home or could not be located; thus, we obtained a response rate of 93.9%.
The data collected offer a substantial amount of information within the limits permitted by face-to-face interviews in a cross-section. The questionnaire included extensive modules on a variety of topics including demographic characteristics, health status and conditions, cognitive and functional performance, anthropometric measurements, and physical performance. Despite the very high response rate, we conducted a follow-up telephone survey of nonrespondents comprising selected demographic, SES, and health questions but found that nonrespondents were not significantly different from respondents along these selected variables.
Measures
Prenatal exposure to poor nutrition
We defined seasonal exposure to poor nutrition and infectious diseases based on birth quarter and the months of the slack or lean season (July–December) in the Puerto Rican sugar cane industry (Clark, 1930; Gayer, Homan, & James, 1938). Mid to late gestation and early infancy may all be periods sensitive to poor nutrition. However, we began with the supposition that late gestation is most relevant (Barker, 1998; Gardiner, 2007). We thus used a more detailed definition of exposure and identified different levels of exposure according to the degree of overlap between the third trimester of gestation (calculated from month of birth) and the months of the slack season defined above. We defined this indicator of exposure to poor nutrition and infectious diseases, level of exposure, as follows: Full exposure (fourth quarter of birth) means that the third trimester fell completely within the slack period, partial exposure means that the third trimester of gestation fell partially within the window defined by the slack months either early (third quarter) or late (first quarter), and no exposure during the third trimester was reserved for those whose third trimester of gestation fell completely outside the window of slack months. We created binary variables to represent levels of exposure, with the reference group being the no exposure group. We also used a broader definition of exposure to poor nutrition and infectious diseases in model estimation called exposure period, which identified whether the respondent had been born during the lean season after the sugar cane harvest (July–December).
Childhood conditions
To assess childhood conditions, we used retrospective questions on childhood health and childhood SES and anthropometric measurement of knee height. Respondents were asked to rate their childhood health using a 5-point scale (“Would you say that your health as a child was excellent, very good, good, fair or poor?”). Although it is true that cultural factors can affect self-reported child health, it is also true that this type of self-report has good reliability and is associated with indicators of poor intrauterine development (Haas, 2007). Respondents were also asked if they had experienced rheumatic fever as a child (yes/no), and we used these responses to create a dichotomous variable because rheumatic fever in childhood is an important risk factor for adult heart disease (Elo & Preston, 1992). Early childhood SES is an important factor affecting later adult health (Hertzman, 1994; Lundberg, 1991; Wadsworth & Kuh, 1997; Wickrama, Conger, & Abraham, 2005). PREHCO respondents were asked to rate their childhood SES based on a 3-point scale (“In general, would you say that the economic conditions in the home in which you were raised were good, fair or poor?”). Previous surveys in the Latin American and Caribbean region (Palloni & Pelaez, 2002) have used this type of question. It has face validity, and preliminary results from the second wave of PREHCO suggest that it is consistent across the PREHCO waves.
There is no consensus in the literature regarding the definition and use of these variables. However, in our study we were most interested in testing hypotheses concerning the effects of poor child health and child SES, and, thus, after examining the distribution of responses, we created a dichotomous variable for poor childhood health where 1 indicated that the respondent rated his or her health during childhood as poor or fair and 0 indicated good. Similarly, a dichotomous variable for poor SES during childhood was defined as 1 if a respondent defined his or her childhood SES as poor and 0 if good or fair.
Knee height was measured in the home of the respondents. We used gender-specific quartiles of knee height as a proxy for early stunting (Eveleth & Tanner, 1976) and a reflection of leg length, which is thought to be particularly sensitive to nutritional status during childhood (Leitch, 1951). In the anthropometric literature, height is often measured using either quartiles or quintiles (Gunnell et al., 1998), and thus we used quartiles of knee height and defined gender-specific dichotomous variables where 1 indicated the lowest quartile of knee height and 0 indicated all other quartiles.
Adult health outcomes
Respondents were asked if a doctor had ever diagnosed them with any of several chronic conditions. We created dichotomous variables for diabetes and heart disease (1 = have condition, 0 = do not have condition). Self-reported health is widely used in population surveys and identifies underlying conditions quite well (Banks, Marmot, Oldfield, & Smith, 2006). However, self-reported chronic conditions are underreported because their identification depends on the degree of access to appropriate health services (Goldman, Lin, Weinstein, & Lin, 2003). Yet, at least for diabetes, differences in prevalence measured with self-reports and with biomarkers produce only slightly different values (Banks et al., 2006; Riosmena & Wong, 2008).
We created a variable to indicate if a family member (parents or siblings) had diabetes because this is an important risk factor for diabetes that can reveal either a genetic component or shared environmental lifestyles (American Diabetes Association, n.d.). By estimating separate models for those with and without a family member with diabetes, we produced conservative estimates of the impact of environmental factors. We also used the indicator to test interaction effects between having a family member with diabetes and indicators of exposure to poor nutrition and infectious diseases.
We calculated body mass index (BMI) as (measured) weight in kilograms divided by (measured) height in meters squared and then defined adult obesity as 1 if BMI was greater than or equal to 30 and 0 otherwise.
Main controls
Gender was a dichotomous variable (female = 1, male = 0). We represented age groups by two binary variables for 65 to 69 and 70 to 74, with the reference group being 60 to 64. We assessed education as the number of years of education completed. We used two criteria to identify respondents who had lived in the countryside as a child: (a) respondent indicated that he or she was born in Puerto Rico and (b) respondent answered affirmatively to a survey question about whether he or she had lived in the countryside as a child before the age of 18.
Models and Estimation
Imputation
We used multiple imputation procedures (Raghunathan, Reiter, & Rubin, 2003; Rubin, 1987; Van Buren, Boshuizen, & Knook, 1999) using IVEware (Raghunathan, Solenberger, & Van Hoewyk, 2007) to ensure that all cases were included. The results obtained with multiple imputation are statistically efficient and avoid systematic biases likely to arise when one deletes cases with missing observations. The validity of our results relies on a weak assumption about missingness (Rubin, 1987). The number of missing responses among the subsample of those born in Puerto Rico who lived in the countryside as a child was small (<0.01 in most study variables; knee height and obesity had about 3% missing). However, we were primarily interested in imputing items about living in the countryside as a child, for which about 14% of the cases were missing primarily because proxies were not asked this question.
Subsample for estimation
We selected a subsample of older adults born in Puerto Rico who responded affirmatively to a survey question that asked them if they had lived for a prolonged period of time in the countryside prior to the age of 18 (n = 1,457). We only considered respondents aged 60 to 74 to generate estimates for the subpopulation that was most at risk of having been affected by harsh early childhood experiences and, simultaneously, had larger probabilities of surviving due to their exposure to the massive deployment of medical technologies and public health measures during the period after 1930. Thus, this cohort may be able to provide some insights into whether early childhood experiences are indeed important in later life because it was less affected by mortality-driven selection than the group of cohorts who preceded it (those aged 75 and older).
