Abstract
Background and Aims
In some lupin species, phosphate deficiency induces cluster-root formation, which enhances P uptake by increasing root surface area and, more importantly, the release of root exudates which enhances P availability.
Methods
Three species of Lupinus, L. albus, L. atlanticus and L. micranthus, with inherently different relative growth rates were cultivated under hydroponics in a greenhouse at four phosphate concentrations (1, 10, 50 and 150 µm) to compare the role of internal P in regulating cluster-root formation.
Key Results
The highest growth rate was observed in L. atlanticus, followed by L. albus and L. micranthus. At 1 µm P, cluster-root formation was markedly induced in all three species. The highest P uptake and accumulation was observed in L. micranthus, followed by L. atlanticus and then L. albus. Inhibition of cluster-root formation was severe at 10 µm P in L. atlanticus, but occurred stepwise with increasing P concentration in the root medium in L. albus.
Conclusions
In L. atlanticus and L. albus cluster-root formation was suppressed by P treatments above 10 µm, indicating a P-inducible regulating system for cluster-root formation, as expected. By contrast, production of cluster roots in L. micranthus, in spite of a high internal P concentration, indicated a lower sensitivity to P status, which allowed P-toxicity symptoms to develop.
Keywords: Cluster roots, lupin, Lupinus, phosphate nutrition, toxicity, uptake
INTRODUCTION
Phosphorus (P) is an essential macronutrient for plant growth, and it is limiting crop production in many regions of the world (Holford, 1997). Global demand for P fertilizer continues to increase while global reserves of P are in decline (Steen, 1998; Cordell et al., 2009). The availability of soil P for plants is related to several plant characters, including the release of carboxylates (Ryan et al., 2001), rhizosphere pH (Curtin et al., 1993; Hinsinger, 2001), morphological traits such as length and surface area of roots (Williamson et al., 2001; Li et al., 2007), root architecture (Lynch, 1995), root hairs (Gahoonia and Nielsen, 1997), mycorrhizas (Smith et al., 2000) and specialized structures such as root clusters (Lambers et al., 2003; Shane and Lambers, 2005). Several investigators have reported that P is a key element that strongly influences the initiation and growth of cluster roots (Shane and Lambers, 2005). In field studies, some grain legumes exhibit higher P-acquisition efficiency than other crops due to cluster-root formation and release of carboxylate (Bolland et al., 1999).
Cluster roots release large quantities of carboxylates in an exudative burst from mature clusters (Watt and Evans, 1999; Shane and Lambers, 2005). On the other hand, adequate P supply to plants inhibits cluster-root formation and so avoids excessive loss of carbon from the root system (Keerthisinghe et al., 1998). Several investigators have reported either initiation or inhibition of cluster-root formation to be linked to internal P concentration in the plant (Shane et al., 2003; Li and Liang, 2005; Shen et al., 2005). However, the exact location of the signal has not yet been fully elucidated; it might be the shoot P concentration, the P concentration in phloem sap or the P concentration in the root (Shane et al., 2003). Recently, Pearse et al. (2006) introduced a model that cluster roots in Lupinus species have a positive effect on P uptake, which enhances P status, but this, in turn, has a negative effect on cluster-root formation and exudation. The P concentration in a plant is balanced by phosphate uptake and plant growth rate. The capacity for P uptake may be affected by several environmentally or genetically controlled factors that differ among Lupinus species (Pearse et al., 2006). Also, a plant's growth rate may be influenced by many ecological or genetic factors. If shoot P concentration is a signal for prevention of cluster-root formation, different species with inherent differences in maximum growth rate and P uptake may differ in the foliar P concentration that either initiates or suppresses cluster-root formation. In this way, those plants that grow quickly, even with a higher uptake of phosphate, may accumulate little P in their shoot and stimulate cluster-root formation. By contrast, species that have an inherently low growth rate may accumulate high levels of P in their shoot, even at a relatively low phosphate supply. It is therefore of interest to evaluate changes in P concentration and cluster-root formation in different plants with inherently different growth rates as affected by phosphate supply in their root medium.
The main objective of the present study was to evaluate the effect of a plant's relative growth rate on its shoot P concentration and cluster-root formation. Based on preliminary experiments, three Lupinus species with inherently different growth rates, L. albus, L. atlanticus and L. micranthus, were grown in hydroponic culture at four P concentrations. These species are all Old World species, which naturally occur in the Mediterranean and North Africa (their origins are: L. albus, Egypt; L. atlanticus, Morocco; L. micranthus, Israel) (Käss and Wink, 1997). The P concentration in shoot and root and plant growth rate were determined to assess possible correlations with cluster-root formation.
