Abstract
Low stringency genomic library screens with genomic fragments from the sex determination gene doublesex identified the Drosophila secreted cuticle protein 73 (dsc73) gene, which encodes an 852-residue protein with an N-terminal signal sequence. In embryos, dsc73 RNA and protein are expressed to high levels in the epidermal cells that secrete the larval cuticle as well as in other cuticle-secreting tissues such as the trachea and salivary duct. Embryonic expression of dsc73 requires Shavenbaby, a transcription factor regulating cuticle formation. Double-labeling experiments with αCrb and αSAS reveal that, as with chitin and other known cuticle proteins, Dsc73 is secreted apically. Zygotic loss of dsc73 results in larval lethality but loss does not result in overt patterning defects or overt morphological defects in the embryonic tissues in which it is expressed. Thus, dsc73 encodes a novel secreted protein, and it is conserved within the Drosophila group. dsc73 may serve as a useful embryonic marker for cuticular patterning.
Keywords: cuticle, Drosophila, secretion
INTRODUCTION
Screens for related genes by low stringency hybridizations of genomic libraries have been successful in identifying several proteins with related functions, perhaps most notably the homeodomain-containing proteins of the Antp-C and Bx-C and their mammalian counterparts, the Hox genes (McGinnis et al., 1984a–c; Scott and Weiner, 1984). The doublesex (dsx) gene encodes two alternatively spliced isoforms DsxM and DsxF that function as transcription factors to control most aspects of male or female somatic sexual development in Drosophila (Belote and Baker, 1983; Baker and Wolfner, 1988; Burtis and Baker, 1989). To identify proteins related to Dsx that could potentially also function in sex determination, we carried out a low-stringency screen of a Drosophila genomic library (Maniatis et al., 1978). Among the six genomic regions isolated in the screen was the region including dsc73, which was originally dubbed doublesex cognate protein in 73A (dsc73) to indicate the manner of its discovery and its cytological position (Andrew, 1987). Based on the findings described in this manuscript, we have renamed the gene Drosophila secreted cuticle protein 73, allowing us to keep the original acronym and to reflect our finding (see below) that the protein is highly conserved only within the Drosophila group. dsc73 encodes a secreted protein with very little similarity to dsx outside of the simple repeat sequences present in the coding regions. Although dsc73 contains a single dsx female-specific consensus splice site (ACATCAATCAACA; Hedley and Maniatis, 1991; Hoshijima et al., 1991; Ryner and Baker, 1991) in the first large intron 54 nucleotides (nt) upstream of the exon 2 splice acceptor site, there is no evidence for alternative splicing of this gene. This study focuses on the expression and localization of Dsc73 and its potential functions in early development.
RESULTS AND DISCUSSION
dsc73 Encodes a Large Secreted Protein
From low-stringency screens of a Drosophila genomic library with several fragments of dsx genomic DNA, we isolated two overlapping phage that mapped to cytological region 73A. We used fragments from the phage DNA to isolate additional genomic DNA by “chromosomal walking” and to screen cDNA libraries (provided by L. Kauvar). Sequencing and mapping of the longest cDNA relative to the genomic DNA revealed that dsc73 encodes a single large primary transcript, spanning ~28.7 kb, with five exons, which vary in size from 78 to 1,534 nt, and four introns, which vary in size from 67 to 23,240 nt. Sequence analysis and mapping of the dsc73 cDNAs isolated by library screening or obtained from the Berkeley Drosophila Genome Project indicate that this is the only splice form. dsc73 corresponds to CG32159 and maps ~18 kb downstream of and distal to argos, which encodes a secreted negative regulator of epithelial growth factor (EGF) signaling (Freeman et al., 1992; Freeman, 1994; Schweitzer et al., 1995; Golembo et al., 1996). Both dsc73 and argos are entirely contained within a large (~63 kb) intron of CG33158, an uncharacterized gene that is transcribed off the other strand.
The dsc73 mRNA has a single large open reading encoding an 852-residue protein with an N-terminal signal sequence and no other hydrophobic regions of sufficient length to span a membrane, suggesting that Dsc73 is secreted (Fig. 1B). The open reading frame (ORF) also contains several consensus sites for N-linked glycosylation and four repeats of CXXPX(1-2)-aromatic. Although the Dsc73 ORF is highly conserved within the Drosophila group, only a single region of 147 amino acid residues is conserved among other insects and arthropods (Fig. 1C). Interestingly, a PONDR (Predictor of Natural Disordered Regions) analysis suggests that the entire protein is highly disordered outside this small conserved region (Romero et al., 1997, 2001; Li et al., 1999).
Fig. 1.
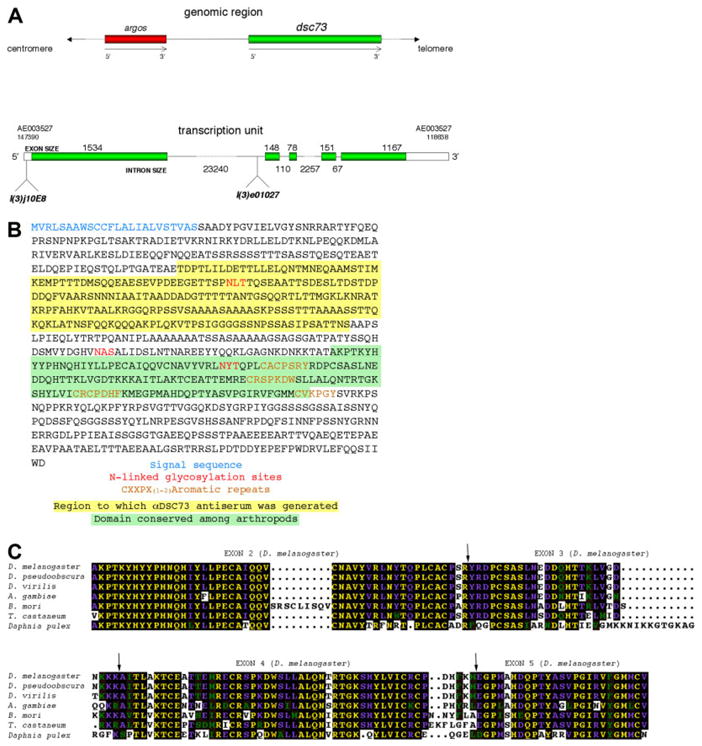
A: dsc73 (green bar, top figure) maps distal to the argos gene (red bar, top figure) and encodes a single transcript with five exons (boxed areas, bottom figure). The open reading frame (green areas inside boxed region, bottom figure) encodes 852 residues. An inactivating P-element insertion in dsc73, l(3)j10E8, maps within the 5′untranslated region and an inactivating Piggy-bac element insertion in dsc73, l(3)e01027, maps ~4.8 kb upstream of the splice acceptor site of exon 2. B: The open reading frame of dsc73 has an N-terminal signal sequence (blue), several N-linked gycosylation consensus sites (red), and four repeats of CXCPX(1-2) aromatic (brown). The sequence with yellow background was used to generate antiserum to Dsc73. The sequence with green background is the most highly conserved and highly structured domain of the protein. C: A 147-residue domain in Dsc73 is conserved in all insects and in the arthropod Daphnia pulex. Of interest, this domain spans four exons in Drosophila melanogaster. The exon boundaries are indicated by arrows.
