Abstract
The recent discovery of angiotensin-converting enzyme 2 (ACE2) and the Mas receptor has resulted in the recognition of a counterregulatory, ACE2/Ang-(1-7)/Mas, axis within the renin-angiotensin system (RAS). Any disturbance in the balance between this and the ACE/AngII/AT1 receptor axis is suggested to lead to the development and progression of cardiovascular disease (CVD). Therefore, activation of the ACE2/Ang-(1-7)/Mas axis has been an obvious target for CVD therapeutics. In this review, we will focus on the current status of the RAS, highlight evidence for the existence of the ACE2/Ang-(1-7)/Mas axis, and discuss, the role of this axis in the pathophysiology of the cardiovascular, renal, pulmonary and central nervous systems and its potential for future CVD therapeutics.
Keywords: ACE2, Angiotensin-(1-7), Mas receptor, Hypertension, Cardio-pulmonary disease prevention/control
Introduction
Despite the tremendous success obtained using the current pharmacotherapy, especially angiotensin-converting enzyme inhibitors (ACEi) and angiotensin (Ang) II receptor blockers (ARBs), the prevalence of cardiovascular diseases (CVDs) remains high around the World. The overall death rate in the United States alone in 2005 was 278.9 per 100,000.1 These unprecedented numbers have stimulated researchers to consider the development of new strategies and targets to control these diseases.
Since its discovery, the renin-angiotensin system (RAS) has been considered an important target to manage the disturbances of the cardiovascular system. In fact, RAS blockers represent the main class of drugs in the treatment of hypertension and CVDs.2 Recent genomic and proteomic studies have led to significant advances in our understanding of the RAS and in experimental methods for studying regulatory mechanisms influenced by the RAS. Thus, demonstrating the existence of a counterregulatory axis within the RAS, constituted by angiotensin-converting enzyme (ACE) 2, Ang-(1-7) and the Mas receptor, has established a new concept for this system, i.e. the classical narrow cascade formed by ACE, AngII and AT1 receptor has been replaced by a flexible hormonal system with many bioactive peptides, receptors, enzymes and interactions among these components.2-4 Consequently, follow-up studies have revealed new possibilities and targets to better control CVD. In this review, we will discuss: (i) the current status of the RAS with an emphasis on evidence for the existence of the ACE2/Ang-(1-7)/Mas axis; (ii) the role of this axis in the pathophysiology of the cardiovascular, renal, pulmonary and central nervous systems; (iii) the potential of this protective axis for the development of novel CVD therapeutics; and (iv) future perspectives.
Auto regulation of the RAS: ACE2/Ang-(1-7)/Mas Receptor Axis
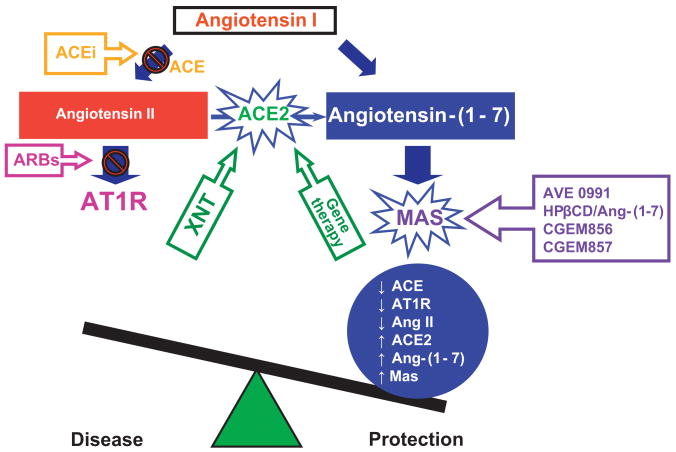
The RAS consists of two distinct and counterregulatory axes (Figure). The classic ACE/AngII/AT1R axis is responsible for the vasoconstrictive, proliferative, hypertensive and fibrotic actions of the RAS. Its hyperactivity is associated with hypertension and CVDs such as cardiac hypertrophy, heart failure, stroke, coronary artery disease and end-stage renal disease. This axis is the primary target for the actions of the RAS blockers. The ACE2/Ang-(1-7)/Mas axis constitutes an alternative axis which represents an intrinsic mechanism to induce vasoprotective actions by counterregulating the ACE/AngII/AT1R axis, thus inducing many beneficial effects in CVDs. AT2 receptors may also contribute to this counterregulation.5 The counterregulatory concept of the RAS is emerging and studies have established a key role for its main component, ACE2.
Figure.
Schematic diagram showing the counterregulatory axes of the RAS: ACE-AngII-AT1 receptor and ACE2-Ang-(1-7)-Mas receptor. The ACEs play a central role in balancing the activity of these axes. While ACE degrades AngI to form AngII, ACE2 hydrolyzes AngII to produce Ang-(1-7). AVE 0991, HPβCD/Ang-(1-7), CGEM856 and CGEM857 are Ang-(1-7) analogues and, consequently, Mas agonists and XNT is an ACE2 activator. ACE: angiotensin-converting enzyme; ACEi: angiotensin-converting enzyme inhibitors; Ang: angiotensin; ARBs: AT1R blockers; AT1R: angiotensin II type 1 receptor.
ACE2 is a monocarboxypeptidase whose active site domain shares approximately 42% homology with the active site of ACE.6 It is found in the plasma membranes of virtually all organs as an ectoenzyme and in the plasma and urine in a soluble active form4. AngI and AngII are the principal substrates for this enzyme. The impact of ACE2 on the metabolism of other cardiovascular relevant peptides has not been completely elucidated.6
ACE2 is one of the principal Ang-(1-7)-forming enzymes.4,6 Because of the higher affinity of ACE2 for Ang II, Ang-(1-7) generation from Ang II is physiologically more relevant than from Ang I.6 As a result, ACE2 plays a crucial role in maintaining the balance between both axes of the RAS, and a chronic and sustained imbalance may lead to pathophysiology of the cardiovascular, renal, pulmonary and central nervous systems. Currently, the physiological signals that tip the balance between the two axes of the RAS to favor a pathological state remain unknown. It is suggested that the equilibrium between the expression/activity of the two ACEs is crucial.7 Furthermore, ACE2 may represent an endogenous autoregulatory mechanism within the RAS since it decreases Ang II levels in favor of Ang-(1-7) formation. The concept that ACE2 is capable of balancing the activities of both axes of the RAS is a central argument in designating this enzyme as a target for the development of novel antihypertensive and cardioprotective drugs.
