Summary
Formation of synaptic connections requires alignment of neurotransmitter receptors on postsynaptic dendrites opposite matching transmitter release sites on presynaptic axons. β-neurexins and neuroligins form a trans-synaptic link at glutamate synapses. We show here that neurexin alone is sufficient to induce glutamate postsynaptic differentiation in contacting dendrites. Surprisingly, neurexin also induces GABA postsynaptic differentiation. Conversely, neuroligins induce presynaptic differentiation in both glutamate and GABA axons. Whereas neuroligins-1, -3, and -4 localize to glutamate postsynaptic sites, neuroligin-2 localizes primarily to GABA synapses. Direct aggregation of neuroligins reveals a linkage of neuroligin-2 to GABA and glutamate postsynaptic proteins, but the other neuroligins only to glutamate postsynaptic proteins. Furthermore, mislocalized expression of neuroligin-2 disperses postsynaptic proteins and disrupts synaptic transmission. Our findings indicate that the neurexin-neuroligin link is a core component mediating both GABAergic and glutamatergic synaptogenesis, and differences in isoform localization and binding affinities may contribute to appropriate differentiation and specificity.
Introduction
During brain development, billions of neurons must connect to one another with extraordinary specificity, creating specialized junctions that transmit signals with high speed and spatial precision. Neurotransmitter-filled vesicles and regulated release machinery in the presynaptic axon must be aligned precisely with appropriate receptors and associated scaffolding and signaling proteins in the postsynaptic dendrite. At the vertebrate neuromuscular junction, agrin from the motoneuron axon induces acetylcholine receptor clustering on the postsynaptic muscle surface (Sanes and Lichtman, 2001). In the mammalian brain, excitatory glutamatergic and inhibitory GABAergic synapses form the basis of most circuits. However, the signals that induce the postsynaptic differentiation of these major CNS synaptic types are unknown.
Recently, neuroligins and synCAMs have been shown to induce presynaptic differentiation in glutamatergic axons (Biederer et al., 2002; Scheiffele et al., 2000). Neuroligins are postsynaptic transmembrane proteins produced from at least four genes, each with several splice variants (Bolliger et al., 2001; Ichtchenko et al., 1996). Intracellularly, neuroligins bind to the PDZ domain of PSD-95 (Irie et al., 1997), a scaffolding protein in excitatory synapses. Extracellularly, neuroligins bind to β-neurexins but not α-neurexins (Ichtchenko et al., 1995). Neurexins are presynaptic transmembrane proteins present in many variants, generated from three genes with two promoters each and many alternative splice sites (Tabuchi and Sudhof, 2002). Neurexins associate with synaptic vesicles by interaction with presynaptic scaffolding proteins such as CASK and Mints and direct binding to synaptotagmin (Biederer and Sudhof, 2000; Hata et al., 1993). Thus, the neurexin-neuroligin link may act as a trans-synaptic bridge bringing vesicles into alignment with the postsynaptic density. This hypothesis gained support when Scheiffele et al. (2000) demonstrated that neuroligin expressed on nonneuronal cells clustered synaptic vesicles in contacting glutamatergic axons. Moreover, antibody-induced clustering of recombinant neurexin directly induced the coclustering of synaptic vesicles (Dean et al., 2003).
There have been no reports of molecules that can induce postsynaptic differentiation of glutamatergic or GABAergic synapses, like agrin does at the neuromuscular junction. Other protein families found at glutamatergic synapses include cadherins, protocadherins, and CNRs, ephrins and Eph receptors, and neuronal activity-regulated pentraxin (Narp) (Craig and Boudin, 2001; Garner et al., 2002; Yamagata et al., 2003). Ephrins binding to EphB receptors can promote NMDA receptor aggregation and spine maturation (Dalva et al., 2000; Henkemeyer et al., 2003) but do not induce clustering of scaffolding proteins such as PSD-95 that normally cluster at developing synapses prior to receptors (Friedman et al., 2000; Rao et al., 1998). Narp appears to have a specialized function in regulating the synaptic density of AMPA receptors on aspiny neurons (O’Brien et al., 1999). Thus, ephrins and Narp are unlikely to be initial triggers for postsynaptic differentiation.
We reasoned that neurexins may be good candidates for inducing postsynaptic differentiation at glutamate synapses. In addition to transducing the signal from neuroligins to mediate synaptic vesicle clustering in the axon, they may actively signal to neuroligins to promote clustering of PSD-95 and glutamate receptors in the dendrite. We show here that not only do neurexins induce glutamatergic postsynaptic differentiation, but surprisingly they also induce clustering of GABA receptors and inhibitory postsynaptic scaffolding molecules via neuroligin family members.
Results
Synaptogenic Activity of Neuroligin
Given that β-neurexins are expressed in both excitatory and inhibitory neurons, as assayed by in situ hybridization (Ullrich et al., 1995), we investigated whether neuroligin could induce presynaptic differentiation in both excitatory and inhibitory axons. We cultured COS cells transfected with neuroligin on top of one-week-old dissociated hippocampal cultures grown by the Banker method (Goslin et al., 1998). The cocultures were immunostained for glutamic acid decarboxylase 65 (GAD65), an enzyme that converts glutamate into GABA in GABAergic axons, and vesicular glutamate transporter 1 (VGlut1), a protein that transports glutamate into glutamatergic synaptic vesicles. Absence of immunoreactivity for postsynaptic antigens distinguished GAD65 and VGlut1 clusters induced by neuroligins from the few endogenous synapses that happen to lie under transfected fibroblasts. Neuroligin-1 and -2 induced clustering of both VGlut1-positive glutamatergic and GAD65-positive GABAergic synaptic vesicles in contacting axons (Figures 1A and 1B and Supplemental Figure S1 at http://www.cell.com/cgi/content/full/119/7/1013/DC1/). Furthermore, recombinant CFP-neurexin-1β localized to presynaptic specializations in both excitatory and inhibitory neurons, coclustering with synaptophysin-YFP and endogenous VGlut1 and GAD (Supplemental Figure S2 on the Cell website). Thus, β-neurexins are expressed by excitatory and inhibitory neurons, tagged neurexin-1β localizes to presynaptic sites in glutamatergic and GABAergic axons, and neuroligins induce clustering of synaptic vesicles in both glutamatergic and GABAergic axons, presumably by binding to neurexins.
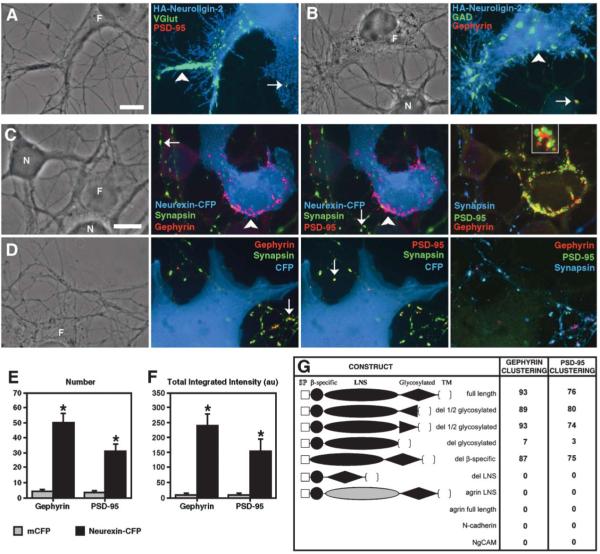
Figure 1.
Neuroligin Clusters Glutamate and GABA Synaptic Vesicles; Neurexin Clusters Glutamate and GABA Postsynaptic Scaffolding Proteins
(A and B) Fibroblasts (F) expressing HA-neuroligin-2 cocultured with hippocampal neurons (N) induced clusters of VGlut1 (A) and GAD (B) in contacting glutamatergic and GABAergic axons. The induced clusters of VGlut1 or GAD lacked postsynaptic PSD-95 or gephyrin immunoreactivity (arrowheads) in contrast to endogenous synaptic clusters (arrows).
(C) Fibroblasts (F) expressing neurexin-1β-CFP cocultured with hippocampal neurons (N) induced clusters of gephyrin and PSD-95 in contacting dendrites. Induced clusters (arrowheads) lacked immunoreactivity for the presynaptic antigen synapsin, unlike endogenous synaptic clusters (arrows). As shown in the magnified inset, induced clusters of gephyrin and PSD-95 were partially overlapping but largely distinct.
(D) Coculture of fibroblasts (F) expressing CFP with neurons resulted in only endogenous synaptic clusters (arrows) but no induced clusters of gephyrin or PSD-95 at contacts with dendrites.
(E and F) Number and total integrated intensity of nonsynaptic clusters of gephyrin and PSD-95 was greater for neuronal processes within areas contacted by fibroblasts expressing neurexin-1β-CFP than mCFP (* p < 0.001, n = 40 cells each).
(G) The neurexin-1β LNS domain was essential for synaptogenic activity, but the β-specific region and glycosylated region were not. Numbers indicate the percentage of fibroblasts expressing the indicated construct (extracellular domain shown) associated with nonsynaptic clusters of gephyrin or PSD-95 in a neuron coculture assay (n = 100 cells counted each). The neurexin LNS domain could not be functionally replaced with the agrin LNS domain. Full-length agrin, N-cadherin, or NgCAM had no synaptogenic activity in this assay.