dsc73 Is Expressed in Cuticle-Secreting Epithelia
dsc73 expression was examined by whole-mount in situ hybridization and Northern blot analysis. RNA is first detected during embryonic mid-stage 12 in precursors to the posterior spiracle (Fig. 2A). By embryonic stage 13, transcript is also detected in patches of epidermal cells and in several domains in the head. At stage 15, dsc73 expression is abundant in the epidermis, as well as in the pharynx, atrium (mouth), esophagus, anterior midgut cells, salivary duct, dorsal trunk cells of the trachea, and a portion of the hindgut. Expression persists in these tissues through the end of embryogenesis, with robust expression in the epidermal cells that form the denticles and hairs, and in what are known as the ventral and lateral “black dots,” cuticularized structures associated with peripheral neurons known as basiconical sensilla. Very similar epidermal expression patterns have been observed with several genes encoding proteins predicted to encode constituents of the larval cuticle (CG1919, CG2555, and CG2560; BDGP: www.fruitfly.org.cgi-bin/ex/ensiut.pl) as well as genes encoding zona pellucida (ZP) domain proteins that are either secreted or anchored to the plasma membrane (miniature, CG17131, CG16798, CG15013, CG17111, CG7802, CG12063, and CG1499; Jazwinska and Affolter, 2004). The high level of expression of dsc73 and these other epithelial genes in the cells that form denticles and hairs suggests a potential structural role for these genes in forming cuticular protrusions. Northern analysis of sex-specific third instar larval, pupal, and adult polyA+-selected RNA revealed a single 3.2-kb transcript that is most highly expressed in pupae (Fig. 2B).
Fig. 2.
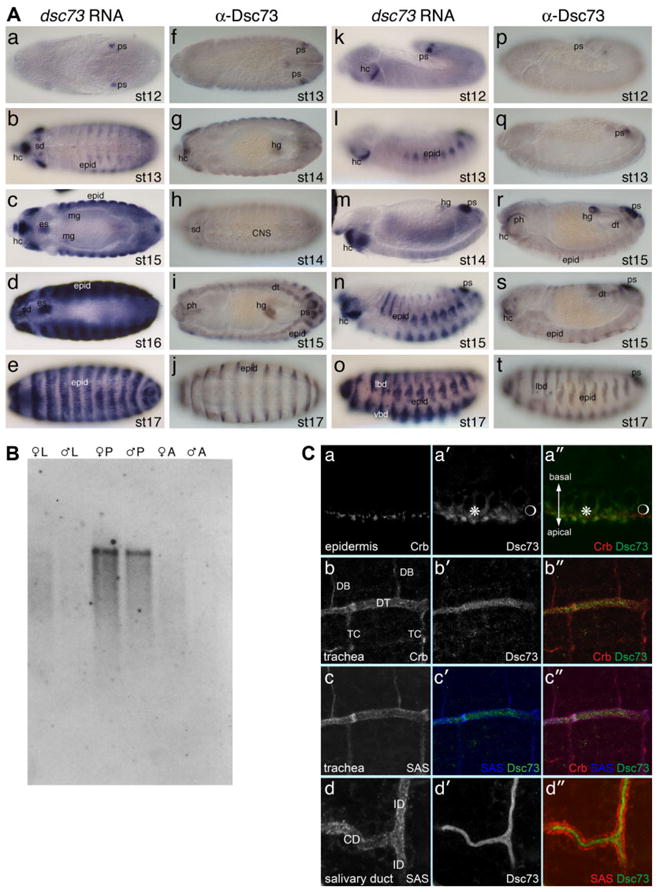
A: dsc73 RNA and protein are expressed to high levels in embryos. a–j show dorsal–ventral views of stage 12 (st 12) through 17 (st 17) embryos. k–t show lateral views of stage 12 through 17 embryos. a–e and k–o were hybridized with an RNA probe corresponding to a dsc73 cDNA. f–j and p–t were immunostained with a rat antiserum directed against a fragment of the Dsc73 protein. Expression is first observed in the posterior spiracle (ps) and limited domains in the head (hc; a,f,k,p). Expression is subsequently observed in epidermal cells (epid) and hindgut (hg; b,g,l,q), with high levels persisting in the head and epidermis through embryogenesis. During stages 15 and 16, expression is also observed in internal tissues, including the pharynx (ph; i,r), dorsal trunk (dt) cells of the trachea (i and s), salivary duct (sd; d and h), esophagus (es; c,d), an anterior subset of midgut cells (mg; c), and a subset of cells in all segments of the central nervous system (j). During stage 17, expression is also observed in the ventral and lateral black dots (vbd and lbd; o,t). Epidermal expression (epid) of dsc73 is highest in cells that form the denticles and hairs (b– e,i,l,j,n,o,s,t). B: Northern analysis of sex-specific polyA+-selected RNA revealed abundant dsc73 expression during the pupal stages (P), with very little expression in third instar larvae (L) and adults (A). C: Colocalization of Dsc73 with known apical proteins (Crb and/or SAS) in epidermal cells (a–a″), dorsal trunk tracheal cells (b– c″), and salivary duct (d– d″) reveals that Dsc73 is secreted apically. Note that levels of Dsc73 are higher in the part of the ventral epidermis that forms the denticles than in the part that forms naked cuticle (* and  , respectively, in a′ and a″). Although Dsc73 is expressed in the dorsal trunk cells of the trachea (DT), it is not detected in the dorsal branch (DB) or transverse connective (TC) (b′, b″, c′ and c″).
