Abstract
Objective
To report the clinical features of anti–N-methyl-D-aspartate receptor (NMDAR) encephalitis in patients ≤ 18 years old.
Methods
Information was obtained by the authors or referring physicians. Antibodies were determined by immunocytochemistry and enzyme-linked immunosorbent assay (ELISA) using HEK293 cells ectopically expressing NR1.
Results
Over an 8-month period, 81 patients (12 male) with anti-NMDAR encephalitis were identified. Thirty-two (40%) were ≤18 years old (youngest 23 months, median 14 years); 6 were male. The frequency of ovarian teratomas was 56% in women >18 years old, 31% in girls ≤18 years old (p = 0.05), and 9% in girls ≤14 years old ( p = 0.008). None of the male patients had tumors. Of 32 patients ≤18 years old, 87.5% presented with behavioral or personality change, sometimes associated with seizures and frequent sleep dysfunction; 9.5% with dyskinesias or dystonia; and 3% with speech reduction. On admission, 53% had severe speech deficits. Eventually, 77% developed seizures, 84% stereotyped movements, 86% autonomic instability, and 23% hypoventilation. Responses to immunotherapy were slow and variable. Overall, 74% had full or substantial recovery after immunotherapy or tumor removal. Neurological relapses occurred in 25%. At the last follow-up, full recovery occurred more frequently in patients who had a teratoma that was removed (5/8) than in those without a teratoma (4/23; p = 0.03).
Interpretation
Anti-NMDAR encephalitis is increasingly recognized in children, comprising 40% of all cases. Younger patients are less likely to have tumors. Behavioral and speech problems, seizures, and abnormal movements are common early symptoms. The phenotype resembles that of the adults, although dysautonomia and hypoventilation are less frequent or severe in children.
Anti–N-methyl-D-aspartate receptor (NMDAR) encephalitis is a recently described disorder with a well defined set of clinical features.1 The associated syndrome has been characterized in adults, frequently young women with teratomas of the ovary who develop changes of mood, behavior, and personality, resembling acute psychosis. The clinical picture usually progresses to include seizures, decreased level of consciousness, dyskinesias, autonomic instability, and hypoventilation.2–5 Despite the severity of the disorder, patients often improve with immunotherapy and removal of the teratoma.1,3,6 These findings and the discovery that all patients have serum and cerebrospinal fluid (CSF) antibodies that react with the cell surface of neurons suggested an immune-mediated pathogenesis.1,7,8 Further studies demonstrated that the target antigen of patients’ antibodies was the NR1 subunit of the NMDAR. Additionally, application of antibodies into cultures of hippocampal neurons resulted in a significant decrease of postsynaptic NMDAR clusters that was reversed after antibody removal.9 A recent series of 100 patients showed that the disorder also occurs in patients without teratoma, and that men and children can be affected. Since then the number of pediatric cases diagnosed at our institution or whose serum and CSF were referred for immunological studies has steadily increased.9 Although this may suggest that in children the phenotype is as characteristic as that of the adults, there are in fact differences in tumor association, neurological presentation, and frequency of symptoms. To facilitate the recognition of this disorder in children, we report eight patients diagnosed over an 8-month period at the Children's Hospital of Philadelphia (CHOP) and review the general clinical features of 24 pediatric cases from other institutions.
Patients and Methods
From May to December of 2008, eight patients admitted to CHOP for new onset of neurological or psychiatric symptoms of unknown etiology were found to have antibodies to the NR1 subunit of the NMDAR (case #1 is described below, the other seven in the Supporting Information). During the same time period, 73 additional patients whose sera or CSF were referred from other institutions were found to have NR1 antibodies. Twenty-four of these patients were 18 years old or younger and along with the eight from CHOP (for a total of 32) are the focus of this study. Information was provided by the referring physicians.