Scale bars 10 μm.
Synaptogenic Activity of Neurexin
To determine whether β-neurexins have synaptogenic activity, we tested the ability of neurexin-1β conjugated with cyan fluorescent protein (CFP) at its intracellular C terminus to cluster postsynaptic proteins in a neuron-fibroblast coculture assay. We found that COS cells expressing neurexin-CFP induced clusters of the PSD-95 family of excitatory postsynaptic scaffolding proteins in contacting dendrites (Figure 1C). Surprisingly, even stronger clustering occurred for gephyrin, a scaffolding protein found exclusively in inhibitory synapses (Figure 1C). The clustering of scaffolding proteins often occurred at the edges of COS cells, where the fibroblast cells were most likely in greatest contact with their environment. The great majority (93%–97%) of gephyrin and PSD-95 clusters associated with COS cells expressing neurexin-CFP lacked synapsin immunoreactivity, indicating that neurexin actively induced the clustering of gephyrin and PSD-95 rather than influencing the formation of endogenous synapses. Although neurexin-induced clustering of gephyrin and PSD-95 often occurred on the same dendrite, gephyrin and PSD-95 clusters were generally distinct, partially overlapping but primarily nonoverlapping (Figure 1C, inset). Such clustering of PSD-95 and gephyrin was never seen at dendrite contacts with COS cells expressing CFP, mCFP (membrane-associated CFP via the GAP-43 fatty acylation signal), or other neuronal cell adhesion proteins N-cadherin or NgCAM/L1 (Figure 1 and Supplemental Figure S3 on the Cell website). Quantitation of synapsin-immunonegative clusters on dendrites underlying transfected fibroblasts revealed a significant increase in cluster number and total intensity of both gephyrin and PSD-95 for cells expressing neurexin-CFP compared with mCFP control (Figures 1E and 1F). The number of synapsin-immunopositive gephyrin and PSD-95 puncta was not different under neurexin-CFP versus CFP transfected fibroblast cells (4.7 ± 1.1 versus 7.1 ± 1.1 respectively, p > 0.1), suggesting that axon and dendrite area under the two conditions was similar.
Induced clustering of gephyrin and PSD-95 by neurexin was consistently observed with rat or mouse hippocampal neurons grown for 2 days to >2 weeks in culture and with either COS or CV1 cells. Even immature neurons lacking endogenous synapses exhibited clusters of gephyrin and PSD-95 precisely colocalized to sites of contact with COS cells expressing neurexin-CFP (Supplemental Figure S4 on the Cell website). Clusters of PSD-95 and gephyrin were observed in >60% of immature 2- to 3-day-old neurons overlapping COS cells expressing neurexin-CFP versus only 15% of sister neurons overlapping COS cells expressing mCFP (n = 20). Neurexin-induced clustering of gephyrin and PSD-95 was observed on dendrites of both pyramidal neurons and interneurons (data not shown). Furthermore, neurexin induced multiple aspects of glutamatergic post-synaptic differentiation (Supplemental Figure S5 on the Cell website), including clustering of GKAP, another glutamatergic postsynaptic scaffolding protein, and Syn-GAP, a glutamatergic postsynaptic signal transducing enzyme (Kennedy, 2000; Sheng and Kim, 2002).
We further assayed the ability of neurexin-1β to cluster neurotransmitter receptors (Figures 2A–2C versus mCFP control in Supplemental Figures S6A–S6C on the Cell website and data not shown). Neurexin induced clustering of GABAA receptor subunits γ2 and α2 just as strikingly as gephyrin. Neurexin also induced clustering of the essential NR1 subunit of NMDA receptors. However, we did not observe clustering of the AMPA receptor subunits GluR1 or GluR2, suggesting that other proteins or cellular mechanisms are required. We quantified the number and total integrated intensity of these clusters and found a significant increase in nonsynaptic GABA receptor and NMDA receptor clusters, but not AMPA receptor clusters, in dendrites associated with fibroblasts expressing neurexin-CFP compared with mCFP (Figures 2D and 2E). To confirm that the induced receptor clusters were on dendrites, separate cocultures were immunolabeled for each of the receptors and the dendrite marker MAP2 (Supplemental Figures S7A–S7C on the Cell website). Using live cell antibody labeling, we found that the nonsynaptic neurexin-induced clusters of GABAA receptor are on the surface of dendrites (Supplemental Figures S7D and S7E on the Cell website) and thus presumably functional.
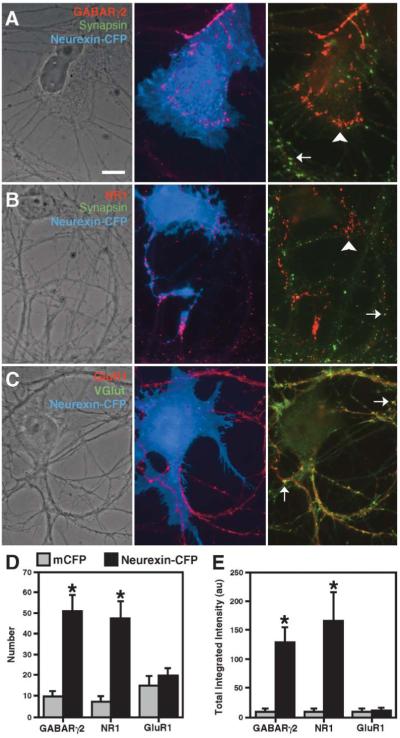
Figure 2.
Neurexin Clusters GABA and NMDA Glutamate Receptors
(A and B) Fibroblasts expressing neurexin-1β-CFP cocultured with hippocampal neurons induced clusters of GABAARγ2 (A) and NR1 (B) in contacting dendrites. Induced clusters (arrowhead) lacked immunoreactivity for synapsin, unlike endogenous synaptic clusters (arrow).
(C) Neurexin-1β-CFP did not induce clustering of GluR1; only endogenous clusters colocalizing with VGlut1 were observed (arrows). Unlike the other receptors, GluR1 levels are relatively high in all dendrite shafts regardless of fibroblast contact.
(D and E) The number and total integrated intensity of nonsynaptic clusters of GABAARγ2 and NR1, but not GluR1, was greater for neuronal processes within areas contacted by fibroblasts expressing neurexin-1β-CFP than mCFP (* p < 0.05, n = 20 cells each).
Scale bar 10 μm.
Neurexin LNS Domain Mediates Synaptogenic Activity
The mature extracellular region of neurexin-1β consists of a 38 aa β-neurexin-specific sequence, a single 178 aa LNS domain, and a 102 aa glycosylated sequence (Ushkaryov et al., 1992). To determine which region(s) are responsible for neurexin-induced clustering of postsynaptic proteins, we created a series of mutations in which each individual region was deleted and assayed synaptogenic activity in the neuron-fibroblast coculture. We found that the LNS domain is responsible for clustering both gephyrin and PSD-95 (Figure 1G). While deletion of the β-neurexin specific region did not affect clustering of either gephyrin or PSD-95, deletion of the LNS domain fully abolished clustering of both proteins. Functional impairment caused by the full deletion of the glycosylated region may have been due to placement of the LNS domain too close to the membrane, rather than loss of essential activity of the glycosylated region since deletion of either half had no impact (a conclusion further supported by Figure 4 below).
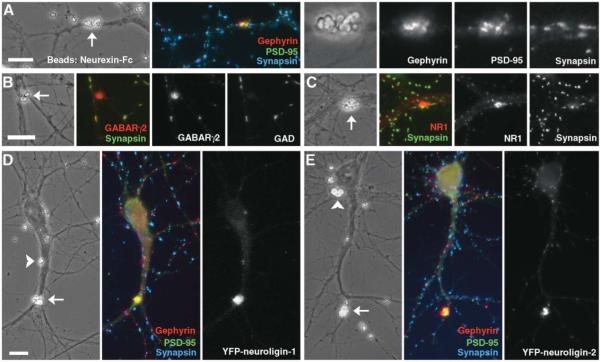
Figure 4.
Purified Neurexin Coclusters Neuroligins with Postsynaptic Proteins
(A–C) Purified neurexin-Fc attached to beads induced clusters of gephyrin, PSD-95 (A), GABA receptor (B), and NR1 (C) at contact sites with dendrites (arrows in phase contrast images). Postsynaptic protein clusters induced by beads bearing neurexin-Fc lacked immunoreactivity for presynaptic proteins synapsin or GAD.
(D, E) In neurons expressing low levels of YFP-neuroligin-1 or YFP-neuroligin-2, purified neurexin-Fc attached to beads induced coclustering of gephyrin and PSD-95 with neuroligins. Dendrite-bead contacts either exhibited strong aggregation of all of YFP-neuroligin, gephyrin, and PSD-95 (arrows) or no detectable aggregation of any of these proteins (arrowheads).
Scale bars 10 μm.
