Abstract
Objective
To determine the burden of chronic obstructive pulmonary disease (COPD) on all-cause mortality in Blacks and Whites from four U.S. communities.
Methods
We determined prospectively the risk of death through December 2004 in relation to baseline (1987–1989) COPD status in 10,333 Black and White participants of the Atherosclerosis Risk in Communities (ARIC) study.
Results
Over a mean follow-up of 15 years (maximum 18 years), 462 deaths occurred in Blacks and 1221 deaths occurred in Whites. Hazard ratios for all-cause mortality among Blacks and Whites were similar (hazard ratio [HR]=1.74 in Blacks and HR=1.59 in Whites), indicating a 59–74% greater risk of mortality for those with COPD. However, for both those with and without COPD, crude death rates were approximately double in Blacks compared to Whites.
Conclusions
Our findings suggest that given COPD, Blacks and Whites have the same proportionate increase in mortality and that the difference in death rates between Blacks and Whites cannot be explained by COPD status. The public health burden of COPD is enormous, and strategies to reduce COPD and smoking could have a large impact on total mortality rates of both Blacks and Whites.
Keywords: Chronic obstructive pulmonary disease, COPD, lung disease, mortality
INTRODUCTION
Chronic obstructive pulmonary disease (COPD), primarily chronic bronchitis and emphysema, is a treatable and preventable disease state characterized by airflow limitation that is not completely irreversible. The airflow limitation is usually progressive and is often associated with an abnormal inflammatory response to environmental exposures, particularly cigarette smoking1, 2. As the abnormalities in small airways increase, pulmonary function deteriorates progressively, and pulmonary function tests distinguish patients with minimal pathologic changes from those with normal airways3, 4. Hence, the defining clinical physiologic characteristic of COPD is airflow obstruction as measured by spirometry1.
COPD is responsible for more than 2.5 million deaths worldwide each year and is the 10th leading cause of disease burden, accounting for 2% of the global burden of disease5. In the U.S., COPD is the 4th leading cause of death6. As of 1999, COPD accounted for 5.1% and 4.8% of deaths in the U.S. in men and women, respectively7. All-cause mortality is higher among individuals with COPD compared to those with normal lung function8. Specific causes of death among COPD patients participating in an international multicenter trial were as follows: respiratory (35%), cardiovascular (27%), cancer (21%), and other/unknown (18%)9.
The aim of our analysis was to describe rates and hazard ratios (HR) for all-cause mortality among those with and without COPD at the baseline examination of the Atherosclerosis Risk in Communities (ARIC) study. Because information on racial differences in all-cause mortality among COPD patients is limited, we also examined rates and hazard ratios for death among Blacks and Whites separately. We hypothesized that all-cause mortality rates would be higher among those with, compared to those without, COPD at baseline, and that the rates and COPD hazard ratios for death may differ between Blacks and Whites.
METHODS
Study Population
The ARIC study recruited for its prospective cohort probability samples of adults aged 45–64 years from four U.S. communities: Forsyth County, North Carolina; Jackson, Mississippi; Minneapolis suburbs, Minnesota; and Washington County, Maryland10. Blacks and Whites were recruited from Forsyth County, only Blacks from Jackson, Mississippi, and predominantly Whites from the other two communities. A total of 15,792 participants (8710 women) enrolled from 1987 to 1989, and completed a home interview and clinic visit. A total of four visits were conducted, each spaced three years apart.
Pulmonary Function Testing
Lung function was measured via the forced vital capacity (FVC) maneuver, in which the maximal volume of air is exhaled during a forced expiration starting from a position of full inspiration and ending at complete expiration. Lung function data were only collected at the baseline visit (1987–89) and first follow-up visit (1990–92). Forced expiratory volume in 1 second (FEV1) is the volume of gas exhaled in the first second of expiration, and FVC is the total volume of gas exhaled. Lung function was measured according to the American Thoracic Society criteria11. Collins Survey II water-seal spirometers (Collins Medical, Inc., Braintree, MA) driven by IBM PC/XT computers and under the control of Pulmo-Screen software (PDS Healthcare Products, Inc., Louisville, CO) were used to assist the technicians with quality control, calculation of pulmonary function variables, and compilation of results for transmission to the ARIC Pulmonary Function Reading Center. Participants performed the FVC maneuver until there were two error-free reproducible maneuvers (FEV1 and FVC within 5%) out of three acceptable maneuvers, with the maneuvers repeated up to eight times if necessary.