Biological actions of the ACE2/Ang-(1-7)/Mas axis
Cardiovascular system
Increasing evidence indicates that the ACE2/Ang-(1-7)/Mas axis plays a critical role in cardiovascular homeostasis, and that alterations in its expression/function are associated with major cardiac and vascular pathophysiologies.8-10 Initially, ACE2 gene disruption on C57BL/6 background leads to severe reduction in cardiac contractility and decreases in aortic and ventricular pressure.8 In addition, transgenic mice overexpressing ACE2 in myocardial cells present cardiac rhythm disturbances.11 Although subsequent studies have shown conflicting results,12 it is now generally accepted that ACE2 deficiency aggravates heart failure (Table). Evidence that Mas deficient mice present marked cardiac fibrosis and impaired cardiac function supports this view.9 Furthermore, many cardiovascular actions of Ang-(1-7) are absent in these animals.9,13,14 Cardiac overexpression of ACE2 in a chronic Ang II-infusion rat model15, in spontaneously hypertensive rats (SHR)16 or in infarcted rat hearts17 protects the heart from cardiac remodeling. In keeping with these observations, data from Mercure et al18 have shown that cardiac overexpression of an Ang-(1-7)-producing fusion protein protects the heart from the deleterious effects of Ang II infusion.18 These findings suggest that transgenic ACE2 overexpression from birth exerts adverse effects while its overexpression after cardiac development completely protects the heart from pathophysiologies. It is likely that embryonic overexpression of ACE2 and/or Ang-(1-7) may induce developmental abnormalities in transgenic animals. Rentzsch et al19 have reported that ACE2 overexpression in the vasculature reduces blood pressure (BP) and improves endothelial function in hypertensive rats. Thus, the injurious effects viewed in ACE2 transgenic mice11 may be tissue specific. Collectively, these observations suggest that the actions of ACE2, as the main Ang-(1-7)-forming enzyme, and the effects of Ang-(1-7) alone in the heart may depend upon the available amount of this heptapeptide since perfusion of isolated hearts with high concentrations of Ang-(1-7) caused cardiac rhythm disturbances,20 while low concentrations induced an antiarrhythmogenic effect.21 In accordance with this observation, Masson et al22 have suggested that the cardiac fibrosis viewed in stroke-prone SHR overexpressing ACE2 in the myocardium, mediated by recombinant adeno-associated virus 6, is due to higher transduction levels achieved using this virus in comparison with lenti-virus.16 In fact, increased ACE2 expression mediated by lenti-virus produces beneficial effects, including anti-fibrosis, in hearts of SHR (Table).
Table.
Summarized phenotypic and the main changes in the RAS observed in some animal models of deficiency or overexpression of ACE2 and Mas.
| Strategy | Strain | Changes in RAS | Phenotype | Reference |
|---|---|---|---|---|
| ACE2 overexpression in smooth muscle cells | SHRSP | ↑aortic ACE2 activity | Improved endothelial function | Rentzsch et al19 |
| ↑Ang-(1-7) plasma/aorta | ||||
| ↑Ang-(1-7)/Ang II | ||||
| Lentiviral ACE2 gene transfer in hearts | SHR | ↑cardiac ACE2 mRNA | BP independent cardioprotection | Diez-Freire et al16 |
| ↑cardiac ACE2 activity | ||||
| ACE2 overexpression in hearts | FVB/NTac | ↑serum ACE2 activity | Cardiac rhythm disturbances and sudden death | Donoghue et al11 |
| ↑cardiac ACE2 expression | ||||
| Adenoviral ACE2 gene transfer in hearts | SHRSP | ↑cardiac ACE2 activity | Severe cardiac fibrosis | Masson et al22 |
| ↑Ang-(1-7) expression | ||||
| ↓ACE activity | ||||
| ACE2 knockout | Mixed background | ↑cardiac/plasma/renal Ang II | ↓BP and impaired systolic function | Crackower et al8 |
| ACE2 knockout | Mixed background | No basal changes | Variable | Gurley et al12 |
| ACE2 knockout | Mixed background | No basal changes | No basal changes | Yamamoto et al61 |
| Mas knockout | C57BL/6 | ↓Ang-(1-7) binding in cardiac myocytes | Impaired heart function and cardiac fibrosis | Santos et al13 |
| Mas knockout | C57BL/6 | Not determined | ↑sympathetic tonus | Walther et al62 |
| Mas knockout | C57BL/6 | Not determined | Impaired Ang-(1-7)-mediated aortic relaxation | Peiró et al63 |
| Mas knockout | FVB/N | Not determined | ↑BP, impaired endothelial function and ↓NO and eNOS | Xu et al64 |
There is clinical evidence to support the role of the ACE2/Ang-(1-7)/Mas axis in cardiovascular pathophysiology. Increased ACE2 expression is observed in cardiac tissue of patients with ischemic heart failure and patients with either idiopathic dilated cardiomyopathy or primary pulmonary hypertension.23 This increase in ACE2 is likely to be a compensatory mechanism to circumvent the chronic activation of the RAS. This view is supported by observations that: (i) blockers of the RAS can prevent the decrease in ACE2 transcription and translation in the late phase of ventricular dysfunction in myocardial infarcted rats,24 (ii) expression of ACE2 and Ang-(1-7) is increased in infarcted Lewis rats following olmesartan treatment,25 and (iii) treatment with ACEi, ARBs or the combined blockade of ACE and AT1 receptors in rats and humans increases plasma Ang-(1-7) levels.26-28 This effect is, at least in part, due to reduced Ang-(1-7) degradation, since this peptide is a substrate for ACE.30 The cellular mechanisms and signaling pathways by which Ang-(1-7) exerts its effects have yet to be elucidated, although Dias-Peixoto et al29 have demonstrated the following in cardiomyocytes of Mas deficient mice on FVB/N background: i) Ang-(1-7) fails to increase nitric oxide (NO) levels; ii) alterations in the expression of proteins that regulate endothelial NO synthase activity; and iii) impaired calcium handling.29 These observations suggest that Mas-deficiency can lead to chronic and sustained ACE/AngII/AT1R effects that may impair Ang-(1-7) signaling and promote cardiac ventricular dysfunction further supporting the concept that activation of the ACE2/Ang-(1-7)/Mas axis promotes protection against cardiovascular diseases (Figure).