To determine whether the LNS domain of neurexin-1β in particular is essential for induced clustering or whether any LNS domain in general will suffice, we tested both full-length agrin and a neurexin-1β construct replacing the LNS domain with the C-terminal LNS domain of agrin containing the 8 aa insert at the z splice site. This agrin LNS domain is responsible for clustering acetylcholine receptors at the neuromuscular junction (Gesemann et al., 1995) and exhibits remarkable structural similarity with the neurexin LNS domain (Rudenko et al., 1999). Expression of either full-length agrin or the neurexin construct swapping in the agrin LNS domain in fibroblasts resulted in a complete lack of clusterinducing activity for gephyrin or PSD-95 at contact sites with dendrites (Figure 1G and Supplemental Figures S8A–S8C on the Cell website; Serpinskaya et al., 1999). Confocal analysis indicated that each of the inactive constructs, including the agrin LNS swap, did reach the cell surface (Supplemental Figures S8D–S8I on the Cell website). Thus, specifically the neurexin-1β LNS domain, and not the LNS domain from agrin or full-length agrin induces clustering of glutamatergic and GABAergic postsynaptic proteins.
Neurexin Clusters Neuroligins but Not Dystroglycan
What is neurexin binding to on the dendrite to mediate postsynaptic differentiation? Neurexin likely clusters PSD-95 and NMDA receptors by binding to neuroligin. Neuroligin-1 localizes specifically to excitatory synapses (Song et al., 1999) and directly binds PSD-95 (Irie et al., 1997). But if it localizes only to glutamatergic synapses, it is a poor candidate for mediating gephyrin and GABA receptor clustering. On the other hand, neurexins are reported to bind to α-dystroglycan (Sugita et al., 2001), a component of a subset of inhibitory synapses (Knuesel et al., 1999; Levi et al., 2002). Thus, we tested whether neurexin can cluster dystroglycan in contacting dendrites. We found that neither α-dystroglycan (Supplemental Figure S9A on the Cell website) nor β-dystroglycan (Figure 3A) was clustered by neurexin in the coculture assay. These results suggest that dystroglycan is not essential for the neurexin-induced clustering of postsynaptic proteins.
Figure 3.
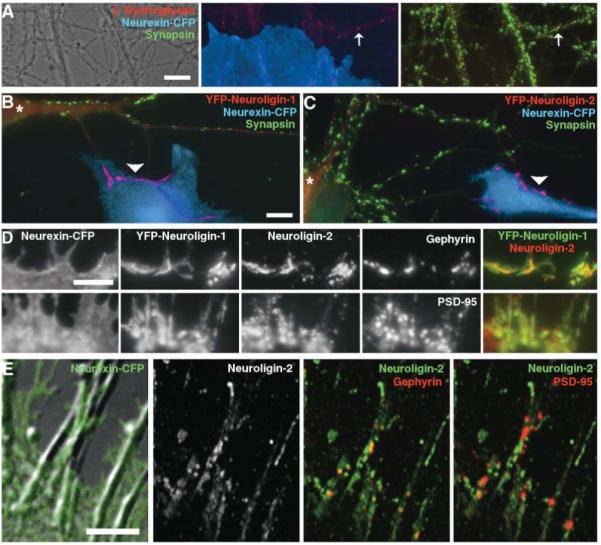
Neurexin Clusters Neuroligins but Not Dystroglycan
(A) Coculture of fibroblasts expressing neurexin-1β-CFP with neurons resulted in only endogenous synaptic clusters (arrows) but no induced clusters of β-dystroglycan at contacts with dendrites.
(B and C) Fibroblasts expressing neurexin-1β-CFP cocultured with hippocampal neurons expressing YFP-neuroligin-1 (B) or YFP-neuroligin-2 (C) induced clusters of YFP-neuroligin lacking immunoreactivity for synapsin in contacting dendrites. The transfected neuron is indicated by *.
(D) Fibroblasts expressing neurexin-1β-CFP cocultured with hippocampal neurons expressing YFP-neuroligin-1 induced overlapping clusters of recombinant YFP-neuroligin-1 and endogenous neuroligin-2 in contacting dendrites. Smaller clusters of gephyrin and PSD-95 were apparent within the neuroligin-positive dendrite regions.
(E) Fibroblasts expressing neurexin-1β-CFP cocultured with hippocampal neurons induced clusters of neuroligin-2, gephyrin, and PSD-95 in contacting dendrites. Within the neurexin contact region as assayed by confocal microscopy, gephyrin clusters were nearly all associated with high concentrations of neuroligin-2, whereas the association of PSD-95 clusters with neuroligin-2-immunopositive regions was more variable.
Scale bars (A)-(C) 10 μm, (D) and (E) 5 μm.
We turned our attention more closely to the role of neuroligins (Figure 3 and Supplemental Figure S9 on the Cell website). We tagged the mature N terminus of neuroligins-1 and -2 with yellow fluorescent protein (YFP), expressed these constructs in neurons at low level, and performed the coculture assay. Indeed, neurexin-1β-CFP presented on COS cells induced clustering of YFP-neuroligins-1 and -2 in contacting dendrites. The dendritic areas of clustering of YFP-neuroligins-1 and -2 overlapped considerably with the sites of clustering of PSD-95 and gephyrin, but were more extensive. This result was confirmed using an antibody against endogenous neuroligin-2: it clustered in dendrites at sites of contact with fibroblasts expressing neurexin-CFP but not mCFP. The dendritic areas of induced clustering of YFP-neuroligin-1 and endogenous neuroligin-2 overlapped extensively. Furthermore, the clusters of gephyrin and PSD-95 tended to occur within the regions of clustered neuroligins. Interestingly, when we compared the degree of overlap of gephyrin or PSD-95 with neuroligin-2 at sites of contact with fibroblasts expressing neurexin-CFP by confocal microscopy, there appeared to be some selectivity (Figure 3E). Whereas 85% ± 2% of gephyrin clusters colocalized with clustered neuroligin-2, only 58% ± 2% of PSD-95 clusters colocalized with clustered neuroligin-2 (n = 19 cells, p < 0.001). These results support the idea that neurexin induces clustering of glutamatergic and GABAergic postsynaptic proteins via binding neuroligins and that different neuroligin isoforms may help mediate specificity.
Purified Neurexin Coclusters Neuroligins with Postsynaptic Proteins
We next purified recombinant soluble neurexin-Fc (the leader sequence and neurexin LNS domain fused to the human Ig constant region), attached this neurexin-Fc to beads, and tested synaptogenic activity. Purified neurexin-Fc on beads induced aggregation of gephyrin, PSD-95, GABA receptor, and NR1 at contact points with neuronal dendrites (Figures 4A–4AC). These induced clusters lacked synapsin immunoreactivity, were generally brighter than the endogenous synaptic clusters, and could often be seen to follow the contour of beads or bead aggregates. About 15%–20% of apparent bead contact sites with dendrites exhibited induced clustering of gephyrin and PSD-95. The induction of postsynaptic protein clustering required the LNS domain of neurexin. Among hundreds of apparent contact sites of dendrites with similarly generated beads bearing neurexin-Fc deleted for the LNS domain, none exhibited nonsynaptic clusters of gephyrin or PSD-95 (data not shown). Thus, purified neurexin alone is synaptogenic and does not require any accessory component from transfected fibroblasts. This experiment also confirms the requirement for the neurexin LNS domain and the lack of requirement for the glycosylated region.
We next added beads bearing neurexin-Fc to neurons expressing low levels of YFP-neuroligin-1 or YFP-neuroligin-2 and immunolabeled for gephyrin and PSD-95 (Figures 4D and 4E). All bead-induced aggregates of YFP-neuroligins coaggregated gephyrin and PSD-95. Conversely, beads in apparent contact with transfected neuron dendrites that did not aggregate YFP-neuroligins did not aggregate gephyrin or PSD-95. Perhaps these particular beads lacked sufficient local concentration of neurexin-Fc or were not in close enough contact with the dendrites. This 1:1 correspondence supports the idea that neuroligins may mediate the ability of neurexin to induce local aggregation of postsynaptic scaffolding proteins and receptors.
Neuroligin Aggregation Alone Is Synaptogenic
Our results to this point are compatible with three general mechanisms for postsynaptic differentiation. One, as suggested above, is that neurexin-induced aggregation of neuroligins may be the primary and only necessary signal for inducing coaggregation of scaffolding proteins and receptors. Alternatively, neuroligins may be passively aggregated along with something else that actively transduces the signal from neurexins to postsynaptic scaffolds and neurotransmitter receptors. A third intermediate scenario is that aggregation of the scaffolding proteins and neurotransmitter receptors may require aggregation of neuroligins plus other pathways set in motion by local presentation of neurexins to dendrites. To differentiate among these possibilities, we locally aggregated neuroligins independently of neurexins. Transfection of neurons with low levels of YFP-neuroligin followed by application of beads bearing anti-YFP antibodies resulted in local clustering of the YFP-neuroligin on the surface of transfected dendrites. Such direct aggregation of YFP-neuroligin-1 resulted in coaggregation of PSD-95 but not gephyrin (Figure 5A). Fluorescence intensity associated with the clustered YFP-neuroligin-1 normalized to the mean dendrite intensity was 2.01 ± 0.14 for PSD-95 and 1.16 ± 0.09 for gephyrin (n = 20). In contrast, direct aggregation of YFP-neuroligin-2 resulted in coaggregation of PSD-95 and gephyrin, gephyrin to a greater extent than PSD-95 (Figure 5B). Fluorescence intensity associated with the clustered YFP-neuroligin-2 normalized to the mean dendrite intensity was 1.67 ± 0.12 for PSD-95 and 2.26 ± 0.24 for gephyrin (n = 20). Gephyrin and PSD-95 could both be aggregated by a single bead but in different subregions of the concentrated YFP-neuroligin-2, with PSD-95 often filling in the smaller portion not occupied by gephyrin.