, respectively, in a′ and a″). Although Dsc73 is expressed in the dorsal trunk cells of the trachea (DT), it is not detected in the dorsal branch (DB) or transverse connective (TC) (b′, b″, c′ and c″).
Antibodies were raised against a 226-residue fragment of Dsc73 in both rats and rabbits (Fig. 1B). Staining with αDsc73 revealed a pattern of accumulation in embryos that paralleled expression of the transcript, with high levels in the posterior spiracle, hindgut, pharynx, atrium, esophagus, and the epidermal cells that secrete the denticles and hairs (Fig. 2A). Lower levels of protein were detected in the salivary duct, tracheal dorsal trunk, and a subset of central nervous system neurons. To learn where the Dsc73 protein localizes in cells, we co-immunostained wild-type embryos with αDsc73 and antibodies that recognize two apical membrane proteins, Crumbs (Crb) (Wodarz et al., 1993) and Stranded at Second (SAS; Schonbaum et al., 1992), and examined these embryos by confocal microscopy. In the epidermis, Dsc73 protein was detected in a domain apical to the domain of Crb accumulation, supporting the molecular data suggesting that Dsc73 is a secreted protein (Fig. 2C, a–a″). Similarly, in both the trachea and the salivary duct, Dsc73 was detected in the apical lumen (Fig. 2C, b– d″, respectively). Thus, in agreement with predictions based on sequence, dsc73 encodes a protein that is secreted apically.
dsc73 Embryonic Expression Requires Svb
The pattern of dsc73 expression in the epidermis parallels that of the Shavenbaby (Shv) transcription factor, which functions downstream of the segment polarity genes and the EGF signaling pathway to regulate formation of denticles and hairs (Payre et al., 1999; Chanut-Delalande et al., 2006), and is a good candidate for regulating expression of dsc73. Indeed, whole-mount in situ hybridization revealed a near complete loss of dsc73 expression in shv mutants (Fig. 3). Notably, dsc73 expression in shv2 homozygotes was limited to the posterior spiracle precursors during embryonic stage 13 and to very low levels in the head region during late embryonic stages.
Fig. 3.
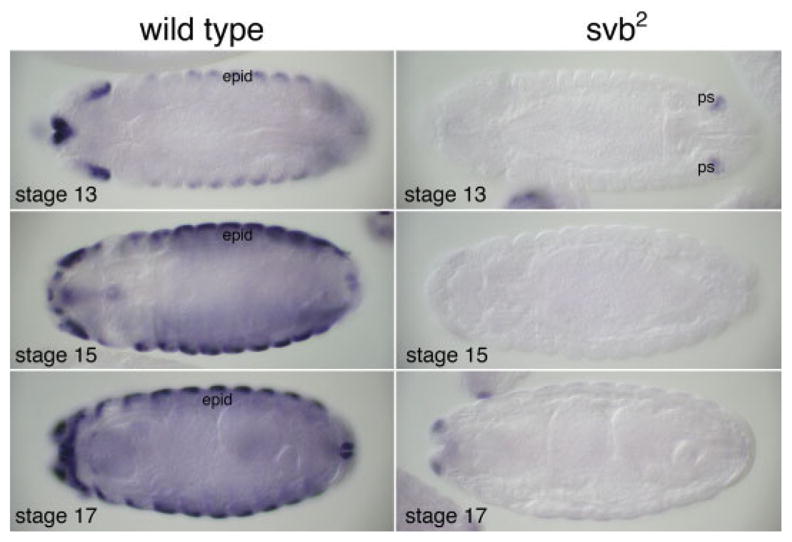
Expression of dsc73 requires ovo/shavenbaby. Wild-type larvae have high levels of dsc73 starting around embryonic stage 13 (left panels), whereas ovo/svb2 homozygotes express very low levels of the transcript (right panels), which is limited to the posterior spiracle precursors during stage 13 (upper right panel) and head structures at later stages (lower two right panels).
dsc73 Mutants Die During the Second Larval Instar Stage
To probe the biological role of dsc73, we obtained two independent P-element lines with insertions in dsc73 (Fig. 1A). One of these lines, l(3)j10E8, had a P-element inserted after nt 31 in the 5′ untranslated region of the mRNA, was homozygous lethal and had a pattern of βgal expression that paralleled the expression of dsc73 (Fig. 4). The second line, l(3)e01027, had a piggyback insertion in the first large intron ~4.8 kb upstream of the splice acceptor site of exon 2 and was homozygous lethal. To generate additional dsc73 alleles and to learn if the lethality in the l(3)j10E8 line is associated with disruption of dsc73, we excised the l(3)j10E8 P-element and obtained both lethal and viable white−(w−) excisants. We focused on two lethal w− excisions (exc64A and exc73A) and one viable w− excision (exc4A). l(3)j10E8 failed to complement exc64A, exc73A, l(3)e01027, and three deficiencies that delete dsc73 and several adjacent genes, and fully complemented exc4A (Table 1). These results indicate that the two insertions disrupt dsc73 function and that the l(3)j10E8 insertion in dsc73 is responsible for the lethality associated with the l(3)j10E8 chromosome. l(3)j10E8 complements the upstream gene argos, indicating that the insertion does not affect argos function, as well as bulge, a gene that has been mapped to the region by complementation but has not been cloned (Wemmer and Klambt, 1995; Table 1). Consistent with the genetic data, Dsc73 protein was undetectable in embryos homozygous for all of the lethal insertion and excision alleles but was at wild-type levels in embryos homozygous for the viable excision allele (Table 2). All of the insertions and excisions of dsc73 and argos could also affect the gene encoded on the other strand because both genes map within the large intron of this gene (CG33158). However, the finding that only the insertions that also affect either dsc73 or argos are lethal and the finding that the lethal P-element insertion in dsc73 complements two argos excision alleles (Table 1), argues against the insertions affecting CG33158 function, although the possibility cannot be ruled out completely until gene-specific mutations in CG33158 become available.
