Abstract
Analogs of N,N-dimethyl-4-(pyrimidin-2-yl)- piperazine-1-sulfonamide possessing either a free radical scavenger group (FRS), chelating groups (CHL), or both (FRS+CHL) have been synthesized. Electrospray ionization mass spectrometry studies indicate that select members of this series bind ions in the relative order of Cu1+= Cu2+ > Fe2+= Fe3+>Zn2+ with no binding of Ca2+ or Mg2+ observed. In vitro evaluation of these compounds in human lens epithelial, human retinal pigmented epithelial, and human hippocampal astrocyte cell lines indicates that all analogs possessing the FRS group as well as the water soluble vitamin E analog 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid protect these cells against decreased cell viability and glutathione levels induced by hydrogen peroxide. In addition, those compounds possessing CHL groups also protected these cells against hydroxyl radicals generated by the Fenton reaction. These compounds are good candidates for the preventive treatment of cataract, age-related macular degeneration (AMD), and Alzheimer's dementia (AD).
Keywords: antioxidants, chelators, 2-amino-4-hydroxy-pyrimidines, age related macular degeneration, cataract, neurodegeneration, Alzheimer's dementia
Introduction
Oxidative stress damages cells and is an accepted determinant of both lifespan and health span. Oxidative stress results from the generation of reactive oxygen species (ROS), primarily H2O2, and is enhanced by the age-related decrease of cellular antioxidant defenses and accumulation of iron and/or copper 1. The increased cellular presence of these redox active metals enhance H2O2 linked ROS by generating hydroxyl radicals through the Fenton reaction 2, 3. Age-related increases in ROS are believed to trigger biochemical cascades that lead to neurodegenerations such as AD 4-6, retinal degenerations such as age-related macular degeneration (AMD) 7-10, and cataract formation 7, 11. While these diseases are pathologically different, studies suggest that they all undergo similar oxidative damage from ROS associated with redox-active metal ions. For example, ROS-associated Aβ in amyloid plaques, which are characteristic of AD 4, 12-15, is also present in AMD 16-18 and age-related cataract 19-21.
The oxidative pathways associated with neurodegenerations and age-related ocular diseases have been targeted as a potential therapeutic approach to the treatment of these age-related diseases 5. A wide variety of antioxidants have been examined to reduce ROS. These range from natural products with antioxidant properties such as aged garlic extract, curcumin, melatonin, resveratrol, Ginkgo biloba extract, green tea, vitamin C, L-carnosine, vitamin E, and cannabinoids to derivatives of lipoic acid, analogs of Coenzyme Q (MitoQ), and “thiol-delivering” glutathione-mimics such as tricyclodecan-9-yl-xanthogenate22-29. Some of these agents have shown limited clinical efficacy with AMD30, 31 but none have demonstrated clinical efficacy in reducing the clinical signs of neurodegeneration or cataract.
Reducing ROS through the chelation of redox-active metals is another approach that has been investigated; however, this approach has primarily been used to treat neurodegenerations. Examples are illustrated in Figure 1. Of these compounds, only the iron chelator desferrioxamine (DFO, N′-{5-[acetyl(hydroxy)amino]pentyl}-N-[5,4-[(5-aminopentyl)(hydroxy)amino]-4-oxobutanoyl}amino)pentyl]-N-hydroxysuccinamide) and clioquinol (5-chloro-7-iodo- quinolin-8-ol) has been investigated for both neurological32, 33 and age-related ocular diseases.34-36 Desferrioxamine is not orally active and does not significantly cross the blood brain barrier37, 38. Clioquinol is an orally active antibiotic that was extensively used as an antibiotic in Asia in the 1960's. In 1971 it was banned in Japan after being linked to sub acute myelo-optic neuropathy (SMON) in over 10,000 Japanese. Its chelating ability was subsequently identified during neurotoxicity studies39 where it has been demonstrated to decrease Cu uptake and counteract Cu efflux activities of the amyloid precursor protein of AD40, disaggregates the metal ion-induced aggregates of Aβ(1-40), and retard fibril growth through a Zn(II)-clioquinol complex formation41. Its efficacy has been demonstrated in vitro, in in vivo animal models, and in several small clinical trials of AD patients15, 42, 43. Because of the promising results of clioquinol, several other 8-hydroxyquinoline analogs have been developed PBT2 (structure not disclosed to date)44, 45, M-30 (5-((methyl (prop-2-ynyl)-amino)methyl)- quinolin-8-ol)46, 47, VK-28 5-((4-(2-hydroxyethyl)piperazin-1-yl) methyl)-quinolin-8-ol)46, HLA-20 (5-((4-(prop-2-ynyl)piperazin-1-yl)methyl)quinolin-8-ol)46, deferasirox (4-(3,5- bis(2-hydroxyphenyl)-1H-1,2,4-triazol-1-yl)benzoic acid)46-48, deferiprone (3-hydroxy- 1,2-dimethylpyridin-4(1H)-one)49, feralex (2-(3-hydroxy-2-methyl-4-oxo-3,4-dihydropyridin-1(2H)-yl)-N-((3R,4R,5S,6R)-2,4,5-trihydroxy-6-(hydroxylmethyl)-tetrahydro-2H-pyran-3-yl)acetamide50, 51, D-penacillamine ((S)-2-amino-3-mercapto- 3-methylbutanoic acid)52 DP-109 (3,3′- (2,2′-(ethane-1,2-diylbis(oxy))- bis(2,1- phenylene))bis(5-(2-(octadecyloxy)ethoxy)-5-oxopentanoic acid)53, and (-)-epigallocatechin-3-gallate (EGCG) ((2R,3R)-5,7-dihydroxy-2-(3,4,5-trihydroxyphenyl)- chroman-3-yl-3,4,5-trihydroxybenzoate)54.
Figure 1.
Structure of antioxidant and chelators evaluated for cataract, AMD and AD.
To date, many research efforts on the treatment of ROS-linked complications have focused on therapeutic targets that enhance cellular antioxidant defenses, demonstrate antioxidant activity, or regulate cellular levels of transition metal ions43, 55. Because multiple mechanisms are involved in the development of ROS-linked disorders, drugs with at least two mechanisms of action targeted at ROS may offer more therapeutic benefit that those only targeting a single mechanism. Toward this end, we have synthesized a series of multifunctional analogs of N,N-dimethyl-4-(pyrimidin-2-yl)-piperazine-1-sulfonamide (1) possessing either a FRS group, (analogs 2, 6), CHL groups (CHL, analogs 3, 7) or both (analogs 4, 8)56. The ring structure of the parent compound 1 was derived from studies investigating the effect of sorbitol dehydrogenase inhibitor (SDI) on sugar cataract formation 57. FRS activity was introduced to 1 by addition of an -OH group in the 5-position of the pyrimidine ring. This was based on a report that 5-pyrimidinols are more effective antioxidants than their corresponding phenols with 2-N,N-Dimethyl-4,6-dimethyl-5-hydroxypyrimidine 5-fold more reactive toward alkyl radicals and essentially equally reactive to peroxy radicals compared to α-tocopherol58. Methoxy, rather than methyl groups, were added to the pyrimidine ring because methoxy groups stabilize the radical scavenger slightly better than the methyl groups and are not as readily subjected to metabolic oxidation as the methyl groups59-61. The ability to chelate was introduced by adding carbonyl groups directly adjacent to the amino group connecting the piperazine ring to the pyrimidine ring. This was based on a report that 2-N-succinamide-1,3-pyrimidine easily forms complexes with transition metals such as Fe3+ and Cu2+62.
Chemistry
Compounds 1, 5 and 6 were synthesized as outlined in Scheme 1. N,N-dimethylpiperazine-1-sulfonamide 10 was obtained from commercially available piperazine 9 according to the literature63. Nucleophilic substitution of 2-chloropyrimidine 11a and 11b with N,N-dimethylpiperazine-1-sulfonamide 10 gave 1 and 5, respectively. Compound 6 was obtained through directed hydroxylation of 5. The aromatic anion of 5, which was generated in the presence of n-BuLi, was treated with oxygen gas to give 6 64.
Scheme 1.
Reagents and conditions: a:ClSO2NMe2/K2CO3/EtOH, room temperature; b:TEA/THF, reflux; c:n-BuLi/THF/O2, -78°C to room temperature.
The 5-hydroxypyrimidine analog, 2 was also initially obtained by nucleophilic substitution of 2-chloro-5-hydroxypyrimidine (15) with N,N-dimethylpiperazine-1-sulfonamide (10) (Scheme 2). Compound 15 was obtained from commercially available 2-chloro-5-nitropyrimidine (12) by first reducing the nitro group to 2-chloro-5-aminoprimidine (13) by refluxing with acetic acid in the presence of iron. Subsequent diazotization of the amine 13 failed; however, 2-chloro-5-hydroxypyrimidine 15 could be obtained in poor yield (33%) by refluxing 13 with 2M sulfuric acid65.
Scheme 2.
Reagents and conditions: a:Fe/AcOH, reflux; b:2M H2SO4, reflux; c:TEA/THF, reflux.