MATERIALS AND METHODS
Seeds of Lupinus albus L. ‘Kiev mutant’, L. micranthus L. ‘P22945’ and L. atlanticus L. ‘P27224’ were scarified by scalpel and hydrated for 5 h. The hydrated seeds were sown in pots filled with washed river sand in a glasshouse on 31 May, 2007 and watered lightly daily. Seedlings were carefully removed and the roots washed free of sand on 8 June, 2007. Healthy seedlings of equal size for each species were selected for hydroponic culture. Each plant was supported in the centre of a grey foam lid, and the roots immersed in a 20-L black plastic container filled with continuously aerated nutrient solution of the following composition (μm): Ca(NO3), 400; K2SO4, 200; MgSO4, 54; MnSO4, 0·24; ZnSO4 0·1; CuSO4, 0·018; H3BO3, 2·4; Na2MoO4, 0·03; Fe-EDTA, 10. De-ionized water was used for making the nutrient solution and the entire solution was replaced daily. The pots were placed in a temperature-controlled root-cooling tank maintained at 18–22 °C. The experiment was carried out in a factorial, completely randomized design. Factor 1 was phosphate concentration in the root medium and factor 2 was species. The cotyledons were removed before starting treatments to limit plants to phosphate absorbed by roots. During the experimental period, the midday irradiance in the glasshouse was approx. 900 µmol photons m−2 s−1, the day/night air temperature was 11/27 °C and the average relative humidity was 67 %.
The experiment from the day of sowing lasted 54 d. Four plants from each species and treatment were harvested randomly at 8, 32, 39, 47 and 54 d after sowing. There was one more harvest for L. albus at day 25 after sowing. Each plant was separated into stems (including petioles), leaves, cluster roots and non-cluster roots, and the components were weighed, dried in an oven for 3 d at 70 °C, re-weighed and ground for determination of P concentration. Leaves, cluster roots and non-cluster roots were scanned before drying (see below) and root mass ratio (root dry mass/total dry mass) was calculated. Relative growth rates (RGRs), based on total dry mass, were calculated using the following equation (Ricker, 1979)
where M2 is the final dry mass (day 54), M1 the initial dry mass (day 8) and T the number of days of growth.
Scanning and image analysis of roots and leaves
Each root sample (cluster roots and non-cluster roots) was dispersed in water in a transparent tray (30 × 20 × 3 cm), and scanned (EPSON Expression 1600, Seiko EPSON Corp., Nagano, Japan) with a resolution of 400 d.p.i. All leaflets of each sample were separated and spread in a transparent tray (without water) and scanned. Where there was a large amount of roots or leaves, up to four trays were used for scanning of one sample. The images were analysed with WinRHIZO software by the method of object separation from background and classification of pixel colours (Regent Instruments Inc., Sainte-Foy, Quebec, Canada). Leaf area, root length and root diameter distribution were measured and leaf/root area ratio (m2 m−2) was calculated.
Determination of total phosphate and phosphate uptake
Concentrations of total P were determined in roots and shoots after digesting approx. 100 mg of dried and powdered plant material in a mixture of concentrated nitric and perchloric acids (3 : 1) at 175 °C. Total P was assayed using a UV-vis spectrophotometer (Shimadzu Corp., Kyoto, Japan) by the malachite green oxalate method (Irving and McLaughlin, 1990).
P uptake rate [J, mg g−1 (root dry mass d−1] was calculated from dry weights and P concentrations for each replicate and was averaged using the following equation (Soon, 1988):
where J is the mean uptake rate (mg g−1 root dry mass d−1), M is the root dry mass (g), P is the amount of P (mg g−1 dry mass) in the plant and T is time (d). For calculation of P uptake based on root area [mg m−2 (root area) day−1], root area (m2) was substituted for root mass in the equation above. The P-uptake rate was calculated for plants between days 39 and 54 after sowing.