Figure 5.
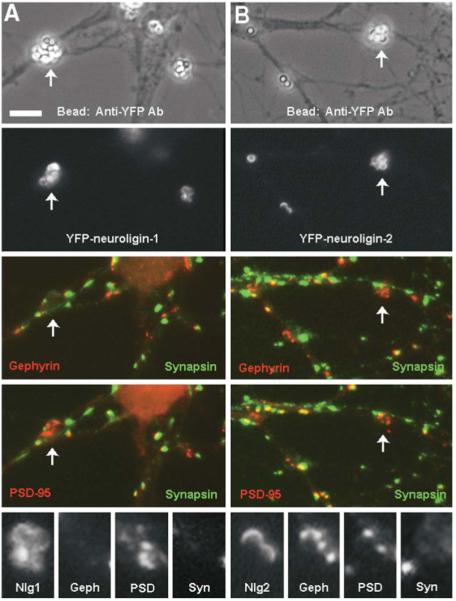
Neuroligin Aggregation Alone Is Synaptogenic
Neurons expressing low levels of YFP-neuroligins were exposed to beads bearing antibodies against YFP to aggregate the neuroligins on dendrites independently of neurexin. Aggregation of YFP-neuroligin-1 resulted in coaggregation of PSD-95 but not gephyrin (A), whereas aggregation of YFP-neuroligin-2 resulted in coaggregation of both PSD-95 and gephyrin (B). Aggregates of the postsynaptic proteins induced by neuroligin aggregation lacked immunoreactivity for the presynaptic protein synapsin. Arrows indicate sites of induced aggregation; other such sites are enlarged below (Nlg1, YFP-neuroligin-1; Geph, gephyrin; PSD, PSD-95; Syn, synapsin; Nlg2, YFP-neuroligin-2). Scale bar 5 μm.
The neuroligin family is comprised of at least four genes (neuroligins-1 to -4 and -4Y in humans; Bolliger et al., 2001; Jamain et al., 2003). To assess the molecular linkages of the other neuroligins, we expressed YFP-neuroligin-3 and YFP-neuroligin-4 in cultured neurons and aggregated these with the anti-YFP beads. Like neuroligin-1, aggregation of either neuroligin-3 or -4 resulted in coaggregation of PSD-95 but not gephyrin (data not shown). This result differs from that of presenting neurexin-Fc on beads; neurexin presumably aggregates all endogenous neuroligins including neuroligin-2, thus leading to local aggregation of both PSD-95 and gephyrin (Figure 4). First, these results indicate that aggregation of neuroligins is sufficient to induce postsynaptic protein clustering; no other events are needed to transduce the signal from neurexins. Second, there is some degree of specificity: neuroligins-1, -3, and -4 link only to glutamatergic postsynaptic proteins, but neuroligin-2 can link to both glutamatergic and GABAergic postsynaptic proteins.
Neuroligins Localize to GABA and Glutamate Synapses
Whereas endogenous neuroligin-1 localizes to glutamate but not GABA synapses in the brain (Song et al., 1999), the subcellular localization of the other neuroligins is not known. We thus determined the distribution of endogenous neuroligin-2 in hippocampal neurons. Neuroligin-2 immunoreactivity was highly punctate in dendrites but did not colocalize with the glutamatergic synaptic proteins PSD-95 or VGlut1 (Figure 6A). Instead, neuroligin-2 coclustered precisely with gephyrin apposed to GAD-immunoreactive terminals (Figure 6B). The selective distributions of the neuroligin isoforms were further confirmed by expression of YFP-neuroligins-1 and -2 in neurons at very low levels. YFP-neuroligin-1 was partially diffuse and partially concentrated at glutamate postsynaptic sites, colocalizing with PSD-95 and VGlut1 (Figure 6C). In contrast, YFP-neuroligin-2 was highly punctate and did not colocalize with PSD-95 or VGlut1 but rather coclustered tightly with gephyrin opposite GAD-immunopositive terminals (Figure 6E). This distribution was unaffected by the presence or absence of splice site B in the acetylcholinesterase-like domain of YFP-neuroligin-2; similarly, spliced forms of YFP-neuroligins-1 and -2 (A2+B+) exhibited the differential localization to glutamate versus GABA postsynaptic sites (data not shown). How is this apparent localization of neuroligin-2 exclusively to GABAergic synapses compatible with its apparent association with PSD-95 and other glutamatergic postsynaptic proteins in our experiments and in previous studies? A very recent report by Prange et al. (2004) suggested a resolution to this question. They found that the level of PSD-95 in a neuron controls the balance between excitatory and inhibitory synapses. For example, overexpressing PSD-95 increased levels of neuroligin-1 at glutamate synapses, reduced the percentage of GABAergic relative to glutamatergic inputs, and reduced mIPSC frequency (Prange et al., 2004). We reasoned that perhaps high levels of PSD-95 in a neuron can recruit neuroligin-2 from GABA to glutamate synapses. Indeed, we found that expression of PSD-95-CFP to increase levels of PSD-95 resulted in a redistribution of YFP-neuroligin-2 from exclusively GABAergic synapses to both GABAergic and glutamatergic synapses (Figure 6F). Thus, depending on the level of endogenous PSD-95, neuroligin-2 may be exclusively clustered at inhibitory synapses or clustered at both inhibitory and excitatory synapses. In contrast, YFP-neuroligin-3 and -4 behaved essentially identically to neuroligin-1; all were partially diffuse and partially clustered at glutamatergic synapses alone, aggregated more at glutamate synapses with higher levels of PSD-95, but not concentrated at GABA synapses (data not shown). Thus, neuroligin-2 appears unique in its ability to associate with GABA synapses and to shuttle between GABA and glutamate synapses.
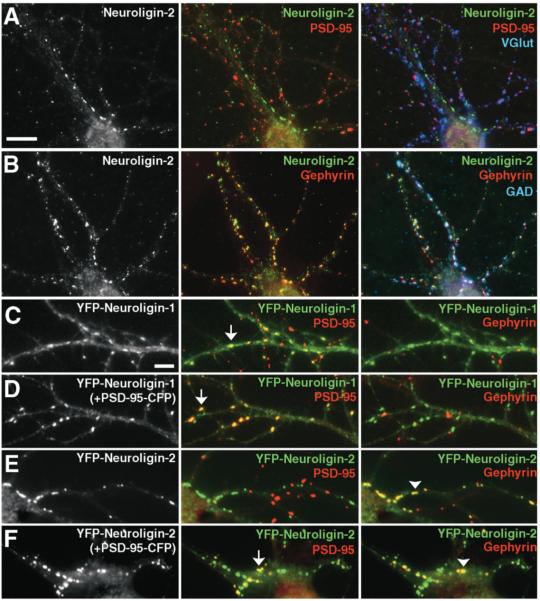
Figure 6.
Neuroligin-2 Localizes Primarily to GABA Synapses but Can Be Recruited to Glutamate Synapses
(A, B) Immunoreactivity for endogenous neuroligin-2 in hippocampal neurons clusters at GABA synapses with gephyrin and GAD, but not at glutamate synapses with PSD-95 or VGlut.
(C) YFP-neuroligin-1 expressed at low level is partially diffuse and partially concentrated at glutamate synapses labeled with PSD-95 (arrow), but not at GABA synapses labeled with gephyrin.
(D) Coexpression of PSD-95-CFP enhances the localization of YFP-neuroligin-1 to glutamate synapses (arrow).
(E) YFP-neuroligin-2 expressed at low level is highly punctate and colocalizes with gephyrin at GABA synapses (arrowhead), but is not detected with PSD-95 at glutamate synapses.
(F) Coexpression of PSD-95-CFP recruits YFP-neuroligin-2 to glutamate synapses (arrow) in addition to its localization with gephyrin at GABA synapses (arrowhead).
Scale bars (A) and (B) 10 μm, (C)–(F) 5 μm.
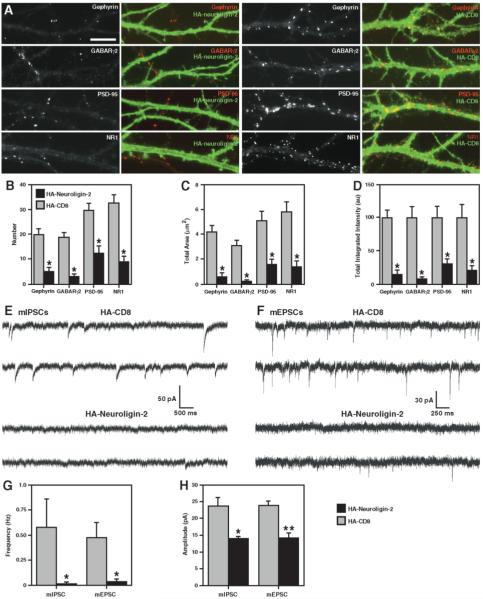
Mislocalized Expression of Neuroligin Disrupts Synapses
We next determined whether neuroligins play an active role in endogenous glutamatergic and GABAergic synapse formation. We expressed neuroligin-2 tagged with HA at its N terminus (HA-neuroligin-2) at high level so that it would mislocalize in cultured neurons to cover the entire dendritic surface. If neuroligin has a key role in synaptogenesis, the excess nonsynaptically localized neuroligin might be expected to disperse other synaptic components away from synapses. Indeed, mislocalized expression of HA-neuroligin-2, but not HA-CD8, used as a control, reduced both GABAergic and glutamatergic dendritic postsynaptic protein clusters, dispersing PSD-95, gephyrin, GABAR, and NR1 (Figure 7A). The number, total area, and total intensity of dendritic clusters of all postsynaptic proteins assayed were significantly reduced in the HA-neuroligin-2 expressing neurons as compared to neurons expressing HA-CD8 (Figures 7B–7D). These neurons still received glutamatergic and GABAergic axon presynaptic contact, often to a greater extent than control due to the synaptogenic activity of neuroligins (data not shown).