Because of the poor yield in obtaining 15, an alternative approach to compound 2 was employed. As outlined in Scheme 3, an initial nucleophilic reaction of N,N-dimethylpiperazine-1-sulfonamide 10 with 2-chloro-5-nitropyrimidine 12, gave the nitro product 16 which was reduced to the amine 17 by refluxing with NH4Cl and iron. Refluxing amine 17 with 2 M sulfuric acid gave 2 in 56% yield.
Scheme 3.
Reagents and conditions: a:TEA/THF, room temperature; b:Fe/NH4Cl/EtOH-H2O, reflux; c:2M H2SO4, reflux.
The piperazine-2,6-dione containing analogs were synthesized by condensation of 2,2′-(N,N-dimethylsulfamoylazanediyl)diacetic acid (19), obtained from commercially available iminodiacetic acid (2,2′-azanediyldiacetic acid) (18) with primary aromatic amines 20a-d (Scheme 4). The piperazine-2, 6-diones 3, 7, 21 and 22, respectively, were obtained by activation of the 2,2′-(N,N-dimethylsulfamoyliminodiacetic acid (19) with acetic anhydride (step b), addition of 20a-d (step c), and subsequent dehydration (step d)66. The benzyl protecting group of 21 and 22 were removed by hydrogenolysis to give final 4 and 8, respectively. Compounds 20a and 20b were commercially available.
Scheme 4.
Reagents and conditions: a:ClSO2NMe2/NaOH/Na2CO3/acetone-H2O, 0°C to room temperature; b:Ac2O, 85°C; c:acetone/toluene, 80°C; d:Ac2O, 80°C; e:Pd-C/H2/EtOAc, room temperature.
To obtain compound 4, the synthesis outlined in Scheme 5 was initially employed. The methoxy 24 was obtained in 60% yield in a 3-step pot reaction from 19 and 23 according to published procedures67. However, attempts at selectively cleaving the methoxy group of 24 failed. In the presence of BBr3, a number of cleavage products were obtained, presumably due to the instability of 2,6-dioxopiperazine in the presence of the strong Lewis acid. Alternatively, no reaction was observed with mild treatment of 24 with TMSI for 12 hours. Condensation of 19 with 2-amino-5-hydroxypyrimidine, 25, the cleaved methoxy product of 23, also did not result in the anticipated amide 27 (Scheme 6). Instead, ester 26 was formed because the 5-hydroxyl group of 2-amino-5-hydroxypyrimidine, 25, is more reactive; however, protection of the hydroxyl group with an easily removable benzyl group gave amine 20c which when condensed with by condensation yielded compound 21. Subsequent hydrogenolysis of 21 gave the desired compound 4.
Scheme 5.
Reagents and conditions: a:Ac2O, 85°C; b:acetone/toluene, 80°C; c:Ac2O, 80°C; d:BBr3/benzene, room temperature; or TMSI/CHCl3 or MeCN.
Scheme 6.
2-Amino-5-(benzyloxy)-4,6-dimethoxypyrimidine (20d) was obtained from commercially available 2-chloro-4,6-dimethoxypyrimidine 11b (Scheme 7). Oxidation of the aromatic anion of 11b generated in the presence of dioxygen gave the hydroxyl 28 in 44% yield. This yield was increased to 94% when dioxygen was replaced with lithium t-butyl peroxide which was generated in situ from t-butylperoxy alcohol and n-butyllithium68. Following benzylation of the hydroxyl group, the 2-chlorine atom was replaced with an amino group. Attempted substitution of the chlorine with an ammonia-methanol solution or ammonia hydroxide was unsuccessful. Refluxing chloride 29 in toluene with benzylamine resulted in the formation of the 2-benzyllamine 30 in 33% yield and a 64% (according to the procedure of preparation of compound 30) with 25% recovery of chloride 29. Replacing toluene with dioxane increased the yield of 30 to 55% with 25% recovery of 29. Attempted selective hydrogenolysis of 30 for 2 hours at room temperature and one atmosphere pressure resulted in 2-benzylamino-4,6-dimethoxy-5-hydroxyprimidine in 90% yield and 2-amino-4,6-dimethoxy-5-hydroxyprimidine 31 in 10% yield. Both benzyl groups were removed to give 31 in 98% yield when hydrogenolysis was increased to 12 hours. The benzyl group was then reintroduced to the 5-hydroxy group to give 20d under conditions similar to those employed for the synthesis of 20c.
Scheme 7.
Reagents and conditions: a:LDA/tBuOOLi/THF, -78°C; b:BnBr/K2CO3/MeCN, room temperature; c:BnNH2/K2CO3/Dioxane, reflux; d:Pd-C/H2/MeOH, room temperature; e:BnBr/K2CO3/MeOH, room temperature.
Chelation Studies
The relative amount of Fe2+, Fe3+, Cu1+, Cu2+, Zn2+, Ca2+, Mg2+ ions bound by compounds 1 - 8 were determined by electrospray ionization mass spectrometry (ESI-MS) by mixing each ion solution with compound in molar ratios of 10:1, 1:1, and 1:10 and then directly infusing each solution under constant ionization energy. Table 1 summarizes the relative percent parent peak observed with a 10:1 metal ion:compound solution for each compound. Under these conditions, a relative decrease in compound parent peak height only occurs with increased ion binding. This is illustrated in Fig. 3 where the parent peak heights of compounds 1 and 3 are compared. Only compound 3 which contains the necessary piperazine-2,6-dione groups required for ion binding shows a decrease in peak height with increasing concentration of Fe+2. Ion binding was only observed with compounds 3, 4, 7 and 8 all of which possess the required piperazine-2,6-dione groups. These compounds bound ions with a relative binding order of Cu1+= Cu2+ > Fe2+=Fe3+>Zn2+. No binding was observed with either Ca2+ or Mg2+.
Table 1.
% Parent peaks remaining at 10 : 1 metal ion solution : compound obtained by ESI-MS.
| lon Solutions | |||||||
|---|---|---|---|---|---|---|---|
| Compd | Fe2+ | Fe3+ | Cu1+ | Cu2+ | Zn2+ | Ca2+ | Mg2+ |
| 1 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 |
| 2 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 |
| 3 | 8.1 | 10.0 | 3.6 | 4.4 | 15.3 | 100.0 | 100.0 |
| 4 | 6.9 | 8.2 | 4.2 | 3.0 | 12.7 | 100.0 | 100.0 |
| 5 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 |
| 6 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 |
| 7 | 7.3 | 7.6 | 4.1 | 3.4 | 13.8 | 100.0 | 100.0 |
| 8 | 7.5 | 8.2 | 4.0 | 3.6 | 14.6 | 100.0 | 100.0 |
Figure 3.
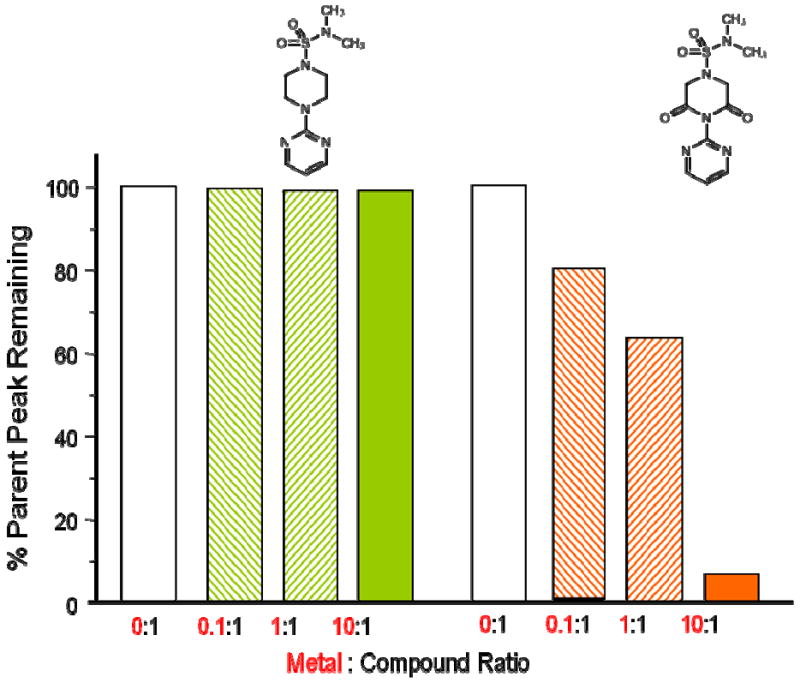
ESI-MS analysis of parent peak of 1-N,N′-dimethylsulfamoyl-4-(2-pyrimidyl)-piperazine (1) and 1-N, N,N-dimethyl-3,5-dioxo-4-(pyrimidin-2-yl)piperazine-1-sulfonamide (3) in the presence of increasing amounts of Fe+2 solutions.