Statistical analysis
All data were analysed by analysis of variance (ANOVA) using SAS statistical software (SAS Institute Inc., Cary, NC, USA). Comparisons of means were performed using Duncan's multiple range analysis test (α = 0·05). Logarithmic curves were fitted using regression analysis to describe the relationship between plant investment in cluster roots and leaf, stem and root P concentration
RESULTS
Deficiency and toxicity symptoms
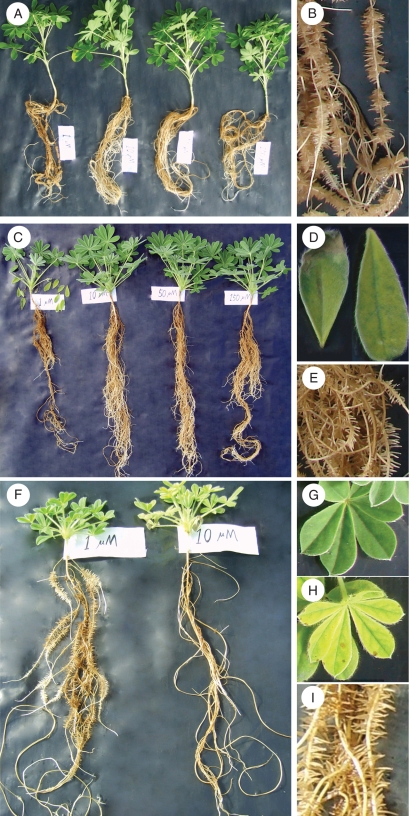
In both L. albus and L. atlanticus, P deficiency at 1 µm phosphate in the nutrient solution caused chlorosis, necrosis and marginal scorching of old leaves, which led to shedding of some of the old leaves (Fig. 1). There were no toxicity symptoms in L. albus and only moderate toxicity symptoms in L. atlanticus, even at 150 µm P. Conversely, in L. micranthus phosphate toxicity was visible at 10 µm P and became more pronounced at higher P concentrations. There were no deficiency symptoms, even at 1 µm P, in L. micranthus, whereas toxicity resulted in interveinal chlorosis and marginal necrosis, leading to shedding of old leaves and leaf desiccation at 50 and 150 µm P.
Fig. 1.
Effect of phosphate concentration in the root environment on plant growth. (A) Lupinus albus whole plants, (B) L. albus cluster root of a plant grown at 1 µm P, (C) L. atlanticus whole plants, (D) L. atlanticus leaves showing P deficiency, (E) L. atlanticus cluster root of a plant grown at 1 µk P, (F) L. micranthus plants, (G) L. micranthus healthy leaf of a plant grown at 1 µm P, (H) L. micranthus leaf showing P toxicity when plants are grown at 10 µm P and (I) L. micranthus cluster root of a plant grown at 1 µm P.
Growth
Based on total plant dry mass, the highest relative growth rate was observed in L. atlanticus, followed by L. albus, and the lowest growth rate was exhibited by L. micranthus (Figs 1 and 2, Table 1).
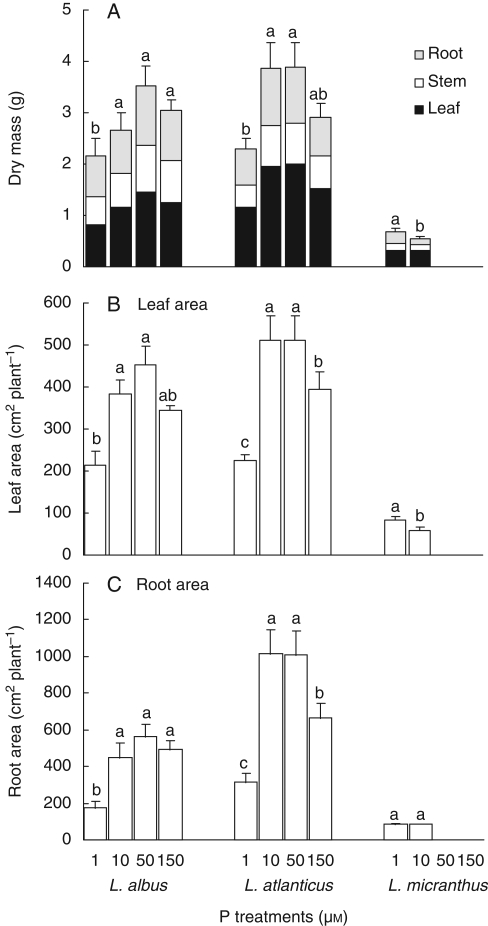
Fig. 2.