FEV1 predicted was calculated as follows for men and women12:
Men: FEV1 predicted = 0.0414(height in cm) − 0.0244(age) − 2.190
Women: FEV1 predicted = 0.0342(height in cm) − 0.0255(age) − 1.578
Because Blacks have lower lung function than Whites13, the FEV1 predictions were multiplied by 0.88 for Blacks in order to adjust the above lung function equations (developed for Whites) for use in Blacks14, 15. We calculated percentage of predicted values for FEV1 for a person by dividing measured FEV1 by predicted FEV1.
COPD Criteria
For this analysis, 3 different sets of criteria were used to define COPD. Spirometric COPD was defined at baseline as having an FEV1 < 80% of the predicted value and an FEV1/FVC ratio of < 70%. Participants meeting only 1 of the 2 criteria for COPD were categorized in the no COPD referent group for analysis. A second definition of COPD included those that had either COPD based on spirometry or chronic bronchitis, defined as having chronic cough most days of the week for at least 3 consecutive months a year for 2 years. Finally, we identified participants with persistent COPD, those having spirometry-defined COPD at both baseline and visit 2.
Epidemiologic and Clinical Data
Body mass index (BMI) was calculated as weight in a scrub suit (in kilograms) divided by height (in meters) squared. Diabetes status was determined by fasting blood glucose ≥126 mg/dL, nonfasting glucose ≥200 mg/dL, or self-report of physician diagnosis or current medication use for diabetes. Otherwise, if fasting glucose was between 110–126 mg/dL, the participant was categorized as having impaired fasting glucose. Blood pressures were measured 3 times in the sitting position after 5 minutes of rest using a random-zero sphygmomanometer. The last 2 blood pressure measurements were averaged, and hypertension was defined as having a systolic blood pressure of ≥140 mmHg, or a diastolic blood pressure of ≥90 mmHg, or self report of medication use for high blood pressure in the previous 2 weeks. Smoking and alcohol amount were determined by self-report. Responses to number of cigarettes smoked per day and duration of smoking were used to calculate pack-years of smoking. The sports index for physical activity during leisure time ranged from 1 (low) to 5 (high), and was based on the questionnaire developed by Baecke et al16.
Ascertainment of Deaths
Deaths among cohort participants were identified through December 2004 via annual telephone calls and by surveillance of local death certificates and obituaries. If a participant was lost to telephone follow-up, a National Death Index search was conducted; this identified less than 4% of deaths in our cohort. Underlying causes of deaths were classified by state health departments according to the International Classification of Diseases, 9th Revision (ICD-9) for those occurring before 1999 and ICD-10 for those occurring in 1999 or later.
Statistical Analysis
All analyses were conducted using SAS version 8.2 (SAS Institute, Cary, NC). Of the 15,792 ARIC participants, we excluded those who were not White or Black (n=103) or who had missing or unacceptable lung function tests (n=4195). As an attempt to account for individuals with a possibly reversible disease, we also excluded individuals with a self-reported history of asthma (n=1161).
Race-specific mortality rates for age groups (45–54, 55–64) were computed, along with age-adjusted mortality rates for additional participant characteristics using Poisson regression. Crude death rates based on COPD status were also calculated among total Blacks, total Whites, and subgroups of Whites stratified by age and gender using Poisson regression models. Life table methods were used to plot the cumulative probability of survival during 18 years of follow-up stratified by race and spirometric COPD status at baseline. Race-specific hazard ratios for death among those with COPD at baseline compared to those without COPD were estimated using Cox regression models after adjustment for age (continuous), gender, center (Forsyth County, NC; Jackson, MS; Minneapolis suburbs, MN; Washington County, MD), BMI (<25, 25 to <30, ≥30 kg/m2), pack-years of smoking (continuous), smoking status (never, former, current), drinking status (never, former, current), diabetes (no, yes), hypertension (no, yes), sports index (ordinal, 1–5), and educational attainment (≤ high school, > high school). Hazard ratios for subgroups of participants were estimated in a similar manner. Multiplicative interactions between COPD and baseline smoking status on risk of death were tested by examining the chi-square statistic for the cross product terms. The log-log survival curves were plotted to show that the proportional hazards assumption was met for our Cox regression models.