To obtain the stoichiometry of the chelator-ion complexes Jobs Plots were prepared for compounds 1-8 with Fe2+, Fe3+, Cu1+, Cu2+, Zn2+, Ca2+, and Mg2+. The maximum absorbance for each compound as a function of the mole fraction of the metal ion was determined and plotted to give linear dependences at high and low molar fractions. The stoichiometry of the formed complexes were determined by the intersections of these lines. The intersection of these lines corresponded to the stoichiometry of the complex formed (Fig 4). Intersecting lines were not observed with compounds 1, 2, 5, or 6, or any compounds incubated with Ca2+ and Mg2+; therefore, no stoichiometry could be obtained for these compounds or ions. This was consistent with the ESI-MS data. Binding stoichiometry for compounds 3,4,6, and 7, summarized in Table 2, was determined to be 1 metal chelator : 2 metal ions for those ions with a tetrahedral/square planar geometry (Cu1+, Cu2+, Zn2+), or 2 metal chelators : 1 metal ion for ions having an octahedral geometry (Fe2+, Fe3+). This stoichiometry was consistent with the data of a similar chelating moiety in 2-(N-succinimidyl) pyrimidine 62.
Figure 4.
Job's plot of compound 4 in acetate solution (1 mM, pH 6.5) at 23 °C. The final concentration of compound 4 and Fe2+ ion was maintained constant at 0.1 mM.
Table 2.
Stoichiometry of metal ion binding to compounds obtained from Job plots. The total concentration of compound to metal ion was maintained at 0.1 mM.
| lon Solutions | |||||||
|---|---|---|---|---|---|---|---|
| Compd. | Fe 2+ | Fe 3+ | Cu1+ | Cu 2+ | Zn 2+ | Mg 2+ | Ca 2+ |
| 1 | 0* | 0* | 0* | 0* | 0* | 0* | 0* |
| 2 | 0* | 0* | 0* | 0* | 0* | 0* | 0* |
| 3 | 2:1.06 | 2:101 | 1:212 | 1:2.03 | 1:1.94 | 0* | 0* |
| 4 | 2:1.08 | 2:096 | 1:194 | 1:1.99 | 1:2.08 | 0* | 0* |
| 5 | 0* | 0* | 0* | 0* | 0* | 0* | 0* |
| 6 | 0* | 0* | 0* | 0* | 0* | 0* | 0* |
| 7 | 2:1.08 | 2:099 | 1:194 | 1:1.99 | 1:1.86 | 0* | 0* |
| 8 | 2:1.10 | 2:099 | 1:190 | 1:2.08 | 1:1.99 | 0* | 0* |
Non-intersecting lines in the Job Plot
In Vitro Evaluation of Antioxidant Activity
Compounds 1-8, were evaluated for their ability to protect SRA-1 human lens epithelium cells (hLECs)69, commercially available ARPE-19 human retinal pigmented epithelial cells (RPEs)70, and HA-h human hippocampal astrocytes (HA-hs). These three cells types are instrumental in the development of cataract, AMD and AD, respectively.
In the first set of studies, the ability of compounds 1-8 to protect cells against ROS-induced cell death was evaluated using a MTS Cell Viability assay. The results were compared to the protective activity of the water soluble vitamin E analog 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid. All cells were exposed for 2 hrs of either 1.0 mM H2O2 or Fenton Reagents (1.0 mM H2O2 and 1.0 mM Fe2+) with/without 1 mM of compounds 1-8 or 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid. As summarized in Fig. 4a, the presence of H2O2, reduced the viability of the hLECs to less that 50%. A similar loss of cell viability was observed for compounds 1, 3, 5 and 7, analogs not possessing the 2-amino-5-hydroxy-1,3-pyrimidine group required for FRS activity. 6-Hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid significantly protected cell viability against ROS and similar protection was also observed with compounds 2, 4, 6 and 8, all compounds possess the 2-amino-5-hydroxy-1,3-pyrimidine FRS group. When ROS was generated by the Fenton reaction (Fig. 5b), in addition to the FRS containing compounds 2, 4, 6 and 8, some protection was now also demonstrated with compounds 3 and 7 which contain the 2,8-dioxopiperazine CHL group. Moreover, the multifunctional compounds possessing both FRS and CHL groups (compounds 4 and 8) demonstrated better protection than 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid. Similar results were observed for the RPE and HA-h cells. The protection afforded by compounds 1 - 8 against Fenton's reagent are summarized in Fig 5C and 5D, respectively.
Figure 5.
In vitro MTS viability assay of HLECs subjected to ROS. A illustrates exposure of HLECs exposed for 2 hr with 1 mM H2O2 with/without the presence of the water soluble vitamin E analog 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid or compounds 1-8. B illustrates 2 hr exposure of HLECs with 1 mM Fenton's reagent with/without the presence of the vitamin E analog or compounds 1-8. C and D illustrate similar 2 hr exposure of hRPE and HA-h cells, respectively, with 1 mM Fenton's reagent with/without the presence of the vitamin E analog or compounds 1-8. All studies were normalized against MTS cell staining obtained without ROS (blank control) and the results represent the mean ± SD of three separate experiments. Significant differences (p>0.05), calculated by ANOVA, were compared to control staining obtained with ROS in the absence of either the vitamin E analog or compounds 1-8.
In these three cell types, the rapid intracellular reduction of cellular glutathione (GSH) is a sensitive marker of oxidative stress. As illustrated in Fig 6A, the presence of 1 mM H2O2 rapidly resulted in a reduction of GSH levels in hLECs and a similar reduction was also observed with compounds 1, 3, 5 and 7, analogs not possessing the FRS group. However, GSH levels were maintained when similar cells were treated with either the water soluble vitamin E analog 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid or compounds 2, 4, 6 and 8 which possess the FRS group. All compounds possess the 2-amino-5-hydroxy-1,3-pyrimidine FRS group. With Fenton generated ROS (Fig. 6b), protection was also afforded by the CHL containing compounds 3 and 7. In addition, the multifunctional compounds possessing both FRS and CHL groups (compounds 4 and 8) demonstrated slightly better protection than 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid. Again, similar results were observed for the RPE and HA-h cells. The protection afforded by compounds 1 - 8 against Fenton's reagent are summarized in Fig 6C and 6D, respectively.
Figure 6.
GSH levels in in vitro cells subjected to ROS. A illustrates exposure of HLECs exposed for 2 hr with 1 mM H2O2 with/without the presence of the water soluble vitamin E analog 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid or compounds 1-8. B illustrates 2 hr exposure of HLECs with 1 mM Fenton's reagent with/without the presence of the vitamin E analog or compounds 1-8. C and D illustrate similar 2 hr exposure of hRPE and HA-h cells, respectively, with 1 mM Fenton's reagent with/without the presence of Trolox or compounds 1-8. GSH levels were expressed as nmol GSH/mg protein, with values normalized to GSH levels in cells not exposed to H2O2 (blank control, 100%). The results represent the mean ± SD of three separate experiments. Significant differences (p>0.05), calculated by ANOVA, were compared to control staining obtained with ROS in the absence of either the vitamin E analog or compounds 1-8.
Experimental Section
NMR spectra were obtained with a Varian 500 MHz spectrometer. EI-MS utilized an Agilent 5973N MSD, and ESI-MS was conducted with a Finnigan MAT LCQ. UV–Visible spectra were measured on a Molecular Devices SpectraMax Plus384 Microplate Spectrophotometer (Molecular Devices, Sunnyvale, CA, USA). Melting points were uncorrected. Column chromatography (CC) utilized Merck silica gel (230-400 mesh). Final compound purities were assessed as ≥ 99% and intermediate compound purities were assessed as ≥ 96% by reverse phase HPLC using a 250×4.6 mm C18 Luna column (5 μ100 Å) with a mobile phase of 75:25 methanol : water at a flow rate of 0.9 mL/min, and detection at 220, 254 and 280 nm. Elemental analyses were performed by M-H-W Laboratories, Phoenix, AZ.
N, N′-dimethylsulfamoylpiperazine 1 was obtained according to the literature63.
General Procedure for the Preparation of 1-N,N′-dimethylsulfamoyl-4-(2-pyrimidyl)-piperazine (1) and 1-N,N′-dimethylsulfamoyl-4-(4,6-dimethoxy-2-pyrimidyl)piperazine (5)
Et3N (0.73 mL, 5.2 mmol) was added to 1.0 g of N, N′-dimethylsulfamoylpiperazine 10, (5.2 mmol) dissolved in 20 mL of THF. 2-Chloro-4,6-dimethoxypyrimidine, 11a, (0.9 g, 5.2 mmol) dissolved in 5 mL of THF was then added and the mixture was refluxed with stirring for 40 hrs. After cooling, THF was removed under vacuum and the remaining yellow solid was dissolved in 300 mL of CHCl3, washed with 0.5 N HCl, water and brine, dried over Na2SO4 and filtered. Removal of solvent and CC with 1:1 CHCl3:hexane gave 1.4 g (81%) of white compound 1, m.p. 157-158° C. 1H NMR (CDCl3) δ8.34 (s, 1H), 8.33 (s, 1H), 6.55 (t, J=4.88 Hz, 1H), 3.92 (appt, J=5.13 Hz, 4H), 3.31 (appt, J=5.13 Hz, 4H), 2.86 (s, 6H); 13C NMR (CDCl3) 161.4, 157.3, 110.5, 46.1, 43.4, 38.2; ESI-MS (m/z) 294 ([M+Na]+); Anal. Calcd. for C10H17N5O2S: C, 44.26; H, 6.31; N, 25.81; S, 11.82. Found: C, 44.12; H, 6.42; N, 25.58; S, 12.03. The yield of Compound 5, m.p. 103-105° C, was 81%. 1H NMR (CDCl3) δ5.41 (s, 1H), 3.86 (s, 6H), 3.89-3.86 (m, 4H), 3.28 (appt, J=4.88Hz, 4H), 2.86 (s, 6H); 13C NMR (CDCl3) 172.0, 160.6, 78.5, 53.5, 46.2, 43.5, 38.2; EI-MS (m/z) 331 (M+); Anal. Calcd. for C12H21N5O4S: C, 43.49; H, 6.39; N, 21.13; S, 9.68. Found: C, 43.60; H, 6.47; N, 21.11; S, 9.85.