Fig. 4.
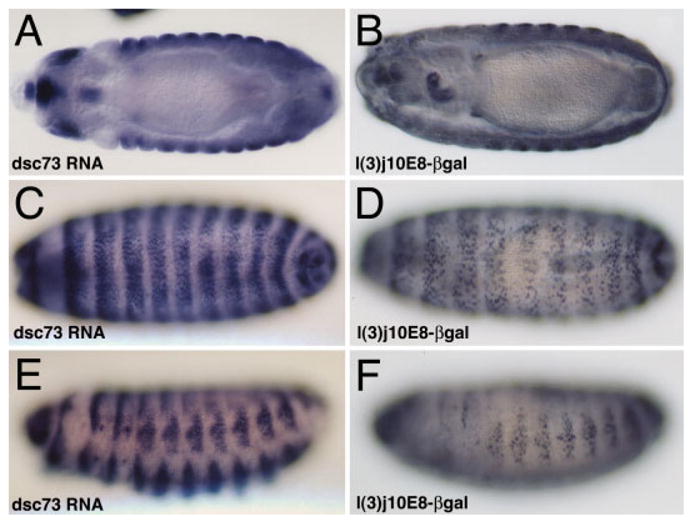
Expression of βgal from the P-element insertion in the l(3)j10E8 line is nuclear and accumulates in the same tissues as the dsc73 transcript. Expression in internal tissues including the esophagus and midgut cells is evident in the top panels, whereas expression in the epidermal cells is evident in the lower four panels.
TABLE 1.
Complementation Tests Between l(3)j10E8 and Insertions, Excisions, Deficiencies, and Mutations in Known Genes That Map to Cytological Region 73Aa
| l(3)j10E8 | argos105 | argos17.1 | bulgeD7 | |
|---|---|---|---|---|
| l(3)j10E8(lethal insertion) | 107:24 | 47:28 | 36:20 | |
| j10E8exc4A(viable excision) | 39:37 | |||
| l(3)j10E8exc64A(lethal excision) | 60:0 | |||
| l(3)j10E8exc73A(lethal excision) | 89:0 | |||
| l(3)e01027(lethal insertion) | 70:0 | |||
| Df(3L)stf13(73A1,2-73B1,2) | 81:0 | |||
| Df(3L)stj7(72D5,6-73A5) | 130:0 | 60:0 | 86:0 | |
| Df(3L)st4(73A1,2-73B1,2) | 54:0 |
Ratios indicate the number of balancer chromosome carrying adults relative to those not carrying a balancer chromosome (the heteroallelic class).
TABLE 2.
dsc73 Lethal Alleles Fail to Make Detectable Levels of Dsc73 Proteina
| Stained embryos stage 13 and older | Unstained embryos stage 13 and older | % of Total embryos stained | |
|---|---|---|---|
| Oregon R (wt) | 126 | 0 | 100% |
| l(3)j10E8/TM3 | 119 | 35 | 77.3% |
| l(3)e01027/TM3 | 99 | 29 | 77.4% |
| exc64A/TM3 | 125 | 44 | 74.0% |
| exc73A/TM3 | 230 | 81 | 74.0% |
| exc4A/exc4A | 157 | 0 | 100% |
Embryos stage 13 and older were scored for staining or absence of staining with αDsc73. All 100% of embryos collected from wild-type and revertant (exc4) flies stained, whereas only approximately 75% of embryos collected from flies heterozygous for the dsc73 lethal alleles stained.
dsc73 homozygous mutant embryos appeared completely normal based on their overall size and tissue morphology when stained with antibodies to Crb, a protein expressed in most of the tissues that express dsc73, including the salivary duct and trachea, or with the 2A12 antibody, which stains the entire tracheal system from stage 14 and later (data not shown). Homozygous dsc73 embryos hatched, crawled around, and survived to the second larval instar transition stage. Patterning of the larval cuticle was normal, and there were no overt defects in denticle or hair morphology (Fig. 5, and data not shown). Gross examination of the homozygous mutant larvae did not reveal an obvious cause of death.
Fig. 5.
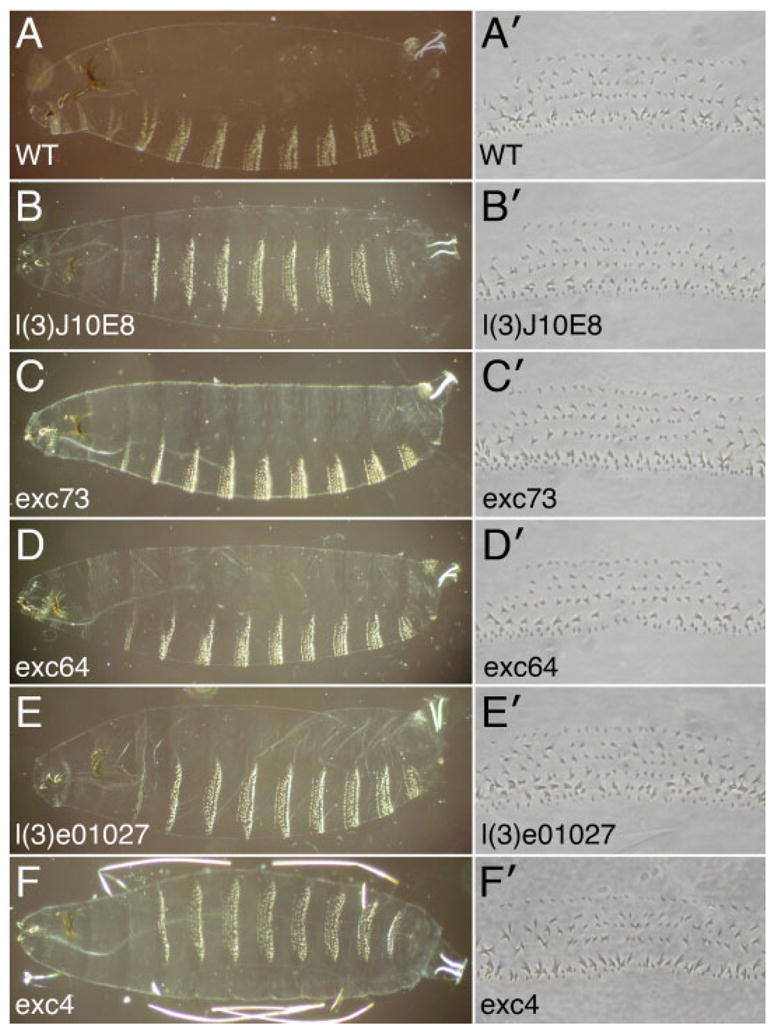
A: A wild-type first instar larval cuticle is shown as well as a magnified view of the denticles from the fourth abdominal segment (A4). B–E: Larval cuticles from dsc73 mutant larvae have a completely normal pattern of denticles and hairs, and the A4 denticles appear morphologically normal. F: Larval cuticles from the viable dsc73 excision allele are also normal.