RESULTS
After exclusions, 10,333 participants (2,047 Blacks and 8,286 Whites) remained for analysis. We included individuals with a self-reported history of cancer or coronary heart disease at baseline, because the results were unchanged when they were removed (data not shown). Blacks compared to Whites had on average more mortality related risk factors: obesity, diabetes, hypertension, current smoking, non-drinking, physical inactivity, and less education (all p-values <0.0001) (Table 1). Additionally, mortality rates were nearly two-fold higher among Blacks than Whites for all of the baseline characteristics, except for individuals who completed education beyond high school.
Table 1.
Race-Specific Prevalence of Baseline Characteristics and Subsequent Age-Adjusted Death Rates, ARIC
| Blacks (n=2047) | Whites (n=8286) | |||
|---|---|---|---|---|
| Prevalence at Baseline (%) | Death Rate per 1000 p-yrs | Prevalence at Baseline (%) | Death Rate per 1000 p-yrs | |
| Age Group | ||||
| 45–54 yrs | 60.2 | 9.3 | 52.7 | 5.3 |
| 55–64 yrs | 39.8 | 26.2 | 47.3 | 14.7 |
| Gender | ||||
| Male | 40.5 | 19.9 | 47.4 | 10.1 |
| Female | 59.5 | 11.5 | 52.6 | 6.1 |
| Center | ||||
| Forsyth | 12.1 | 13.3 | 29.8 | 7.3 |
| Jackson | 87.9 | 15.0 | 0 | -- |
| Minneapolis | 0 | -- | 36.0 | 7.7 |
| Washington | 0 | -- | 34.2 | 8.8 |
| BMI, kg/m2 | ||||
| < 25 | 21.8 | 19.6 | 36.9 | 7.1 |
| 25 to <30 | 37.9 | 13.2 | 40.4 | 7.3 |
| > 30 | 40.4 | 13.9 | 22.7 | 10.6 |
| Diabetes | ||||
| No | 81.7 | 12.7 | 91.7 | 7.2 |
| Yes | 18.3 | 25.9 | 8.3 | 17.2 |
| Hypertension | ||||
| No | 45.6 | 11.0 | 73.3 | 6.9 |
| Yes | 54.4 | 17.8 | 26.7 | 10.9 |
| Smoking Status | ||||
| Never | 44.7 | 9.2 | 39.9 | 4.6 |
| Former | 24.8 | 14.6 | 36.1 | 7.6 |
| Current | 30.5 | 24.1 | 24.0 | 14.3 |
| Drinking Status | ||||
| Never | 44.5 | 11.5 | 17.0 | 6.1 |
| Former | 23.1 | 20.8 | 17.1 | 11.4 |
| Current | 32.4 | 15.7 | 65.9 | 7.6 |
| Sport Index | ||||
| 1.0–2.5 | 77.1 | 14.2 | 54.9 | 6.8 |
| > 2.5–5.0 | 22.9 | 14.9 | 45.1 | 8.9 |
| Education | ||||
| ≤ High School | 58.3 | 18.3 | 50.0 | 8.8 |
| > High School | 41.7 | 9.8 | 50.0 | 7.1 |
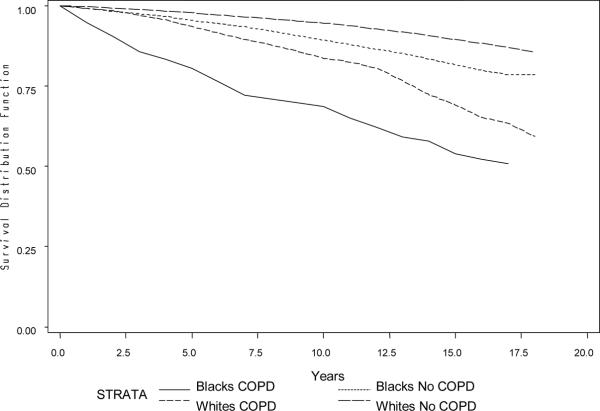
The prevalence of spirometric COPD was 13.1% in Black males, 4.9% in Black females, 15.2% in White males, and 7.4% in White females. Over a mean follow-up of 15 years (maximum 18 years), there were 462 deaths in Blacks and 1221 in Whites. The proportion of deaths with underlying cause coded as respiratory, cancer, cardiovascular disease, and other were 6.5%, 39.9%, 36.3%, and 17.3%, respectively. The percentage of deaths due to respiratory disease was 16.5% in those with baseline COPD and 3.3% in those without (data not shown). Figure 1 depicts that individuals with spirometric COPD had lower survival than those who did not have spirometric COPD at baseline. Whites had better survival than Blacks.