1-N,N′-dimethylsulfamoyl-4-(4,6-dimethoxy-5-hydroxy-2-pyrimidyl)piperazine (6)
To a solution of compound 5 (1.66g, 5 mmol) in 40 mL of THF at -78°C and under an Ar atmosphere was added 0.3 mL of n-BuLi (7 mmol). After 3 hrs stirring at -60°C, the Ar atmosphere was replaced with oxygen. The reaction mixture was then gradually warmed to room temperature (RT) and stirring was continued for an additional 6 hrs. After adjusting the pH to 6-7 with dilute HCl, THF was removed by evaporation and the remaining aqueous layer was extracted with CHCl3. The CHCl3 layers were washed with brine, dried over Na2SO4 and filtered. Solvent evaporation gave a yellow solid, which was purified by CC with 1:1:2 CHCl3:EtOAc:hexane to yield 530 mg (30%) of compound 6, m.p. 113-114° C. The structure was confirmed by 1H NMR (CDCl3) δ4.21 (s, 1H), 3.95 (s, 6H), 3.76 (s, 4H), 3.29 (s, 4H), 2.86 (s, 6H); EI-MS (m/z) 347 (M+); Anal. Calcd. for C12H21N5O5S: C, 41.49; H, 6.09; N, 21.16; S, 9.23. Found: C, 43.61; H, 6.13; N, 19.96; S, 9.18.
N,N-dimethyl-4-(5-nitropyrimidin-2-yl)piperazine-1-sulfonamide (16)
Triethylamine (7.2 mL, 51.8 mmol) was added to a solution of 10, (10 g, 51.8 mmol) dissolved in 400 mL of THF. 2-Chloro-5-nitropyrimidine, 12, (7.7 g, 48.3 mmol) dissolved in 20 mL of THF was added to the stirred mixture. After 24 hrs stirring at RT, THF was removed under vacuum and 600 mL of CHCl3 was added to the residue. The CHCl3 layer was washed with 0.5 N HCl, water and brine, dried over Na2SO4, and filtered. Solvent evaporation gave 14.1 g (92%) of straw yellow solid 16. 1H NMR (CDCl3) δ9.08 (s, 2H), 4.09 (appt, J=5.13 Hz, 4H), 3.35 (appt, J=5.13 Hz, 4H), 2.87 (s, 6H).
4-(5-aminopyrimidin-2-yl)-N,N-dimethylpiperazine-1-sulfonamide (17)
NH4Cl (1.43 g, 26.76 mmol) was added to a suspension of 16 (14.1 g, 44.6 mmol) in a mixture of EtOH (175 mL) and H2O (48 mL), followed by iron powder (7.5 g, 133.9 mmol). After refluxing 1 hr, the reaction mixture was filtered and concentrated to give a brown solid which was dissolved in 800 mL of CHCl3, washed with NaHCO3 and brine, dried over Na2SO4, and filtered. Solvent evaporation gave a yellow solid which after CC with 100:1 CHCl3: MeOH gave 11.5 g (90%) of solid yellow 17. 1H NMR (CDCl3) δ7.98 (s, 2H), 3.76 (t, J=4.88 Hz, 4H), 3.30 (t, J=4.88 Hz, 4H), 2.86 (s, 6H); EI-MS (m/z) 286 (M+).
4-(5-hydroxypyrimidin-2-yl)-N,N-dimethylpiperazine-1-sulfonamide (2)
A suspension of 17 (5.76 g, 20.2 mmol) in 100 mL of 2 M H2SO4 was refluxed for 1.5 hrs until the suspension became clear. After cooling to RT, the solution pH was adjusted to 6-7 with 10 N NaOH, and the cloudy mixture was extracted with EtOAc. The combined organic layers were washed with brine, dried over Na2SO4, and filtered. Solvent evaporation gave a yellow solid, which was purified by CC using 1:1 EtOAc:hexane to yield 3.23 g (56%) of white solid compound 2, m.p.134-135° C. 1H NMR (CDCl3) δ8.08 (s, 2H), 3.78 (appt, J=5.13 Hz, 4H), 3.29 (appt, J=5.13 Hz, 4H), 2.86 (s, 6H); 13C NMR (CDCl3) 156.9, 145.8, 143.5, 46.0, 44.3, 38.1; EI-MS (m/z) 287 (M+); Anal. Calcd for C10H17N5O3S: C, 41.80; H, 5.96; N, 24.37; S, 11.16. Found: C, 41.60; H, 5.98; N, 24.16; S, 11.02.
5-hydroxy-2-aminopyrimidine (25)
Under Argon at RT, BBr3 (2.6 mL, 50 mmol) was added drop-wise to a solution of 23 (1.25 g, 10 mmol) in 60 mL of benzene and the mixture was refluxed for 6 hrs. After standing overnight at rt, the solvent was evaporated and the residue was carefully treated with 10 mL of ice cold H2O. The pH of the solution was adjusted to 6-7 with 6 N NaOH and then extracted with EtOAc. The EtOAc layers were washed with brine, dried over Na2SO4, and filtered. Solvent evaporation gave a yellow solid, which after CC using 20:1 CHCl3: MeOH gave 840 mg (75%) of white solid 5-hydroxy-2-amino-pyrimidine 25. 1H NMR (DMSO-d6) δ8.90 (s, 1H), 7.86 (s, 2H), 5.91 (s, 2H); EI-MS (m/z) 111 (M+).
5-benzyloxy-2-aminopyrimidine (20c)
K2CO3 (276 mg, 2 mmol) was added to 222 mg of 25 (2 mmol) in 5 mL of methanol, followed by BnBr (0.24 mL, 2 mmol). After 14 hrs stirring at RT, the reaction was stopped by addition of water. Methanol was evaporated the remaining aqueous layer was extracted with CHCl3. The combined CHCl3 layers were washed with brine, dried over Na2SO4, and filtered. Removal of the solvent followed by CC using 67:1 CHCl3:MeOH to yield 300 mg (75%) of white solid 20c. 1H NMR (CDCl3) δ 8.08 (s, 2H), 7.40-7.32 (m, 5H), 5.03 (s, 2H), 4.77 (br s, 2H).
2-chloro-5-hydroxy-4,6-dimethoxypyrimidine (28)
To a stirred suspension of 10.44 g 2-chloro-4,6-dimethoxypyrimidine 11b, (60 mmol) in 200 mL of THF under argon at -78°C was added drop-wise a solution of LDA (2 M in THF/heptane/ethyl benzene, 60 mL, 120 mmol). To a separate round-bottomed flask -78°C under argon containing t-butyl hydroperoxide (5.5 M in decane, 22.7 mL,125 mmol) in 150 mL of THF was added a solution of n-BuLi (1.6 M in hexane, 78 mL, 125 mmol). Then, after stirring for 1 hr, the hydroperoxide anion solution was added via a double-ended needle to the aromatic anion. The temperature was gradually raised to 0°C with stirring continued an additional 3 hrs. The reaction was quenched with 6N HCl until a pH of 7 was obtained. After THF evaporation the remaining aqueous layer was extracted with CHCl3. The CHCl3 layers were washed with brine, dried over Na2SO4, and filtered. Removal of solvent and subsequent purification by CC using 100:1 CHCl3: MeOH gave 10.67 g (93.4%) of white solid 28. 1H NMR (CDCl3) δ 4.93 (s, 1H), 4.05 (s, 6H); 13C NMR (CDCl3) 158.1, 146.7, 123.0, 55.1.
2-chloro-5-(benzyloxy)-4,6-dimethoxypyrimidine (29)
K2CO3 (10.0 g, 70 mmol) was added to 28 (13.24 g, 69.5 mmol) dissolved in 200 mL of MeCN followed by BnBr (8.3 mL, 69.5 mmol). After 14 hrs stirring at RT, the reaction was stopped by addition of water. MeCN was evaporated the remaining aqueous layer was extracted with CHCl3. The combined CHCl3 layers were washed with brine, dried over Na2SO4, and filtered. Removal of the solvent followed by CC using 25:1 hexane:EtOAc gave 16.57 g (85%) of benzyloxy 29. 1H NMR (CDCl3) δ 7.41-7.31 (m, 5H), 5.00 (s, 2H), 3.98 (s, 6H); 13C NMR (CDCl3) 163.6, 150.5, 136.4, 128.5, 128.4, 128.3, 124.4, 74.8, 54.0.