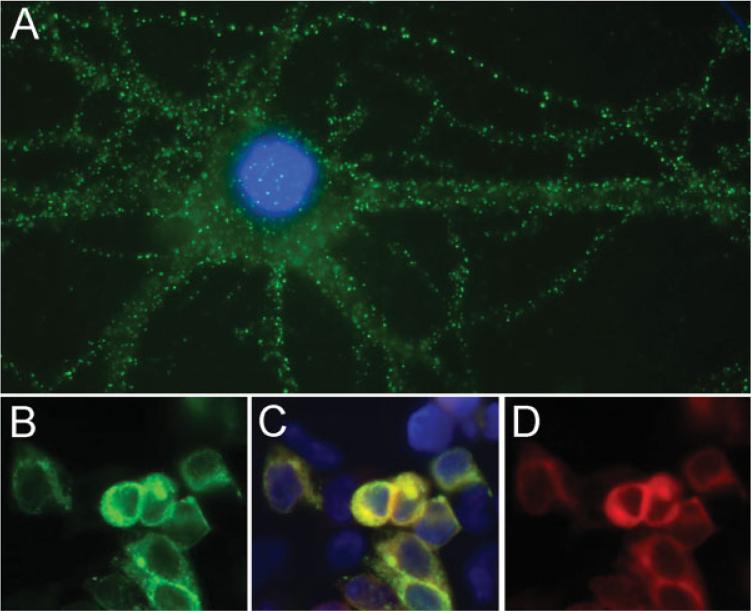
Most patients underwent extensive diagnostic studies including magnetic resonance imaging (MRI) of the brain (18 cases reviewed by the authors), electroencephalogram (EEG), serum and CSF viral studies, toxicological screening, and testing for autoimmune and metabolic disorders (see Supporting Information). Using reported techniques,9 all patients’ serum or CSF fulfilled three criteria: (1) characteristic immunohistochemical reactivity with the neuropil of rat hippocampus (not shown here; previously described1,9); (2) cell surface immunolabeling of nonpermeabilized neurons (Fig 1A); and (3) specific recognition of the NR1 subunit of the NMDAR (Fig 1B–D). Antibody titers were measured by enzyme-linked immunosorbent assay (ELISA), as reported.9
Fig 1.
Criteria of anti-NR1 antibodies. (A) The reactivity of a patient's CSF antibodies with neuronal cell surface antigens. (B–D) HEK293 cells transfected with the NR1 subunit of the NMDAR. (B) The reactivity of a patient's CSF is similar to the reactivity of a rabbit polyclonal antibody to NR1 (D); (C) merges both types of immunolabeling. Technical details previously reported.9 (A) Immunofluorescence method using nonpermeabilized rat hippocampal neurons and patient's CSF diluted 1:10; ×800 oil lens. (B–D) Immunofluorescence method using permeabilized HEK293 cells transfected with NR1 and colabeled with patient's CSF (diluted 1:10) and a rabbit polyclonal antibody to NR1 (1:1,000, AB9864; Chemicon, Temecula, CA); ×400.
Patients were considered to have “full neurological recovery” if they were able to return to all their activities; “substantial improvement” if they returned to their homes with mild deficits and were improving; and “limited improvement” if they were at home, in the hospital, or rehabilitation center with minimal change in the neurological status 3 months after neurological symptom presentation. Studies were approved by the University of Pennsylvania Institutional Review Board.
Statistical Analysis
Differences in outcome among patients with or without teratoma were analyzed with Fisher's two-sided exact test. Differences among antibody titers and the presence or absence of a teratoma were analyzed with the Mann-Whitney test.
Patient 1
This 3-year-old girl presented to the hospital when her family noted dragging of the left leg, flexion and extension movements of the left fingers, and twisting wrist motions that occurred at rest. She had a recent history of upper respiratory symptoms and low-grade fever. At the emergency department, she was noted to have chewing movements and teeth grinding. The movements of her left arm were characterized as chorea. On admission, EEG demonstrated right-sided slowing without epileptiform discharges; MRI of the brain was normal. The CSF revealed 10 white blood cells (WBC)/μl with normal protein and glucose concentrations. She became increasingly irritable and confused with awakenings during sleep every 10 minutes. She asked for her socks to be taken off when her feet were bare. She screamed when an unfamiliar person walked into the room and had episodes of kicking, biting, and thrashing. The chorea persisted and she developed twitching movements in the left face and shoulder. Mild tachycardia and slight elevations of blood pressure were recorded. A repeat EEG showed right-sided background slowing. One week after symptom onset, she was empirically treated with a 5-day pulse of methylprednisolone. Three days later it was evident that her encephalopathy and dystonia were improving, with no further behavioral outbursts, and she was discharged on a steroid taper. As the steroids were weaned, her irritability returned and she became hyperoral, biting her father and a plastic object with such force she damaged her teeth. NMDAR antibodies were identified and she was readmitted and treated with intravenous immunoglobulin (IVIg) on day 15 of the illness. During this treatment the encephalopathy resolved. She was seen in follow-up 2 months after onset, with complete resolution of symptoms.