Preparation for model estimation
Before model estimation, we first examined associations between all predictor and independent variables either through chi-square, Pearson correlations, or analysis of variance. We used a Tukey-type multiple comparison test for proportions to examine differences between prevalence rates of heart disease and diabetes and quarter of birth (Zar, 1999). We examined potential confounding and mediating variables that could help explain associations between exposure to poor nutrition and infectious diseases (season of birth) and heart disease or diabetes. Potential confounding variables in our study that are risk factors for heart disease and diabetes are age, gender, SES (related to lifestyle risk factors), and BMI. Rheumatic fever is also an important potential confounding variable for heart disease (Elo & Preston, 1992; Kuh & Ben-Shlomo, 2004). Because early childhood health has an effect on adult health (Davey Smith & Lynch, 2004; Elo & Preston, 1992; Kuh & Ben-Shlomo, 2004; Lundberg, 1991; O'Rand & Hamil-Luker, 2005), childhood health and knee height may be mediating variables that explain the association between level of exposure to poor nutrition and infectious diseases and heart disease.
We next examined the validity of birth quarter as an indicator of poor nutritional status by testing the statistical significance of the association between prevalence of heart disease and diabetes according to birth quarter and place of residence during childhood. In the case of diabetes, we also compared the prevalence of the disease for those respondents with and without family members with diabetes. Finally, we examined the convergent validity of self-reported childhood health by testing the statistical association between self-reported child health and a series of childhood illnesses and periods of health problems as a child that was asked of respondents in the survey. We examined the convergent validity of childhood SES by examining the association between this question and fathers' education level and occupational status, also obtained in the survey. We tested these associations using both the complete scale (child health, five items; child SES, three items) and the dichotomous variables created for childhood health and SES.
Estimation
We estimated a series of logistic regression models for heart disease and then both with and without the variable denoting family members with diabetes. The purpose of the models was to better assess the total and net effects of exposure to poor nutrition and infectious diseases (season of birth), childhood health, childhood SES, rheumatic fever (in the case of heart disease), and low knee height. In particular, we were interested in examining the degree to which the effects of childhood conditions such health and knee height are attenuated by level of exposure. The baseline models estimated the effects of the more detailed definition of exposure to poor nutrition and infectious diseases, level of exposure, on health including controls for only age and gender. We then estimated models with childhood health and knee height controlling for age and gender. We then expanded these to ultimately include education, season of birth, poor childhood health, poor childhood SES, knee height, and adult obesity (the latter included as a control in the models for diabetes and heart disease).
We tested for interactions between childhood conditions (health, SES, important infectious diseases such as malaria and dengue), adult obesity, and family member with diabetes (diabetes models only) by including suitable interaction terms in our final models. We also examined results by estimating models with the broader definition of exposure to poor nutrition and infectious diseases, exposure period (July–December). We finally compared the best model with a similar one estimated using respondents who had not lived in the countryside as a child. We compared imputed and non-imputed results to ensure that there were no important discrepancies in model estimation. To assess the actual magnitude of effects of season of birth, we calculated predicted probabilities of experiencing heart disease or diabetes for an individual with average attributes and then added poor childhood conditions and adult obesity. The typical respondent was 66 years old, had 7 years of education, was not obese, reported that he or she was not poor and did not have poor health as a child, and had not experienced rheumatic fever (in the case of heart disease).
Results
Associations Between Exposure, Heart Disease, and Diabetes
We found no statistically significant associations between exposure to poor nutrition and infectious diseases (season of birth) and potentially confounding (gender, age, education, rheumatic fever, SES, BMI) or mediating (childhood health, knee height) variables (see Table 1). However, we did find significant associations between exposure levels and heart disease, χ2(3) = 11.44, p = .010, and diabetes for those respondents with no family members with diabetes, χ2(3) = 9.48, p = .024. In the case of heart disease, those who experienced full exposure (October–December) during late gestation (third trimester) had a larger prevalence of heart disease than did those with partial exposure and those with no exposure to poor nutrition and infectious diseases during late gestation. A Tukey-type multiple comparison test for proportions (Zar, 1999) suggested that full exposure was significantly different from all other levels of exposure and that the most significant difference appeared with those who experienced no exposure period (p < .001). In the case of diabetes, those respondents with no family members with diabetes who experienced partial early exposure (July–September) had the largest prevalence of diabetes. There were statistical differences between partial early exposure and partial late exposure (p < .05) but no clear pattern of statistical differences between other levels of exposure. Associations among predictor variables did not reveal any anomalies.
Table 1.
Characteristics of the Sample
| Level of Exposure During Late Gestation |
|||||
|---|---|---|---|---|---|
| Characteristic | None | Partial Early | Full | Partial Late | All Cases |
| Season of birth | April–June | July–September | October–December | January–March | |
| Demographic | |||||
| Female (%) | 50 | 47 | 56 | 53 | 51 |
| Age (years) | 66 (4) | 66 (4) | 66 (4) | 66 (4) | 66 (4) |
| Education (years) | 7 (5) | 8 (5) | 7 (5) | 7 (4) | 7 (5) |
| Childhood conditions | |||||
| Health (%) | |||||
| Excellent | 22 | 22 | 24 | 23 | 23 |
| Very good | 14 | 8 | 14 | 16 | 13 |
| Good | 41 | 41 | 34 | 42 | 39 |
| Fair | 19 | 25 | 22 | 16 | 21 |
| Poor | 2 | 5 | 6 | 3 | 4 |
| Rheumatic fever (%) | 2 | 2 | 1 | 2 | 2 |
| SES (%) | |||||
| Good | 14 | 20 | 18 | 21 | 18 |
| Fair | 49 | 40 | 49 | 37 | 44 |
| Poor | 36 | 39 | 33 | 42 | 38 |
| Knee height (cm) | 46 (5) | 47 (5) | 46 (5) | 46 (5) | 46 (5) |
| Adult risk (%) | |||||
| BMI | |||||
| <18.5 | 1 | 1 | 5 | 2 | 2 |
| 18.5 ≤ BMI < 25 | 30 | 32 | 26 | 25 | 29 |
| 25 ≤ BMI < 30 | 41 | 42 | 45 | 42 | 42 |
| ≥30 | 29 | 25 | 24 | 31 | 27 |
| Family member with diabetes | 61 | 59 | 59 | 62 | 60 |
| Adult health outcomes (%) | |||||
| Heart disease | 12 | 16 | 24 | 19 | 18 |
| Diabetes | 28 | 32 | 33 | 32 | 31 |
| Distribution of births (total number) | 394 | 353 | 369 | 341 | 1,457 |
Notes: Standard deviations appear in parentheses. Totals do not always sum to 100 due to rounding. The total number of cases by level of exposure is also the distribution of births by birth quarter for the selected sample. SES = socioeconomic status; BMI = body mass index.
Source: Puerto Rican Elderly: Health Conditions project (PREHCO, 2007), imputed and weighted; all 60- to 74-year-olds born in Puerto Rico and who lived in the countryside as a child.