N-benzyl-5-(benzyloxy)-4,6-dimethoxypyrimidin-2-amine (30)
A mixture of 29 (3.18 g, 11.33mmol), BnNH2 (2.2 mL, 20 mmol) and K2CO3 (1.66g, 12 mmol) in dioxane (60 mL) was refluxed for 4 days. The reaction was filtered and the filtrate concentrated in vacuo to give a yellow oil, which after CC with 25:1 to 10:1 hexane:EtOAc gave 2.54 g (64%) of white solid 30. 1H NMR (CDCl3) δ 7.45-7.24 (m, 10H), 5.10 (br s, 1H), 4.85 (s, 2H), 4.55 (d, J=5.5 Hz, 2H), 3.86 (s, 6H); 13C NMR (CDCl3) 163.5, 156.0, 139.7, 137.5, 128.5, 128.4, 128.1, 127.8, 127.6, 127.1, 117.7, 75.2, 53.7, 45.9.
2-amino-4,6-dimethoxypyrimidin-5-ol (31)
Compound 30 (2.54 g, 7.24 mmol) in 40 mL of MeOH was hydrogenated for 12 hrs at RT in the presence of 635 mg of 10% Pd/C catalyst. After filtration, and solvent evaporation, a white solid was obtained which after CC using 50:1 CHCl3:MeOH yielded 1.21 g (98%) of 31. 1H NMR (CDCl3) δ 4.50 (s, 2H), 4.30 (s, 1H), 3.93 (s, 6H).
5-(benzyloxy)-4,6-dimethoxypyrimidin-2-amine (20d)
To 4.16 g of 31 (24.3 mmol) in MeOH (240 mL) was added K2CO3 (3.5 g, 25 mmol), and followed by BnBr (3.0 mL, 25 mmol). After 12 hrs stirring at RT, water was added to the reaction mixture. MeOH was evaporated and the water layer was extracted with CHCl3. The combined CHCl3 layers were washed with brine, dried over Na2SO4, and filtered. Removal of CHCl3 gave a yellow solid, which after purification by CC with 10:1 to 2:1hexane: EtOAc yielded 5.14 g (81%) of 2-amine-4,6-dimethoxy-5-benzyloxypyrimidine, 20d. 1H NMR (CDCl3) δ 7.44-7.27 (m, 5H), 4.86 (s, 2H), 4.60 (s, 2H), 3.87 (s, 6H).
2,2′-(N,N-dimethylsulfamoylazanediyl)diacetic acid (19)
26.62 g of the iminodiacetic acid 18, (200 mmol) was added to 16.0 g of NaOH (400 mmol) in 50 mL of H2O at 0°C, followed by 21.2 g of Na2CO3 (200 mmol) in 100 mL of H2O. Then dimethylsulfamoyl chloride (200 mmol) dissolved in 50 mL of acetone was slowly added with stirring and the stirring was continued at RT. After 48 hrs, the reaction mixture was extracted with EtOAc. The pH of the aqueous layer was adjusted to 2 with 12 N HCl and extracted again with EtOAc. The organic layer was dried over Na2SO4, and filtered. Solvent evaporation gave 28.75 g (60%) 19, as a white powder which was recrystallization from EtOAc. The structure was confirmed by 1H NMR (DMSO-d6) δ12.81 (br s, 2H), 3.99 (s, 4H), 2.71 (s, 6H); 13C NMR (DMSO-d6) 170.9, 49.7, 37.8.
General Procedure of Preparation of 1-N, N,N-dimethyl-3,5-dioxo-4-(pyrimidin-2-yl) piperazine-1-sulfonamide (3), 4-(4,6-dimethoxypyrimidin-2-yl)-N,N-dimethyl-3,5-dioxopiperazine-1-sulfonamide (7), 4-(5-(benzyloxy)pyrimidin-2-yl)-N,N-dimethyl- 3, 5-dioxopiperazine-1-sulfonamide (21) and 4-(5-(benzyloxy)-4,6-dimethoxypyrimidin-2-yl)-N,N-dimethyl-3,5-dioxopiperazine-1-sulfonamide (22)
A suspension of 1.44 g of diacid 19 (6 mmol) in 8.0 mL of Ac2O was heated to 85°C for 5 min until the solution became clear. After excess Ac2O was removed under vacuum at 60°C, the resulting anhydride was obtained as a yellow oil. The resulting anhydride, in 10 mL of toluene, was then reacted with 475 mg of 2-aminopyrimidine, 20a, (5 mmol) dissolved in 7 mL of acetone by heating the mixture to 80°C for 6 hours. Solvent evaporation gave the amide as a yellow oil. Heating amide for 4 hrs at 80 °C with 8 mL of Ac2O resulted into the final piperidine-2,6-ring product as a red clear solution. Removal of the remaining Ac2O under vacuum at 60°C followed by CC with 3:1:1 hexane:EtOAc:CHCl3 gave 760 mg (50.8%) of compound 3, m.p. 179-181°C. 1H NMR (CDCl3) δ8.90 (d, J=4.88Hz, 2H), 7.45 (t, J=4.88Hz, 1H), 4.31 (s, 4H), 2.96 (s, 6H); 13C NMR (CDCl3) 167.0, 159.5, 154.8, 121.4, 49.5, 38.3; ESI-MS (m/z) 322 ([M+Na]+); Anal. Calcd. for C10H13N5O4S: C, 40.13; H, 4.38; N, 23.40; S, 10.71. Found: C, 40.40; H, 4.54; N, 23.41; S, 10.66.
The white solid of compound 7, m.p. 200-202° C was obtained in 57.9% yield. 1H NMR (CDCl3) δ6.10 (s, 1H), 4.30 (s, 4H), 3.92 (s, 6H), 2.967 (s, 6H); 13C NMR (CDCl3) 172.8, 166.7, 152.8, 90.5, 54.7, 49.4, 38.2; ESI-MS (m/z) 382 ([M+Na]+); Anal. Calcd for C12H17N5O6S: C, 40.11; H, 4.77; N, 19.49; S, 8.92. Found: C, 40.31; H, 4.84; N, 19.28; S, 9.10.
Crude Compound 21 was purified by CC with 100:1 CHCl3:MeOH, and white solid of 21 was obtained in 69% yield. 1H NMR (CDCl3) δ 8.56 (s, 2H), 7.44-7.26 (m, 5H), 5.20 (s, 2H), 4.29 (s, 4H), 2.95 (s, 6H).
Crude compound 22 was purified by CC using 100:1 CHCl3: MeOH, and pale yellow solid of 22 was obtained in 41% yield. 1H NMR (CDCl3) δ 7.46-7.32 (m, 5H), 5.05 (s, 2H), 4.29 (s, 4H), 3.95 (s, 6H), 2.96 (s, 6H);
General Procedure of preparation of 4-(5-hydroxypyrimidin-2-yl)-N,N-dimethyl-3,5-dioxopiperazine-1-sulfonamide (4) and 4-(5-hydroxy-4,6-dimethoxypyrimidin-2-yl)-N,N-dimethyl-3,5-dioxopiperazine-1- sulfonamide (8)
Compound 21 (2.0 g, 4.9 mmol) dissolved in 100 mL of EtOAc was hydrogenated with 500 mg of 10% Pd/C catalyst at RT for 12 hrs. After filtration and solvent evaporation, compound 4, recrystallized from EtOAc to give 1.83 g (80%) of a white powder, m.p. 225-226° C. 1H NMR (DMSO-d6) δ11.0 (s, 1H), 8.45 (s, 2H), 4.39 (s, 4H), 2.85 (s, 6H); 13C NMR (CDCl3) 168.9, 152.7, 146.9, 146.1, 50.0, 38.5; ESI-MS (m/z) 338 ([M+Na]+); Anal. Calcd for C10H13N5O5S: C, 38.09; H, 4.16; N, 22.21; S, 10.17. Found: C, 38.18; H, 4.35; N, 21.98; S, 9.93.
Crude compound 8 was purified by CC using 80:1 CHCl3:MeOH to yield 1.15 g (91%) of white solid 8, m.p. 222-224°C. 1H NMR (CDCl3) δ 9.67 (s, 1H), 4.44 (s, 4H), 3.88 (s, 6H), 2.51 (s, 6H); 13C NMR (CDCl3) 168.1, 159.4, 140.9, 124.7, 54.7, 49.3, 37.9; ESI-MS (m/z) 398 ([M+Na]+); Anal. Calcd for C12H17N5O7S: C, 38.40; H, 4.56; N, 18.66; S, 8.54. Found: C, 38.60; H, 4.66; N, 18.49; S, 8.58.