Results
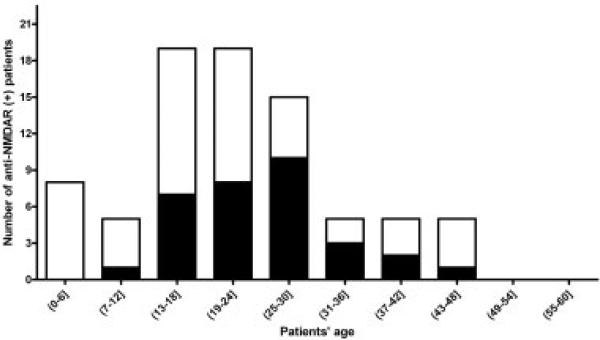
The main neuropsychiatric symptoms, duration of hospitalization, treatment, and outcome of the eight patients from CHOP are summarized in Table 1. The age distribution and tumor association of these patients and 73 cases whose sera or CSF were referred from other institutions are shown in Fig 2. Twelve patients were male; none of them had an underlying tumor. Thirty-two patients (40%) were 18 years old or younger; six were male. The frequency of ovarian teratomas was 56% (24/43) in women >18 years old, 31% (8/26) in girls ≤18 years old (p = 0.05), and 9% (1/11) in girls ≤14 years old (p = 0.008).
Table 1.
Main Neuropsychiatric Features, Hospital Stay, Treatment, and Outcome of Eight Patients Seen at the Children's Hospital of Philadelphia
| Case# | Sex/Age (years) | Neuropsychiatric Symptoms | Movement Abnormalities | Autonomic Dysfunction | Hospital Stay (days) | Treatment | Improvement |
|---|---|---|---|---|---|---|---|
| 1 |
F/3 |
Agitation; staff phobia; hyperoral |
Orofacial dyskinesias; unilateral dystonia, chorea |
Hypertensiona; enuresis; sleep dysfunction |
Inpatient 21 |
Solumedrol; IVIg |
Full |
| 2 |
F/2 |
Mood lability; speech dysfunction; focal seizures; hyperreflexia |
Orofacial dyskinesias; unilateral dystonia; stereotyped movements |
Enuresis; tachycardiaa; hypothermiaa; sleep dysfunction |
Inpatient 24 |
Solumedrol; IVIg |
Full |
| 3 |
F/16 |
Hallucinations; paranoia; agitation; focal seizures; hyperreflexia |
Orofacial dyskinesias; rigidity; catatonia; stereotyped movements |
Tachycardia; hypertension; enuresis; sleep dysfunction |
Inpatient 38; rehab 18 |
Solumedrol; IVIg |
Substantial |
| 4 |
M/6 |
Agitation; disinhibition; speech dysfunction; hyperreflexia |
Orofacial dyskinesias; chorea; stereotyped movements |
Enuresis; drooling; hyperthermiaa; hypoventilationa; sleep dysfunction |
ICU 8; inpatient 31; rehab 147 |
Solumedrol; IVIg |
Substantial |
| 5 |
M/6 |
Agitation; paranoia; disinhibition; hypersexual; focal seizures; speech dysfunction |
Orofacial dyskinesias; opisthotonus; dystonia; chorea |
Drooling; tachycardiaa; enuresis; sleep dysfunction |
ICU 2; inpatient 45; rehab 31 |
Solumedrol; IVIg |
Substantial |
| 6 |
F/14 |
Arm pain/numbness; speech dysfunction; agitation; paranoia; staff phobia; hyperoral; hypersexual; hyperreflexia |
Orofacial dyskinesias; dystonia; stereotyped movements; rigidity; opisthotonus |
Enuresis; drooling; hyperthermia; hypoventilationa; sleep dysfunction |
ICU 20; inpatient 4; rehab 40 |
Solumedrol; IVIg |
Substantial |
| 7 |
F/16 |
Agitation; mood lability; hallucinations; delusions; hypersexual; speech dysfunction |
Orofacial dyskinesias; stereotyped movements; catatonia |
Hypertension; tachycardia; pupil dilation; sleep dysfunction; enuresis; hypoventilationa |
Inpatient 30 |
Solumedrol; IVIg |
Substantial |
| 8 | M/9 | Agitation; speech dysfunction | Unilateral dystonia; Rigidity; opisthotonus; stereotyped movements | Pupil dilation; hypoventilation; hypertension; enuresis; hyperthermiaa; sleep dysfunction | ICU 19; inpatient 58 | Solumedrol; IVIg | Limitedb |
Episodic and associated with episodes of agitation.
Limited (currently treated).
ICU = intensive care unit; IVIg = intravenous immunoglobulin.
Fig 2.
Distribution of age and tumors in 81 patients with anti-NMDA receptor encephalitis. Black bars indicate the number of patients with tumors (in all instances ovarian teratomas). White bars indicate the number of patients without tumors. Younger patients were less likely to have a tumor.