Figure 7.
Mislocalized Expression of Neuroligin Disrupts Postsynaptic Protein Localization and Synaptic Transmission
(A) High level extrasynaptic expression of HA-neuroligin-2 (left panels) but not HA-CD8 control (right panels) in hippocampal neurons disrupts dendritic clustering of gephyrin, GABA receptor, PSD-95, and NMDA receptor. Most of the clusters seen in the left panels are from untransfected dendrites.
(B–D) The number, total area, and total integrated intensity of clusters per dendrite length of gephyrin, GABA receptor, PSD-95, and NMDA receptor are reduced in neurons expressing mislocalized HA-neuroligin-2 compared with HA-CD8 control (* p < 0.001, n = 20 cells each).
(E and F) Representative recordings show reduction in mIPSCs (E) and mEPSCs (F) in neurons expressing mislocalized HA-neuroligin-2 compared to HA-CD8 control.
(G and H) The frequency (G) and amplitude (H) of both mIPSCs and mEPSCs is reduced in neurons expressing high levels of HA-neuroligin-2 compared to HA-CD8 control (* p < 0.01, ** p < 0.05, Kolmogorov-Smirnov test, n = 11–13 cells each; only the neurons exhibiting PSCs were included in the amplitude comparison).
Scale bar 10 μm.
To further characterize the role of neuroligins in functional synaptogenesis, we performed whole-cell patch clamp recordings in voltage clamp mode for neurons expressing either HA-neuroligin-2 or HA-CD8. Neurons were cotransfected with YFP to mark expressing cells; separate experiments confirmed that all of 50 neurons assayed expressing YFP coexpressed mislocalized HA-neuroligin-2. Mislocalized expression of HA-neuroligin-2, but not HA-CD8, significantly reduced frequency and amplitude of both AMPA-mediated miniature excitatory postsynaptic currents (mEPSCs) and GABAA-mediated miniature inhibitory postsynaptic currents (mIPSCs) (Figures 7E–7H). Several cells expressing HA-neuroligin-2 had no detectable events in 5 min of recording, and the others fell below or in the lowest range of the control group frequency. For the neuroligin-expressing cells exhibiting mPSCs, the amplitudes were reduced, with the mean values per cell all falling below the control range. Thus, mislocalized expression of HA-neuroligin-2 in neurons disrupts synaptic protein localization and synaptic transmission. These results suggest that neuroligins are an essential postsynaptic component for maintaining both glutamatergic and GABAergic synapses.
Discussion
We present here evidence that neurexins and neuroligins are essential for GABAergic and glutamatergic synaptogenesis and sufficient to trigger this process. Neurexins at glutamate synapses actively signal to dendrites to trigger clustering of glutamate postsynaptic scaffolding proteins, signal transducing enzymes, and NMDA glutamate receptors. The signaling mechanism occurs by simple aggregation of neuroligins. Disruption of the neurexin-neuroligin system results in disassembly of existing glutamate synapses and loss of transmission. Moreover, the role of neurexins and neuroligins is not restricted to glutamate synapses. Although we initially assessed GABAergic synapses as an expected negative control, the evidence pointing to a central role for neurexins and neuroligins at GABA synapses is as compelling as for glutamate synapses. We conclude that neurexins and neuroligin-2 mediate GABAergic synaptogenesis based on the following findings: (1) neuroligin induces clustering of GAD and vesicular GABA transporter (VGAT) presynaptically, (2) neurexin induces clustering of gephyrin and GABAA receptors postsynaptically, (3) neurexin concentrates at GABAergic boutons, (4) neuroligin-2 concentrates at GABAergic postsynaptic sites, (5) direct aggregation of neuroligin-2 induces coaggregation of gephyrin, and (6) mislocalized expression of neuroligin-2 to disrupt localized function disperses existing GABAA receptors and gephyrin and reduces mIPSC frequency and amplitude.
Mechanisms of Postsynaptic Differentiation: Glutamate Synapses
Our results support a simple mechanism underlying neurexin induction of postsynaptic differentiation by a protein-protein interaction cascade (Supplemental Figure S10 on the Cell website). Focal presentation of neurexin aggregates neuroligins, which can bind and thus aggregate PSD-95 and the closely related MAGUKs, which can bind and thus aggregate NR2, SynGAP, and GKAP. Further interactions of each of these components with additional proteins could lead to local aggregation of nearly all known components of glutamatergic postsynaptic sites (Kennedy, 2000; Sheng and Kim, 2002). Such a mechanism would be fundamentally different from the mechanism of acetylcholine receptor clustering at the neuromuscular junction, where agrin binding to a coreceptor activates MuSK tyrosine kinase activity, and this kinase activity is essential for postsynaptic differentiation (Sanes and Lichtman, 2001).
There are a couple of additional refinements to the model necessary to explain all of the experimental observations. First, neurexins induced clustering of PSD-95 but not AMPA receptors, even though stargazin family proteins that can link PSD-95 and AMPA receptors are expressed by these neurons. Neuroligins are likely necessary for proper AMPA receptor localization as indicated by the reduction in mEPSC frequency and amplitude with mislocalized neuroligin expression. Thus, some other signal presented by the glutamatergic axon, in addition to neurexin, is necessary to cluster AMPA receptors. Second, the area of clustering of the scaffolding proteins and receptors was often smaller than the area of clustering of the neuroligins, both in dendrites contacting COS cells expressing neurexins (Figures 3D and 3E) and in clusters of YFP-neuroligins induced by anti-YFP beads (Figure 5). These data suggest that the presence of concentrated neuroligin on the dendrite surface is necessary but not sufficient to induce completely colocalized aggregation of a postsynaptic complex. Other intracellular mechanisms within the dendrite may limit the size of aggregates of other postsynaptic proteins within the neuroligin-positive dendrite regions.
Mechanisms of Postsynaptic Differentiation: GABA Synapses
We show here that neurexins and neuroligins function directly at GABAergic synapses, not just at glutamatergic synapses as previously thought. A simple model is that local aggregation of neuroligin-2 in dendrites induced by local presentation of neurexins on GABA axons mediates GABAergic synaptogenesis (Supplemental Figure S10 on the Cell website). Neuroligin-2 is not known to bind gephyrin or GABAA receptors, and we could find no evidence for direct interaction in cotransfected fibroblasts, in contrast to evidence for interaction of neuroligins with PSD-95 (data not shown). Further studies will be required to determine how neuroligin-2 localizes to GABA synapses, and what intermediary proteins form a link from neuroligin-2 to gephyrin and GABA receptors. As for glutamatergic synapses, the simplest model would be coaggregation of postsynaptic components by high-affinity protein-protein interactions. In addition, even though α-neurexins are unable to bind to neuroligins (Ichtchenko et al., 1995), they may still play some role in GABAergic synaptogenesis. Neurexophilins, secreted proteins that bind to the second LNS domain of α-neurexins, are selectively expressed by inhibitory interneurons (Missler et al., 1998; Petrenko et al., 1996). Furthermore, mice lacking all three α-neurexins, while maintaining expression of the three β-neurexins, develop a normal density of glutamate synapses but only half the normal density of GABA synapses (Missler et al., 2003).
Much less is known about mechanisms of GABAergic compared with glutamatergic synaptogenesis. A major neurexin binding protein associated with GABA synapses is dystroglycan, but its role is poorly understood. Neurexins induced aggregation of gephyrin and GABA receptors but not dystroglycan. Thus, our current data are in agreement with previous studies indicating that dystroglycan is not essential for GABAeric synaptogenesis (Levi et al., 2002; Moore et al., 2002). Gephyrin itself contributes but is not essential for GABAergic synaptogenesis (Fischer et al., 2000; Levi et al., 2004). Other identified GABAA receptor interacting proteins including GABARAP and Plic-1 ubiquitin-like protein are involved more in regulation of GABAA receptor trafficking outside the synapse (Luscher and Keller, 2004). Identification here of neuroligin-2 as a major player at GABAergic postsynaptic sites may be a first step toward identifying interacting partners essential for GABAergic synaptogenesis.
Neurexins and Neuroligins: Specific or Generic Synaptogenic Signals?