Renal system
ACE2, Ang-(1-7) and Mas expression is abundant throughout renal structures and has significant pathophysiological consequences.14,30 Deletion of ACE2 gene in mice leads to the development of glomerulosclerosis with deposition of type I and III collagen, and fibronectin; and increased albuminuria.31 ACE2 expression is altered in kidneys of hypertensive rats,32 diabetic animals30 and, more importantly, in kidneys of humans with renal disease.33 The renal damages observed in streptozotocin-induced diabetic mice worsens with treatment with the ACE2 inhibitor, MLN4760.34 Diabetic SHR chronically treated with Ang-(1-7) present an improvement in renal endothelial function, renal function, and a reduction in nicotinamide adenine dinucleotide phosphate (NADPH)-mediated oxidative stress.35 The protective role of this axis in renal function and structure is further supported by the genetic deletion of Mas which leads to a reduction in urine volume, sodium retention, microalbuminuria and reduced renal blood flow.36 Kidneys from these animals exhibit a reduction in glomerular tuff diameter; increased expression of collagen type III and IV, and of fibronectin; and increased renal AT1R.36 In contrast, Shao et al37 have demonstrated that chronic Ang-(1-7) treatment of streptozotocin-induced male rats accelerates diabetic renal injury. The contrasting data may be due to local levels of Ang-(1-7), strain variability, age, gender, dose, and/or treatment duration.
Patients with diabetic kidney disease exhibit an imbalance between the expression of ACE and ACE2 mRNA and protein in the kidney.38,39 This finding reinforces the hypothesis that the balance between the two ACEs is crucial to the development of hypertension, CVDs, and renal diseases (Figure). The predominance of ACE effects over those of ACE2 progresses to disease states, while the tipping the balance towards ACE2 restores the equilibrium and promotes the attenuation or reversal of pathologies.
Pulmonary system
The role of the ACE2/Ang-(1-7)/Mas axis in lung pathophysiology has been pursued since the identification of ACE2 in pulmonary tissues. Penninger's group has demonstrated that ACE2 deletion worsens acute lung injury induced by acid aspiration or sepsis.40 Replacement of ACE2 using recombinant human ACE2 improves the pulmonary damage in ACE2 knockout mice.40 In addition, severe lung failure on an ACE2 knockout background was rescued by inactivation of ACE, and genetic loss of AT1a improved lung function.40 Along the same line of investigation, our studies have shown that activation of endogenous ACE2, using an ACE2 activator designated as XNT ([1-[[2-(dimethylamino)ethyl]amino]-4-(hydroxymethyl)-7-[[(4-methylphenyl)sulfonyl]oxy]-9H-xanthen-9-one]),41 prevents the development of pulmonary hypertension (PH). This small molecule was also able to abolish the development of PH-associated damages such as right ventricular and pulmonary vessel hypertrophy. These effects were completely blunted by the Ang-(1-7) antagonist, A-779, indicating that the ACE2/Ang-(1-7)/Mas branch of the pulmonary RAS has important protective effects in PH.7 Further evidence that pulmonary ACE2 is important in maintaining a balance in the RAS is derived from ACE2 overexpression studies. Lenti-ACE2 particles injected into the trachea of mice were able to protect lungs from PH. In addition, this strategy is also effective in the partial reversal of PH.42
Central nervous system
Robust expression of ACE2 and Mas, and actions of Ang-(1-7) has been reported in the brain of both animals and humans. Centrally mediated cardiovascular effects of Ang-(1-7) include baroreflex facilitation, hypotension, bradykinin (Bk) potentiation, vasopressin, Bk and NO release and prevention of norepinephrine release.43
In addition, there are many studies establishing an altered expression of central ACE2 in pathological conditions.43-45 For example, a 40% decrease in ACE2 expression has been reported in the rostral ventrolateral medulla (RVLM), a cardiovascular nucleus of the brainstem, of SHR. A bilateral injection of lenti-ACE2 in this area resulted in a long-term expression of transgenic ACE2 with concomitant decrease in BP and heart rate (HR).45 In addition to RVLM, ACE2 is shown to regulate cardiovascular functions in other brain nuclei. For instance, microinjections of MNL4760 into the nucleus tractus solitarius, NTS, reduced the baroreceptor reflex sensitivity in rats.46 Furthermore, overexpression of ACE2 in the subfornical organ downregulates AT1 receptors and attenuates the vasopressor and drinking responses elicited by intracerebroventricular infusion of Ang II.47
More recent studies have focused on the role of the ACE2/Ang-(1-7)/Mas axis in cerebrovascular disease. Studies from our group have shown that activation of Mas with chronic or acute administration of Ang-(1-7) increases cerebral blood flow, and selective Ang-(1-7) antagonism decreases flow.48 This increase in blood flow might be explained by evidence that central administration of Ang-(1-7) shortly following stroke onset increases Bk levels in the brain, as well as upregulating Bk receptors.49 In addition, NO release and endothelial NO synthase expression are increased with Ang-(1-7) treatment.50 In fact, our data suggest that chronic central administration of Ang-(1-7) reduces the infarct area and provided cerebroprotection in a rodent stroke model.51 Together, these observations point toward a potential mechanism of cerebroprotection mediated by Ang-(1-7) stimulation of Mas and subsequent release of Bk leading to stimulation of Bk receptors, and release of NO, which could increase the cerebrovascular reserve during an ischemic insult.
ACE2/Ang-(1-7)/Mas axis is a potential target for future CVDs therapeutics
It is evident from the above discussion that significant conceptual progress has been made in the last several years that lead us to conclude that this vasoprotective axis of the RAS could serve as a new direction for improved therapeutics for CVDs. In animal models, two different strategies have been followed in this regard.