Concluding Remarks
The relationship between dsx and dsc73 is not clear, especially because the two proteins localize to very different cellular locations and statistical analysis of the relatedness of the two proteins by a “jumble analysis” (Doolittle, 1986) indicates only a marginal degree of similarity. The cross-hybridization between genomic DNA and cDNAs from dsx and dsc73 at low stringency can be explained in part by the sequences encoding the runs of alanines found in both proteins, because the calculated melting temperatures (Tms) for the DNA encoding these runs of homology (McConaughy et al., 1969; Britten et al., 1974; Wetmur, 1976) are theoretically high enough to allow duplex formation under the conditions used for the screen. Thus, our attempt to find true dsx homologues was unsuccessful, likely because of the background signals from the polyamino acid stretches encoded by many Drosophila genes. The more recent completion of the genome sequence (Adams et al., 2000) has revealed several genes encoding proteins with significant homology to Dsx and its Caenorhabditis elegans homologue Mab5, including dmrt99B, dmrt11E, and dmrt93B, which like dsx appear to encode nuclear proteins. Also among the genes that can be identified through a blast search of the Drosophila genome with the Dsx protein sequence is dissatisfaction (dsf), a gene known to regulate sex-specific behavior and neuronal development (Finley et al., 1997, 1998). Later genomic library screens using tandem repeats of the female-specific splice regulatory sites of dsx did identify fruitless (fru), whose splicing is regulated by the upstream components of the sex determination pathway and has clear roles in sex-specific behavior and morphology (Ryner et al., 1996). Of interest, this subsequent screen also identified dsc73, which contains a single copy of the female splice consensus site near the exon 2 splice acceptor site (L. Ryner and B.S. Baker, unpublished observations). The finding that only a single-size transcript is observed on Northern blots and that none of the many dsc73 cDNAs that have been characterized contain alternative exons, however, indicates that the female splice consensus site is unlikely to be functional.
During embryogenesis, dsc73 is expressed to highest levels in cuticle forming tissues and the protein is secreted (Fig. 2), suggesting that Dsc73 may be a structural component of the cuticle. The subsequent high levels of expression during the pupal stages (Fig. 2B) are consistent with this role because the adult cuticle is formed in pupae. A cuticle-specific function is also supported by the finding that homologues to Dsc73 appear to be limited to arthropods based on blast searches of all of the available genome databases at NCBI. The changes in dsc73 expression in svb mutants reflect the larval cuticle defects observed with loss-of-function mutations in svb (Payre et al., 1999; Delon et al., 2003) and indicate that dsc73 functions downstream of svb and the patterning genes. Mutations in genes encoding other components of the larval and adult cuticle have variable effects on organism viability, ranging from lethality at hatching, which is observed with mutations in genes required for chitin synthesis and/or organization (Ostrowski et al., 2002; Moussian et al., 2005a,b), to full viability and fertility, which is observed with mutations in yellow, which encodes a secreted protein required for pigment melanization (Walter et al., 1991; Kornezos and Chia, 1992). Lethality of dsc73 mutants at the first larval molt suggests a subtle, but critical role for this gene in cuticle-forming tissues, the basis of which may be evident only through ultrastructural analysis. In conclusion, dsc73 encodes a secreted cuticle protein essential for viability and is a useful embryonic marker for patterning read-out.
EXPERIMENTAL PROCEDURES
Library Screens
Genomic phage were isolated from a Canton S wild-type recombinant library cloned into Charon 4A (Maniatis et al., 1978). Filters containing the library were hybridized at 42°C in 29% formamide, 5× SSPE, 1× Denhardt’s solution, 0.2% sodium dodecyl sulfate (SDS), and 250 μg/ml carrier salmon sperm DNA using as probes approximately 5 × 105 cpm/ml of nick translated fragments of genomic DNA from the dsx gene. Filters were washed in 1× SSPE, 0.1% SDS at 50°C. Theoretically, these conditions allow for a mismatch of 15% (McConaughy et al., 1969).
Northern Blotss
RNA was prepared by homogenizing animals in 50 mM Tris-HCl pH 7.5, 50 mM NaCl, 10 mm ethylenediaminetetraacetic acid (EDTA) and 0.5% SDS with 250 μg/ml proteinase K. Samples were then incubated 1 hr at 37°C, phenol-chloroform extracted several times and ethanol precipitated. RNA was resuspended in DEPC-treated water and poly A+-selected using a 5- to 10-fold excess of oligo-dT cellulose. Samples were size fractionated on 1% agarose, 6% formaldehyde gels (prepared in 50 mM Hepes, 1 mM EDTA buffer, pH 8); transferred to nitrocellulose; and probed with nick translated DNA. Large numbers of unisexual populations of larvae, pupae, and adults for RNA preparation were generated using the cinnamon mutation as described by McKeown et al. (1987). As a control for the amount of poly A+RNA in each track, a replica Northern blot prepared from the same RNA samples was hybridized with the ribosomal protein gene probe rp49 (O’Connell and Rosbash, 1984).