An interesting issue raised by our findings is to what degree neurexins and neuroligins specify glutamatergic versus GABAergic synapse formation versus function as generic factors in assembly of both synapse types. There exist up to 3908 different neurexin isoforms (Tabuchi and Sudhof, 2002) and up to 40 neuroligin isoforms (Bolliger et al., 2001). Although detailed characterizations of the potential interactions are still in progress, it is clear that assorted neurexin isoforms have different binding affinities to the various neuroligins (Ichtchenko et al., 1996). One extreme view would be that neurexins and neuroligins participate in synaptogenesis but have nothing to do with specifying synaptic type. This view is not well supported by our data; it seems incompatible with the localization of neuroligin-2 but not the other neuroligins to GABAergic synapses (Figure 6), and incompatible with the ability of neuroligin-2 but not the other neuroligins to aggregate gephyrin (Figure 5). The other extreme possibility is that neurexins and neuroligins specify synapse type. One could imagine that GABAergic neurons express specific sets of neurexins with greatest affinity for neuroligin-2. This could lead to local aggregation of neuroligin-2 triggering local GABAergic postsynaptic differentiation. In an analogous way, glutamatergic synaptic differentiation could be specified by expression of neurexins with greatest affinity for neuroligins-1, -3, and -4. Current data support this idea in part but indicate that the situation is more complicated. Recombinant neuroligins-1 and -2 bind apparently equally well to PSD-95 (Irie et al., 1997 and data not shown). Furthermore, we show here a link between neuroligin-2 and PSD-95 or glutamatergic synapses in neurons, albeit under artificial conditions of induced neuroligin-2 aggregation or mislocalized expression or PSD-95 overexpression (Figures 5-7). These data suggest that some additional mechanism is needed to specify GABAergic postsynaptic differentiation. It is tempting to speculate that such an additional mechanism may involve proteins that interact directly with the neurexin-neuroligin system.
It is interesting that excitatory and inhibitory neurons would utilize a shared family of proteins for glutamatergic and GABAergic synapse formation. The existence of a shared synaptogenic family of proteins with different affinities of interactions was suggested previously based on the finding of junctions with incorrectly apposed pre- and postsynaptic components in cultured hippocampal neurons receiving only either glutamatergic or GABAergic input (Rao et al., 2000). Key future studies will be to determine the phenotype of neurons lacking specific neuroligins and specific neurexins. Viability of mice lacking neuroligin-1 (Song et al., 1999) indicates some redundancy in the system. Our data also reveal an apparent similarity in neuroligins-1, -3, and -4 with respect to localization and coaggregation with PSD-95. However, linkage of mutations in the X-linked genes encoding neuroligins-3 and -4 to autism and mental retardation (Jamain et al., 2003; Laumonnier et al., 2004) reveals some selectivity in function of neuroligin isoforms for human brain development and cognition.
While our model suggests a specific role for different neurexin and neuroligin isoforms, we further propose that this specificity may be highly dependent on the stoichiometric ratios of the major molecular players, and loss or overexpression of individual components may have more widespread effects. For example, in the cocultures or on beads, the presentation of neurexin-1β at high levels to dendrites presumably resulted in interactions with multiple neuroligins, thus inducing both glutamatergic and GABAergic postsynaptic differentiation. Furthermore, increasing the level of PSD-95 recruits all neuroligins, including neuroligin-2, to glutamate synapses and increases glutamatergic synaptic input while reducing GABAergic synaptic input (Prange et al., 2004; Figure 6). Perhaps the action of PSD-95 recruiting neuroligin-2 away from GABA synapses may be what leads to GABA synapse disassembly in this scenario. Neurons may utilize this shared synaptogenic family of neurexins and neuroligins, and particularly the ability of neuroligin-2 to shuttle between synapse types, to set the balance of excitatory and inhibitory inputs to their dendritic trees.
Experimental Procedures
Cell Culture
Hippocampal neuron cultures were prepared from E18 rat embryos using previously described methods (Goslin et al., 1998). Neurons were grown on coverslips inverted over an astroglia feeder layer in serum-free media. Neurons were treated with either 100 μM APV (Research Biochemicals) or 10 μM MK-801 (Alexis) beginning on day 7. Neurons were transfected at 9 DIV using Lipofectamine 2000 (Invitrogen).
COS-7, CV1, and HEK cell lines were maintained in DMEM supplemented with 10% FBS and 1% penicillin/streptomycin. For fibroblast-neuron cocultures, COS-7 or CV1 cells were transfected with Effectene (Qiagen) and trypsinized 24 hr later. Cells were washed twice, plated onto neurons, and the cocultures were maintained for 24 hr before fixation.
Bead Experiments
Soluble neurexin-Fc (human IgG) or neurexinΔLNS-Fc were expressed in COS cells. Protein was collected in serum-free media, purified with protein A columns from an ImmunoPure IgG purification kit (Pierce), and concentrated in PBS with Centricon filters (Millipore). For bead experiments, nonfluorescent Neutravidin labeled FluoSpheres (Molecular Probes) with a diameter of 1 μm were incubated with either biotin conjugated anti-GFP (here called anti-YFP; Rockland Immunochemicals) or biotin conjugated anti-human IgG (Jackson ImmunoResearch). The beads were rinsed in PBS, and the anti-human IgG bound beads were further incubated in either soluble neurexin-Fc or soluble neurexinΔLNS-Fc. One day after beads had attached to the neurons, the cells were fixed and stained.
DNA Constructs
The expression vector for soluble neurexin-1β-Fc containing aa 1–262 of mouse neurexin-1β was received from P. Scheiffele (Columbia University; Scheiffele et al., 2000). Soluble neurexinΔLNS-Fc was created by deleting the entire LNS domain (aa 84–261). To create neurexin-1β-CFP, aa 262–438 of neurexin-1β were cloned by PCR from Marathon rat brain cDNA (Clontech). The resulting product was fused to aa 1–262 from neurexin-1β-Fc and inserted into pECFP-N1 (Clontech) to create a full-length neurexin-1β construct with CFP attached to the C terminus. This form of neurexin-1β lacks any insert at splice site 4. The β-neurexin-specific region was deleted by removing aa 47–83. To delete the LNS domain, aa 84–261 were excised. The entire glycosylated region was deleted by removing aa 262–356. Removal of either aa 262–311 or 312–356 created partial deletions of the glycosylated region. To replace the neurexin LNS domain with the agrin LNS domain, aa 84-261 of neurexin-1β were replaced with aa 1863-2043 of human agrin (XM_372195; cDNA provided by J. Sanes, Harvard University). To determine synaptic localization of CFP-neurexin in neurons, the CFP tag was removed from the C terminus and inserted into the extracellular β-neurexin-specific region between residues 63 and 64. Expression vector for full-length agrin from rat was a gift from M. Ferns (University of California, Davis), for N-cadherin-YFP was a gift from D. Benson (Mount Sinai School of Medicine), and chick NgCAM was a gift from Peter Sonderegger (University of Zurich). NgCAM-YFP was made by attaching YFP to the C-terminal end of the protein. The HA-tagged neuroligins-1 and -2 (gift from P. Scheiffele; Scheiffele et al., 2000) both contain the neuroligin-1 signal sequence followed by the HA tag (YPYDVPDYA) and the mature N terminus of each of the neuroligins inserted into the pNice expression vector. YFP-neuroligin-1 and -2 were created by removing the HA epitope in HA-neuroligin-1 and -2 and inserting YFP in between the signal peptide and the mature N terminus. YFP-neuroligin-3 and -4 were created by replacing the neuroligin-2 sequence following YFP with the mature neuroligin-3 and -4 sequences from human neuroligin-3 (BC051715, Open Biosystems) and human neuroligin-4 (BC034018, Open Biosystems). Rat PSD-95 (gift from M. Sheng, MIT) was fused at its C terminus with CFP and expressed from the Clontech pECFP-N1 vector. The HA-CD8 construct contains the neuroligin-1 signal sequence followed by the HA-tag (YPYDVPDYA) and the entire mature human CD8α sequence (NM_001768).
Antibodies
The following mouse monoclonal antibodies were used: gephyrin (mAb7a; IgG1; 1:500; Alexis), PSD-95 (although the term PSD-95 is used for simplicity, this antibody recognizes PSD-95, PSD-93, SAP102, and SAP97; 6G6-1C9; IgG2a; 1:500; Affinity Bioreagents), NR1 (54.1; IgG2a; 1:1000; PharMingen), α-dystroglycan (VIA4.1; IgG1; 1:50; Upstate Biotechnology), β-dystroglycan (43DAG1/8D5; IgG2a; 1:25; Novocastra), GAD65 (GAD6; IgG2a; 1:100; Developmental Studies Hybridoma Bank), MAP2 (IgG1; 1:500; Chemicon), HA (IgG2b; 1:500; Roche). Rabbit antibodies were used against synapsin (1:1000; Chemicon), MAP2 (1:20,000; gift from S. Halpain, Scripps Research Institute), the γ2 subunit of GABAA receptors (1:200; Alomone), GluR1 (1:5000; Upstate Biotechnology), SynGAP (1:1000; Affinity Bioreagents), and GKAP (1:300; gift from M. Sheng, MIT). Guinea pig antibodies were used against the α2 and γ2 subunits of GABAA receptors (1:2000; gift from J.M. Fritschy, University of Zurich) and VGlut 1 (1:2000; Chemicon). Goat antibodies were used against neuroligin-2 (D-15; 1:200; Santa Cruz Biotechnology) and agrin (1:1000; R&D Systems). The specificity of this neuroligin-2 antibody was confirmed by immunoreactivity against COS cells expressing YFP-neuroligin-2 but not YFP-neuroligin-1 (data not shown). Secondary antibodies were Alexa488, Alexa568, and Alexa647 conjugated anti-mouse IgG1, anti-mouse IgG2a, anti-mouse IgG2b, anti-rabbit, anti-guinea pig, and anti-goat (Molecular Probes), and AMCA anti-rabbit (Vector Laboratories).