Validity of Season of Birth, Childhood Health, and SES
The results of checking the validity of season of birth as an indicator of exposure to poor nutrition and infectious diseases showed that there were statistically significant differences between prevalence of heart disease by level of exposure to poor nutrition and infectious diseases for those who lived in the countryside as a child, χ2(3)=11.44, p=.010, as one would expect, no such associations existed among those who did not live in the countryside as a child (see Table 2). In the case of diabetes, significant associations appeared only in the case of those respondents who had no family member with diabetes, χ2(3) = 9.48, p = .024.
Table 2.
Prevalence of Heart Disease and Diabetes According to Seasonal Exposure and Place Lived During Childhood
| Level of Exposure (Birth Quarter) |
||||||
|---|---|---|---|---|---|---|
| Condition | None (2nd) | Partial Early (3rd) | Full (4th) | Partial Late (1st) | χ2(df) | p |
| Heart disease | ||||||
| Countryside | 14 | 19 | 24 | 20 | 11.44 (3) | .010 |
| Not countryside | 14 | 16 | 15 | 13 | 1.72 (3) | .632 |
| Diabetes—No family member with diabetes | ||||||
| Countryside | 17 | 26 | 21 | 13 | 9.48 (3) | .024 |
| Not countryside | 12 | 13 | 14 | 15 | 0.30 (3) | .960 |
| Diabetes—Family member with diabetes | ||||||
| Countryside | 41 | 40 | 42 | 41 | 0.35 (3) | .951 |
| Not countryside | 43 | 40 | 35 | 38 | 2.03 (3) | .565 |
Notes: These results are unweighted and thus show slightly different prevalence rates than those presented in Table 1, whose results use sample weights. Sample sizes: lived as a child in the countryside (n = 1,457), did not live as a child in the countryside (n = 1,147), lived in the countryside as a child and had a family member (parents or siblings) with/without diabetes (n = 869, n = 588), did not live in the countryside as a child and had a family member (parents or siblings) with/without diabetes (n = 626, n = 521).
Source: Puerto Rican Elderly: Health Conditions project (PREHCO, 2007), imputed, unweighted; all 60- to 74-year-olds born in Puerto Rico.
The results of checking the convergent validity of self-reported child health with other measures of childhood diseases showed that self-reported child health (5-item scale) was statistically associated with several serious childhood diseases and with the more prevalent serious diseases in Puerto Rico from the 1920s through the early 1940s such as malaria, χ2(4)=25.87, p < .001; typhoid fever, χ2(4) = 9.52, p ≤ .05; and dengue fever, χ2(4) = 23.73, p ≤ .001 (see Table 3). This was also true when we dichotomized self-reported child health.
Table 3.
Associations Between Self-Reported Child Health and Childhood Illnesses, and Self-Reported Childhood SES and Father Education and Occupation
| Variable | Scalea | Scale (0/1) |
|---|---|---|
| Self-reported childhood healthb | ||
| Periods of deprivations due to healthc | 173.46 (8)*** | 114.55 (2)*** |
| Highly prevalent illnesses in Puerto Rico | (1920s–1930s) | |
| Malaria | 25.87 (4)*** | 11.48 (1)*** |
| Tuberculosisd | 4.78 (4) | 4.63 (1)* |
| Typhoid | 9.52 (4)* | 4.42 (1)* |
| Other serious illnesses | ||
| Asthma | 40.04 (4)*** | 39.84 (1)*** |
| Bronchitis | 24.49 (4)*** | 17.77 (1)*** |
| Dengue fever | 23.73 (4)*** | 17.92 (1)*** |
| Hepatitis | 3.99 (4) | 1.33 (1) |
| Pneumonia | 25.33 (4)*** | 22.15 (1)*** |
| Polio | 1.04 (4) | 0.23 (1) |
| Rheumatic fever | 30.44 (4)*** | 21.78 (1)*** |
| Smallpox | 22.94 (4)*** | 18.37 (1)*** |
| Self-reported childhood SES | ||
| Father educatione | 36.58 (4)*** | 30.84 (2)*** |
| Father occupationf | 17.61 (6)*** | 14.73 (3)*** |
Notes: Data are chi-square (df). SES = socioeconomic status.
Source: Puerto Rican Elderly: Health Conditions project (PREHCO, 2007), imputed and unweighted; all 60- to 74-year-olds born in Puerto Rico and who lived in the countryside as a child.
Scale is 1–5 for self-reported childhood health and 1–3 for self-reported childhood SES.
Respondents were asked if they had ever experienced a list of childhood illnesses (yes/no). We report only the more serious self-reported illnesses. However, there were no associations or marginal associations between self-reported childhood health and the more common childhood illnesses such as mumps, measles, and chickenpox. The source for identification of diseases with very high prevalence rates during the 1920s and 1930s was Clark (1930).
Frequent, some, none.
The prevalence of tuberculosis was very high in urban areas of Puerto Rico. It was also a disease more prevalent among Puerto Rican adults than children (Clark, 1930, p. 62).
None, eighth grade, more than eighth grade.
Respondents were asked the occupation of their father using an open-ended question, and responses were coded into occupational categories (i.e., agricultural, urban service, professional, other) according to standards used by the International Labor Organization (2004).
p < .05
p < .001.
Heart Disease and Diabetes
Table 4 shows nested models for heart disease. The effects of level of exposure to poor nutrition and infectious diseases were only modestly altered when we compared the baseline model including age, gender, and level of exposure (Model 1) to a model that added other early childhood conditions. The best fitting model was Model 4, based on a comparison of the Bayesian information criterion statistic for each model using each individual imputed data set. Model 4 showed that the odds of self-reporting heart disease in Puerto Rico for those born during the worst expected time (fourth quarter, full exposure) were about 1.89 times (95% confidence interval [CI] = 1.30–2.75) that of those born in the hypothesized better time (second quarter, no exposure). Self-reported childhood health status had moderate to strong effects on heart disease (odds ratio [OR] = 1.40, 95% CI = 1.03–1.91). Obesity also contributed to heart disease (OR = 1.89, 95% CI = 1.41–2.52), and childhood rheumatic fever had a very strong effect on heart disease (OR = 3.44, 95% CI = 1.67–7.09). We obtained similar results when we used the broader indicator of exposure, exposure period (July–December), to predict heart disease (Model 5). In addition, there were no significant effects of exposure to poor nutrition and infectious diseases when we estimated a similar model for those respondents who did not live in the countryside as children (Model 6). Non-imputed data led to analogous inferences, although the effects of season of birth were somewhat stronger for heart disease (results not shown).
Table 4.