Pharmacotherapy
Development of a small molecule ACE2 activator, XNT, is currently being pursued to translate the vasoprotective concept into an effective cardiovascular therapy. XNT has been shown to decrease BP and to cause improvements in cardiac function and reversal of myocardial and perivascular fibrosis.41 Similar beneficial effects of XNT are observed in PH induced by monocrotaline treatment in rats.7
Mas agonists, such as AVE0991 and HPβCD/Ang-(1-7), are also under investigation. AVE0991 was the first orally active Ang-(1-7) analogue capable of mimicking Ang-(1-7) actions.52 The protective effects of AVE0991 are qualitatively comparable to those of Ang-(1-7) in the endothelium,52 blood vessels,53 kidneys14 and heart.54 Recently, Lula et al55 have proposed that complexing Ang-(1-7) with hydroxipropyl-β-cyclodextrin (HPβCD) renders Ang-(1-7) to a more stable complex thus allowing a formulation for oral administration of Ang-(1-7) with increased half-life.55 Initial testing has revealed that HPβCD/Ang-(1-7) reduces the deleterious effects induced by myocardial infarction on cardiac function and reduces infarct size. Furthermore, this compound attenuates the isoproterenol effects on cardiac function as well as decreasing the cardiac hypertrophy and the cardiac damage induced by isoproterenol without any change in the systemic BP.56 Independently, Kluskens et al57 have reported a stable cyclized derivative of Ang-(1-7) whose vasodilator activity is 2-fold higher when compared to the normal Ang-(1-7).57 Finally, two more Mas agonists have been discovered (CGEM856 and CGEM857) and initial data demonstrate that these peptides produce endothelium-dependent vasorelaxation, cardioprotection and anti-hypertensive effects in SHR.58
Gene Transfer Therapy
A target gene therapy strategy holds significant potential to translate the available fundamental research of ACE2 into therapeutics. In fact, initial animal experiments have been extremely encouraging. For example, viral-mediated ACE2 overexpression in the heart has been shown to protect the animals from myocardial injuries;15 to decrease high BP; and attenuate perivascular cardiac fibrosis.16 This strategy also preserves cardiac function, left ventricular wall motion and contractility, and attenuates left ventricular wall thinning induced by myocardial infarction.17 That the efficacy of ACE2 gene transfer is not limited to the heart is illustrated by ACE2 overexpression studies in the brain.45,47 ACE2 overexpression in the RVLM causes significant decreases in BP and HR.45 Additionally, its overexpression in the lungs protects mice from PH.42 Similar beneficial effects are observed with Ang-(1-7) fusion protein.59
Taken together, this exciting and rapidly evolving field of investigation is likely to provide a better understanding of the role of both RAS axes in CVD pathophysiology and will position us to develop new strategies for cardiovascular therapeutics. Compared with ACEi and ARBs, we believe that targeting ACE2 has the following therapeutic advantages: (i) it degrades Ang II to generate Ang-(1-7). Thus, targeting ACE2 would not only produce the vasoprotective/antiproliferative peptide Ang-(1-7) but also influence the vasoconstrictive/proliferative effects of the ACE-AngII-AT1R axis; (ii) it is a multifunctional enzyme with many biologically active substrates. The effects of ACE2 on substrates other than Ang I and Ang II still need to be determined. They could hold an unidentified relevance for the treatment of CVD; (iii) unlike ARB/ACEi therapy, ACE2 is an endogenous regulator of the RAS; (iv) it is a part of the vasodilatory/antiproliferative axis of the RAS that seems to be effective in the control of fibrosis and structural remodeling without changes in systemic BP which could prove to be extremely beneficial for PH; (v) although treatment with ACEi or ARBs indirectly increases ACE2 expression, direct activation of this enzyme could result in a better outcome in CVDs because ACE2 is not the main target for ACEi and ARBs.
Future directions
The above discussion supports the concept that regulated increases in the activity of the vasoprotective axis of the RAS can produce beneficial outcomes in CVDs. Both pharmacotherapy and gene therapy strategies have shown promise in animal experiments. Moreover, it has been reported that treatment with ACEi or ARBs increases plasma Ang-(1-7) levels in humans,28 and that increased soluble ACE2 activity in humans correlates with worsened cardiac performance. Therefore, it has prognostic value as a predictor of adverse events.60 However, many issues and potential pitfalls must be addressed in order to take this leap. Some of these issues are as follows: (i) the mechanism of an inter-dependent regulation of both axes of the RAS need to be elucidated. Co-localization of ACE and ACE2 may provide some indication if the expression of one enzyme could directly regulate the other enzyme's activity. The possibility that the co-regulation could be at the level of signaling by the AT1R and Mas should be considered. (ii) we must understand the mechanisms of action of ACE2/Ang-(1-7) at the tissue/cellular levels. Is the target for ACE2/Ang-(1-7)/Mas endothelial cells or do they act in paracrine/autocrine manner involving multiple vascular cells to control the RAS? (iii) ACE2 is a ubiquitous enzyme as well as the receptor for SARS virus. We must determine the consequences of a long-term activation of ACE2. The effects of ACE2 activators on the immune competence of animals and their vulnerability to SARS infection must be tested before these molecules are ready for preclinical trials. (iv) Can genetically-modified endothelial progenitor cells overexpressing ACE2 or Ang-(1-7) be a better approach for targeting disease-induced endothelial damage? This combinational approach is exciting and offers a faster-track for testing the concept in patients. (v) RAS has been shown to play a role in stroke. Since ARBs produce limited beneficial outcomes, it would be interesting to determine if activation of the ACE2/Ang-(1-7)/Mas axis provides an improved protection and reversal from ischemia-induced neural damage. (vi) Is the benefit of ACE2 intervention organ specific? We must establish a consensus about the influence of gender, strain, organ, dose, timing and methodology on ACE2 effects. (vii) gene therapy strategy holds great promise because it could be targeted to the diseased organ, but is not without inherent pitfalls. For example, an ideal vector that can produce high levels of transduction without immune or other adverse effects is not currently available, but is within reach in near future. One must develop a transduction system that can be turned on/off on demand in the event of adverse effects of a long-term transgene expression.
Perspectives
It is becoming evident that normal cardiovascular homeostasis is a result of a balance between the activities of the vasoconstrictive, hypertrophic, proliferative and anti-fibrotic (ACE/AngI/AT1R) and the vasoprotective [ACE2/Ang-(1-7)/Mas] axes of the RAS. Any chronic imbalance leads to major cardiovascular pathophysiology. Both animal and human studies provide strong conceptual support for this view. They also demonstrate that pharmacological or genetic intervention in restoring this balance by increasing the activity of the ACE2/Ang-(1-7)/Mas axis produces impressive outcomes against cardiovascular and pulmonary diseases. Thus, it is reasonable to suggest that the vasoprotective axis offers a novel target for the development of improved and more successful therapy for cardio-pulmonary diseases.