Clinical Features
The clinical features of the 32 patients ≤18 years old are shown in Table 2. Twenty-six patients were female, median age = 14 years (youngest 23 months). Prodromal symptoms such as fever, headache, upper respiratory symptoms, vomiting, or diarrhea were noted in 48% of patients. Twenty-eight patients presented with mood, behavior, or personality changes. In addition to psychiatric symptoms, six patients had early seizures, and three had hypersomnia, enuresis, limb pain, and numbness, or dyskinesias. In four patients the first symptom was not psychiatric: two had limb dystonia (cases #1 and #8), one had dyskinesia, and one had decrease of speech. Most patients became increasingly anxious or agitated, sometimes combative and paranoid, with inappropriate laughing or crying, and progressive reduction of speech. Visual or auditory hallucinations were common. By the time of admission, 17 patients had severe speech problems, including reduction of verbal output or mutism, mumbling, or perseveration.
Table 2.
Clinical Features of 32 Children with Anti-NMDAR Encephalitis
| Characteristic | Total |
|---|---|
| Female sex, n (%) |
26 (81) |
| Age (years), range (median) |
23 months–18 years (14) |
| Prodromal symptoms/assessable cases, n (%) |
15/31 (48) |
| Symptom presentation, n (%) |
|
| Behavior/personality |
19 (59) |
| Behavior/personality and seizures |
6 (19) |
| Behavior/personality and othera |
3 (9.5) |
| Dystonia or dyskinesias |
3 (9.5) |
| Speech reduction |
1 (3) |
| Seizuresb/assessable cases, n (%) |
23/30 (77) |
| Movement disorders/assessable cases, n (%) |
26/31 (84) |
| Any type |
18 (58) |
| Orolinguofacial dyskinesias |
14 (45) |
| Choreoathetoid |
10 (32) |
| Dystonic postures, muscle rigidity, or increased tone |
9 (29) |
| Complex, stereotyped movements (extremities, abdomen, pelvis) |
|
| Otherc |
6 (19) |
| Autonomic instability/assessable cases, n (%)d |
24/28 (86) |
| Central hypoventilation/assessable cases, n (%) | 7/31 (23) |
One patient had hypersomnia and enuresis; 1 arm pain and numbness, and 1 dyskinesias.
Nineteen patients had partial motor or complex seizures; 2 generalized tonic-clonic seizures, 1 status epilepticus, and 1 partial seizures and status epilepticus.
Two had myoclonic movements, 2 oculogyric crisis, and 2 opisthotonos-like postures. When involving face and mouth, the abnormal movements were described as kissing, pouting, frowning, smiling, fish-like motions, and tongue protruding and rolling, and when involving the extremities, as dancing, milking, bicycling, floating, flailing, pill-rolling, and pelvic thrusting.
Sixteen (57%) tachycardia, 12 (43%) hyperthermia, 9 (32%) hypertension, 2 (7%) hypothermia, 1 (4%) hypotension. Enuresis, sleep dysfunction, and drooling were not assessed in patients from other institutions; all 8 patients from CHOP had enuresis and sleep dysfunction, and 3 had prominent drooling.
NMDAR = N-methyl-D-aspartate receptor; CHOP = Children's Hospital of Philadelphia.
During the course of the disease 77% of patients developed seizures, usually partial motor or complex seizures, 84% movement disorders (Fig 3), and 86% autonomic instability, predominantly tachycardia, hyperthermia, and hypertension. Twenty-three percent of patients required intubation for central hypoventilation, 19% for airway protection, and 16% had transient episodes of oxygen desaturation or apnea that did not require mechanical support.
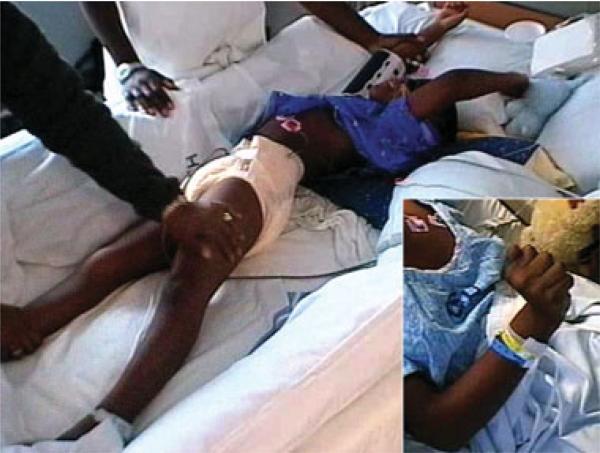
Fig 3.
Abnormal postures in patients with anti-NMDAR encephalitis. Patient #5 developed periods of agitation with opisthotonic posturing (as shown), purposeless opening of the mouth, choreic movements with hands and arms, pressured speech, and tachycardia alternating with periods of calm. The inset corresponds to an abnormal hand posture of patient #6; this posture and associated movements were described as pill-rolling.