Effects of Seasonal Exposure During Late Gestation and Other Childhood Conditions on Adult Heart Disease
| Model 1a | Model 2a | Model 3a | Model 4a | Model 5a | Model 6b | |
|---|---|---|---|---|---|---|
| Variable | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) |
| Female | 1.21 (0.93–1.59) | 1.21 (0.92–1.58) | 1.23 (0.94–1.62) | 1.11 (0.83–1.47) | 1.11 (0.83–1.47) | 1.04 (0.72–1.48) |
| Age 60–64 (ref) | ||||||
| Age 65–69 | 1.41 (1.02–1.94) | 1.42 (1.03–1.96) | 1.42 (1.03–1.96) | 1.45 (1.04–2.01) | 1.44 (1.04–1.99) | 1.04 (0.68–1.58) |
| Age 70–74 | 1.66 (1.19–2.33) | 1.74 (1.24–2.43) | 1.71 (1.22–2.40) | 1.72 (1.21–2.43) | 1.68 (1.19–2.39) | 1.29 (0.84–1.96) |
| Education (years) | 0.99 (0.96–1.02) | 0.98 (0.95–1.02) | 0.98 (0.95–1.02) | |||
| Obesity | 1.89 (1.41–2.52) | 1.90 (1.42–2.53) | 1.20 (0.83–1.73) | |||
| Poor child healthc | 1.50 (1.11–2.02) | 1.37 (1.01–1.86) | 1.40 (1.03–1.91) | 1.39 (1.02–1.90) | 1.47 (0.95–2.27) | |
| Rheumatic feverc | 3.29 (1.60–6.76) | 3.44 (1.67–7.09) | 3.50 (1.70–7.21) | 0.39 (0.04–3.75) | ||
| Low knee heightc | 1.03 (0.75–1.42) | 1.03 (0.75–1.42) | 1.04 (0.75–1.45) | 1.05 (0.76–1.46) | 0.82 (0.53–1.27) | |
| Poor child SES | 1.15 (0.87–1.51) | 1.11 (0.84–1.47) | 1.12 (0.85–1.49) | 1.00 (0.68–1.47) | ||
| No exposure (ref) | ||||||
| Partial early exposure | 1.38 (0.94–2.05) | 1.41 (0.94–2.10) | 1.16 (0.72–1.87) | |||
| Full exposure | 1.83 (1.26–2.62) | 1.89 (1.30–2.75) | 1.11 (0.69–1.79) | |||
| Partial late exposure | 1.38 (0.93–2.04) | 1.36 (0.91–2.02) | 0.97 (0.59–1.59) | |||
| Exposure periodd | 1.42 (1.08–1.86) | |||||
| Range of log likelihoode,f | (−716, −696) | (−713, −694) | (−712, −694) | (−695, −678) | (−689, −675) | (−474, −452) |
| Range of BICe | 1,453–1,482 | 1,439–1,476 | 1,445–1,482 | 1,451–1,485 | 1,429–1,459 | 995–1,028 |
Notes: OR = odds ratio; CI = confidence interval; SES = socioeconomic status; BIC = Bayesian information criterion.
Source: Puerto Rican Elderly: Health Conditions project (PREHCO, 2007), imputed; all 60- to 74-year-olds. Results shown are based on combining multiple imputation results.
Models 1–5 use the subsample of respondents 60 to 74 years old born in Puerto Rico and who lived in the countryside as a child (n = 1,457).
Model 6 uses respondents 60 to 74 years old born in Puerto Rico and who did not live in the countryside as a child (n = 1,147). None of the models tested for heart disease those not living in the countryside during childhood were significantly different from the null model.
We were particularly interested in the mediating effects of other childhood conditions such as health, which included childhood health and knee height, and so included only these in Model 2 controlling for age and gender. Childhood SES and rheumatic fever, potential confounding variables, appeared in Model 3.
We also estimated models with a broader definition of exposure, exposure period, which indicated if the respondent was born during the lean season (July–December).
The multiple imputation procedure required us to work with five alternative completed data sets. In this case it was not clear how to calculate conventional statistics, such as chi square, BIC, or Akaike's information criterion, all of which are functions of data-specific log-likelihood functions. As a partial resolution to the conundrum, we present in this table the range of values for the chosen statistics obtained after estimating models for each of the imputed data sets.
All models were highly significantly different from the null model (p < .001) with the following exceptions: estimated models for persons not living in the countryside as children (Model 6).
The full model for heart disease (Model 4) suggested that the probability of heart disease for the average male respondent (i.e., 65–69 years of age with 7 years of education) in Puerto Rico was of the order of 0.10 for unexposed and 0.18 for fully exposed men. For women the figures were 0.12 and 0.20, respectively (see Figure 2). Thus, for the average respondent, a shift from the best to the worst exposure seasonal category increased the probability of self-reported heart disease by about 80% for men and about 66% for women. The predicted probability of heart disease increased when we added poor childhood conditions (health, knee height, SES) and adult obesity. The increase became even more pronounced when we introduced rheumatic fever as a risk factor. Including interaction terms in Model 4 for childhood conditions, adult obesity, and rheumatic fever with exposure to poor nutrition and infectious diseases produced no indications of statistically significant interaction effects.
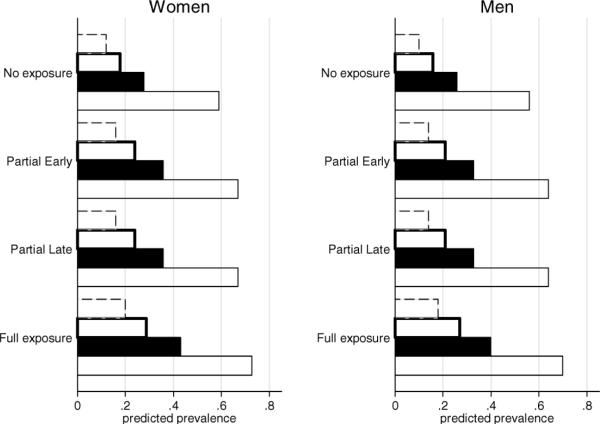
Figure 2.
Predicted prevalence of heart disease by level of exposure during late gestation. The predicted probabilities corresponding to the (a) typical respondent (between the ages of 65 and 69 with 7 years of education), (b) plus poor childhood conditions (poor child health, poor child socioeconomic status, low knee height), (c) plus adult obesity, and (d) plus rheumatic fever during childhood follow. Women: no exposure (0.12, 0.18, 0.28, 0.59), partial early exposure (0.16, 0.24, 0.36, 0.67), partial late exposure (0.16, 0.24, 0.36, 0.67), full exposure (0.20, 0.29, 0.43, 0.73). Men: no exposure (0.10, 0.16, 0.26, 0.56), partial early exposure (0.14, 0.21, 0.33, 0.64), partial late exposure (0.14. 0.21, 0.33, 0.64), full exposure (0.18, 0.27, 0.40, 0.70). Source: Puerto Rican Elderly: Health Conditions project (PREHCO, 2007), 60–74 years, lived in countryside as a child, imputed (n = 1,457). Legend (from top to bottom bar): Dashed bar corresponds to a typical respondent; bolded bar is respondent who had poor childhood SES; solid black bar is respondent who is also obese and bottom bar is respondent who also had childhood rheumatic fever.