Chelation Studies
The relative amounts of ions bound by compounds 1 - 8 were determined by electrospray ionization mass spectrometry (ESI-MS) according to the method of Baron and Hering 71. Stock solutions (1 mM) were prepared with double distilled water from ammonium iron (II) sulfate hexahydrate, iron (III) nitrate nonahydrate, copper (I) chloride, copper (II) chloride dihydrate, zinc chloride, magnesium chloride and calcium chloride. Each ion solution was mixed with compound 1- 8, respectively, in ratios of 10:1, 1:1, and 1:10 metal ion:compound so that a final compound concentration of 50 μM was maintained. Forty-five minutes after mixing, each solution was directly infused into the MS under constant ionization energy.
The stoichiometry of the complexes of compounds 1-8 with Fe2+, Fe3+, Cu1+, Cu2+, Zn2+, Ca2+, and Mg2+ were determined using the method of continuous variation (Job plots) 72. Due to their low solubility, compounds 1-8 were first dissolved in acetonitrile (HPLC grade). These concentrated solutions were then diluted to 0.1 mM stock solutions with 1 mM acetate, pH 6.5). In a typical experiment (at 23 °C), several solutions of the compound and metal ion of interest were prepared at a constant total concentration of compound and ion, but with a different mole fraction of one component. After an equilibration period of 45 minutes, the change in UV absorbance was monitored from 210 to 310 nm. The maximum absorbance for each compound was then recorded as a function of the mole fraction of the metal ion. Two linear dependences were obtained, at low and high molar fractions, which intersect at a mole fraction value that corresponds to the stoichiometry of the complex.
Cell Culture Studies
Human lens epithelial cells (SRA-1), obtained as a gift from Dr. Reddy69 were cultured in Dulbecco's Modified Eagle's Medium (DMEM) media containing 10% fetal bovine serum (FBS), 4 mM L-glutamine, 1.5g/L NaHCO3, and 1% penicillin/streptomycin solution. Human retinal pigmented epithelial cells (ARPE-19), obtained from the American Type Culture Collection (Manassas, VA), were cultured in a 1:1 mixture of Dulbecco's Modified Eagle's Medium and Ham's F12 (DMEM/F12) medium with 2.5 mM L-glutamine and 15 mM HEPES buffer (Gibco, Carlsbad, CA) containing 10% heat-inactivated FBS and 1% penicillin/streptavidin solution. Human hippocampal astrocytes (HA-h), obtained from the ScienCell Research Laboratories (Carlsbad, CA) were cultured on poly-L-lysine coated containers (2 μg/cm2) with astrocyte medium (ScienCell, Carlsbad, CA) composed of 500 ml of basal medium, 5 ml of astrocyte growth supplement and 5 ml of penicillin/streptomycin solution. All cells were cultured at 37°C in a humidified atmosphere of 5% CO2 and 95% air until subconfluent (2-4 days). Cells were passaged when 80-90% confluent by treatment with trypsin-EDTA and then plated at a density of 1 × 104 cells onto 96-well plates or 1 × 106 cells onto 150-mm dishes. For compound evaluation, FBS-free media (blank and control) or FBS-free media containing 100 μM of either compound 1 – 8 or the water soluble vitamin E analog 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox) were added to each individual well/dish. After 1 hr, an additional aliquot of FBS-free media was added to the blank well/dish, and a similar volume of FBS-free media containing 1 mM H2O2 was added to all remaining wells/dishes. For the Fenton studies, addition of H2O2 was immediately followed by addition of 1 mM iron (II) chloride. After 2 hr exposure, all media was removed and the cells were washed at room temperature with PBS. All studies were conducted in triplicate.
Cell viability studies were conducted in 96-well plates using the CellTiter 96® AQueous One Solution Cell Proliferation Assay (MTS, Promega, Madison, WI). Briefly, after 2 hr exposure to either H2O2 or Fenton reagents, media was removed from all wells and each well was washed with PBS. After removal of the PBS wash, 100 μL of blank media was added to each well followed by addition of 20 μL of the MTS solution. The cells were further incubated at 37° C for 1.5 hr and then spectrophotometrically evaluated at 490 nm in a plate reader. The results were normalized to blank control (100%) of cells not treated with H2O2.
GSH measurements were conducted on cells cultured in either 100 or 150 mm dishes. After 2 hr exposure to either H2O2 or Fenton reagents, the media was removed and the cells were washed with PBS. The cells were removed from each dish by scraping and homogenized in glass homogenizers. Following centrifugation at 4°C, protein levels in the supernatant were measured according to Bradford 73. The cell homogenates were then deproteinized with equal volumes of 20% trichloroacetic acid (TCA) and GSH levels were measured at 412 nm according to the DTNB method 74. GSH levels were expressed as nmol GSH/mg protein, with values subsequently normalized to GSH levels in cells not exposed to H2O2 (blank control, 100%).
Figure 2.
Analgs of of 1-N,N′-dimethylsulfamoyl-4-(2-pyrimidyl)piperazine (1) synthesized and evaluated.
Acknowledgments
Support: NIH EY016460
Abbreviations
- AD
Alzheimer's dementia
- AMD
age-related macular degeneration
- BSA
bovine serum albumin
- CHL
chelating groups
- FRS
free radical scavenger groups
- hLECs
human lens epithelium cells
- HA-h
human hippocampal astrocytes
- ROS
reactive oxygen
- SDI
sorbitol dehydrogenase inhibitor
References
- 1.Brewer GJ. Iron and copper toxicity in diseases of aging, particularly atherosclerosis and Alzheimer's disease. Exp Biol Med (Maywood) 2007;232:323–35. [PubMed] [Google Scholar]
- 2.Gilca M, Stoian I, Atanasiu V, Virgolici B. The oxidative hypothesis of senescence. J Postgrad Med. 2007;53:207–13. doi: 10.4103/0022-3859.33869. [DOI] [PubMed] [Google Scholar]
- 3.Stadtman ER, Oliver CN, Levine RL, Fucci L, Rivett AJ. Implication of protein oxidation in protein turnover, aging, and oxygen toxicity. Basic Life Sci. 1988;49:331–9. doi: 10.1007/978-1-4684-5568-7_50. [DOI] [PubMed] [Google Scholar]
- 4.Allsop D, Mayes J, Moore S, Masad A, Tabner BJ. Metal-dependent generation of reactive oxygen species from amyloid proteins implicated in neurodegenerative disease. Biochem Soc Trans. 2008;36:1293–8. doi: 10.1042/BST0361293. [DOI] [PubMed] [Google Scholar]
- 5.Smith DG, Cappai R, Barnham KJ. The redox chemistry of the Alzheimer's disease amyloid beta peptide. Biochim Biophys Acta. 2007;1768:1976–90. doi: 10.1016/j.bbamem.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 6.Valko M, Morris H, Cronin MT. Metals, toxicity and oxidative stress. Curr Med Chem. 2005;12:1161–208. doi: 10.2174/0929867053764635. [DOI] [PubMed] [Google Scholar]
- 7.Brennan LA, Kantorow M. Mitochondrial function and redox control in the aging eye: role of MsrA and other repair systems in cataract and macular degenerations. Exp Eye Res. 2009;88:195–203. doi: 10.1016/j.exer.2008.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dunaief JL, Richa C, Franks EP, Schultze RL, Aleman TS, Schenck JF, Zimmerman EA, Brooks DG. Macular degeneration in a patient with aceruloplasminemia, a disease associated with retinal iron overload. Ophthalmology. 2005;112:1062–5. doi: 10.1016/j.ophtha.2004.12.029. [DOI] [PubMed] [Google Scholar]
- 9.Richer S, Rudy D, Statkute L, Karofty K, Frankowski J. Serum iron, transferrin saturation, ferritin, and dietary data in age-related macular degeneration. Am J Ther. 2002;9:25–8. doi: 10.1097/00045391-200201000-00006. [DOI] [PubMed] [Google Scholar]
- 10.Dunaief JL. Iron induced oxidative damage as a potential factor in age-related macular degeneration: the Cogan Lecture. Invest Ophthalmol Vis Sci. 2006;47:4660–4. doi: 10.1167/iovs.06-0568. [DOI] [PubMed] [Google Scholar]