Ancillary Tests and NMDA Receptor Antibodies
MRI, EEG, and CSF findings are shown in Table 3 and Fig 4. All patients had antibodies in CSF or serum that reacted with extracellular epitopes of NR1. Using normalized IgG concentrations from paired CSF and serum samples available from 21 patients, all patients had stronger antibody reactivity in CSF. At the time of initial assessment, seven patients who had been empirically treated with plasma exchange or IVIg had positive CSF, but negative serum reactivity (both normalized at 15μg IgG/100μl). Patients with teratoma had higher CSF titers (median 115,312 ELISA relative fluorescence units/microliter [rfu/μl]; range, 8,257–468,544) than those without teratoma (median 10,300 rfu/μl; range, 68–124,445) (Mann-Whitney, p = 0.01); values in 20 control samples were <10 rfu/μl.
Table 3.
Ancillary Tests, Treatment, and Outcome
| Characteristic | Subjects |
|---|---|
| Brain MRI, n (%) |
|
| Total with abnormal findingsa |
10 (31) |
| EEG in 25 assessable cases, n (%) |
|
| Total with abnormal findings |
25 (100) |
| Focal or diffuse delta/theta wave or disorganized activity |
22 (88) |
| Epileptic activity |
7 (28) |
| CSF in 31 assessable cases, n (%) |
|
| Total with abnormal findings |
29 (94) |
| Lymphocytic pleocytosis |
27 (87) |
| Range, cells/μl |
5–200 |
| Median |
17 |
| Protein concentration >45 mg/dl |
4 (13) |
| Oligoclonal bands (assessed in 6 patients) |
5 (83) |
| Tumor,b n (%) |
|
| Teratoma of the ovary |
8 |
| Treatment in 31 assessable cases, n (%) |
|
| Tumor resection |
8 (27) |
| Immunotherapyc |
30 (97) |
| Electroconvulsive therapy |
2 (7) |
| Supportive care |
1 (3) |
| Outcome in 31 assessable patients, n (%) |
|
| Full recovery |
9 (29) |
| Substantial improvement |
14 (45) |
| Limited improvement | 8 (26) |
Five patients had transient FLAIR hyperintensity in one or more areas (medial temporal lobe, periventricular, cerebellar); 2 transient leptomeningeal enhancement, 1 transient cortical enhancement, sulcal abnormalities, 1 mild mesial temporal atrophy, 1 multifocal parenchymal enhancement.
None of the male patients had evidence of systemic tumor.
Twenty-six patients received intravenous corticosteroids, 23 IVIg, 14 plasma exchange, 2 rituximab, 1 cyclophosphamide, and 4 both rituximab and cyclophosphamide.
MRI = magnetic resonance imaging; EEG = electroencephalography; CSF = cerebrospinal fluid; FLAIR = fluid attenuation inversion recovery; IVIg = intravenous immunoglobulin.
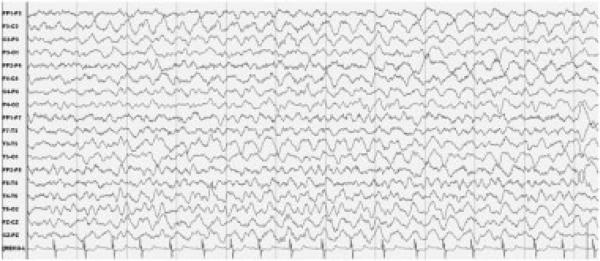
Fig 4.
EEG activity in a patient with anti-NMDAR encephalitis. The EEG from patient #4 demonstrates continuous activity with bilateral, left predominant, frontal-central-temporal rhythmic 2–3Hz delta activity. No correlate was found between the EEG activity and patient's abnormal movements, including shoulder writhing, dystonic neck extension, tongue thrusting, drooling, chewing, and “kissing” mouth movements. The EEG findings did not change after administration of levetiracetam.
Treatment and Outcome
Tumor removal was performed in the eight patients with evidence of ovarian teratoma (two bilateral); all had a mature cystic teratoma. Two tumors were examined for expression of NMDAR and antibody binding and both were positive (data not shown). Of 31 patients, 30 (97%) had immunotherapy that usually consisted of a combination of corticosteroids, IVIg, or plasma exchange. Seven patients refractory to these treatments received rituximab (two cases), cyclophosphamide (one case), or both (four cases). These treatments were well tolerated; four patients started to improve shortly after treatment (one with cyclophosph-amide, three with cyclophosphamide and rituximab); the other three had slow progressive improvement not clearly related to the treatments.