Acknowledgments
Source of funding: This work was supported by the NIH Grants HL56921 and HL33610.
Footnotes
Conflict of Interest/Disclosure Statement: Robson AS Santos is a consultant of COMPUGEN – Israel.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lloyd-Jones D, Adams R, Carnethon M, De Simone G, Ferguson TB, Flegal K, Ford E, Furie K, Go A, Greenlund K, Haase N, Hailpern S, Ho M, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott M, Meigs J, Mozaffarian D, Nichol G, O'Donnell C, Roger V, Rosamond W, Sacco R, Sorlie P, Stafford R, Steinberger J, Thom T, Wasserthiel-Smoller S, Wong N, Wylie-Rosett J, Hong Y, American Heart Association Statistics Committee and Stroke Statistics Subcommittee Heart disease and stroke statistics-2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:480–486. doi: 10.1161/CIRCULATIONAHA.108.191259. [DOI] [PubMed] [Google Scholar]
- 2.Schmieder RE, Hilgers KF, Schlaich MP, Schmidt BM. Renin-angiotensin system and cardiovascular risk. Lancet. 2007;369:1208–1219. doi: 10.1016/S0140-6736(07)60242-6. [DOI] [PubMed] [Google Scholar]
- 3.Ferrario CM, Trask AJ, Jessup JA. Advances in biochemical and functional roles of angiotensin-converting enzyme 2 and angiotensin-(1-7) in regulation of cardiovascular function. Am J Physiol Heart Circ Physiol. 2005;289:H2281–H2290. doi: 10.1152/ajpheart.00618.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferreira AJ, Raizada MK. Are we poised to target ACE2 for the next generation of antihypertensives? J Mol Med. 2008;86:685–690. doi: 10.1007/s00109-008-0339-x. [DOI] [PubMed] [Google Scholar]
- 5.Carey RM. Update on the role of the AT2 receptor. Curr Opin Nephrol Hypertens. 2005;14:67–71. doi: 10.1097/00041552-200501000-00011. [DOI] [PubMed] [Google Scholar]
- 6.Vickers C, Hales P, Kaushik V, Dick L, Gavin J, Tang J, Godbout K, Parsons T, Baronas E, Hsieh F, Acton S, Patane M, Nichols A, Tummino P. Hydrolysis of biological peptides by human angiotensin-converting enzyme-related carboxypeptidase. J Biol Chem. 2002;277:14838–14843. doi: 10.1074/jbc.M200581200. [DOI] [PubMed] [Google Scholar]
- 7.Ferreira AJ, Shenoy V, Yamazato Y, Sriramula S, Francis J, Yuan L, Castellano RK, Ostrov DA, Oh SP, Katovich MJ, Raizada MK. Evidence for angiotensin-converting enzyme 2 as a therapeutic target for the prevention of pulmonary hypertension. Am J Respir Crit Care Med. 2009;179:1048–1054. doi: 10.1164/rccm.200811-1678OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crackower MA, Sarao R, Oudit GY, Yagil C, Kozieradzki I, Scanga SE, Oliveira-dos-Santos AJ, da Costa J, Zhang L, Pei Y, Scholey J, Ferrario CM, Manoukian AS, Chappell MC, Backx PH, Yagil Y, Penninger JM. Angiotensin-converting enzyme 2 is an essential regulator of heart function. Nature. 2002;417:822–828. doi: 10.1038/nature00786. [DOI] [PubMed] [Google Scholar]
- 9.Santos RAS, Castro CH, Gava E, Pinheiro SV, Almeida AP, Paula RD, Cruz JS, Ramos AS, Rosa KT, Irigoyen MC, Bader M, Alenina N, Kitten GT, Ferreira AJ. Impairment of in vitro and in vivo heart function in angiotensin-(1-7) receptor MAS knockout mice. Hypertension. 2006;47:996–1002. doi: 10.1161/01.HYP.0000215289.51180.5c. [DOI] [PubMed] [Google Scholar]
- 10.Grobe JL, Mecca AP, Lingis M, Shenoy V, Bolton TA, Machado JM, Speth RC, Raizada MK, Katovich MJ. Prevention of angiotensin II-induced cardiac remodeling by angiotensin-(1-7) Am J Physiol Heart Circ Physiol. 2007;292:H736–H742. doi: 10.1152/ajpheart.00937.2006. [DOI] [PubMed] [Google Scholar]
- 11.Donoghue M, Wakimoto H, Maguire CT, Acton S, Hales P, Stagliano N, Fairchild-Huntress V, Xu J, Lorenz JN, Kadambi V, Berul CI, Breitbart RE. Heart block, ventricular tachycardia, and sudden death in ACE2 transgenic mice with downregulated connexins. J Mol Cell Cardiol. 2003;35:1043–1053. doi: 10.1016/s0022-2828(03)00177-9. [DOI] [PubMed] [Google Scholar]
- 12.Gurley SB, Allred A, Le TH, Griffiths R, Mao L, Philip N, Haystead TA, Donoghue M, Breitbart RE, Acton SL, Rockman HA, Coffman TM. Altered blood pressure responses and normal cardiac phenotype in ACE2-null mice. J Clin Invest. 2006;116:2218–2225. doi: 10.1172/JCI16980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Santos RAS, Simões e Silva AC, Maric C, Silva DM, Machado RP, de Buhr I, Heringer-Walther S, Pinheiro SV, Lopes MT, Bader M, Mendes EP, Lemos VS, Campagnole-Santos MJ, Schultheiss HP, Speth R, Walther T. Angiotensin-(1-7) is an endogenous ligand for the G protein-coupled receptor Mas. Proc Natl Acad Sci U S A. 2003;100:8258–8263. doi: 10.1073/pnas.1432869100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pinheiro SV, Simões e Silva AC, Sampaio WO, Paula RD, Mendes EP, Bontempo ED, Pesquero JB, Walther T, Alenina N, Bader M, Bleich M, Santos RAS. Nonpeptide AVE 0991 is an angiotensin-(1-7) receptor Mas agonist in the mouse kidney. Hypertension. 2004;44:490–496. doi: 10.1161/01.HYP.0000141438.64887.42. [DOI] [PubMed] [Google Scholar]