Figure 1. Survival curves by race and COPD status* at baseline during up to 18 years of follow-up in the Atherosclerosis Risk in Communities (ARIC) study.
*COPD defined by spirometry at baseline, as having an FEV1 < 80% of the predicted value and an FEV1/FVC ratio < 70%.
Death rates and hazard ratios are provided for total Blacks and total Whites, as well as subgroups of Whites, in whom a significant 3-way interaction between spirometric COPD, gender, and age group (45–54, 55–64) was found (p<0.01) (Table 2). The adjusted hazard ratios for all-cause mortality were similar among Blacks (HR=1.74) and Whites (HR=1.59), indicating a 59–74% greater risk of mortality for those with spirometric COPD. However, consistent with the survival curves, crude death rates were nearly double in Blacks compared to Whites within COPD categorization. When the Whites were stratified, the hazard ratios for mortality in relation to spirometric COPD ranged from 1.31 in older White men to 2.76 in younger White women (Table 2).
Table 2.
Race-Specific Death Rates and Hazard Ratios for Death by COPD Status*, ARIC
| Number of Deaths | Person-years | Death Rate† | Hazard Ratio‡ | 95% CI | |
|---|---|---|---|---|---|
| Total Blacks | |||||
| No COPD | 379 | 27792 | 13.6 | 1.00 | Ref. |
| COPD | 83 | 1990 | 41.7 | 1.74 | (1.34–2.25) |
| Total Whites | |||||
| No COPD | 898 | 114467 | 7.8 | 1.00 | Ref. |
| COPD | 323 | 12897 | 25.0 | 1.59 | (1.37–1.84) |
| White Men 45–54 | |||||
| No COPD | 154 | 27852 | 5.5 | 1.00 | Ref. |
| COPD | 51 | 2807 | 18.2 | 1.79 | (1.24–2.58) |
| White Women 45–54 | |||||
| No COPD | 131 | 36446 | 3.6 | 1.00 | Ref. |
| COPD | 29 | 1951 | 14.9 | 2.76 | (1.78–4.33) |
| White Men 55–64 | |||||
| No COPD | 361 | 23109 | 15.6 | 1.00 | Ref. |
| COPD | 173 | 5474 | 31.6 | 1.31 | (1.06–1.60) |
| White Women 55–64 | |||||
| No COPD | 252 | 27061 | 9.3 | 1.00 | Ref. |
| COPD | 70 | 2665 | 26.3 | 1.81 | (1.34–2.45) |
COPD defined by spirometry at baseline, as having an FEV1 < 80% of the predicted value and an FEV1/FVC ratio < 70%.
Crude death rates per 1000 person-years.
Hazard ratios are adjusted for age, center, BMI, pack-years of smoking, smoking status, drinking status, diabetes, hypertension, sports index, and education. Hazard ratios for total Blacks and total Whites are additionally adjusted for gender.
A 3-way interaction between COPD, gender, and age group was significant among Whites only; therefore, estimates are also given for Whites stratified on gender and age group.