Immunocytochemistry
Generally, neurons were fixed for 15 min in warm PBS with 4% paraformaldehyde and 4% sucrose and permeabilized with 0.25% Triton X-100. They were incubated in 10% BSA (30 min, 37°C), appropriate primary antibody (Ab) in PBS with 3% BSA (overnight, 20°C) and secondary Ab (45 min, 37°C). Coverslips were mounted in elvanol (Tris-HCl, glycerol, and polyvinyl alcohol with 2% 1,4-diazabicyclo[2,2,2]octane). Coverslips used for NR1, β-dystroglycan, or neuroligin-2 immunostaining were fixed with methanol for 10 min at −20°C. When used with other antibodies, neuroligin-2 immunostaining was performed sequentially, with neuroligin-2 antibody first. Fluorescence and phase-contrast images were captured with a Photometrics Sensys or Diagnostics Instruments SPOT-RT cooled CCD camera mounted on a Zeiss Axioscope or Axioplan microscope with a 63× 1.4 NA oil objective using Metamorph imaging software. To determine surface localization of mutated neurexin-CFP proteins in transfected HEK cells and colocalization of induced neuroligin-2 with gephyrin and PSD-95, optical sections were captured using an Olympus Fluoview FV500 confocal on a BX61W microscope with a 60× 1.4 NA oil objective. Imaging in sequential scan mode with 442, 488, 568, and 633 nm laser lines and customized filters was used for separate detection of fluorophores. Images were prepared using Adobe Photoshop 5.5 software.
Electrophysiology
Neurons were transfected at 9 DIV to express HA-neuroligin-2 or HA-CD8 along with YFP. Neurons were identified for recording by expression of YFP and without knowledge of the cotransfected protein. Spontaneous mPSCs were recorded at 12–14 DIV at room temperature in the whole-cell voltage-clamp configuration. The extracellular solution contained: 168 mM NaCl, 2.4 mM KCl, 10 mM HEPES, 10 mM D-glucose, 1.3 mM CaCl2, and 1.3 mM MgCl2 (pH 7.4). mEPSCs were recorded in the presence of 0.5 μM TTX, 100 μM APV, and 10 μM SR95531. For mEPSCs, the intracellular solution contained: 140 mM Kgluconate, 10 mM HEPES, 8 mM NaCl, 2 mM Na2ATP, 0.1 mM Na2GTP, 10 mM EGTA, 6.23 mM CaCl2, and 2 mM MgCl2 (pH 7.4). mIPSCs were recorded in the presence of 0.5 μM TTX, 100 μM APV, and 5 μM NBQX. For mIPSCs, the intracellular solution contained: 144 mM CsCl2, 10 mM HEPES, 5 mM Na2ATP, 1.1 mM EGTA, 0.1 mM CaCl2, and 5 mM MgCl2 (pH 7.4). Recordings were performed with an Axopatch-1D amplifier and Axograph software (Axon Instruments). Records were filtered at 5 kHz and acquired at 10 kHz. Neurons were clamped at −70 mV. Series resistance values were <10 MΩ, input resistance >150 MΩ, and these parameters did not differ between groups. mPSCs were detected with Axograph software event detection using an optimally scaled sliding template and criteria of three times the SD.
Image Analysis
Sets of cells used for quantification were stained simultaneously. Images were randomized prior to quantification so the experimenter was blind to the treatment group. For the neuron-fibroblast coculture assay, transfected COS cells were chosen randomly based on phase contrast showing significant contact of COS cells with dendrites. Images were taken of the postsynaptic proteins, the presynaptic protein, and the transfected COS cell (neurexin-CFP or mCFP) using the same exposure time for both experimental and control conditions. Images of the pre- and postsynaptic proteins were thresholded and the area for measuring defined by the perimeter of the transfected COS cell. For each postsynaptic protein cluster, the area and total gray value was measured. A region was drawn around each cluster and thresholded synapsin or VGlut was measured through these regions to determine which clusters were synaptic. Only values from postsynaptic protein clusters that were not apposed to synapsin or VGlut were used in the final quantification.
For assessing colocalization of induced clusters of neuroligin-2 with gephyrin and PSD-95, neurexin-CFP transfected COS cells with induced clusters of gephryin and PSD-95 were selected. Gephyrin and PSD-95 images were randomized and thresholded, and regions were drawn around each cluster. Images of neuroligin-2 were thresholded, and the percentage of gephyrin and PSD-95 puncta that exhibited any pixel overlap with thresholded neuroligin-2 was quantified.
To determine the amount of gephyrin and PSD-95 localized to regions of clustered YFP-neuroligin-1 or -2 by anti-YFP bound beads, transfected neurons exhibiting bead-induced clusters of YFP-neuroligin-1 or -2 were selected by scanning in phase contrast and the YFP channel. YFP-neuroligin images were thresholded to define regions of neuroligin clustered by beads. The average gray value of unthresholded gephyrin and PSD-95 was measured within these regions, and the average gray value of the off-cell background subtracted. These values were then normalized to mean dendrite gephyrin and PSD-95 average gray value, also subtracted for off-cell background, so that final values reflect relative concentrations of gephyrin or PSD-95 above a mean dendrite value of 1.
For quantification of postsynaptic proteins in neurons transfected with HA-neuroligin-2, neurons overexpressing HA-neuroligin-2 or HA-CD8 were chosen randomly based on health and level of expression. Images were thresholded and postsynaptic protein puncta were delineated by the perimeter of the transfected neuron. Random regions of dendrite were selected and the number, area, and total gray value of the thresholded postsynaptic protein puncta per 100 μm length of dendrite were measured.
Analysis was performed using Metamorph, Microsoft Excel, Stat-View, and Cricket-Graph. Statistical comparisons of immunofluorescence were made using Student’s unpaired t test. All data are reported as mean ± SEM.
Supplementary Material
Acknowledgments
We thank Huaiyang Wu for excellent preparation of neuron cultures, Anushka Hauner, Yunhee Kang, Chenghua Wu, and Ji-Young Kim for additional assistance, and Dr. Peter Scheiffele for gifts of cDNA. This work was supported by NIH NS34448 and NS33184 (A.M.C.) and a NSF predoctoral fellowship (E.R.G.).