The outcome was assessable in 31 patients and one was lost to follow-up. Nine patients (29%) had full recovery; 14 (45%) substantial improvement, all currently recovering with mild deficits; and eight (26%) limited improvement with all showing signs of slow recovery from severe deficits. The median time from symptom presentation to initial signs of improvement was 6 weeks (range, 2–28 weeks). The median follow-up was 4.5 months (range, 2–14.5 months). At the last follow-up, full recovery occurred more frequently in patients who had a teratoma that was removed (5/8) than in those without teratoma (4/23) (p = 0.03).
Neurological Relapses
Eight patients (25%) had a history of one episode (five patients) or several episodes (three patients) of a similar type of encephalitis 1 to 96 months (median, 24 months) before the diagnosis of anti-NMDAR encephalitis. None of them had an underlying tumor at presentation or at relapse. In four patients relapses occurred while being tapered from corticosteroids or after completing immunotherapy; the other four relapsed more than 1 year after full recovery (two patients) or partial recovery (two patients) from the previous episode of encephalitis. From one of these patients (case #3 in Supporting Information), archived CSF obtained during the first episode of encephalitis was available for study. This CSF contained NMDAR antibodies (2,451 rfu/μl); the patient had full clinical recovery and relapsed 3 years later, at which time the CSF NMDAR antibody titer was 69,410 rfu/μl.
Discussion
Anti-NMDAR encephalitis is a recently described disorder that appears to be mediated by antibodies to the NR1 subunit of the receptor.9 In the current study, the diagnosis of eight pediatric patients over 8 months in a single institution suggests the disorder in children is more frequent than previously thought. A similar increase in the recognition of the disorder in children has been noted in referrals from other institutions. In a previous series of 100 patients, 22 were 18 years old or younger and of these 55% had an underlying tumor. In the current study of 81 patients, 40% were 18 years old or younger and of these only 25% (31% if only females were considered) had a tumor, all ovarian teratomas.9 The younger the patient the less likely a tumor was to be identified. Our findings also indicate the disorder is increasingly recognized in young boys.
In most respects the clinical picture of pediatric patients with anti-NMDAR encephalitis is similar to that of adults although there are differences in the frequency and manifestation of some symptoms. In the first series, which mostly included adults, 85% initially presented to psychiatrists for anxiety, agitation, paranoia, and visual or auditory hallucinations.9 In the current series, most adolescents had similar symptoms, but the recognition of psychosis in younger children posed more of a challenge. Parents described temper tantrums, behavioral change, agitation, aggression, and progressive speech deterioration as initial symptoms; however, these behaviors in children can initially be overlooked. Three patients had dystonia or dyskinesias as the presenting symptom with no behavioral change. Yet, as occurred in adults, most children eventually developed abnormal movements, including complex stereotyped motions involving the face, limbs, trunk, or abdomen, among other movements (see descriptions in Table 2).2,10 Some of these movements were suspicious for seizures. Although electrographic seizures were verified in several children, the ictal changes were often subclinical and some movements causing concern for seizures had no EEG correlate. These findings caution against overtreating these patients with antiepileptic medications before a thorough video EEG evaluation.
The autonomic manifestations in children appear to be less severe than in adults. While 66% of patients in the original series exhibited central hypoventilation usually requiring weeks of mechanical support,9 only 23% of patients of the current series had hypoventilation. It is unclear whether these differences are the result of earlier diagnosis, or if they reflect developmental differences in the brainstem control of breathing or expression of NMDAR. Other signs of autonomic lability were more common and, in fact, all patients from CHOP had urinary incontinence and sleep dysfunction, including erratic sleep patterns, insomnia, and less frequently hypersomnia. Episodes of hypertension, tachycardia, or hyperthermia were frequent and often correlated with agitation, reminiscent of autonomic storming. However, different from adults, severe cardiac dysrhythmia (eg, requiring a pacemaker) did not occur in any of the children.4
Given the neuropsychiatric manifestations of the disorder, the differential diagnosis can be broad as evidenced by the extensive testing performed on the patients. Viral encephalitis is often the first presumptive diagnosis, suggested by the acute neurological change, CSF pleocytosis, and occasional hyperthermia.11 In a review of encephalitis by investigators of the California Encephalitis Project, 63% of cases ultimately had no identified etiology, suggesting that some subtypes could be immune-mediated.12 This hypothesis gains support with the identification of NMDAR antibodies in 10 of 19 (53%) of their cases that after extensive viral studies had been classified as “idiopathic encephalitis with psychiatric manifestations or dyskinesias” (C.A.G. and J.D., unpublished results). Another disorder frequently considered is neuroleptic malignant syndrome.4 However, symptoms of muscle rigidity, hyperthermia, elevated serum levels of creatine kinase (eg, case #8) and rhabdomyolysis may occur in patients with anti-NMDAR encephalitis that have not been treated with neuroleptics or antipsychotics.