- 15.Huentelman MJ, Grobe JL, Vazquez J, Stewart JM, Mecca AP, Katovich MJ, Ferrario CM, Raizada MK. Protection from angiotensin II-induced cardiac hypertrophy and fibrosis by systemic lentiviral delivery of ACE2 in rats. Exp Physiol. 2005;90:783–790. doi: 10.1113/expphysiol.2005.031096. [DOI] [PubMed] [Google Scholar]
- 16.Díez-Freire C, Vázquez J, Correa de Adjounian MF, Ferrari MF, Yuan L, Silver X, Torres R, Raizada MK. ACE2 gene transfer attenuates hypertension-linked pathophysiological changes in the SHR. Physiol Genomics. 2006;27:12–19. doi: 10.1152/physiolgenomics.00312.2005. [DOI] [PubMed] [Google Scholar]
- 17.Der Sarkissian S, Grobe JL, Yuan L, Narielwala DR, Walter GA, Katovich MJ, Raizada MK. Cardiac overexpression of angiotensin converting enzyme 2 protects the heart from ischemia-induced pathophysiology. Hypertension. 2008;51:712–718. doi: 10.1161/HYPERTENSIONAHA.107.100693. [DOI] [PubMed] [Google Scholar]
- 18.Mercure C, Yogi A, Callera GE, Aranha AB, Bader M, Ferreira AJ, Santos RAS, Walther T, Touyz RM, Reudelhuber TL. Angiotensin(1-7) blunts hypertensive cardiac remodeling by a direct effect on the heart. Circ Res. 2008;103:1319–1326. doi: 10.1161/CIRCRESAHA.108.184911. [DOI] [PubMed] [Google Scholar]
- 19.Rentzsch B, Todiras M, Iliescu R, Popova E, Campos LA, Oliveira ML, Baltatu OC, Santos RAS, Bader M. Transgenic angiotensin-converting enzyme 2 overexpression in vessels of SHRSP rats reduces blood pressure and improves endothelial function. Hypertension. 2008;52:967–973. doi: 10.1161/HYPERTENSIONAHA.108.114322. [DOI] [PubMed] [Google Scholar]
- 20.Neves LA, Almeida AP, Khosla MC, Campagnole-Santos MJ, Santos RAS. Effect of angiotensin-(1-7) on reperfusion arrhythmias in isolated rat hearts. Braz J Med Biol Res. 1997;30:801–809. doi: 10.1590/s0100-879x1997000600016. [DOI] [PubMed] [Google Scholar]
- 21.Ferreira AJ, Santos RAS, Almeida AP. Angiotensin-(1-7): cardioprotective effect in myocardial ischemia/reperfusion. Hypertension. 2001;38:665–668. doi: 10.1161/01.hyp.38.3.665. [DOI] [PubMed] [Google Scholar]
- 22.Masson R, Nicklin SA, Craig MA, McBride M, Gilday K, Gregorevic P, Allen JM, Chamberlain JS, Smith G, Graham D, Dominiczak AF, Napoli C, Baker AH. Onset of experimental severe cardiac fibrosis is mediated by overexpression of Angiotensin-converting enzyme 2. Hypertension. 2009;53:694–700. doi: 10.1161/HYPERTENSIONAHA.108.122333. [DOI] [PubMed] [Google Scholar]
- 23.Zisman LS, Keller RS, Weaver B, Lin Q, Speth R, Bristow MR, Canver CC. Increased angiotensin-(1-7)-forming activity in failing human heart ventricles: evidence for up regulation of the angiotensin-converting enzyme homologue ACE2. Circulation. 2003;108:1707–1712. doi: 10.1161/01.CIR.0000094734.67990.99. [DOI] [PubMed] [Google Scholar]
- 24.Ocaranza MP, Godoy I, Jalil JE, Varas M, Collantes P, Pinto M, Roman M, Ramirez C, Copaja M, Diaz-Araya G, Castro P, Lavandero S. Enalapril attenuates downregulation of Angiotensin-converting enzyme 2 in the late phase of ventricular dysfunction in myocardial infarcted rat. Hypertension. 2006;48:572–578. doi: 10.1161/01.HYP.0000237862.94083.45. [DOI] [PubMed] [Google Scholar]
- 25.Ishiyama Y, Gallagher PE, Averill DB, Tallant EA, Brosnihan KB, Ferrario CM. Upregulation of angiotensin-converting enzyme 2 after myocardial infarcte by blockade of angiotensin II receptors. Hypertension. 2004;43:970–976. doi: 10.1161/01.HYP.0000124667.34652.1a. [DOI] [PubMed] [Google Scholar]
- 26.Chappell MC, Pirro NT, Sykes A, Ferrario CM. Metabolism of angiotensin-(1-7) by angiotensin-converting enzyme. Hypertension. 1998;31:362–367. doi: 10.1161/01.hyp.31.1.362. [DOI] [PubMed] [Google Scholar]
- 27.Iyer SN, Chappell MC, Averill DB, Diz DI, Ferrario CM. Vasodepressor actions of angiotensin-(1-7) unmasked during combined treatment with lisinopril and losartan. Hypertension. 1998;31:699–705. doi: 10.1161/01.hyp.31.2.699. [DOI] [PubMed] [Google Scholar]
- 28.Kocks MJ, Lely AT, Boomsma F, de Jong PE, Navis G. Sodium status and angiotensin-converting enzyme inhibition: effects on plasma angiotensin-(1-7) in healthy man. J Hypertens. 2005;23:597–602. doi: 10.1097/01.hjh.0000160217.86597.b6. [DOI] [PubMed] [Google Scholar]
- 29.Dias-Peixoto MF, Santos RAS, Gomes ER, Alves MN, Almeida PW, Greco L, Rosa M, Fauler B, Bader M, Alenina N, Guatimosim S. Molecular mechanisms involved in the angiotensin-(1-7)/Mas signaling pathway in cardiomyocytes. Hypertension. 2008;52:542–548. doi: 10.1161/HYPERTENSIONAHA.108.114280. [DOI] [PubMed] [Google Scholar]
- 30.Ye M, Wysocki J, Naaz P, Salabat MR, LaPointe MS, Batlle D. Increased ACE2 and decreased ACE protein in renal tubules from diabetic mice: a renoprotective combination? Hypertension. 2004;43:1120–1125. doi: 10.1161/01.HYP.0000126192.27644.76. [DOI] [PubMed] [Google Scholar]