Effects of phosphate concentration in the root environment on dry mass (A), leaf area (B) and root area (C) of Lupinus albus, L. atlanticus and L. micranthus plants after 54 d in hydroponics. Treatment means marked with the same lower-case letter are not significantly different within each group using a one-way ANOVA for each harvest followed by Duncan's multiple test (P < 0·05).
Table 1.
Effects of phosphate treatments on root mass ratio (total dry mass/root dry mass), leaf/root area ratio, relative growth rate and P uptake in Lupinus albus, L. atlanticus and L. micranthus plants during 54 d in hydroponics culture
| P uptake |
||||||
|---|---|---|---|---|---|---|
| P treatment (μm) | Root mass ratio, day 54 (g g−1) | Leaf/root area ratio, Day 54 (cm2 cm−2) | Relative growth rate, days 8/54 (d−1) | mg g−1 d−1 | mg m−2 d−1 | |
| L. albus | ||||||
| 1 | 0·37a | 0·46b | 0·039b | 0·06c | 0·83c | |
| 10 | 0·32b | 0·70a | 0·044ab | 0·36c | 4·55c | |
| 50 | 0·33b | 0·64a | 0·050a | 4·90a | 77·44a | |
| 150 | 0·32b | 0·60a | 0·047ab | 3·35b | 49·72b | |
| Mean | 0·33a | 0·60a | 0·045b | 2·45b | 33·14b | |
| L. atlanticus | ||||||
| 1 | 0·30a | 0·34b | 0·049b | 0·09c | 0·91c | |
| 10 | 0·29a | 0·51ab | 0·061a | 2·43b | 25·43b | |
| 50 | 0·28a | 0·51ab | 0·060a | 5·52a | 62·20a | |
| 150 | 0·27a | 0·66a | 0·057a | 6·13a | 63·54a | |
| Mean | 0·28b | 0·52b | 0·057a | 3·54a | 38·02a | |
| L. micranthus | ||||||
| 1 | 0·33a | 0·30b | 0·048a | 0·09b | 0·70b | |
| 10 | 0·21b | 0·58a | 0·036b | 4·81a | 44·71a | |
| 50 | ||||||
| 150 | ||||||
| Mean | 0·27 | 0·44 | 0·042 | 2·17 | 22·21 | |
Treatments marked with the same lower-case letter are not significantly different within each species using one-way ANOVA followed by Duncan's multiple test (P < 0·05). Treatment means indicated a difference among species using two-way ANOVA followed by Duncan's multiple test (P < 0·05); not relevant for L. micranthus because there were no data for 50 and 150 µm P.
In L. albus, P deficiency, first visible after 32 d, caused more than 60 % reduction of dry mass at 1 µm P compared with 50 µm P (Fig. 1). Dry mass was highest at 50 µm P and harvested on day 54 (Fig. 2). Similarly, root and leaf area were higher at 50 µm P and lower at 1 µm P, compared with other treatments at the final harvest (Fig. 3, Table 1).
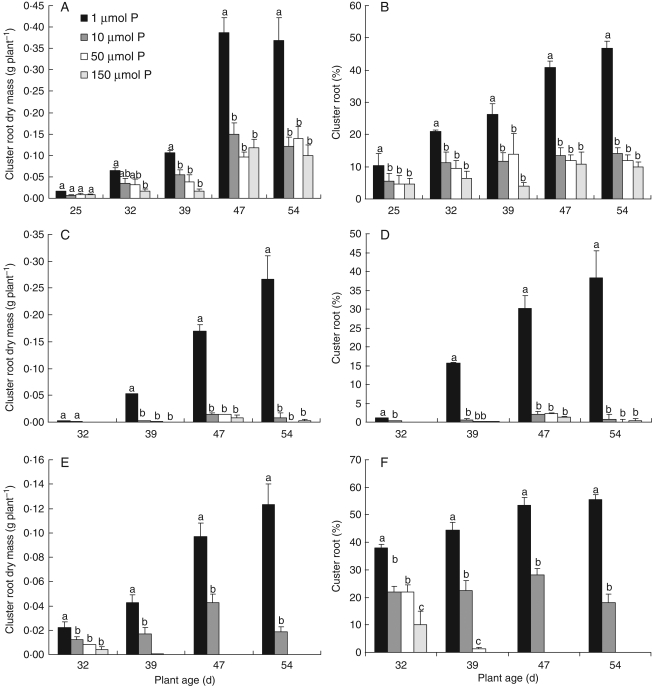
Fig. 3.
