Abstract
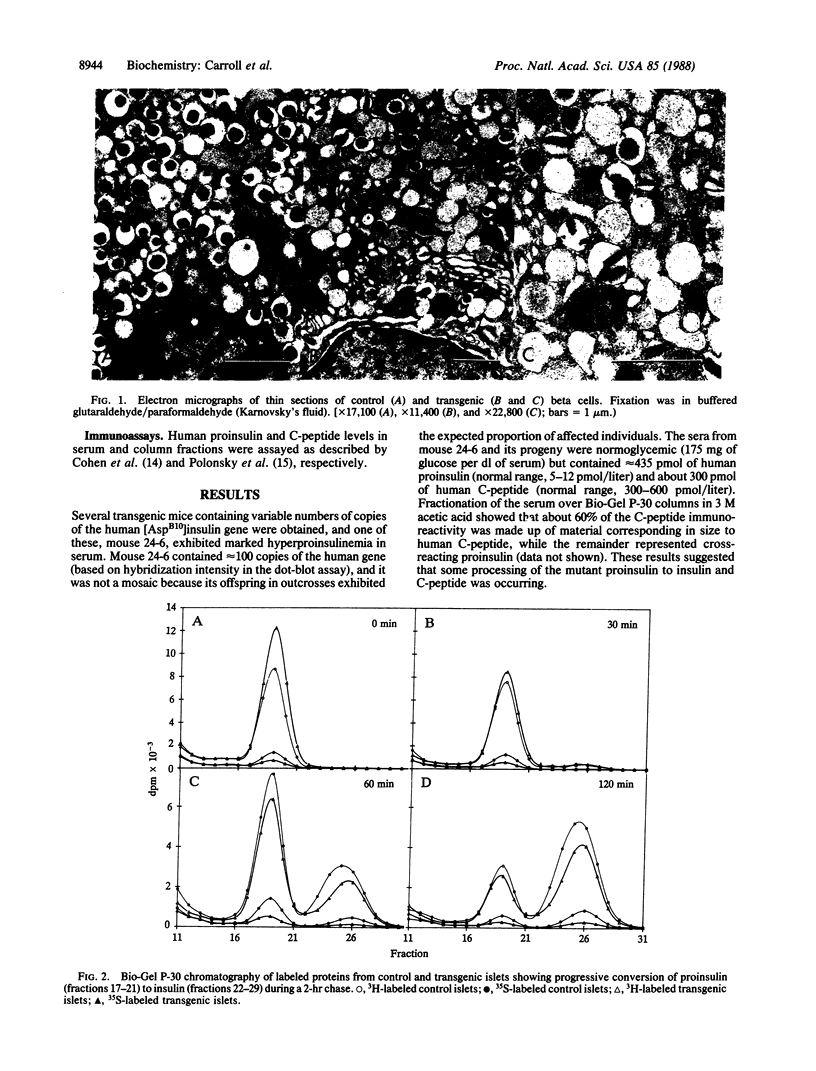
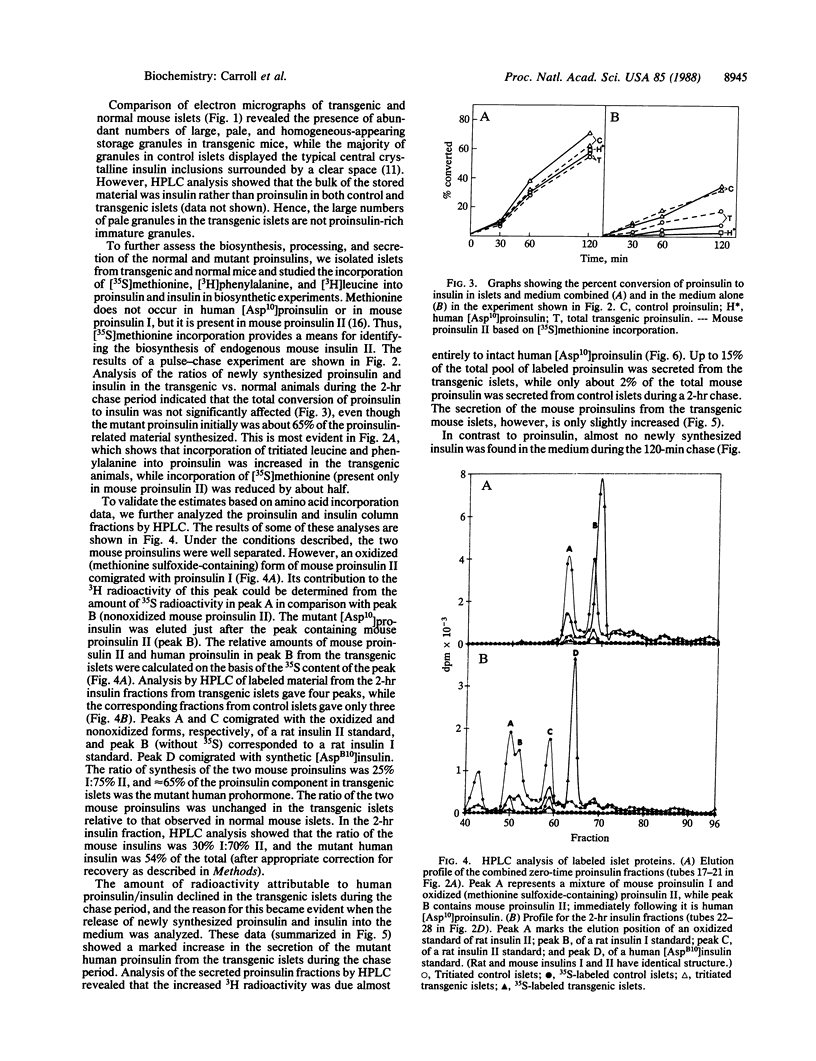
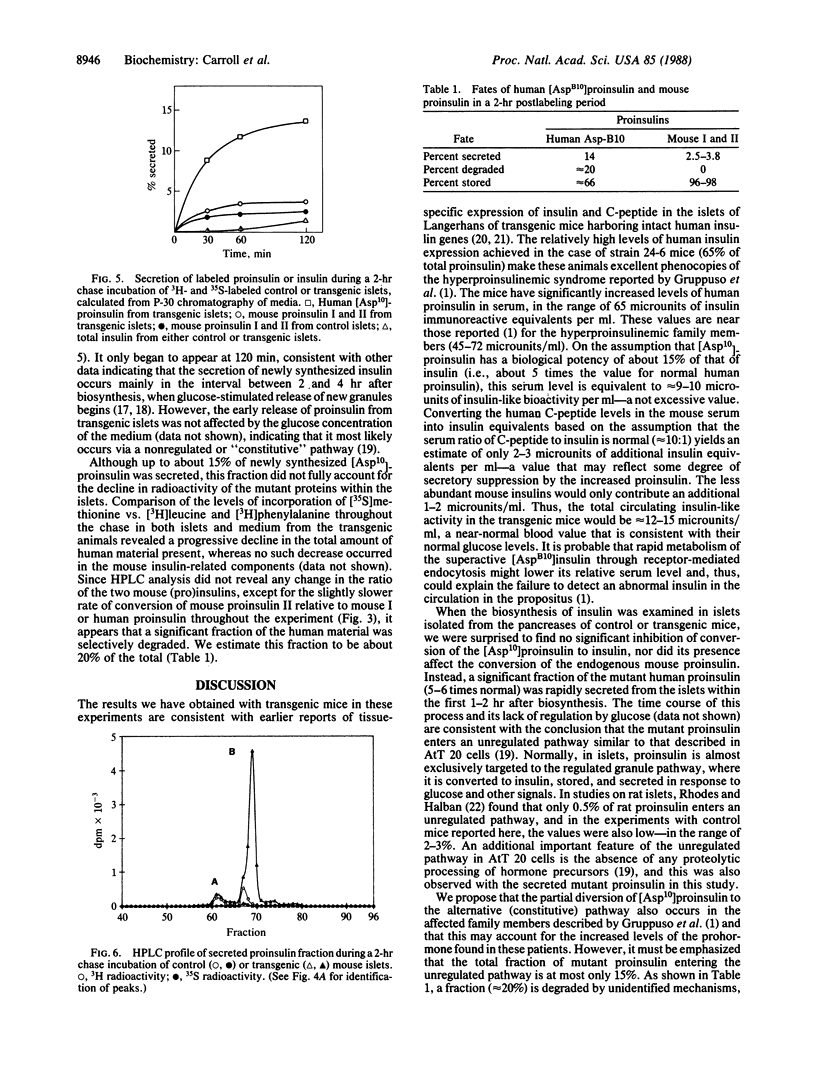
A coding mutation in the human insulin gene (His-B10----Asp) is associated with familial hyperproinsulinemia. To model this syndrome, we have produced transgenic mice that express high levels of the mutant prohormone in their islets of Langerhans. Strain 24-6 mice, containing about 100 copies of the mutant gene, were normoglycemic but had marked increases of serum human proinsulin immunoreactive components. Biosynthetic studies on isolated islets revealed that approximately 65% of the proinsulin synthesized in these mice was the human mutant form. Unlike the normal endogenous mouse proinsulin, which was almost exclusively handled via a regulated secretory pathway, up to 15% of the human [Asp10]proinsulin was rapidly secreted after synthesis via an unregulated or constitutive pathway, and approximately 20% was degraded within the islet cells. The secreted human [Asp10]proinsulin was not processed proteolytically. However, the processing of the normal mouse and human mutant proinsulins within the islets from transgenic mice was not significantly impaired. These findings suggest that the hyperproinsulinemia of the patients is the result of the continuous secretion of unprocessed mutant prohormone from the islets via this alternative unregulated pathway.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brange J., Ribel U., Hansen J. F., Dodson G., Hansen M. T., Havelund S., Melberg S. G., Norris F., Norris K., Snel L. Monomeric insulins obtained by protein engineering and their medical implications. Nature. 1988 Jun 16;333(6174):679–682. doi: 10.1038/333679a0. [DOI] [PubMed] [Google Scholar]