- 31.Oudit GY, Herzenberg AM, Kassiri Z, Wong D, Reich H, Khokha R, Crackower MA, Backx PH, Penninger JM, Scholey JW. Loss of angiotensin-converting enzyme-2 leads to the late development of angiotensin II-dependent glomerulosclerosis. Am J Pathol. 2006;168:1808–1820. doi: 10.2353/ajpath.2006.051091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tikellis C, Cooper ME, Bialkowski K, Johnston CI, Burns WC, Lew RA, Smith AI, Thomas MC. Developmental expression of ACE2 in the SHR kidney: a role in hypertension? Kidney Int. 2006;70:34–41. doi: 10.1038/sj.ki.5000428. [DOI] [PubMed] [Google Scholar]
- 33.Lely AT, Hamming I, van Goor H, Navis GJ. Renal ACE2 expression in human kidney disease. J Pathol. 2004;204:587–593. doi: 10.1002/path.1670. [DOI] [PubMed] [Google Scholar]
- 34.Soler MJ, Wysocki J, Ye M, Lloveras J, Kanwar Y, Batlle D. ACE2 inhibition worsens glomerular injury in association with increased ACE expression in streptozotocin-induced diabetic mice. Kidney Int. 2007;72:614–623. doi: 10.1038/sj.ki.5002373. [DOI] [PubMed] [Google Scholar]
- 35.Benter IF, Yousif MH, Dhaunsi GS, Kaur J, Chappell MC, Diz DI. Angiotensin-(1-7) prevents activation of NADPH oxidase and renal vascular dysfunction in diabetic hypertensive rats. Am J Nephrol. 2008;28:25–33. doi: 10.1159/000108758. [DOI] [PubMed] [Google Scholar]
- 36.Pinheiro SV, Ferreira AJ, Kitten GT, Silveira KD, Silva DA, Santos SH, Gava E, Castro CH, Magalhães JA, Mota RK, Botelho-Santos GA, Bader M, Alenina N, Santos RAS, Simões e Silva AC. Genetic deletion of the angiotensin-(1-7) receptor Mas leads to glomerular hyperfiltration and microalbuminuria. Kidney Int. 2009;75:1184–1193. doi: 10.1038/ki.2009.61. [DOI] [PubMed] [Google Scholar]
- 37.Shao Y, He M, Zhou L, Yao T, Huang Y, Lu LM. Chronic angiotensin-(1-7) injection accelerates STZ-induced diabetic renal injury. Acta Pharmacol Sin. 2008;29:829–837. doi: 10.1111/j.1745-7254.2008.00812.x. [DOI] [PubMed] [Google Scholar]
- 38.Reich HN, Oudit GY, Penninger JM, Scholey JW, Herzenberg AM. Decreased glomerular and tubular expression of ACE2 in patients with type 2 diabetes and kidney disease. Kidney Int. 2008;74:1610–1616. doi: 10.1038/ki.2008.497. [DOI] [PubMed] [Google Scholar]
- 39.Mizuiri S, Hemmi H, Arita M, Ohashi Y, Tanaka Y, Miyagi M, Sakai K, Ishikawa Y, Shibuya K, Hase H, Aikawa A. Expression of ACE and ACE2 in individuals with diabetic kidney disease and healthy controls. Am J Kidney Dis. 2008;51:613–623. doi: 10.1053/j.ajkd.2007.11.022. [DOI] [PubMed] [Google Scholar]
- 40.Imai Y, Kuba K, Rao S, Huan Y, Guo F, Guan B, Yang P, Sarao R, Wada T, Leong-Poi H, Crackower MA, Fukamizu A, Hui CC, Hein L, Uhlig S, Slutsky AS, Jiang C, Penninger JM. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436:112–116. doi: 10.1038/nature03712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hernández-Prada JA, Ferreira AJ, Katovich MJ, Shenoy V, Qi Y, Santos RAS, Castellano RK, Lampkins AJ, Gubala V, Ostrov DA, Raizada MK. Structure-based identification of small molecule ACE2 activators as novel antihypertensives. Hypertension. 2008;51:1312–1317. doi: 10.1161/HYPERTENSIONAHA.107.108944. [DOI] [PubMed] [Google Scholar]
- 42.Yamazato Y, Ferreira AJ, Hong KH, Sriramula S, Francis J, Yamazato M, Yuan L, Bradford CN, Shenoy V, Oh SP, Katovich MJ, Raizada MK. Prevention of pulmonary hypertension by angiotensin-converting enzyme 2 gene transfer. Hypertension. 2009;54:365–371. doi: 10.1161/HYPERTENSIONAHA.108.125468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xia H, Lazartigues E. Angiotensin-converting enzyme 2 in the brain: properties and future directions. J Neurochem. 2008;107:1482–1494. doi: 10.1111/j.1471-4159.2008.05723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Doobay MF, Talman LS, Obr TD, Tian X, Davisson RL, Lazartigues E. Differential expression of neuronal ACE2 in transgenic mice with overexpression of the brain renin-angiotensin system. Am J Physiol Regul Integr Comp Physiol. 2007;292:R373–R381. doi: 10.1152/ajpregu.00292.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yamazato M, Yamazato Y, Sun C, Diez-Freire C, Raizada MK. Overexpression of angiotensin-converting enzyme 2 in the rostral ventrolateral medulla causes long-term decrease in blood pressure in the spontaneously hypertensive rats. Hypertension. 2007;49:926–931. doi: 10.1161/01.HYP.0000259942.38108.20. [DOI] [PubMed] [Google Scholar]
- 46.Diz DI, Garcia-Espinosa MA, Gegick S, Tommasi EN, Ferrario CM, Ann Tallant E, Chappell MC, Gallagher PE. Injections of angiotensin-converting enzyme 2 inhibitor MLN4760 into nucleus tractus solitarii reduce baroreceptor reflex sensitivity for heart rate control in rats. Exp Physiol. 2008;93:694–700. doi: 10.1113/expphysiol.2007.040261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Feng Y, Yue X, Xia H, Bindom SM, Hickman PJ, Filipeanu CM, Wu G, Lazartigues E. Angiotensin-converting enzyme 2 overexpression in the subfornical organ prevents the angiotensin II-mediated pressor and drinking responses and is associated with angiotensin II type 1 receptor downregulation. Circ Res. 2008;102:729–736. doi: 10.1161/CIRCRESAHA.107.169110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sampaio WO, Nascimento AA, Santos RAS. Systemic and regional hemodynamic effects of angiotensin-(1-7) in rats. Am J Physiol Heart Circ Physiol. 2003;284:H1985–H1994. doi: 10.1152/ajpheart.01145.2002. [DOI] [PubMed] [Google Scholar]