The mechanisms that initiate this disorder are unknown. In a subgroup of patients, the presence of a tumor, usually a teratoma of the ovary that expresses NMDAR, likely contributes in triggering the immune response.1 The paradigm is similar to other autoimmune disorders such as myasthenia gravis or the Lambert-Eaton syndrome, which may occur with or without tumor association.13,14 Four patients of the current study had serum antibodies to antinuclear antibody (ANA), thyroid peroxidase, or both, which may suggest a propensity to autoimmunity. Extensive studies for a common infectious agent were negative, making an immune response by molecular mimicry unlikely (Supporting Information). It should be noted that five patients had positive mycoplasma serologies but negative CSF polymerase chain reaction (PCR); however, the significance of this finding is unclear given the high prevalence of positive mycoplasma serologies in most series of pediatric encephalitis.11
Although in young girls with anti-NMDAR encephalitis the frequency of a teratoma is low compared with that of the adults, it does raise the question of what screening to perform and at what interval. Considering the age-related limitations of some tests (eg, transvaginal ultrasound; exposure to radiation with repeat CT scans), we recommend ultrasound and MRI of abdomen and pelvis. Based on the experience with some adults whose initial teratoma or second teratoma was identified many months after the first episode of encephalitis or at a relapse,2 periodic MRI or ultrasound studies for at least 2 years appears prudent.15 At this time, the number of male patients is too small for recommendations on tumor surveillance. Our initial series showed that two of nine male patients had tumors (one bilateral testicular teratoma and seminoma,16 and one small-cell lung cancer),9 but none of 12 subsequently diagnosed patients (six children) had tumors.
At CHOP, the experience with immunotherapy has been mixed. Some children appeared to respond rapidly to IVIg or methylprednisolone; however, this was not universal and, at times, even those who responded to an initial course did not seem to benefit after repeat treatment; some of these patients continued to slowly improve independent of the treatments used. A similar experience regarding the varied response to corticosteroids, IVIg, or plasma exchange was noted in patients from other institutions. This could relate to the fact that most children did not have tumors. Indeed, as described in adults,9 children who had a teratoma and were treated with tumor removal and immunotherapy had more frequent full recovery (p = 0.03) and less relapses than those without a tumor. Nevertheless, 23 of 31 (74%) assessable patients had either full recovery (29%) or substantial improvement (45%). Although these results are notable, one should keep in mind the short follow-up of the current study. It is likely that many patients with mild deficits will attain full recovery, as seen in adults, but some may have relapses with or without residual deficits.
Based on this and previous studies,9 anti-NMDAR encephalitis should be suspected in children with acute behavioral change, seizures, dystonia, or dyskinesias, usually accompanied by (1) CSF lymphocytic pleocytosis or oligoclonal bands; (2) EEG with infrequent epileptic activity, but frequent slow, disorganized activity that does not correlate with most abnormal movements; and (3) brain MRI that is often normal or shows transient fluid attenuation inversion recovery (FLAIR) or contrast-enhancing abnormalities. Detection of antibodies to NR1 subunits of the NMDAR confirms the diagnosis. Patients should be examined for a tumor, usually an ovarian teratoma, and treated with immunotherapy. The recovery is slow, frequently over months, and requires a multidisciplinary team including physical rehabilitation and psychiatric management of the protracted behavioral symptoms.
Despite substantial progress in the recognition of this disorder, many questions remain. The trigger of the immune response is unknown, particularly in patients without teratoma; human leukocyte antigen (HLA) profiling to determine a genetic predisposition to autoimmunity should be considered in future studies. After tumor removal or immunotherapy, patients who do not improve usually have persistent high CSF antibody titers even though serum titers may be substantially decreased (J.D., personal observation). Prospective analysis of paired serum and CSF antibody titers in patients who do and do not improve is needed to determine the optimal clinical-immunological follow-up. It is unclear whether the early use of other immunotherapy (eg, cyclophosphamide, rituximab)6 to control the immune response within the central nervous system (CNS) would shorten the duration of symptoms. Given that early relapses appear to occur while tapering the immunotherapy and in patients without teratoma, the effectiveness of long-term immunosuppression should be assessed.
Supplementary Material
Acknowledgments
This work was supported by in part by National Cancer Institute, National Institute of Health 2RO1CA89054 (J.D.) and National Cancer Institute, National Institute of Health RO1CA107192 (J.D.); National Institute of Health NSR56-45986 (D.L.), National Institute of Health NSR01-45986 (D.L.), Foederer Foundation of the Children's Hospital of Philadelphia (D.L.); and National Institutes of Health (NIH) National Institute of Mental Health (NIMH) F31MH083395 (A.J.G.).