In the case of diabetes there were no significant effects when we used the more detailed indicator of exposure to poor nutrition and infectious diseases, level of exposure, for models including all those born in the countryside. However, the broader indicator of exposure, exposure period (July–December), produced strong and significant effects (Table 5, Model 5, OR = 1.81, 95% CI = 1.15–2.85) in this subsample. A comparable set of models for respondents who did not live in the countryside did not produce significant association with either exposure period or level of exposure.
Table 5.
Effects of Seasonal Exposure During Late Gestation and Other Childhood Conditions on Adult Diabetes
| Model 1a | Model 2a | Model 3a | Model 4a | Model 5a | Model 6b | |
|---|---|---|---|---|---|---|
| Variable | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) |
| Female | 1.56 (1.00–2.43) | 1.52 (0.98–2.36) | 1.53 (0.98–2.39) | 1.46 (0.93–2.31) | 1.44 (0.91–2.28) | 1.07 (0.84–1.37) |
| Age 60–64 (ref) | ||||||
| Age 65–69 | 1.22 (0.71–2.08) | 1.22 (0.72–2.09) | 1.22 (0.71–2.07) | 1.23 (0.72–2.11) | 1.24 (0.72–2.13) | 1.20 (0.91–1.59) |
| Age 70–74 | 1.66 (0.98–2.82) | 1.69 (1.00–2.87) | 1.67 (0.99–2.84) | 1.67 (0.97–2.88) | 1.71 (0.99–2.94) | 1.28 (0.95–1.72) |
| Education (years) | 0.99 (0.94–1.04) | 0.98 (0.94–1.04) | 0.98 (0.96–1.01) | |||
| Obesity | 1.44 (0.88–2.38) | 1.44 (0.88–2.37) | 1.45 (1.09–1.93) | |||
| Poor child healthc | 1.04 (0.62–1.75) | 1.00 (0.59–1.71) | 1.00 (0.59–1.69) | 1.01 (0.60–1.72) | 1.20 (0.92–1.58) | |
| Low knee heightc | 0.99 (0.56–1.75) | 0.98 (0.55–1.74) | 0.95 (0.54–1.68) | 0.96 (0.54–1.69) | 1.29 (0.98–1.70) | |
| Poor child SES | 1.19 (0.76–1.86) | 1.14 (0.72–1.82) | 1.14 (0.72–1.80) | 1.02 (0.78–1.33) | ||
| Family member diabetes | 3.94 (2.65–5.85) | |||||
| No exposure (ref) | ||||||
| Partial early exposured | 1.79 (0.99–3.26) | 1.81 (0.98–3.33) | ||||
| Full exposure | 1.34 (0.72–2.50) | 1.35 (0.72–2.54) | ||||
| Partial late exposure | 0.74 (0.38–1.45) | 0.73 (0.37–1.44) | ||||
| Exposure periode | 1.81 (1.15–2.85) | 1.78 (1.14–2.77) | ||||
| Family Member × Exposure Period interaction | 0.57 (0.33–0.96) | |||||
| Range of log likelihoodf | (−289, −273) | (−292, −277) | (−292, −277) | (−279, −271) | (−278, −271) | (−837, −866) |
| Range of BICf | 591–622 | 592–623 | 598–629 | 618–634 | 605–638 | 1,762–1,820 |
Notes: OR = odds ratio; CI = confidence interval; SES = socioeconomic status; BIC = Bayesian information criterion.
Source: Puerto Rican Elderly: Health Conditions project (PREHCO, 2007), imputed; 60- to 74-year-olds born in Puerto Rico and who lived in the countryside as a child (n = 1,457). Results shown are based on combining multiple imputation results.
Models 1–5 include only respondents with no family member with diabetes.
Model 6 shows the results when we examined models with the same predictors as Model 5 but (a) included all respondents living in the countryside and (b) estimated the effects of having a family member with diabetes and interaction effects with this family indicator and the broad definition of exposure, exposure period.
We were particularly interested in the mediating effects of other childhood conditions such as health, which included childhood health and knee height, and so included only these in Model 2 controlling for age and gender. Childhood SES, a potential confounding variable, appeared in Model 3.
The p value for partial early exposure was .056. When we estimated models for each individual imputed file, we obtained a range of results from significant (p < .05) to not significant.
We also estimated models with a broader definition of exposure, exposure period, which indicated if the respondent was born during the lean season (July–December). We estimated Model 5 for those who did not live in the countryside and had no family members with diabetes and found no significant effects of exposure period on diabetes (results not shown). None of the models tested for diabetes for those not living in the countryside during childhood were significantly different from the null model.
The multiple imputation procedure required us to work with five alternative completed data sets. In this case it was not clear how to calculate conventional statistics, such as chi square, BIC, or Akaike's information criterion, all of which are functions of data-specific log-likelihood functions. As a partial resolution to the conundrum, we present in this table the range of values for the chosen statistics obtained after estimating models for each of the imputed data sets. The only models significantly different from the null model were Models 1, 4, 5, and 6.
When we estimated a new model on the sample of all respondents who lived in the countryside as a child (Model 6)—one that included the indicator of family diabetes as an additive term and an interaction term between it and exposure period—we found significant effects for the indicator, exposure period (OR = 1.78, 95% CI = 1.14–2.77); the family indicator of diabetes (OR = 3.94, 95% CI = 2.65–5.85); and the interaction term (OR = 0.57, 95% CI = 0.33–0.96), suggesting that effects of exposure period were much lower among those with a family member who experienced diabetes.
We found that predicted probabilities for diabetes for the typical male respondent in Puerto Rico (using Model 5) were 0.11 for those born during the harvest season and 0.19 for those born during the lean season. For women the figures were 0.15 and 0.24, respectively (see Figure 3). Thus, for the average respondent a shift from the best to the worst exposure category increased the probability of self-reported diabetes by about 73% for men and 60% for women. When we added childhood conditions (health, knee height, SES) and obesity, the predicted probability of diabetes increased slightly, although there were no significant interaction effects between exposure period, childhood conditions, and adult obesity.
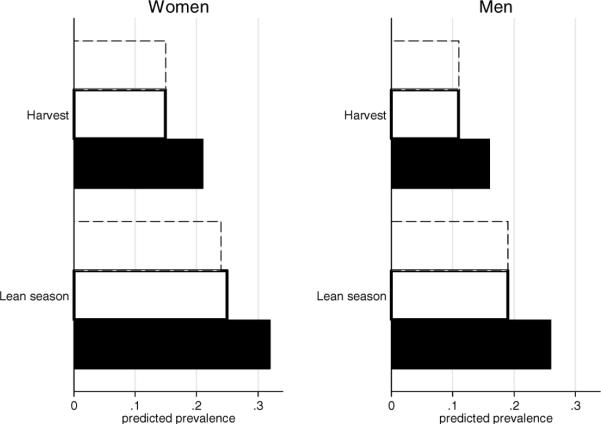
Figure 3.