Effects of phosphate concentration in the root environment on cluster-root dry mass and percentage of cluster roots as compared with total roots based on dry mass in Lupinus species during 54 d in hydroponics. L. albus (A, B), L. atlanticus (C, D) and L. micranthus (E, F). Treatment means marked with the same lower-case letter are not significantly different within each group using a one-way ANOVA for each harvest followed by Duncan's multiple test (P < 0·05).
In L. atlanticus, P deficiency caused a significant reduction in growth at 1 µm P after 32 d. Also, phosphate toxicity resulted in a marked reduction in growth at 150 µm P (Fig. 2). At the final harvest, maximum growth was observed at 10 and 50 µm P. Root and leaf area were higher at 10 and 50 µm P and lower at 1 µm P in comparison with other treatments at the final harvest (Fig. 3). Comparing the 50 and 150 µm P treatments, phosphate toxicity was more severe, showing more than 25 % reduction in dry mass in L. atlanticus in comparison with less than 15 % in L. albus.
L. micranthus was very sensitive to phosphate toxicity, showing significant reduction of fresh and dry mass at 50 and 150 µm P from the first harvest onwards (day 32), and subsequently (after 39 d of treatment) desiccation of the leaves (Fig. 1). The highest fresh and dry mass was observed at 1 µm P, and even 10 µm P caused mild toxicity (Figs 1 and 2). The same result was found for leaf area, where 1 µm P gave the highest leaf area (Fig. 3).
The leaf/root area ratio was higher in L. albus than in L. atlanticus and L. micranthus, and this ratio increased for all species with increasing P supply (Table 1).
The root mass ratio (root dry mass/total dry mass) varied significantly among species, and, for some species, between treatments. The mass of roots was significantly less in L. albus than in L. atlanticus and L. micranthus, on average representing 33, 28 and 26 % of the whole plant on day 54, respectively. In L. albus and L. micranthus, but not in L. atlanticus, allocation of biomass to roots compared with whole plants increased significantly when supplied with the higher level of P (37 % at 1 µm P to 32 % at 10 µm P in L. albus and 33 % at 1 µm P to 21 % at 1 µm P in L. micranthus) (Table 1).
Cluster-root formation
The highest dry mass and area of cluster roots was exhibited at 1 µm P in all species; increasing P concentration in the root environment decreased cluster-root formation (Figs 1 and 3, Table 1). The most severe inhibition of cluster-root formation by P was observed in L. atlanticus, where the dry mass of cluster roots decreased from 38 % of total root mass at 1 µm P to less than 1 % at 150 µm P. The highest percentage of cluster roots was shown by L. micranthus followed by L. albus (Fig. 3). These differences coincided with the apparent rate at which clusters developed: on day 32, the generally slow-growing L. micranthus at 1 µm P already had 44 % cluster roots, whereas the faster-growing L. albus and L. atlanticus had 21 and 15 %, respectively.
Phosphate uptake and concentration
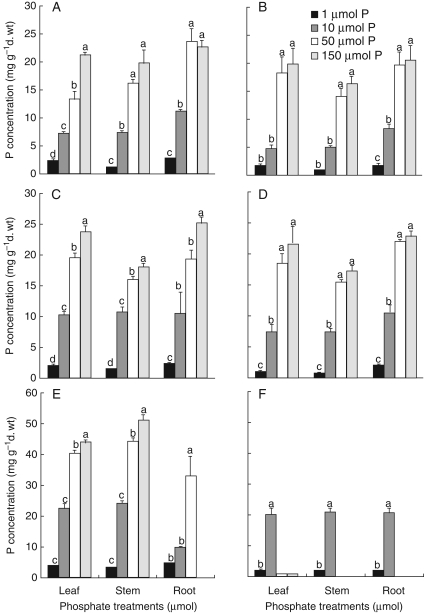
The P concentration in plant tissues was very low at 1 µm P, but increased gradually with increasing P concentration in the root environment (Fig. 4). The highest concentration of P was observed in L. micranthus (50 mg g−1 d. wt). The rate of P uptake increased at higher P concentrations in all three species. Comparing the three species at 10 µm P, the highest P-uptake rate was observed for L. micranthus, followed by L. atlanticus and L. albus.
Fig. 4.