References
- Biederer T, Sudhof TC. Mints as adaptors. Direct binding to neurexins and recruitment of munc18. J. Biol. Chem. 2000;275:39803–39806. doi: 10.1074/jbc.C000656200. [DOI] [PubMed] [Google Scholar]
- Biederer T, Sara Y, Mozhayeva M, Atasoy D, Liu X, Kavalali ET, Sudhof TC. SynCAM, a synaptic adhesion molecule that drives synapse assembly. Science. 2002;297:1525–1531. doi: 10.1126/science.1072356. [DOI] [PubMed] [Google Scholar]
- Bolliger MF, Frei K, Winterhalter KH, Gloor SM. Identification of a novel neuroligin in humans which binds to PSD-95 and has a widespread expression. Biochem. J. 2001;356:581–588. doi: 10.1042/0264-6021:3560581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AM, Boudin H. Molecular heterogeneity of central synapses: afferent and target regulation. Nat. Neurosci. 2001;4:569–578. doi: 10.1038/88388. [DOI] [PubMed] [Google Scholar]
- Dalva MB, Takasu MA, Lin MZ, Shamah SM, Hu L, Gale NW, Greenberg ME. EphB receptors interact with NMDA receptors and regulate excitatory synapse formation. Cell. 2000;103:945–956. doi: 10.1016/s0092-8674(00)00197-5. [DOI] [PubMed] [Google Scholar]
- Dean C, Scholl FG, Choih J, DeMaria S, Berger J, Isacoff E, Scheiffele P. Neurexin mediates the assembly of presynaptic terminals. Nat. Neurosci. 2003;6:708–716. doi: 10.1038/nn1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer F, Kneussel M, Tintrup H, Haverkamp S, Rauen T, Betz H, Wassle H. Reduced synaptic clustering of GABA and glycine receptors in the retina of the gephyrin null mutant mouse. J. Comp. Neurol. 2000;427:634–648. doi: 10.1002/1096-9861(20001127)427:4<634::aid-cne10>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Friedman HV, Bresler T, Garner CC, Ziv NE. Assembly of new individual excitatory synapses: Time course and temporal order of synaptic molecule recruitment. Neuron. 2000;27:57–69. doi: 10.1016/s0896-6273(00)00009-x. [DOI] [PubMed] [Google Scholar]
- Garner CC, Zhai RG, Gundelfinger ED, Ziv NE. Molecular mechanisms of CNS synaptogenesis. Trends Neurosci. 2002;25:243–251. doi: 10.1016/s0166-2236(02)02152-5. [DOI] [PubMed] [Google Scholar]
- Gesemann M, Denzer AJ, Ruegg MA. Acetylcholine receptor-aggregating activity of agrin isoforms and mapping of the active site. J. Cell Biol. 1995;128:625–636. doi: 10.1083/jcb.128.4.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goslin K, Asmussen H, Banker G. Rat hippocampal neurons in low-density culture. In: Banker G, Goslin K, editors. Culturing Nerve Cells. MIT Press; Cambridge: 1998. pp. 339–370. [Google Scholar]
- Hata Y, Davletov B, Petrenko AG, Jahn R, Sudhof TC. Interaction of synaptotagmin with the cytoplasmic domains of neurexins. Neuron. 1993;10:307–315. doi: 10.1016/0896-6273(93)90320-q. [DOI] [PubMed] [Google Scholar]
- Henkemeyer M, Itkis OS, Ngo M, Hickmott PW, Ethell IM. Multiple EphB receptor tyrosine kinases shape dendritic spines in the hippocampus. J. Cell Biol. 2003;163:1313–1326. doi: 10.1083/jcb.200306033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichtchenko K, Hata Y, Nguyen T, Ullrich B, Missler M, Moo-maw C, Sudhof TC. Neuroligin 1: A splice site-specific ligand for beta-neurexins. Cell. 1995;81:435–443. doi: 10.1016/0092-8674(95)90396-8. [DOI] [PubMed] [Google Scholar]
- Ichtchenko K, Nguyen T, Sudhof TC. Structures, alternative splicing, and neurexin binding of multiple neuroligins. J. Biol. Chem. 1996;271:2676–2682. doi: 10.1074/jbc.271.5.2676. [DOI] [PubMed] [Google Scholar]
- Irie M, Hata Y, Takeuchi M, Ichtchenko K, Toyoda A, Hirao K, Takai Y, Rosahl TW, Sudhof TC. Binding of neuroligins to PSD-95. Science. 1997;277:1511–1515. doi: 10.1126/science.277.5331.1511. [DOI] [PubMed] [Google Scholar]
- Jamain S, Quach H, Betancur C, Rastam M, Colineaux C, Gillberg IC, Soderstrom H, Giros B, Leboyer M, Gillberg C, Bourgeron T. Mutations of the X-linked genes encoding neuroligins NLGN3 and NLGN4 are associated with autism. Nat. Genet. 2003;34:27–29. doi: 10.1038/ng1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy MB. Signal-processing machines at the postsynaptic density. Science. 2000;290:750–754. doi: 10.1126/science.290.5492.750. [DOI] [PubMed] [Google Scholar]
- Knuesel I, Mastrocola M, Zuellig RA, Bornhauser B, Schaub MC, Fritschy JM. Short communication: altered synaptic clustering of GABAA receptors in mice lacking dystrophin (mdx mice) Eur. J. Neurosci. 1999;11:4457–4462. doi: 10.1046/j.1460-9568.1999.00887.x. [DOI] [PubMed] [Google Scholar]
- Laumonnier F, Bonnet-Brilhault F, Gomot M, Blanc R, David A, Moizard MP, Raynaud M, Ronce N, Lemonnier E, Calvas P, et al. X-linked mental retardation and autism are associated with a mutation in the NLGN4 gene, a member of the neuroligin family. Am. J. Hum. Genet. 2004;74:552–557. doi: 10.1086/382137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi S, Grady RM, Henry MD, Campbell KP, Sanes JR, Craig AM. Dystroglycan is selectively associated with inhibitory GABAergic synapses but is dispensable for their differentiation. J. Neurosci. 2002;22:4274–4285. doi: 10.1523/JNEUROSCI.22-11-04274.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi S, Logan SM, Tovar KR, Craig AM. Gephyrin is critical for glycine receptor clustering but not for the formation of functional GABAergic synapses in hippocampal neurons. J. Neurosci. 2004;24:207–217. doi: 10.1523/JNEUROSCI.1661-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscher B, Keller CA. Regulation of GABAA receptor trafficking, channel activity, and functional plasticity of inhibitory synapses. Pharmacol. Ther. 2004;102:195–221. doi: 10.1016/j.pharmthera.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Missler M, Hammer RE, Sudhof TC. Neurexophilin binding to alpha-neurexins. A single LNS domain functions as an independently folding ligand-binding unit. J. Biol. Chem. 1998;273:34716–34723. doi: 10.1074/jbc.273.52.34716. [DOI] [PubMed] [Google Scholar]
- Missler M, Zhang W, Rohlmann A, Kattenstroth G, Hammer RE, Gottmann K, Sudhof TC. Alpha-neurexins couple Ca2+ channels to synaptic vesicle exocytosis. Nature. 2003;424:939–948. doi: 10.1038/nature01755. [DOI] [PubMed] [Google Scholar]
- Moore SA, Saito F, Chen J, Michele DE, Henry MD, Messing A, Cohn RD, Ross-Barta SE, Westra S, Williamson RA, et al. Deletion of brain dystroglycan recapitulates aspects of congenital muscular dystrophy. Nature. 2002;418:422–425. doi: 10.1038/nature00838. [DOI] [PubMed] [Google Scholar]
- O’Brien RJ, Xu D, Petralia RS, Steward O, Huganir RL, Worley P. Synaptic clustering of AMPA receptors by the extracellular immediate-early gene product Narp. Neuron. 1999;23:309–323. doi: 10.1016/s0896-6273(00)80782-5. [DOI] [PubMed] [Google Scholar]
- Petrenko AG, Ullrich B, Missler M, Krasnoperov V, Rosahl TW, Sudhof TC. Structure and evolution of neurexophilin. J. Neurosci. 1996;16:4360–4369. doi: 10.1523/JNEUROSCI.16-14-04360.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prange O, Wong TP, Gerrow K, Wang YT, El-Husseini A. A balance between excitatory and inhibitory synapses is controlled by PSD-95 and neuroligin. Proc. Natl. Acad. Sci. USA. 2004;101:13915–13920. doi: 10.1073/pnas.0405939101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao A, Kim E, Sheng M, Craig AM. Heterogeneity in the molecular composition of excitatory postsynaptic sites during development of hippocampal neurons in culture. J. Neurosci. 1998;18:1217–1229. doi: 10.1523/JNEUROSCI.18-04-01217.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao A, Cha EM, Craig AM. Mismatched appositions of presynaptic and postsynaptic components in isolated hippocampal neurons. J. Neurosci. 2000;20:8344–8353. doi: 10.1523/JNEUROSCI.20-22-08344.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudenko G, Nguyen T, Chelliah Y, Sudhof TC, Deisenhofer J. The structure of the ligand-binding domain of neurexin Ibeta: Regulation of LNS domain function by alternative splicing. Cell. 1999;99:93–101. doi: 10.1016/s0092-8674(00)80065-3. [DOI] [PubMed] [Google Scholar]
- Sanes JR, Lichtman JW. Induction, assembly, maturation and maintenance of a postsynaptic apparatus. Nat. Rev. Neurosci. 2001;2:791–805. doi: 10.1038/35097557. [DOI] [PubMed] [Google Scholar]
- Scheiffele P, Fan J, Choih J, Fetter R, Serafini T. Neuroligin expressed in nonneuronal cells triggers presynaptic development in contacting axons. Cell. 2000;101:657–669. doi: 10.1016/s0092-8674(00)80877-6. [DOI] [PubMed] [Google Scholar]
- Serpinskaya AS, Feng G, Sanes JR, Craig AM. Synapse formation by hippocampal neurons from agrin-deficient mice. Dev. Biol. 1999;205:65–78. doi: 10.1006/dbio.1998.9112. [DOI] [PubMed] [Google Scholar]
- Sheng M, Kim MJ. Postsynaptic signaling and plasticity mechanisms. Science. 2002;298:776–780. doi: 10.1126/science.1075333. [DOI] [PubMed] [Google Scholar]
- Song JY, Ichtchenko K, Sudhof TC, Brose N. Neuroligin 1 is a postsynaptic cell-adhesion molecule of excitatory synapses. Proc. Natl. Acad. Sci. USA. 1999;96:1100–1105. doi: 10.1073/pnas.96.3.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugita S, Saito F, Tang J, Satz J, Campbell K, Sudhof TC. A stoichiometric complex of neurexins and dystroglycan in brain. J. Cell Biol. 2001;154:435–445. doi: 10.1083/jcb.200105003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabuchi K, Sudhof TC. Structure and evolution of neurexin genes: insight into the mechanism of alternative splicing. Genomics. 2002;79:849–859. doi: 10.1006/geno.2002.6780. [DOI] [PubMed] [Google Scholar]
- Ullrich B, Ushkaryov YA, Sudhof TC. Cartography of neurexins: More than 1000 isoforms generated by alternative splicing and expressed in distinct subsets of neurons. Neuron. 1995;14:497–507. doi: 10.1016/0896-6273(95)90306-2. [DOI] [PubMed] [Google Scholar]
- Ushkaryov YA, Petrenko AG, Geppert M, Sudhof TC. Neurexins: synaptic cell surface proteins related to the alpha-latrotoxin receptor and laminin. Science. 1992;257:50–56. doi: 10.1126/science.1621094. [DOI] [PubMed] [Google Scholar]
- Yamagata M, Sanes JR, Weiner JA. Synaptic adhesion molecules. Curr. Opin. Cell Biol. 2003;15:621–632. doi: 10.1016/s0955-0674(03)00107-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.