We additionally estimated hazard ratios and death rates in total Blacks and total Whites 1) including those self-reporting symptoms of chronic bronchitis in the COPD category, and 2) for those with persistent COPD, defined as having COPD at both visits 1 and 2 based on spirometry (Table 3). Rates were lower for COPD including chronic bronchitis and for persistent COPD, while hazard ratios were similar, compared with COPD defined by spirometry at baseline. Again, hazard ratios for COPD were similar in Whites and Blacks for these additional categorizations of COPD, but absolute death rates were higher in Blacks. Among those with COPD including chronic bronchitis, the death rates were 29.9 in Blacks and 18.5 per 1000 person-years in Whites; among those with persistent COPD, the death rates were 31.3 in Blacks and 25.9 per 1000 person-years in Whites.
Table 3.
Race-Specific Death Rates and Hazard Ratios for Death by COPD with Chronic Bronchitis and Persistent COPD, ARIC
| Number of Deaths | Person-years | Death Rate* | Hazard Ratio† | 95% CI | |
|---|---|---|---|---|---|
| COPD including Chronic Bronchitis‡ | |||||
| Blacks | |||||
| No COPD | 354 | 26167 | 13.5 | 1.00 | Ref. |
| COPD | 108 | 3615 | 29.9 | 1.50 | (1.19–1.90) |
| Whites | |||||
| No COPD | 781 | 103615 | 7.5 | 1.00 | Ref. |
| COPD | 440 | 23749 | 18.5 | 1.42 | (1.25–1.62) |
| Persistent COPD§ | |||||
| Blacks | |||||
| No COPD | 197 | 15099 | 13.0 | 1.00 | Ref. |
| COPD | 16 | 511 | 31.3 | 1.31 | (0.75–−2.30) |
| Whites | |||||
| No COPD | 614 | 77566 | 7.9 | 1.00 | Ref. |
| COPD | 173 | 6674 | 25.9 | 1.58 | (1.30–1.91) |
Crude death rates per 1000 person-years.
Hazard ratios are adjusted for age, gender, center, BMI, pack-years of smoking, smoking status, drinking status, diabetes, hypertension, sports index, and education.
COPD based on spirometry and/or those with self-reported chronic bronchitis (having chronic cough most days of the week for at least 3 consecutive months a year for 2 years).
Participants who met the definition of COPD based on spirometry at both the baseline and second examinations.
The interaction between spirometric COPD and baseline smoking status on risk of death was not statistically significant for both Blacks and Whites (data not shown, p>0.20 for both races). Among current-smokers, both Blacks (HR=2.18, 95%CI 1.57–3.04) and Whites (HR=1.73, 95% CI 1.42–2.10) with spirometric COPD had an increased risk of death compared to those without spirometry-defined COPD. In former-smokers, the increased risk of mortality with spirometric COPD remained statistically significant in Whites (HR=1.49, 95% CI 1.17–1.89), but not Blacks (HR=1.29, 95% CI 0.72–2.24). The association of increased risk of death among those with COPD did not hold among never-smokers at baseline (HR=1.16, 95% CI 0.56–2.41 for Blacks and HR=1.12, 95% CI 0.58–2.20 for Whites). However, this may be due to the small number of never-smoking participants who had COPD at baseline.
A sensitivity analysis was conducted including those with either FEV1 < 80% of the predicted value or FEV1/FVC ratio < 70%, but not both criteria, in the referent no spirometric COPD category. The hazard ratios did not differ from those in Table 2 (data not shown).
DISCUSSION
In this population-based prospective study with up to 18 years of follow-up, we found 59–74% higher mortality rates among individuals with spirometric COPD compared to those without spirometric COPD, and this hazard ratio was similar for Blacks and Whites. In contrast, for both those with and without COPD, death rates were approximately double in Blacks compared to Whites.
Although it is well established that COPD is a risk factor for premature mortality, data are limited on whether COPD carries comparable relative risks of all-cause mortality in Blacks and Whites. Our findings suggest that given COPD, Blacks and Whites have the same proportionate increase in mortality, with a 59–74% increase in mortality for individuals with COPD compared to those without COPD. The public health burden of COPD is enormous, and strategies to reduce COPD and smoking could have a large impact on total mortality rates of both Blacks and Whites. Our data suggest that reducing the burden of COPD may have a similar mortality impact on both race/ethnic groups.