- Brinster R. L., Chen H. Y., Trumbauer M. E., Yagle M. K., Palmiter R. D. Factors affecting the efficiency of introducing foreign DNA into mice by microinjecting eggs. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4438–4442. doi: 10.1073/pnas.82.13.4438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucchini D., Ripoche M. A., Stinnakre M. G., Desbois P., Lorès P., Monthioux E., Absil J., Lepesant J. A., Pictet R., Jami J. Pancreatic expression of human insulin gene in transgenic mice. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2511–2515. doi: 10.1073/pnas.83.8.2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess T. L., Kelly R. B. Constitutive and regulated secretion of proteins. Annu Rev Cell Biol. 1987;3:243–293. doi: 10.1146/annurev.cb.03.110187.001331. [DOI] [PubMed] [Google Scholar]
- Chan S. J., Seino S., Gruppuso P. A., Schwartz R., Steiner D. F. A mutation in the B chain coding region is associated with impaired proinsulin conversion in a family with hyperproinsulinemia. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2194–2197. doi: 10.1073/pnas.84.8.2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C. W., Thomas C. A., Jr Recovery of DNA segments from agarose gels. Anal Biochem. 1980 Jan 15;101(2):339–341. doi: 10.1016/0003-2697(80)90197-9. [DOI] [PubMed] [Google Scholar]
- Cohen R. M., Given B. D., Licinio-Paixao J., Provow S. A., Rue P. A., Frank B. H., Root M. A., Polonsky K. S., Tager H. S., Rubenstein A. H. Proinsulin radioimmunoassay in the evaluation of insulinomas and familial hyperproinsulinemia. Metabolism. 1986 Dec;35(12):1137–1146. doi: 10.1016/0026-0495(86)90027-2. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Brown M. S., Anderson R. G., Russell D. W., Schneider W. J. Receptor-mediated endocytosis: concepts emerging from the LDL receptor system. Annu Rev Cell Biol. 1985;1:1–39. doi: 10.1146/annurev.cb.01.110185.000245. [DOI] [PubMed] [Google Scholar]
- Gruppuso P. A., Gorden P., Kahn C. R., Cornblath M., Zeller W. P., Schwartz R. Familial hyperproinsulinemia due to a proposed defect in conversion of proinsulin to insulin. N Engl J Med. 1984 Sep 6;311(10):629–634. doi: 10.1056/NEJM198409063111003. [DOI] [PubMed] [Google Scholar]