Predicted prevalence of diabetes by exposure period. The predicted probabilities corresponding to the (a) typical respondent (between the ages of 65 and 69 with 7 years of education, (b) plus poor childhood conditions (poor child health, poor child socioeconomic status, low knee height), and (c) plus obesity follow. Women: harvest (0.15, 0.15, 0.21), lean season (0.24, 0.25, 0.32). Men: harvest (0.11, 0.11, 0.16), lean season (0.19, 0.19, 0.26). Source: Puerto Rican Elderly: Health Conditions project (PREHCO, 2007), 60–74 years, lived in countryside as a child, no family history of diabetes, imputed (n = 1,457). Legend (from top to bottom bar): Dashed bar corresponds to typical respondent; bolded bar is respondent who had poor childhood SES; and black bar is respondent who is also obese.
Discussion
This study used season of birth as an indicator of poor nutrition and impaired intrauterine and infant growth on adult health among the Puerto Rican older adult population who had lived a prolonged period of time in the countryside prior to the age of 18. We highlight four main findings: First, there were significant associations between self-reported heart disease and diabetes and season of birth for those living in the countryside as a child, whereas none of those associations materialized for those who did not live in the countryside as a child. These are important findings because they corroborate the validity of season of birth as a measure of early nutritional and infectious disease exposure. The literature has not clearly shown the validity of season of birth as a measure of early nutritional or infectious disease exposure (Bengtsson & Lindstrom, 2003; Costa, 2005; Doblhammer, 2004; Gavrilov & Gavrilova, 2005). In addition, early childhood SES, although an important factor affecting later adult health (Hertzman, 1994; Lundberg, 1991; Wadsworth & Kuh, 1997; Wickrama et al., 2005), was not associated with season of birth. This further provides evidence regarding the belief that season of birth is largely independent of social class of origin (Doblhammer, 2004) and thus may be a very useful indicator for disentangling the effects of early exposure to poor nutrition from other childhood conditions.
Second, we also found significant associations between self-reported child health and childhood illnesses, and between self-reported child SES and measures of fathers' educational and occupational status. These findings also demonstrate the convergent validity of self-reported child health at least in terms of the dimensions of health reflected in the illnesses obtained from the PREHCO study.
Third, the effects of seasonal exposure to poor nutrition and infectious diseases using birth quarter on self-reported heart disease are remarkably strong, robust, and in the expected direction. Those born toward the end of the harvest period (second quarter, no exposure) have lower probabilities of self-reporting heart disease, and those born prior to the beginning of the harvest (fourth quarter, full exposure) have higher probabilities of self-reporting heart disease. As confirmed by examination of predicted values of self-reported heart disease, the actual magnitude of the effects is far from trivial. The strong associations found in Puerto Rico provide some support for the idea that there is a relation between early nutrition during pregnancy and early infancy and later chronic conditions (Barker, 1998; Gluckman & Hanson, 2006). Although maternal infections could be playing a role as well, it is less likely that infections during the first few months of infancy are of significance because the peak of the season for more serious infectious diseases during the early 20th century in Puerto Rico occurred in the second and third quarter (Rigau Pérez, 2000).
The findings for diabetes are more fragile. We found no significant effects when we used the more detailed indicator for exposure to poor nutrition and infectious diseases, level of exposure, using season of birth. However, when we used the broader definition for exposure to poor nutrition and infectious diseases, namely exposure period, we found that being born during the pertinent period (July–December) increased the odds of having adult diabetes. Furthermore, a model with interaction effects between exposure period and having a family member with diabetes suggests that the effect of being born in the lean period is very small among persons who have a family member with diabetes.
What are we to make of the weak results for diabetes? It may be that our results using level of exposure to poor nutrition and infectious diseases reflect differences in the timing of critical periods for heart disease and diabetes and that we are not able to adequately represent all of the factors that determine the latter, such as the genetic component that may play a stronger role for diabetes than for heart disease. A hint that this is precisely what may be happening is conveyed by our models including the indicator of family members who experience diabetes. In those models, both the exposure to poor nutrition and infectious diseases (season of birth) indicator and the indicator of family diabetes exert powerful influences in the expected direction. The fact that the presence of relatives with diabetes continues to have an influence on the likelihood of diabetes even after we control for seasonal exposure suggests one of two things: Either there is an independent genetic component not captured at all by season of birth, or, alternatively, the onset of diabetes is due to environmental factors other than those captured by season of birth, including adult shared lifestyles. The fact that effects of seasonal exposure to poor nutrition and infectious diseases are much larger among those whose closest kin have not experienced diabetes (interaction term between season of birth and family indicator) suggests, in addition, that there might be strong gene-environment interactions (if the family indicator reflects genetic predisposition) or early adult-environment interactions (if the family indicator reflects shared adult lifestyles).
The results provide insight into the relative importance of exposure to poor nutrition and infectious diseases and other childhood conditions and important risk factors. In the case of heart disease, the effects of early exposure are similar in magnitude to those of obesity and much more important than childhood health (as measured in our study), although they were dwarfed in comparison with the effects of rheumatic fever. It appears that exposure to poor nutrition and infectious diseases is more important than obesity in predicting diabetes, whereas the effects of childhood health are negligent. Thus, the magnitude of effects of early exposure for heart disease and diabetes reflects an arguably substantial impact. At the population level, however, its impact must be rather tenuous because the distribution of births by season is unlikely to change much from year to year, except after or before social and economic upheavals or population crises of one sort or another.
There are other plausible reasons for these findings. It may be the case, for example, that the pathway to adult disease is confounded by infectious diseases during pregnancy or early infancy (malaria in particular). The hurricane season peaks from August to October and brings with it a long period of hot and humid weather that augments exposure to diseases such as dysentery, diarrhea, malaria, and dengue fever (Rigau Pérez, 2000). However, there was no confounding or interaction between season of birth and malaria or dengue fever. Thus, although we cannot completely discard the possibility of the importance of infectious disease interacting with poor nutrition, our data appear to indicate that this is not a strong part of the story, at least not for malaria or dengue fever.
It may also be that there are traits that vary across cohorts that operate to attenuate the linkages between early exposure to poor nutrition and infectious diseases, and adult health status. Thus, researchers suggest that differences in immune functions and exposure to other infections or environmental risks (none of which we measured in our study) is one factor that explains the heterogeneous importance of season of birth between populations (Moore et al., 1999; Simondon et al., 2004). Other factors such as differences between cohorts' experiences may reduce the effects of season of birth on adult health.
Another complicating factor is breastfeeding and weaning patterns in Puerto Rico. Although we do not have data to examine the specific variations in breastfeeding in the rural countryside in Puerto Rico during the 1930s and 1940s, we do know that the use of bottled formula became prevalent only beginning in the late 1940s (Becerra & Smith, 1990). Thus, breastfeeding probably followed traditional norms (nearly universal and of long duration) in the rural countryside. If this was the case, and the nutritional status of the mother influences the production as well as the quality of the mother's milk (John et al., 1987), then children whose mothers experience infection/malnutrition right before the children's birth and sometime thereafter could be affected. Therefore, breastfeeding could be a mechanism (not a confounder) through which season of birth affects early nutritional status and growth.