Antibody Production, Immunostaining, and Whole-Mount In Situ Hybridization
αDsc73 antiserum was raised in rats to a βgal fusion protein using the λgt11 vector as described by Carroll and Laughon (Carroll et al., 1988). In situ hybridization and antibody staining were performed as previously described (Reuter and Scott, 1990; Lehmann and Tautz, 1994), with the exception that, for antibody staining with αDsc73 (used at 1:200), Bouins fixative (Sigma; St. Louis, MO) was used in place of the 4% formaldehyde in 1× phosphate buffered saline during the heptane phase. For fluorescent staining, αDsc73 was used at a dilution of 1:50, αCrb was used at a dilution of 1:10 and αSAS was used at a dilution of 1:200. With the svb2 mutation, which was not balanced over a lacZ- or GFP-tagged balancer chromosome, levels of dsc73 transcript were compared among all embryos stage 13 and older that were reacted in the same tube. Significant loss of RNA signal was observed in 26.8% (77/287) of embryos at these stages compared with wild-type where 1.4% (4/285) of embryos at these stages had no signal. Similarly, because Dsc73 immunostaining works well only with Bouins fixation, which does not work well for detection of either βgal or green fluorescent protein (GFP), we compared Dsc73 staining in all embryos stage 13 and older. Dsc73 staining was detectable in approximately 75% of embryos from each of the dsc73 mutant stocks, whereas wild-type and exc4A had detectable staining in 100% of embryos stage 13 and older (Table 2). For staining and analysis of dsc73 mutants with αCrb (1:100) and α2A12 (1:100), embryos were also stained with αβgal antiserum (1:5,000; Promega; Madison, WI) to allow unambiguous identification of homozygous dsc73 mutants, which do not stain with αβgal.
Determination of Lethal Phase and Cuticle Preparations
Homozygous late stage dsc73 mutant embryos were selected from collections from GFP-tagged balancer stocks based on the absence of GFP fluorescence. Embryos were placed in a vial and monitored over the next several days. All dsc73 homozygotes hatched and survived until the point where wild-type larvae would undergo the first larval molt, at the transition to the second larval instar stage. Wild-type and exc4A homozygotes treated in the same manner survived to become adults. For cuticle preparations, non-GFP tagged late-stage embryos were selected, aged 12–16 hr, mounted on a slide in Hoyer’s medium, and photographed under dark-field (×10 magnification) or phase contrast (×40 magnification) microscopy on a Zeiss Axiophot.
Acknowledgments
Grant sponsor: NIH; Grant number: RO1 DE12873; Grant number: RO1 GM22345.
We thank both J.W. Posakony and M.P. Scott, in whose laboratories a portion of this work was carried out. We also thank A. Haberman and B. Kerman for technical assistance on the project. We thank R. Fox and S.-Y. Chung and two anonymous reviewers for careful reading and critiquing of this manuscript. Both authors were funded by the NIH.
References
- Adams MD, Celniker SE, Holt RA, Evans CA, Gocayne JD, Amanatides PG, Scherer SE, Li PW, Hoskins RA, Galle RF, George RA, Lewis SE, Richards S, Ashburner M, Henderson SN, Sutton GG, Wortman JR, Yandell MD, Zhang Q, Chen LX, Brandon RC, Rogers YH, Blazej RG, Champe M, Pfeiffer BD, Wan KH, Doyle C, Baxter EG, Helt G, Nelson CR, Gabor GL, Abril JF, Agbayani A, An HJ, Andrews-Pfannkoch C, Baldwin D, Ballew RM, Basu A, Baxendale J, Bayraktaroglu L, Beasley EM, Beeson KY, Benos PV, Berman BP, Bhandari D, Bolshakov S, Borkova D, Botchan MR, Bouck J, Brokstein P, Brottier P, Burtis KC, Busam DA, Butler H, Cadieu E, Center A, Chandra I, Cherry JM, Cawley S, Dahlke C, Davenport LB, Davies P, de Pablos B, Delcher A, Deng Z, Mays AD, Dew I, Dietz SM, Dodson K, Doup LE, Downes M, Dugan-Rocha S, Dunkov BC, Dunn P, Durbin KJ, Evangelista CC, Ferraz C, Ferriera S, Fleischmann W, Fosler C, Gabrielian AE, Garg NS, Gelbart WM, Glasser K, Glodek A, Gong F, Gorrell JH, Gu Z, Guan P, Harris M, Harris NL, Harvey D, Heiman TJ, Hernandez JR, Houck J, Hostin D, Houston KA, Howland TJ, Wei MH, Ibegwam C, Jalali M, Kalush F, Karpen GH, Ke Z, Kennison JA, Ketchum KA, Kimmel BE, Kodira CD, Kraft C, Kravitz S, Kulp D, Lai Z, Lasko P, Lei Y, Levitsky AA, Li J, Li Z, Liang Y, Lin X, Liu X, Mattei B, McIntosh TC, McLeod MP, McPherson D, Merkulov G, Milshina NV, Mobarry C, Morris J, Moshrefi A, Mount SM, Moy M, Murphy B, Murphy L, Muzny DM, Nelson DL, Nelson DR, Nelson KA, Nixon K, Nusskern DR, Pacleb JM, Palazzolo M, Pittman GS, Pan S, Pollard J, Puri V, Reese MG, Reinert K, Remington K, Saunders RD, Scheeler F, Shen H, Shue BC, Siden-Kiamos I, Simpson M, Skupski MP, Smith T, Spier E, Spradling AC, Stapleton M, Strong R, Sun E, Svirskas R, Tector C, Turner R, Venter E, Wang AH, Wang X, Wang ZY, Wassarman DA, Weinstock GM, Weissenbach J, Williams SM, Woodage T, Worley KC, Wu D, Yang S, Yao QA, Ye J, Yeh RF, Zaveri JS, Zhan M, Zhang G, Zhao Q, Zheng L, Zheng XH, Zhong FN, Zhong W, Zhou X, Zhu S, Zhu X, Smith HO, Gibbs RA, Myers EW, Rubin GM, Venter JC. The genome sequence of Drosophila melanogaster. Science. 2000;287:2185–2195. doi: 10.1126/science.287.5461.2185. [DOI] [PubMed] [Google Scholar]