We thank the physicians, patients, and family members that provided clinical information.
Footnotes
Potential conflict of interest: Nothing to report.
Additional Supporting Information may be found in the online version of this article.
References
- 1.Dalmau J, Tuzun E, Wu HY, et al. Paraneoplastic anti-N-methyl-D-aspartate receptor encephalitis associated with ovarian teratoma. Ann Neurol. 2007;61:25–36. doi: 10.1002/ana.21050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iizuka T, Sakai F, Ide T, et al. Anti-NMDA receptor encephalitis in Japan: long-term outcome without tumor removal. Neurology. 2008;70:504–511. doi: 10.1212/01.wnl.0000278388.90370.c3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seki M, Suzuki S, Iizuka T, et al. Neurological response to early removal of ovarian teratoma in anti-NMDAR encephalitis. J Neurol Neurosurg Psychiatry. 2008;79:324–326. doi: 10.1136/jnnp.2007.136473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sansing LH, Tuzun E, Ko MW, et al. A patient with encephalitis associated with NMDA receptor antibodies. Nat Clin Pract Neurol. 2007;3:291–296. doi: 10.1038/ncpneuro0493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tonomura Y, Kataoka H, Hara Y, et al. Clinical analysis of paraneoplastic encephalitis associated with ovarian teratoma. J Neurooncol. 2007;84:287–292. doi: 10.1007/s11060-007-9372-9. [DOI] [PubMed] [Google Scholar]
- 6.Ishiura H, Matsuda S, Higashihara M, et al. Response of anti-NMDA receptor encephalitis without tumor to immunotherapy including rituximab. Neurology. 2008;71:1921–1923. doi: 10.1212/01.wnl.0000336648.43562.59. [DOI] [PubMed] [Google Scholar]
- 7.Vitaliani R, Mason W, Ances B, et al. Paraneoplastic encephalitis, psychiatric symptoms, and hypoventilation in ovarian teratoma. Ann Neurol. 2005;58:594–604. doi: 10.1002/ana.20614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shimazaki H, Ando Y, Nakano I, Dalmau J. Reversible limbic encephalitis with antibodies against the membranes of neurones of the hippocampus. J Neurol Neurosurg Psychiatry. 2007;78:324–325. doi: 10.1136/jnnp.2006.104513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dalmau J, Gleichman AJ, Hughes EG, et al. Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol. 2008;7:1091–1098. doi: 10.1016/S1474-4422(08)70224-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kleinig TJ, Thompson PD, Matar W, et al. The distinctive movement disorder of ovarian teratoma-associated encephalitis. Mov Disord. 2008;23:1256–1261. doi: 10.1002/mds.22073. [DOI] [PubMed] [Google Scholar]
- 11.Lewis P, Glaser CA. Encephalitis. Pediatr Rev. 2005;26:353–363. doi: 10.1542/pir.26-10-353. [DOI] [PubMed] [Google Scholar]
- 12.Glaser CA, Honarmand S, Anderson LJ, et al. Beyond viruses: clinical profiles and etiologies associated with encephalitis. Clin Infect Dis. 2006;43:1565–1577. doi: 10.1086/509330. [DOI] [PubMed] [Google Scholar]
- 13.Wirtz PW, Nijnuis MG, Sotodeh M, et al. The epidemiology of myasthenia gravis, Lambert-Eaton myasthenic syndrome and their associated tumours in the northern part of the province of South Holland. J Neurol. 2003;250:698–701. doi: 10.1007/s00415-003-1063-7. [DOI] [PubMed] [Google Scholar]
- 14.Wirtz PW, Smallegange TM, Wintzen AR, Verschuuren JJ. Differences in clinical features between the Lambert-Eaton myasthenic syndrome with and without cancer: an analysis of 227 published cases. Clin Neurol Neurosurg. 2002;104:359–363. doi: 10.1016/s0303-8467(02)00054-9. [DOI] [PubMed] [Google Scholar]
- 15.Graus F, Delattre JY, Antoine JC, et al. Recommended diagnostic criteria for paraneoplastic neurological syndromes. J Neurol Neurosurg Psychiatry. 2004;75:1135–1140. doi: 10.1136/jnnp.2003.034447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eker A, Saka E, Dalmau J, et al. Testicular teratoma and anti-N-methyl-D-aspartate receptor-associated encephalitis. J Neurol Neurosurg Psychiatry. 2008;79:1082–1083. doi: 10.1136/jnnp.2008.147611. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.