Effects of phosphate concentration in the root environment on P concentration in Lupinus species during 54 d of hydroponics culture. L. albus day 39 (A), L. albus day 54 (B), L. atlanticus day 39 (C), L. atlanticus day 54 (D), L. micranthus day 39 (E), L. micranthus day 54 (F). Treatment means marked with the same lower-case letter are not significantly different within each group using a one-way ANOVA for each harvest followed by Duncan's multiple test (P < 0·05).
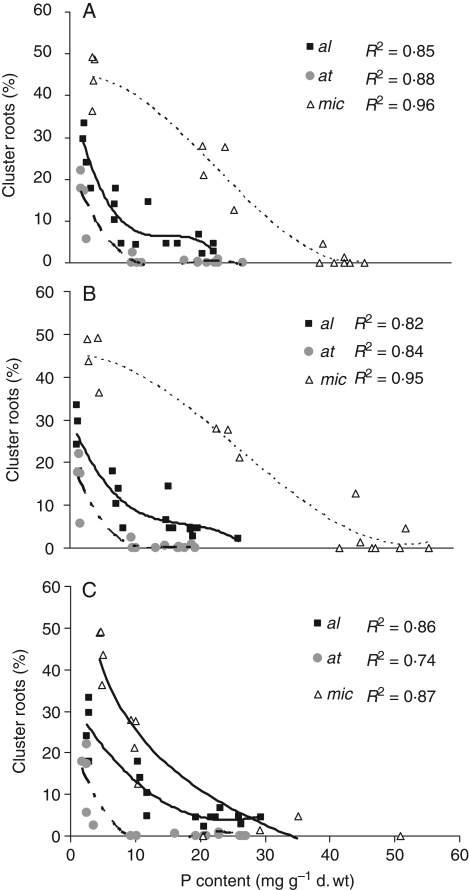
Relationship between P concentration and cluster-root formation
Based on regression analysis, there was a significant correlation between cluster-root formation and P concentration in all species and for P concentration in all plant organs. The best curves for exhibition of these correlations were polynomial. Cluster-root formation decreased sharply as P concentrations in roots, stems and leaves of all three species increased (Fig. 5). The highest inhibition of cluster-root formation by P concentration in leaves was in L. atlanticus, where cluster-roots decreased to less than 1 % by increasing P concentration of leaves to about 20 mg g−1 d. wt in comparison with 5 and 27 % in L. albus and L. micranthus, respectively. The differences among species in cluster-root formation as affected by P concentration were even more obvious when comparing P concentrations in stems, showing a reduction of cluster-roots to 5 % at 6, 21 and 43 mg P g−1 d. wt in L. atlanticus, L. albus and L. micranthus, respectively.
Fig. 5.
Relationship between percentage of cluster roots as compared with total roots (based on dry mass) and phosphate concentration in leaves (A), stems (B) and roots (C) of Lupinus species. L. albus = al, L. atlanticus = at and L. micranthus = mic.
DISCUSSION
The main objective of the present study was to assess the effect of phosphate supply on cluster-root formation in three lupin species with different relative growth rates and to test the model proposed by Pearse et al. (2006). The results indicate major differences in growth and P status in response of the three Lupinus species to P supply. Relative growth rates increased from L. micranthus to L. albus followed by L. atlanticus (Table 2). Conversely, the highest specific rate of P uptake (P uptake per unit root mass or area) was observed for L. micranthus, followed by L. atlanticus and L. albus (Fig. 4, Table 2). At 1 µm P in the root environment, all plants produced cluster roots, but cluster-root formation decreased with increasing rate of P concentration in the nutrient solution (Fig. 3), consistent with previous findings for L. albus (e.g. Keerthisinghe et al., 1998; Watt and Evans, 1999) and L. atlanticus (Pearse et al., 2006). Below, we provide critical insights into the differences in response among species.
Table 2.