A higher all-cause mortality rate in Blacks compared to Whites is well known17–20 and in our study this was also observed regardless of COPD status. This ethnic disparity is undoubtedly attributable to lifestyle factors, comorbidities, access to care, and socioeconomic status. In our sample, a larger proportion of Blacks were overweight, inactive, diabetic, hypertensive, and less well educated than Whites. Less access to medical care among Blacks compared to Whites21 is also a likely key factor in the increased mortality among Blacks in our cohort compared to Whites. Yet, our data suggest that the difference in death rates between Blacks and Whites cannot be explained by COPD status.
Our study included a large community-based sample of Blacks and Whites. However, there were some limitations to this study. First, in the ARIC cohort, the Blacks were all from Jackson, MS or Forsyth County, NC, whereas the Whites were from Forsyth County, NC, Minneapolis suburbs, MN, and Washington County, MD. Thus, geography and ethnicity are confounded in ARIC and our results should not be generalized to the whole U.S. Second, we excluded a large number of participants who had missing or unacceptable lung function tests (N=4195). Not unexpectedly, these excluded participants were less healthy than the remaining cohort. Those excluded were more likely to be Black, female, older, less physically active, and had more diabetes and hypertension than the participants remaining in our analysis (data not shown). Additionally, those excluded also had higher crude rates of death, by approximately 4 per 1000 person-years, than those in our analysis (data not shown). Therefore, it is possible that the race-specific associations between COPD and mortality would have been somewhat different had acceptable lung function tests been obtained from these participants. Third, we defined our exposure as the presence or absence of COPD. There were too few individuals with moderate to severe COPD in our dataset to stratify based on COPD severity. Had we been able to further stratify on COPD severity, we may have been able to determine if the shape of the COPD severity curve with mortality differed for Blacks and Whites. Finally, there may have been some misclassification based on our secondary COPD definition using self-reported symptoms of chronic bronchitis.
In conclusion, although death rates were 2-fold higher in Blacks compared to Whites regardless of COPD status, the increased hazard ratio of death in relation to COPD was similar in Blacks and Whites. Thus, COPD does not seem to convey greater risk of death in one race/ethnic group than the other. The prevention of COPD, particularly through decreasing smoking, should be viewed as equally important in reducing mortality in both Blacks and Whites.
ACKNOWLEDGMENT
The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts N01-HC-55015, N01-HC-55016, N01-HC-55018, N01-HC-55019, N01-HC-55020, N01-HC-55021, and N01-HC-55022. The authors thank the staff and participants of the ARIC study for their important contributions. AMC was supported by NHLBI grant T32-HL-007779.
Footnotes
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Rabe KF, Hurd S, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007;176:532–555. doi: 10.1164/rccm.200703-456SO. [see comment] [DOI] [PubMed] [Google Scholar]
- 2.De Palo VA. Pulmonary disease: Pneumonia, chronic obstructive pulmonary disease, asthma, and thromboembolic disease. J Am Podiatr Med Assoc. 2004;94:157–167. doi: 10.7547/87507315-94-2-157. [DOI] [PubMed] [Google Scholar]
- 3.Petty TL, Silvers GW, Stanford RE, Baird MD, Mitchell RS. Small airway pathology is related to increased closing capacity and abnormal slope of phase III in excised human lungs. Am Rev Respir Dis. 1980;121:449–456. doi: 10.1164/arrd.1980.121.3.449. [DOI] [PubMed] [Google Scholar]
- 4.Saetta M, Ghezzo H, Kim WD, et al. Loss of alveolar attachments in smokers. A morphometric correlate of lung function impairment. Am Rev Respir Dis. 1985;132:894–900. doi: 10.1164/arrd.1985.132.4.894. [DOI] [PubMed] [Google Scholar]