- 11.Garner B, Roberg K, Qian M, Brunk UT, Eaton JW, Truscott RJ. Redox availability of lens iron and copper: implications for HO* generation in cataract. Redox Rep. 1999;4:313–5. doi: 10.1179/135100099101535007. [DOI] [PubMed] [Google Scholar]
- 12.Ecroyd H, Carver JA. Crystallin proteins and amyloid fibrils. Cell Mol Life Sci. 2009;66:62–81. doi: 10.1007/s00018-008-8327-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Narayanan S, Kamps B, Boelens WC, Reif B. alphaB-crystallin competes with Alzheimer's disease beta-amyloid peptide for peptide-peptide interactions and induces oxidation of Abeta-Met35. FEBS Lett. 2006;580:5941–6. doi: 10.1016/j.febslet.2006.09.063. [DOI] [PubMed] [Google Scholar]
- 14.Lee S, Carson K, Rice-Ficht A, Good T. Hsp20, a novel alpha-crystallin, prevents Abeta fibril formation and toxicity. Protein Sci. 2005;14:593–601. doi: 10.1110/ps.041020705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith DG, Cappai R, Barnham KJ. The redox chemistry of the Alzheimer's disease amyloid beta peptide. Biochimica Et Biophysica Acta. 2007;1768:1976–90. doi: 10.1016/j.bbamem.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 16.Anderson DH, Talaga KC, Rivest AJ, Barron E, Hageman GS, Johnson LV. Characterization of beta amyloid assemblies in drusen: the deposits associated with aging and age-related macular degeneration. Experimental Eye Research. 2004;78:243–56. doi: 10.1016/j.exer.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 17.Bruban J, Glotin AL, Dinet V, Chalour N, Sennlaub F, Jonet L, An N, Faussat AM, Mascarelli F. Amyloid-beta(1-42) alters structure and function of retinal pigmented epithelial cells. Aging Cell. 2009;8:162–77. doi: 10.1111/j.1474-9726.2009.00456.x. [DOI] [PubMed] [Google Scholar]
- 18.Wang J, Ohno-Matsui K, Yoshida T, Shimada N, Ichinose S, Sato T, Mochizuki M, Morita I. Amyloid-beta up-regulates complement factor B in retinal pigment epithelial cells through cytokines released from recruited macrophages/microglia: Another mechanism of complement activation in age-related macular degeneration. J Cell Physiol. 2009;220:119–28. doi: 10.1002/jcp.21742. [DOI] [PubMed] [Google Scholar]
- 19.Surolia I, Sinha S, Sarkar DP, Reddy PY, Reddy GB, Surolia A. Concurrence of Danish dementia and cataract: insights from the interactions of dementia associated peptides with eye lens alpha-crystallin. PLoS One. 2008;3:e2927. doi: 10.1371/journal.pone.0002927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goldstein LE, Muffat JA, Cherny RA, Moir RD, Ericsson MH, Huang X, Mavros C, Coccia JA, Faget KY, Fitch KA, Masters CL, Tanzi RE, Chylack LT, Jr, Bush AI. Cytosolic beta-amyloid deposition and supranuclear cataracts in lenses from people with Alzheimer's disease. Lancet. 2003;361:1258–65. doi: 10.1016/S0140-6736(03)12981-9. [DOI] [PubMed] [Google Scholar]
- 21.Meehan S, Berry Y, Luisi B, Dobson CM, Carver JA, MacPhee CE. Amyloid fibril formation by lens crystallin proteins and its implications for cataract formation. J Biol Chem. 2004;279:3413–9. doi: 10.1074/jbc.M308203200. [DOI] [PubMed] [Google Scholar]
- 22.Frank B, Gupta S. A review of antioxidants and Alzheimer's disease. Annals Of Clinical Psychiatry: Official Journal Of The American Academy Of Clinical Psychiatrists. 2005;17:269–86. doi: 10.1080/10401230500296428. [DOI] [PubMed] [Google Scholar]
- 23.Garcia-Arencibia M, Gonzalez S, de Lago E, Ramos JA, Mechoulam R, Fernandez-Ruiz J. Evaluation of the neuroprotective effect of cannabinoids in a rat model of Parkinson's disease: importance of antioxidant and cannabinoid receptor-independent properties. Brain Research. 2007;1134:162–70. doi: 10.1016/j.brainres.2006.11.063. [DOI] [PubMed] [Google Scholar]
- 24.Bolognesi ML, Minarini A, Tumiatti V, Melchiorre C. Lipoic acid, a lead structure for multi-target-directed drugs for neurodegeneration. Mini Reviews In Medicinal Chemistry. 2006;6:1269–74. doi: 10.2174/138955706778742731. [DOI] [PubMed] [Google Scholar]
- 25.Binienda Z, Przybyla-Zawislak B, Virmani A, Schmued L. L-carnitine and neuroprotection in the animal model of mitochondrial dysfunction. Annals Of The New York Academy Of Sciences. 2005;1053:174–82. doi: 10.1196/annals.1344.015. [DOI] [PubMed] [Google Scholar]
- 26.Tauskela JS. MitoQ--a mitochondria-targeted antioxidant. Idrugs: The Investigational Drugs Journal. 2007;10:399–412. [PubMed] [Google Scholar]
- 27.Perluigi M, Joshi G, Sultana R, Calabrese V, De Marco C, Coccia R, Butterfield DA. In vivo protection by the xanthate tricyclodecan-9-yl-xanthogenate against amyloid beta-peptide (1-42)-induced oxidative stress. Neuroscience. 2006;138:1161–70. doi: 10.1016/j.neuroscience.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 28.Skulachev VP, Anisimov VN, Antonenko YN, Bakeeva LE, Chernyak BV, Erichev VP, Filenko OF, Kalinina NI, Kapelko VI, Kolosova NG, Kopnin BP, Korshunova GA, Lichinitser MR, Obukhova LA, Pasyukova EG, Pisarenko OI, Roginsky VA, Ruuge EK, Senin II, Severina II, Skulachev MV, Spivak IM, Tashlitsky VN, Tkachuk VA, Vyssokikh MY, Yaguzhinsky LS, Zorov DB. An attempt to prevent senescence: a mitochondrial approach. Biochim Biophys Acta. 2009;1787:437–61. doi: 10.1016/j.bbabio.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 29.Gupta SK, Selvan VK, Agrawal SS, Saxena R. Advances in pharmacological strategies for the prevention of cataract development. Indian J Ophthalmol. 2009;57:175–83. doi: 10.4103/0301-4738.49390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Evans J. Antioxidant supplements to prevent or slow down the progression of AMD: a systematic review and meta-analysis. Eye (Lond) 2008;22:751–60. doi: 10.1038/eye.2008.100. [DOI] [PubMed] [Google Scholar]
- 31.Evans JR, Henshaw K. Antioxidant vitamin and mineral supplements for preventing age-related macular degeneration. Cochrane Database Of Systematic Reviews (Online) 2008:CD000253. doi: 10.1002/14651858.CD000253.pub2. [DOI] [PubMed] [Google Scholar]
- 32.House E, Collingwood J, Khan A, Korchazkina O, Berthon G, Exley C. Aluminium, iron, zinc and copper influence the in vitro formation of amyloid fibrils of Abeta42 in a manner which may have consequences for metal chelation therapy in Alzheimer's disease. J Alzheimers Dis. 2004;6:291–301. doi: 10.3233/jad-2004-6310. [DOI] [PubMed] [Google Scholar]
- 33.CNS DRug DElivey. NIH Monogram [Google Scholar]
- 34.Wong RW, Richa DC, Hahn P, Green WR, Dunaief JL. Iron toxicity as a potential factor in AMD. Retina. 2007;27:997–1003. doi: 10.1097/IAE.0b013e318074c290. [DOI] [PubMed] [Google Scholar]
- 35.Avunduk AM, Yardimci S, Avunduk MC, Kurnaz L. Cataractous changes in rat lens following cigarette smoke exposure is prevented by parenteral deferoxamine therapy. Arch Ophthalmol. 1999;117:1368–72. doi: 10.1001/archopht.117.10.1368. [DOI] [PubMed] [Google Scholar]
- 36.Melov S, Wolf N, Strozyk D, Doctrow SR, Bush AI. Mice transgenic for Alzheimer disease beta-amyloid develop lens cataracts that are rescued by antioxidant treatment. Free Radic Biol Med. 2005;38:258–61. doi: 10.1016/j.freeradbiomed.2004.10.023. [DOI] [PubMed] [Google Scholar]
- 37.Cuajungco MP, Lees GJ. Diverse effects of metal chelating agents on the neuronal cytotoxicity of zinc in the hippocampus. Brain Research. 1998;799:97–107. doi: 10.1016/s0006-8993(98)00482-x. [DOI] [PubMed] [Google Scholar]
- 38.Finefrock AE, Bush AI, Doraiswamy PM. Current status of metals as therapeutic targets in Alzheimer's disease. J Am Geriatr Soc. 2003;51:1143–8. doi: 10.1046/j.1532-5415.2003.51368.x. [DOI] [PubMed] [Google Scholar]
- 39.Clifford Rose F, Gawel M. Clioquinol neurotoxicity: an overview. Acta Neurologica Scandinavica Supplementum. 1984;100:137–45. [PubMed] [Google Scholar]