- Lernmark A., Nathans A., Steiner D. F. Preparation and characterization of plasma membrane-enriched fractions from rat pancreatic islets. J Cell Biol. 1976 Nov;71(2):606–623. doi: 10.1083/jcb.71.2.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael J., Carroll R., Swift H. H., Steiner D. F. Studies on the molecular organization of rat insulin secretory granules. J Biol Chem. 1987 Dec 5;262(34):16531–16535. [PubMed] [Google Scholar]
- Owerbach D., Bell G. I., Rutter W. J., Shows T. B. The insulin gene is located on chromosome 11 in humans. Nature. 1980 Jul 3;286(5768):82–84. doi: 10.1038/286082a0. [DOI] [PubMed] [Google Scholar]
- Polonsky K. S., Given B. D., Hirsch L., Shapiro E. T., Tillil H., Beebe C., Galloway J. A., Frank B. H., Karrison T., Van Cauter E. Quantitative study of insulin secretion and clearance in normal and obese subjects. J Clin Invest. 1988 Feb;81(2):435–441. doi: 10.1172/JCI113338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes C. J., Halban P. A. Newly synthesized proinsulin/insulin and stored insulin are released from pancreatic B cells predominantly via a regulated, rather than a constitutive, pathway. J Cell Biol. 1987 Jul;105(1):145–153. doi: 10.1083/jcb.105.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivier J., McClintock R. Reversed-phase high-performance liquid chromatography of insulins from different species. J Chromatogr. 1983 Sep 23;268(1):112–119. doi: 10.1016/s0021-9673(01)95395-6. [DOI] [PubMed] [Google Scholar]
- Robbins D. C., Shoelson S. E., Rubenstein A. H., Tager H. S. Familial hyperproinsulinemia. Two cohorts secreting indistinguishable type II intermediates of proinsulin conversion. J Clin Invest. 1984 Mar;73(3):714–719. doi: 10.1172/JCI111264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sando H., Borg J., Steiner D. F. Studies on the secretion of newly synthesized proinsulin and insulin from isolated rat islets of Langerhans. J Clin Invest. 1972 Jun;51(6):1476–1485. doi: 10.1172/JCI106944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sando H., Grodsky G. M. Dynamic synthesis and release of insulin and proinsulin from perifused islets. Diabetes. 1973 May;22(5):354–360. doi: 10.2337/diab.22.5.354. [DOI] [PubMed] [Google Scholar]