Finally, it could be that the identifying question regarding living in the countryside as a child before the age of 18 was not specific enough to identify more precisely the actual time frame for the birth and the early infancy period, and thus we were not able to discriminate between early life exposure to poor nutrition and infectious diseases and other events happening in later childhood. Be that as it may, this problem would lead us to underestimate the effects of season of birth, not exaggerate them.
Our fourth finding is that the effects of other indicators of early childhood health change only slightly when we include season of birth in the models for both heart disease and diabetes. Similarly, the effects of season of birth remain invariant when one controls for the other, secondary measures of early childhood conditions. It is possible that early life exposure to poor nutrition and infectious diseases (as proxied by season of birth) has direct effects on adult heart disease that are not mediated through childhood health. However, this explanation is inconsistent with a body of literature documenting the influence of early childhood health on adult health (Davey Smith & Lynch, 2004; Elo & Preston, 1992; Kuh & Ben-Shlomo, 2004; Lundberg, 1991; O'Rand & Hamil-Luker, 2005). A more likely situation is that season of birth and the other indicators of child health status/nutrition are tapping different and unrelated dimensions. Thus, our results suggest that it is not the case that the effects of self-reported childhood health status are inflated due to correlations across individuals' life cycle conditions that determine the adult onset of chronic disease. An alternative and more pessimistic assessment is that season of birth is a poor indicator of early nutritional status and that its apparent relevance for heart disease in Puerto Rico is due to other factors.
Alternatively, self-reported childhood health status could be a mediocre indicator because the question on which it is based is not centered on any specific age and, in spite of findings suggesting the opposite in this and other studies, its validity and reliability may be questionable. Our measures of both childhood health and season of birth may be too coarse and may not coincide with the critical time for everyone. Focusing exclusively on the third trimester may miss the mark. More important, and as shown by our convergent validity check, it may be that self-reported childhood health reflects dimensions of childhood health status that are not strongly associated with early exposure in utero and early infancy. Finally, self-reported childhood health may be more closely associated with current adult health status than it is with early life exposure to poor nutrition and infectious diseases. By the same token, month of birth could be an excessively coarse measure of intrauterine environment except under the most extreme conditions (Bengtsson & Lindstrom, 2003; Costa, 2005; Doblhammer, 2004; Gavrilov & Gavrilova, 2005), which may not have applied to the rural population of Puerto Rico during the early 20th century, despite the strong seasonality in food supply.
In spite of these limitations, the strong associations with heart disease and even the mixed results for diabetes give us reason to believe that further examination of the effects of season of birth on adult health in Puerto Rico and among similar populations has merit.
Acknowledgments
This research was supported by National Institute on Aging Grant R01 AG16209 (Puerto Rican Elderly: Health Conditions) and Grant K25 AG027239. Research work for University of Wisconsin–Madison researchers was supported by core grants to the Center for Demography and Ecology at the University of Wisconsin (R24 HD47873) and to the Center for Demography of Health and Aging at the University of Wisconsin (P30 AG017266). We would like to acknowledge comments from early discussions of birth month and adult health made by Robert Hauser, Zhen Zeng, and James Raymo; and library assistance from John Carlson and editorial assistance from Sarah Moen and Janet Clear from the Center for Demography and Ecology, University of Wisconsin–Madison. We are also very grateful for the helpful comments made by the anonymous reviewers.
M. McEniry planned the study, performed all statistical data analyses, and wrote the paper. A. Palloni helped plan the study, participated in the interpretation of data analyses, and contributed to revising the paper. A. L. Dávila and A. García Gurucharri helped revise the manuscript.
References
- American Diabetes Association The genetics of diabetes [Fact sheet] n.d. Retrieved August 4, 2008, from www.diabetes.org/genetics.jsp.
- Banks J, Marmot M, Oldfield Z, Smith J. Disease and disadvantage in the United States and in England. Journal of the American Medical Association. 2006;295:2037–2045. doi: 10.1001/jama.295.17.2037. [DOI] [PubMed] [Google Scholar]
- Barker DJP. Mothers, babies and health in later life. 2nd ed. Churchill Livingstone; Edinburgh, Scotland: 1998. [Google Scholar]
- Becerra JE, Smith JC. Breastfeeding patterns in Puerto Rico. American Journal of Public Health. 1990;80:694–697. doi: 10.2105/ajph.80.6.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengtsson T, Lindstrom M. Airborne infectious diseases during infancy and mortality in later life in southern Sweden, 1766–1894. International Journal of Epidemiology. 2003;32:286–294. doi: 10.1093/ije/dyg061. [DOI] [PubMed] [Google Scholar]
- Clark VS. Porto Rico and its problems. Brookings Institution; Washington, DC: 1930. [Google Scholar]
- Costa DL. Becoming oldest-old: Evidence from historical U.S. data. Genus. 2005;LXI(1):125–161. [Google Scholar]
- Davey Smith G, Greenwood D, Gunnell RD, Sweetnam P, Yarnell J, Elwood P. Leg length, insulin resistance, and coronary heart disease risk: The Caerphilly Study. Journal of Epidemiology and Community Health. 2001;55:867–872. doi: 10.1136/jech.55.12.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey Smith G, Lynch J. Life course approaches to socioeconomic differentials in health. In: Smith GD, Lynch J, editors. A life course approach to chronic disease epidemiology. Oxford University Press; Oxford, England: 2004. pp. 77–115. [Google Scholar]
- Doblhammer G. The late life legacy of very early life. Springer; Berlin, Germany: 2004. [Google Scholar]
- Elo IT, Preston SH. Effects of early-life conditions on adult mortality: A review. Population Index. 1992;58(2):186–212. [PubMed] [Google Scholar]
- Eveleth PB, Tanner JM. Worldwide variation in human growth. Cambridge University Press; Cambridge, England: 1976. [Google Scholar]
- Gardiner HM. Early environmental influences on vascular development. Early Human Development. 2007;83:819–823. doi: 10.1016/j.earlhumdev.2007.09.011. [DOI] [PubMed] [Google Scholar]
- Gavrilov LA, Gavrilova NS. Mortality of centenarians: A study based on the Social Security Administration death master file. Paper presented at the annual meeting of the Population Association of America; Philadelphia, PA. March/April, 2005. [Google Scholar]
- Gayer AD, Homan PT, James EK. The sugar economy of Puerto Rico. Columbia University Press; New York: 1938. [Google Scholar]
- Gluckman E, Hanson M. Developmental origins of health and disease. Cambridge University Press; Cambridge, England: 2006. [Google Scholar]
- Goldman N, Lin I, Weinstein M, Lin Y. Evaluating the quality of self-reports of hypertension and diabetes. Journal of Clinical Epidemiology. 2003;56:148–154. doi: 10.1016/s0895-4356(02)00580-2. [DOI] [PubMed] [Google Scholar]
- Gunnell DJ, Davey Smith G, Holly JMP, Frankel S. Leg length and risk of cancer in the Boyd Orr cohort. British Medical Journal. 1998;317:1350–1351. doi: 10.1136/bmj.317.7169.1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas SA. The long-term effects of poor childhood health. Demography. 2007;44(1):113–135. doi: 10.1353/dem.2007.0003. [DOI] [PubMed] [Google Scholar]
- Hertzman C. The lifelong impact of childhood experiences: A population health perspective. Daedalus. 1994;123(4):167–180. [Google Scholar]
- Huxley R, Neil A, Collins R. Unraveling the fetal origins hypothesis: Is there really an inverse relationship between birth weight and subsequent blood pressure? Lancet. 2002;360:659–665. doi: 10.1016/S0140-6736(02)09834-3. [DOI] [PubMed] [Google Scholar]
- International Labor Organization ISCO, International Standard Classification of Occupations. 2004 Retrieved August 4, 2008, from www.ilo.org/public/english/bureau/stat/isco/index.htm.