- 40.Treiber C, Simons A, Strauss M, Hafner M, Cappai R, Bayer TA, Multhaup G. Clioquinol mediates copper uptake and counteracts copper efflux activities of the amyloid precursor protein of Alzheimer's disease. J Biol Chem. 2004;279:51958–64. doi: 10.1074/jbc.M407410200. [DOI] [PubMed] [Google Scholar]
- 41.Raman B, Ban T, Yamaguchi K, Sakai M, Kawai T, Naiki H, Goto Y. Metal ion-dependent effects of clioquinol on the fibril growth of an amyloid {beta} peptide. J Biol Chem. 2005;280:16157–62. doi: 10.1074/jbc.M500309200. [DOI] [PubMed] [Google Scholar]
- 42.Jenagaratnam L, McShane R. Clioquinol for the treatment of Alzheimer's Disease. Cochrane Database Of Systematic Reviews (Online) 2006 doi: 10.1002/14651858.CD005380.pub2. [DOI] [PubMed] [Google Scholar]
- 43.Crouch PJ, Barnham KJ, Bush AI, White AR. Therapeutic treatments for Alzheimer's disease based on metal bioavailability. Drug News & Perspectives. 2006;19:469–74. doi: 10.1358/dnp.2006.19.8.1021492. [DOI] [PubMed] [Google Scholar]
- 44.Lannfelt L, Blennow K, Zetterberg H, Batsman S, Ames D, Harrison J, Masters CL, Targum S, Bush AI, Murdoch R, Wilson J, Ritchie CW. Safety, efficacy, and biomarker findings of PBT2 in targeting Abeta as a modifying therapy for Alzheimer's disease: a phase IIa, double-blind, randomised, placebo-controlled trial. Lancet Neurol. 2008;7:779–86. doi: 10.1016/S1474-4422(08)70167-4. [DOI] [PubMed] [Google Scholar]
- 45.Adlard PA, Cherny RA, Finkelstein DI, Gautier E, Robb E, Cortes M, Volitakis I, Liu X, Smith JP, Perez K, Laughton K, Li QX, Charman SA, Nicolazzo JA, Wilkins S, Deleva K, Lynch T, Kok G, Ritchie CW, Tanzi RE, Cappai R, Masters CL, Barnham KJ, Bush AI. Rapid restoration of cognition in Alzheimer's transgenic mice with 8-hydroxy quinoline analogs is associated with decreased interstitial Abeta. Neuron. 2008;59:43–55. doi: 10.1016/j.neuron.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 46.Kupershmidt L, Weinreb O, Amit T, Mandel S, Carri MT, Youdim MB. Neuroprotective and neuritogenic activities of novel multimodal iron-chelating drugs in motor-neuron-like NSC-34 cells and transgenic mouse model of amyotrophic lateral sclerosis. FASEB J. 2009;23:3766–79. doi: 10.1096/fj.09-130047. [DOI] [PubMed] [Google Scholar]
- 47.Weinreb O, Mandel S, Bar-Am O, Yogev-Falach M, Avramovich-Tirosh Y, Amit T, Youdim MB. Multifunctional neuroprotective derivatives of rasagiline as anti-Alzheimer's disease drugs. Neurotherapeutics. 2009;6:163–74. doi: 10.1016/j.nurt.2008.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Silver H, Chertkow Y, Weinreb O, Danovich L, Youdim M. Multifunctional pharmacotherapy: what can we learn from study of selective serotonin reuptake inhibitor augmentation of antipsychotics in negative-symptom schizophrenia? Neurotherapeutics. 2009;6:86–93. doi: 10.1016/j.nurt.2008.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Forni GL, Balocco M, Cremonesi L, Abbruzzese G, Parodi RC, Marchese R. Regression of symptoms after selective iron chelation therapy in a case of neurodegeneration with brain iron accumulation. Mov Disord. 2008;23:904–7. doi: 10.1002/mds.22002. [DOI] [PubMed] [Google Scholar]
- 50.Kruck TP, Burrow TE. Synthesis of feralex a novel aluminum/iron chelating compound. J Inorg Biochem. 2002;88:19–24. doi: 10.1016/s0162-0134(01)00372-5. [DOI] [PubMed] [Google Scholar]
- 51.Kruck TP, Percy ME, Lukiw WJ. Metal sulfate-mediated induction of pathogenic genes and repression by phenyl butyl nitrone and Feralex-G. Neuroreport. 2008;19:245–9. doi: 10.1097/WNR.0b013e3282f4cb7e. [DOI] [PubMed] [Google Scholar]
- 52.Brewer GJ. The risks of free copper in the body and the development of useful anticopper drugs. Curr Opin Clin Nutr Metab Care. 2008;11:727–32. doi: 10.1097/MCO.0b013e328314b678. [DOI] [PubMed] [Google Scholar]
- 53.Lee JY, Friedman JE, Angel I, Kozak A, Koh JY. The lipophilic metal chelator DP-109 reduces amyloid pathology in brains of human beta-amyloid precursor protein transgenic mice. Neurobiol Aging. 2004;25:1315–21. doi: 10.1016/j.neurobiolaging.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 54.Scott LE, Orvig C. Medicinal inorganic chemistry approaches to passivation and removal of aberrant metal ions in disease. Chemical Reviews. 2009;109:4885–910. doi: 10.1021/cr9000176. [DOI] [PubMed] [Google Scholar]
- 55.Bush AI, Tanzi RE. Therapeutics for Alzheimer's disease based on the metal hypothesis. Neurotherapeutics. 2008;5:421–32. doi: 10.1016/j.nurt.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kador PF, Jin H. Multifunctional Compounds and Methods of Use Thereof Patent Pending [Google Scholar]
- 57.Kador PF, Inoue J, Blessing K. Anticataract activity of analogs of a sorbitol dehydrogenase inhibitor. J Ocul Pharmacol Ther. 2004;20:333–44. doi: 10.1089/1080768041725281. [DOI] [PubMed] [Google Scholar]
- 58.Gino A, DiLabio GA, Brigati G, Pedulli GF, Valgimigli L. 5-Pyrimidinols: Novel chain-breaking antioxidants more effective than phenols. J Am Chem Soc. 2001;123:4625–26. doi: 10.1021/ja005679l. [DOI] [PubMed] [Google Scholar]
- 59.Bakalbassis EG, Lithoxoidou AT, Vafiadis AP. Theoretical Calculation of Accurate Absolute and Relative Gas- and Liquid-Phase O-H Bond Dissociation Enthalpies of 2-Mono- and 2,6-Disubstituted Phenols, Using DFT/B3LYP. J Physical Chem A. 2003;107:8594–8606. [Google Scholar]
- 60.Jinno S, Okita T. Synthesis and structure-activity relationships of phaffiaol and related antioxidants. Chemical & Pharmaceutical Bulletin. 1998;46:1688–94. doi: 10.1248/cpb.47.1276. [DOI] [PubMed] [Google Scholar]
- 61.Pedulli G, Franco LM. Thermochemical and kinetic studies of antioxidants. Trends in Org Chem. 2001;9:97–105. doi: 10.1021/jo015653s. [DOI] [PubMed] [Google Scholar]
- 62.Zaidi SA, Shahjahan A, Sharif M, Siddiqi KS. Transition metal complexes of 2-(N-succinimidyl)pyrimidine. Synthesis and Reactivity Inorganic and Metal-Organic Chemistry. 1993;23:1571–84. [Google Scholar]
- 63.Fourmaintraux E, Depreux P, Lesieur I. Synthesis of New Phthalazinyl Compounds as Potential Inhibitors of Aldose Reductase and Sorbitol Dehydrogenase. Heterocyclic Communications. 1999;33:313–316. [Google Scholar]
- 64.Parker KA, Koziski KA. Directed Hydroxylation of Aromatics. Journal Organic Chemistry. 1987;51:674–676. [Google Scholar]
- 65.Kizner TA, Mikhaleva MA, Serebryakova ES. Selective Acid Hydrolysis of 2-Substituted-5-Dimethylaminomethyleneaminopyrimidines to 5-amino-and 5-hydroxypyrimidines. Chemistry of Heterocyclic Compounds. 1990;6:668–670. [Google Scholar]
- 66.Henry DWA. Facile Synthesis of Piperazines from Primary Amines (1) Journal Heterocyclic Chemistry. 1966;3:503–511. [Google Scholar]
- 67.Priewe H, Gutsche K. 5-Alkoxy-2-aminopyrimidines. 1963 [Google Scholar]
- 68.Julia M, Pfeuty-Saint Jalmes V, Ple K, Verpeaux JN. Hydroxylation of carbanions with lithium tert-butyl peroxide acting as an oxenoid. Bull Soc Chim Fr. 1996;133:15–24. [Google Scholar]
- 69.Ibaraki N, Chen SC, Lin LR, Okamoto H, Pipas JM, Reddy VN. Human lens epithelial cell line. Exp Eye Res. 1998;67:577–85. doi: 10.1006/exer.1998.0551. [DOI] [PubMed] [Google Scholar]
- 70.Dunn KC, Aotaki-Keen AE, Putkey FR, Hjelmeland LM. ARPE-19, a human retinal pigment epithelial cell line with differentiated properties. Exp Eye Res. 1996;62:155–69. doi: 10.1006/exer.1996.0020. [DOI] [PubMed] [Google Scholar]
- 71.Baron D, Hering JG. Analysis of Metal-EDTA Complexes by Electrospray Mass. Spectrometry J Environ Qual. 1998;27:844–850. [Google Scholar]
- 72.Huang CY. Determination of binding stoichiometry by the continuous variation method: the Job plot. Methods Enzymol. 1982;87:509–25. doi: 10.1016/s0076-6879(82)87029-8. [DOI] [PubMed] [Google Scholar]
- 73.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 74.Lou MF, Dickerson JE., Jr Protein-thiol mixed disulfides in human lens. Exp Eye Res. 1992;55:889–96. doi: 10.1016/0014-4835(92)90015-k. [DOI] [PubMed] [Google Scholar]