- Schwartz G. P., Burke G. T., Katsoyannis P. G. A superactive insulin: [B10-aspartic acid]insulin(human). Proc Natl Acad Sci U S A. 1987 Sep;84(18):6408–6411. doi: 10.1073/pnas.84.18.6408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selden R. F., Skośkiewicz M. J., Howie K. B., Russell P. S., Goodman H. M. Regulation of human insulin gene expression in transgenic mice. 1986 May 29-Jun 4Nature. 321(6069):525–528. doi: 10.1038/321525a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibasaki Y., Kawakami T., Kanazawa Y., Akanuma Y., Takaku F. Posttranslational cleavage of proinsulin is blocked by a point mutation in familial hyperproinsulinemia. J Clin Invest. 1985 Jul;76(1):378–380. doi: 10.1172/JCI111973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tager H. S., Rubenstein A. H., Steiner D. F. Methods for the assessment of peptide precursors. Studies insulin biosynthesis. Methods Enzymol. 1975;37:326–345. doi: 10.1016/s0076-6879(75)37030-4. [DOI] [PubMed] [Google Scholar]
- Terris S., Steiner D. F. Retention and degradation of 125I-insulin by perfused livers from diabetic rats. J Clin Invest. 1976 Apr;57(4):885–896. doi: 10.1172/JCI108365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wentworth B. M., Schaefer I. M., Villa-Komaroff L., Chirgwin J. M. Characterization of the two nonallelic genes encoding mouse preproinsulin. J Mol Evol. 1986;23(4):305–312. doi: 10.1007/BF02100639. [DOI] [PubMed] [Google Scholar]
- Wood S. P., Blundell T. L., Wollmer A., Lazarus N. R., Neville R. W. The relation of conformation and association of insulin to receptor binding; x-ray and circular-dichroism studies on bovine and hystricomorph insulins. Eur J Biochem. 1975 Jul 15;55(3):531–542. doi: 10.1111/j.1432-1033.1975.tb02190.x. [DOI] [PubMed] [Google Scholar]