- 49.Lu J, Zhang Y, Shi J. Effects of intracerebroventricular infusion of angiotensin-(1-7) on bradykinin formation and the kinin receptor expression after focal cerebral ischemia-reperfusion in rats. Brain Res. 2008;1219:127–135. doi: 10.1016/j.brainres.2008.04.057. [DOI] [PubMed] [Google Scholar]
- 50.Zhang Y, Lu J, Shi J, Lin X, Dong J, Zhang S, Liu Y, Tong Q. Central administration of angiotensin-(1-7) stimulates nitric oxide release and upregulates the endothelial nitric oxide synthase expression following focal cerebral ischemia/reperfusion in rats. Neuropeptides. 2008;42:593–600. doi: 10.1016/j.npep.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 51.Mecca AP, O'Connor TE, Dooies A, Katovich MJ, Sumners C. Central administration of Angiotensin 1-7 is cerebroprotective in a rat model of ischemic stroke. FASEB J. 2009;23:947.1. Abstract. [Google Scholar]
- 52.Wiemer G, Dobrucki LW, Louka FR, Malinski T, Heitsch H. AVE 0991, a nonpeptide mimic of the effects of angiotensin-(1-7) on the endothelium. Hypertension. 2002;40:847–852. doi: 10.1161/01.hyp.0000037979.53963.8f. [DOI] [PubMed] [Google Scholar]
- 53.Faria-Silva R, Duarte FV, Santos RAS. Short-term angiotensin(1-7) receptor MAS stimulation improves endothelial function in normotensive rats. Hypertension. 2005;46:948–952. doi: 10.1161/01.HYP.0000174594.17052.33. [DOI] [PubMed] [Google Scholar]
- 54.Ferreira AJ, Jacoby BA, Araujo CA, Macedo FA, Silva GAB, Almeida AP, Caliari MV, Santos RAS. The nonpeptide angiotensin-(1-7) receptor Mas agonist AVE 0991 attenuates heart failure induced by myocardial infarction. Am J Physiol Heart Circ Physiol. 2007;292:H1113–H1119. doi: 10.1152/ajpheart.00828.2006. [DOI] [PubMed] [Google Scholar]
- 55.Lula I, Denadai AL, Resende JM, Sousa FB, Lima GF, Pilo-Veloso D, Heine T, Duarte HA, Santos RAS, Sinisterra RD. Study of angiotensin-(1-7) vasoactive peptide and its beta-cyclodextrin inclusion complexes: complete sequence-specific NMR assignments and structural studies. Peptides. 2007;28:2199–2210. doi: 10.1016/j.peptides.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 56.Marques FD, Ferreira AJ, Sinisterra RD, Jacoby BA, Silva GAB, Almeida AP, Caliari MV, Oliveira PB, Sousa FB, Santos RAS. An oral formulation of Angiotensin-(1-7) produces cardioprotective effects in infarcted and isoproterenol-treated rats. Hypertension. 2008;52:e34–e131. doi: 10.1161/HYPERTENSIONAHA.110.167346. Abstract. [DOI] [PubMed] [Google Scholar]
- 57.Kluskens LD, Nelemans A, Rink R, de Vries L, Meter-Arkema A, Wang Y, Walther T, Kuipers A, Moll GN, Haas M. Angiotensin-(1-7) with thioether-bridge: an ACE-resistant, potent Ang-(1-7) analogue. J Pharmacol Exp Ther. 2009;328:849–854. doi: 10.1124/jpet.108.146431. [DOI] [PubMed] [Google Scholar]
- 58.Santos RAS, Savergnini SQ, Paula-Carvalho V, Rotman G, Cojocaru G, Cohen Y, Cohen-Dayag A, Zauberman A, Irigoyen MC, Cândido G, Almeida AP, Beiman M. Antihypertensive effect of the novel receptor Mas agonist CGEN-856S. Hypertension. 2009;54:e110. Abstract. [Google Scholar]
- 59.Shenoy V, Ferreira AJ, Qi Y, Dooies KA, Raizada MK, Katovich MJ. Lenti-viral mediated overexpression of ACE2 or Angiotensin-(1-7) prevents bleomycin-induced pulmonary fibrosis. FASEB J. 2009;23:770.7. Abstract. [Google Scholar]
- 60.Epelman S, Shrestha K, Troughton RW, Francis GS, Sen S, Klein AL, Tang WH. Soluble angiotensin-converting enzyme 2 in human heart failure: relation with myocardial function and clinical outcomes. J Card Fail. 2009;15:565–571. doi: 10.1016/j.cardfail.2009.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yamamoto K, Ohishi M, Katsuya T, Ito N, Ikushima M, Kaibe M, Tatara Y, Shiota A, Sugano S, Takeda S, Rakugi H, Ogihara T. Deletion of angiotensin-converting enzyme 2 accelerates pressure overload-induced cardiac dysfunction by increasing local angiotensin II. Hypertension. 2006;47:718–726. doi: 10.1161/01.HYP.0000205833.89478.5b. [DOI] [PubMed] [Google Scholar]
- 62.Walther T, Wessel N, Kang N, Sander A, Tschöpe C, Malberg H, Bader M, Voss A. Altered heart rate and blood pressure variability in mice lacking the Mas protooncogene. Braz J Med Biol Res. 2000;33:1–9. doi: 10.1590/s0100-879x2000000100001. [DOI] [PubMed] [Google Scholar]
- 63.Peiró C, Vallejo S, Gembardt F, Azcutia V, Heringer-Walther S, Rodríguez-Mañas L, Schultheiss HP, Sánchez-Ferrer CF, Walther T. Endothelial dysfunction through genetic deletion or inhibition of the G protein-coupled receptor Mas: a new target to improve endothelial function. J Hypertens. 2007;25:2421–2425. doi: 10.1097/HJH.0b013e3282f0143c. [DOI] [PubMed] [Google Scholar]
- 64.Xu P, Santos RA, Bader M, Alenina N. Alterations in gene expression in the testis of angiotensin-(1-7)-receptor Mas-deficient mice. Regul Pept. 2007;138:51–55. doi: 10.1016/j.regpep.2006.11.017. [DOI] [PubMed] [Google Scholar]