- Andrew DJ. PhD Thesis. UC; San Diego: 1987. A search for new genes regulating sex determination in Drosophila melanogaster. [Google Scholar]
- Baker BS, Wolfner MF. A molecular analysis of doublesex, a bifunctional gene that controls both male and female sexual differentiation in Drosophila melanogaster. Genes Dev. 1988;2:477–489. doi: 10.1101/gad.2.4.477. [DOI] [PubMed] [Google Scholar]
- Belote JM, Baker BS. The dual functions of a sex determination gene in Drosophila melanogaster. Dev Biol. 1983;95:512–517. doi: 10.1016/0012-1606(83)90054-4. [DOI] [PubMed] [Google Scholar]
- Britten RJ, Graham DE, Neufeld BR. Analysis of repeating DNA sequences by reassociation. Methods Enzymol. 1974;29:363–418. doi: 10.1016/0076-6879(74)29033-5. [DOI] [PubMed] [Google Scholar]
- Burtis KC, Baker BS. Drosophila doublesex gene controls somatic sexual differentiation by producing alternatively spliced mRNAs encoding related sex-specific polypeptides. Cell. 1989;56:997–1010. doi: 10.1016/0092-8674(89)90633-8. [DOI] [PubMed] [Google Scholar]
- Carroll SB, Laughon A, Thalley BS. Expression, function, and regulation of the hairy segmentation protein in the Drosophila embryo. Genes Dev. 1988;2:883–890. doi: 10.1101/gad.2.7.883. [DOI] [PubMed] [Google Scholar]
- Chanut-Delalande H, Fernandes I, Roch F, Payre F, Plaza S. Shavenbaby couples patterning to epidermal cell shape control. PLoS Biol. 2006;4:e290. doi: 10.1371/journal.pbio.0040290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delon I, Chanut-Delalande H, Payre F. The Ovo/Shavenbaby transcription factor specifies actin remodelling during epidermal differentiation in Drosophila. Mech Dev. 2003;120:747–758. doi: 10.1016/s0925-4773(03)00081-9. [DOI] [PubMed] [Google Scholar]
- Doolittle RF. A primer on how to analyze derived amino acid sequences. Mill Valley, CA: University Science Books; 1986. Of URFS and ORFS; pp. 3–24. [Google Scholar]
- Finley KD, Taylor BJ, Milstein M, McKeown M. dissatisfaction, a gene involved in sex-specific behavior and neural development of Drosophila melanogaster. Proc Natl Acad Sci U S A. 1997;94:913–918. doi: 10.1073/pnas.94.3.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finley KD, Edeen PT, Foss M, Gross E, Ghbeish N, Palmer RH, Taylor BJ, McKeown M. Dissatisfaction encodes a tailless-like nuclear receptor expressed in a subset of CNS neurons controlling Drosophila sexual behavior. Neuron. 1998;21:1363–1374. doi: 10.1016/s0896-6273(00)80655-8. [DOI] [PubMed] [Google Scholar]
- Freeman M. Misexpression of the Drosophila argos gene, a secreted regulator of cell determination. Development. 1994;120:2297–2304. doi: 10.1242/dev.120.8.2297. [DOI] [PubMed] [Google Scholar]
- Freeman M, Klambt C, Goodman CS, Rubin GM. The argos gene encodes a diffusible factor that regulates cell fate decisions in the Drosophila eye. Cell. 1992;69:963–975. doi: 10.1016/0092-8674(92)90615-j. [DOI] [PubMed] [Google Scholar]
- Golembo M, Schweitzer R, Freeman M, Shilo BZ. Argos transcription is induced by the Drosophila EGF receptor pathway to form an inhibitory feedback loop. Development. 1996;122:223–230. doi: 10.1242/dev.122.1.223. [DOI] [PubMed] [Google Scholar]
- Hedley ML, Maniatis T. Sex-specific splicing and polyadenylation of dsx pre-mRNA requires a sequence that binds specifically to tra-2 protein in vitro. Cell. 1991;65:579–586. doi: 10.1016/0092-8674(91)90090-l. [DOI] [PubMed] [Google Scholar]
- Hoshijima K, Inoue K, Higuchi I, Sakamoto H, Shimura Y. Control of doublesex alternative splicing by transformer and transformer-2 in Drosophila. Science. 1991;252:833–836. doi: 10.1126/science.1902987. [DOI] [PubMed] [Google Scholar]
- Jazwinska A, Affolter M. A family of genes encoding zona pellucida (ZP) domain proteins is expressed in various epithelial tissues during Drosophila embryogenesis. Gene Expr Patterns. 2004;4:413–421. doi: 10.1016/j.modgep.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Kornezos A, Chia W. Apical secretion and association of the Drosophila yellow gene product with developing larval cuticle structures during embryogenesis. Mol Gen Genet. 1992;235:397–405. doi: 10.1007/BF00279386. [DOI] [PubMed] [Google Scholar]
- Lehmann R, Tautz D. In situ hybridization to RNA. Methods Cell Biol. 1994;44:575–598. doi: 10.1016/s0091-679x(08)60933-4. [DOI] [PubMed] [Google Scholar]
- Li X, Romero P, Rani M, Dunker AK, Obradovic Z. Predicting protein disorder for N-, C-, and internal regions. Genome Inform Ser Workshop Genome Inform. 1999;10:30–40. [PubMed] [Google Scholar]
- Maniatis T, Hardison RC, Lacy E, Lauer J, O’Connell C, Quon D, Sim GK, Efstratiadis A. The isolation of structural genes from libraries of eucaryotic DNA. Cell. 1978;15:687–701. doi: 10.1016/0092-8674(78)90036-3. [DOI] [PubMed] [Google Scholar]
- McConaughy BL, Laird CD, McCarthy BJ. Nucleic acid reassociation in formamide. Biochemistry. 1969;8:3289–3295. doi: 10.1021/bi00836a024. [DOI] [PubMed] [Google Scholar]