- John AM, Menken JA, Chowdhury AKMA. The effects of breastfeeding and nutrition on fecundability in rural Bangladesh: A hazards-model analysis. Population Studies. 1987;41:433–446. [Google Scholar]
- Joseph KS, Kramer MS. Review of the evidence of fetal and early childhood antecedents of adult chronic disease. Epidemiologic Review. 1996;18(2):158–174. doi: 10.1093/oxfordjournals.epirev.a017923. [DOI] [PubMed] [Google Scholar]
- Kevan SM. Season of life—Season of death. Social Science & Medicine. 1979;13D:227–232. doi: 10.1016/0160-8002(79)90042-x. [DOI] [PubMed] [Google Scholar]
- Kuh D, Ben-Shlomo Y, editors. A life course approach to chronic disease epidemiology. Oxford University Press; Oxford, England: 2004. [PubMed] [Google Scholar]
- Leitch I. Growth and health. British Journal of Nutrition. 1951;5:142–151. doi: 10.1079/bjn19510017. [DOI] [PubMed] [Google Scholar]
- Lundberg O. Childhood living conditions, health status, and social mobility: A contribution to the health selection debate. European Sociological Review. 1991;7(2):149–162. [Google Scholar]
- Moore SE, Cole TJ, Collinson AC, Poskitt EME, McGregor IA, Prentice AM. Prenatal or early postnatal events predict infectious deaths in young adulthood in rural Africa. International Journal of Epidemiology. 1999;28:1088–1095. doi: 10.1093/ije/28.6.1088. [DOI] [PubMed] [Google Scholar]
- Murray CJL, Lopez AD, editors. Global health statistics: Global burden of disease and injury series. Vol. 2. Harvard School of Public Health; Boston: 1996. [Google Scholar]
- O'Rand AM, Hamil-Luker J. Processes of cumulative adversity: Childhood disadvantage and increased risk of heart attack across the life course. Journal of Gerontology: Social Sciences. 2005;60B:S117–S124. doi: 10.1093/geronb/60.special_issue_2.s117. [DOI] [PubMed] [Google Scholar]
- Palloni A, McEniry M, Dávila AL, García Gurucharri A. The influence of early conditions on health status among elderly Puerto Ricans. Social Biology. 2005;52(34):132–164. doi: 10.1080/19485565.2005.9989106. [DOI] [PubMed] [Google Scholar]
- Palloni A, Pelaez M. Survey of Health and Well-Being of Elders (Final report) Pan American Health Organization; Washington, DC: 2002. [Google Scholar]
- PREHCO . Proyecto PREHCO I 2002–2003 Informe metodológico [Methods report, PREHCO I Project 2002–2003] University of Wisconsin, Center for Demography and Ecology; and University of Puerto Rico, Graduate School of Public Health; Madison: 2007. Available at: www.ssc.wisc.edu/cdha/projects/projects.html. [Google Scholar]
- Raghunathan TE, Reiter JP, Rubin DE. Multiple imputation for disclosure limitation. Journal of Official Statistics. 2003;19:1–16. [Google Scholar]
- Raghunathan TE, Solenberger PW, Van Hoewyk J. IVEware: Imputation and variance estimation [Computer software] 2007 Retrieved July 31, 2007, from www.isr.umich.edu/src/smp/ive/
- Ravelli AC, van der Meulen JH, Michels RP, Osmond C, Barker DJ, Hales CN, et al. Glucose tolerance in adults after prenatal exposure to the Dutch famine. Lancet. 1998;351:173–177. doi: 10.1016/s0140-6736(97)07244-9. [DOI] [PubMed] [Google Scholar]
- Rigau Pérez JG. La salud en Puerto Rico en el Siglo XX [Health in Puerto Rico in the twentieth century] Puerto Rican Health Sciences Journal. 2000;19(4):357–368. [PubMed] [Google Scholar]
- Riosmena F, Wong R. Health selectivity and SES-health gradients in Mexico-U.S. migration and return: A bi-national perspective on older adults. Paper presented at the annual meeting of the Population Association of America; New Orleans, LA. Apr, 2008. [Google Scholar]
- Rubin D. Multiple imputation for nonresponse in surveys. Wiley; New York: 1987. [Google Scholar]
- Simondon KB, Elguero E, Marra A, Diallo A, Aaby P, Simondon F. Quarter of birth is not associated with risk of early adult death in rural Senegal. International Journal of Epidemiology. 2004;33:130–136. doi: 10.1093/ije/dyg279. [DOI] [PubMed] [Google Scholar]
- U.S. Census Bureau . Outlying territories and possessions. U.S. Government Printing Office; Washington, DC: 1932. [Google Scholar]
- Van Buren S, Boshuizen H, Knook D. Multiple imputation of missing blood pressure covariates in survival analysis. Statistics in Medicine. 1999;18:681–694. doi: 10.1002/(sici)1097-0258(19990330)18:6<681::aid-sim71>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Wadsworth M, Hardy R, Paul A, Marshall S, Cole T. Leg and trunk length at 43 years in relation to childhood health, diet and family circumstances: Evidence from the 1946 national birth cohort. International Journal of Epidemiology. 2002;31:383–390. [PubMed] [Google Scholar]
- Wadsworth M, Kuh D. Childhood influences on adult health: A review of recent work from the British 1946 national birth cohort study, the MRC National Survey of Health and Development. Paediatric and Perinatal Epidemiology. 1997;11:2–20. doi: 10.1046/j.1365-3016.1997.d01-7.x. [DOI] [PubMed] [Google Scholar]
- Wickrama KAS, Conger RD, Abraham WT. Early adversity and later health: The intergenerational transmission of adversity through mental disorder and physical illness. Journal of Gerontology: Social Sciences. 2005;60B:S125–S129. doi: 10.1093/geronb/60.special_issue_2.s125. [DOI] [PubMed] [Google Scholar]
- World Health Organization . Obesity: Preventing and managing the global epidemic. Author; Geneva, Switzerland: 2000. [PubMed] [Google Scholar]
- Zar J. Biostatistical analysis. 4th ed. Prentice Hall; Upper Saddle River, NJ: 1999. [Google Scholar]