- 5.Lopez AD, Shibuya K, Rao C, et al. Chronic obstructive pulmonary disease: Current burden and future projections. Eur Respir J. 2006;27:397–412. doi: 10.1183/09031936.06.00025805. [DOI] [PubMed] [Google Scholar]
- 6.Kung HC, Hoyert DL, Xu JQ, Murphy SL. National vital statistics reports. vol 56. National center for health statistics; Hyattsville, MD: 2008. Deaths: Final data for 2005. no 10. [PubMed] [Google Scholar]
- 7.Kazerouni N, Alverson CJ, Redd SC, Mott JA, Mannino DM. Sex differences in COPD and lung cancer mortality trends--united states, 1968–1999. J Women's Health. 2004;13:17–23. doi: 10.1089/154099904322836410. [DOI] [PubMed] [Google Scholar]
- 8.Mannino DM, Doherty DE, Sonia Buist A. Global initiative on obstructive lung disease (GOLD) classification of lung disease and mortality: Findings from the atherosclerosis risk in communities (ARIC) study. Respir Med. 2006;100:115–122. doi: 10.1016/j.rmed.2005.03.035. [DOI] [PubMed] [Google Scholar]
- 9.McGarvey LP, John M, Anderson JA, Zvarich M, Wise RA, TORCH Clinical Endpoint C Ascertainment of cause-specific mortality in COPD: Operations of the TORCH clinical endpoint committee. Thorax. 2007;62:411–415. doi: 10.1136/thx.2006.072348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.The ARIC investigators The atherosclerosis risk in communities (ARIC) study: Design and objectives. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 11.American Thoracic Society ATS statement--snowbird workshop on standardization of spirometry. Am Rev Respir Dis. 1979;119:831–838. doi: 10.1164/arrd.1979.119.5.831. [DOI] [PubMed] [Google Scholar]
- 12.Crapo RO, Morris AH, Gardner RM. Reference spirometric values using techniques and equipment that meet ATS recommendations. Am Rev Respir Dis. 1981;123:659–664. doi: 10.1164/arrd.1981.123.6.659. [DOI] [PubMed] [Google Scholar]
- 13.Harik-Khan RI, Fleg JL, Muller DC, Wise RA. The effect of anthropometric and socioeconomic factors on the racial difference in lung function. Am J Respir Crit Care Med. 2001;164:1647–1654. doi: 10.1164/ajrccm.164.9.2106075. [DOI] [PubMed] [Google Scholar]
- 14.American Thoracic Society Lung function testing: Selection of reference values and interpretative strategies. Am Rev Respir Dis. 1991;144:1202–1218. doi: 10.1164/ajrccm/144.5.1202. [DOI] [PubMed] [Google Scholar]
- 15.Glindmeyer HW, Lefante JJ, McColloster C, Jones RN, Weill H. Blue-collar normative spirometric values for caucasian and african-american men and women aged 18 to 65. Am J Respir Crit Care Med. 1995;151:412–422. doi: 10.1164/ajrccm.151.2.7842200. [DOI] [PubMed] [Google Scholar]
- 16.Baecke J, Burema J, Frijters J. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr. 1982;36:936–942. doi: 10.1093/ajcn/36.5.936. [DOI] [PubMed] [Google Scholar]
- 17.Levine RS, Foster JE, Fullilove RE, et al. Black-white inequalities in mortality and life expectancy, 1933–1999: Implications for healthy people 2010. Public Health Rep. 2001;116:474–483. doi: 10.1093/phr/116.5.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cooper RS. Health and the social status of blacks in the united states. Ann Epidemiol. 1993;3:137–144. doi: 10.1016/1047-2797(93)90126-o. [DOI] [PubMed] [Google Scholar]
- 19.Margellos H, Silva A, Whitman S. Comparison of health status indicators in chicago: Are black-white disparities worsening? Am J Public Health. 2004;94:116–121. doi: 10.2105/ajph.94.1.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barnett E, Armstrong DL, Casper ML. Social class and premature mortality among men: A method for state-based surveillance. Am J Public Health. 1997;87:1521–1525. doi: 10.2105/ajph.87.9.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mayberry RM, Mili F, Ofili E. Racial and ethnic differences in access to medical care. Med Care Res Rev. 2000;57:108–145. doi: 10.1177/1077558700057001S06. [DOI] [PubMed] [Google Scholar]