- McGinnis W, Garber RL, Wirz J, Kuroiwa A, Gehring WJ. A homologous protein-coding sequence in Drosophila homeotic genes and its conservation in other metazoans. Cell. 1984a;37:403–408. doi: 10.1016/0092-8674(84)90370-2. [DOI] [PubMed] [Google Scholar]
- McGinnis W, Hart CP, Gehring WJ, Ruddle FH. Molecular cloning and chromosome mapping of a mouse DNA sequence homologous to homeotic genes of Drosophila. Cell. 1984b;38:675–680. doi: 10.1016/0092-8674(84)90262-9. [DOI] [PubMed] [Google Scholar]
- McGinnis W, Levine MS, Hafen E, Kuroiwa A, Gehring WJ. A conserved DNA sequence in homoeotic genes of the Drosophila Antennapedia and bithorax complexes. Nature. 1984c;308:428–433. doi: 10.1038/308428a0. [DOI] [PubMed] [Google Scholar]
- McKeown M, Belote JM, Baker BS. A molecular analysis of transformer, a gene in Drosophila melanogaster that controls female sexual differentiation. Cell. 1987;48:489–499. doi: 10.1016/0092-8674(87)90199-1. [DOI] [PubMed] [Google Scholar]
- Moussian B, Schwarz H, Bartoszewski S, Nüsslein-Volhard C. Involvement of chitin in exoskeleton morphogenesis in Drosophila melanogaster. J Morphol. 2005a;264:117–130. doi: 10.1002/jmor.10324. [DOI] [PubMed] [Google Scholar]
- Moussian B, Tang E, Tonning A, Helms S, Schwarz H, Nüsslein-Volhard C, Uv AE. Drosophila Knickkopf and Retroactive are needed for epithelial tube growth and cuticle differentiation through their specific requirement for chitin filament organization. Development. 2005b;133:163–171. doi: 10.1242/dev.02177. [DOI] [PubMed] [Google Scholar]
- O’Connell PO, Rosbash M. Sequence, structure, and codon preference of the Drosophila ribosomal protein 49 gene. Nucleic Acids Res. 1984;12:5495–5513. doi: 10.1093/nar/12.13.5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrowski S, Dierick HA, Bejsovec A. Genetic control of cuticle formation during embryonic development of Drosophila melanogaster. Genetics. 2002;161:171–182. doi: 10.1093/genetics/161.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payre F, Vincent A, Carreno S. ovo/svb integrates Wingless and DER pathways to control epidermis differentiation. Nature. 1999;400:271–275. doi: 10.1038/22330. [DOI] [PubMed] [Google Scholar]
- Reuter R, Scott MP. Expression and function of the homoeotic genes Antennapedia and Sex combs reduced in the embryonic midgut of Drosophila. Development. 1990;109:289–303. doi: 10.1242/dev.109.2.289. [DOI] [PubMed] [Google Scholar]
- Romero P, Obradovic Z, Dunker AK. Sequence data analysis for long disordered regions prediction in the Calcineurin family. Genome Inform Ser Workshop Genome Inform. 1997;8:110–124. [PubMed] [Google Scholar]
- Romero P, Obradovic Z, Li X, Garner EC, Brown CJ, Dunker AK. Sequence complexity of disordered protein. Proteins. 2001;42:38–48. doi: 10.1002/1097-0134(20010101)42:1<38::aid-prot50>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Ryner LC, Baker BS. Regulation of doublesex pre-mRNA processing occurs by 3′-splice site activation. Genes Dev. 1991;5:2071–2085. doi: 10.1101/gad.5.11.2071. [DOI] [PubMed] [Google Scholar]
- Ryner LC, Goodwin SF, Castrillon DH, Anand A, Villella A, Baker BS, Hall JC, Taylor BJ, Wasserman SA. Control of male sexual behavior and sexual orientation in Drosophila by the fruitless gene. Cell. 1996;87:1079–1089. doi: 10.1016/s0092-8674(00)81802-4. [DOI] [PubMed] [Google Scholar]
- Schonbaum CP, Organ EL, Qu S, Cavener DR. The Drosopila melanogaster stranded at second (sas) gene encodes a putative epidermal cell surface receptor required for larval development. Dev Biol. 1992;151:431–445. doi: 10.1016/0012-1606(92)90183-h. [DOI] [PubMed] [Google Scholar]
- Schweitzer R, Howes R, Smith R, Shilo BZ, Freeman M. Inhibition of Drosophila EGF receptor activation by the secreted protein Argos. Nature. 1995;376:699–702. doi: 10.1038/376699a0. [DOI] [PubMed] [Google Scholar]
- Scott MP, Weiner AJ. Structural relationships among genes that control development: sequence homology between the Antennapedia, Ultrabithorax, and fushi tarazu loci of Drosophila. Proc Natl Acad Sci U S A. 1984;81:4115–4119. doi: 10.1073/pnas.81.13.4115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter MF, Black BC, Afshar G, Kermabon A-Y, Wright TRF, Biessman H. Temporal and spatial expression of the yellow gene in correlation with cuticle formation and DOPA decarboxylase activity in Drosophila development. Dev Biol. 1991;147:32–45. doi: 10.1016/s0012-1606(05)80005-3. [DOI] [PubMed] [Google Scholar]
- Wemmer T, Klambt C. A genetic analysis of the Drosophila closely linked interacting genes bulge, argos and soba. Genetics. 1995;140:629–641. doi: 10.1093/genetics/140.2.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetmur JG. Hybridization and renaturation kinetics of nucleic acids. Annu Rev Biophys Bioeng. 1976;5:337–361. doi: 10.1146/annurev.bb.05.060176.002005. [DOI] [PubMed] [Google Scholar]
- Wodarz A, Grawe F, Knust E. CRUMBS is involved in the control of apical protein targeting during Drosophila epithelial development. Mech Dev. 1993;44:175–187. doi: 10.1016/0925-4773(93)90066-7. [DOI] [PubMed] [Google Scholar]