Comparison of three Lupinus species based on growth rate, P uptake and P accumulation, and initiation or suppression of cluster-root formation
| L. micranthus | L. albus | L. atlanticus | |
|---|---|---|---|
| Growth rate | Low | Medium | High |
| Optimum phosphate concentration for the highest growth (μm) | 1 | 10 | 10–50 |
| Induction of cluster root at 1 µm P supply | High | High | High |
| Inhibition of cluster roots by higher phosphate supply | Stepwise | Stepwise | Sharply at 10 µmol |
| Foliar phosphate concentration below 10 µm P in solution (mg g−1 d. wt) | 20–22 | 4–7 | 7–10 |
| P uptake rate at 10 µm P in solution (mg P g−1 d. wt d−1) | 4·81 | 0·36 | 2·43 |
| P uptake rate at 1 µm P in solution (mg P g−1 d. wt d−1) | 0·09 | 0·06 | 0·09 |
| Reduction in cluster-root formation at 10 µm P compared with 1 µm P (%) | 68 | 70 | 98 |
Sensitivity to P supply differed among the present Lupinus species. At 1 µm P, on day 54 the concentration of P in leaves of L. albus and L. atlanticus was 1·68 and 0·93 mg g−1 d. wt, respectively which caused P-deficiency symptoms (Figs 1 and 4). In L. micranthus, on day 39 the concentration of P in leaves at 1 µm P was 3·62 mg g−1 d. wt with no symptoms of P deficiency; however, on day 54 the concentration of P in leaves was only 2·26 mg g−1 d. wt, above the critical leaf P concentration previously determined for L. albus (Bolland et al., 1999). The P concentration on day 54 increased up to 19·9 and 21·6 mg g−1 d. wt in leaves of L. albus and L. atlanticus, respectively, at 150 µm P without any visual signs of toxicity such as chlorosis of leaves; however, root and leaf area and total dry mass decreased markedly compared with that at 50 µm P. (Fig. 4). The P concentration of L. micranthus increased from 40·23 mg g−1 d. wt at 50 µm P to 43·7 mg g−1 d. wt at 150 µm P on day 39 with visible symptoms of P toxicity, leaf desiccation and drying of plants (Figs 1, 2 and 4). Development of foliar symptoms of phosphate toxicity, even at very low rhizosphere [P], has been reported in Proteaceae (e.g. in Banksia ericifolia, Ozanne and Specht, 1981; Parks et al., 2000; B. grandis, Lambers et al., 2002; Hakea prostrata, Shane et al., 2004).
The percentage cluster roots at the lowest P concentration (1 µm) decreased from L. micranthus to L. albus, followed by L. atlanticus (Fig. 3). In L. micranthus, greater cluster-root formation in this treatment from day 32 may have resulted in more efficient P uptake and accumulation, and consequently adequate P supply (Fig. 4, Tables 1 and 2). In L. albus and L. atlanticus, formation of cluster roots at 1 µm P during growth was not as high as in L. micranthus; P concentration was not adequate and deficiency symptoms were shown (Figs 1, 3 and 4).
In L. atlanticus, induction of cluster-root formation was high at 1 µm P, but decreased drastically at higher concentrations when cluster-root dry weight decreased by 98 % from 1 to 10 µm P (Fig. 3, Table 2). In spite of a high growth rate, the relatively high P-uptake rate resulted in intermediate accumulation of P in plants (Table 2, Fig. 4). As a result, cluster-root formation suppressed at 10 µm P (7–10 mg g−1 d. wt in leaves). Regression analysis also indicated that highest inhibition of cluster-root formation by P concentration was in L. atlanticus (Fig. 4). This species provides a good example for confirmation of the model proposed by Pearse et al. (2006), although the P treatments in the study by Pearse et al. (2006) were not low enough to see such a great change in proportion of cluster roots. Inhibition of cluster-root formation has been reported in Banksia ericifolia at >5 mg g−1 shoot d. wt P (Handreck, 1991) and Banksia grandis (Lambers et al., 2002), whereas cluster roots were abundant at <1 mg P g−1 d. wt. Cluster-root formation is drastically inhibited when P concentrations reach a threshold. However, different Lupinus species show a different threshold for inhibition of cluster-root formation.
In L. albus, with an intermediate growth rate and low P-uptake rate, cluster-root formation was extremely high at 1 µm P, and decreased stepwise at higher P concentrations (Table 1, Figs 3 and 5). Shane et al. (2003), Li and Liang (2005) and Shen et al. (2005) have reported that P concentrations in shoot and phloem sap of white lupin increased at higher P supply, associated with decreased cluster-root formation. Li et al. (2008) reported that the formation of cluster roots in L. albus is regulated by shoot P concentration, with a critical level of 2–3 mg g−1. Also, a stepwise reduction of cluster roots at increasing P concentration in the root environment and plant tissues in white lupin has been reported by Keerthisinghe et al. (1998). The result for this species, although not as strong as in L. atlanticus, confirmed the model by Pearse et al. (2006).
In L. micranthus, with the lowest relative growth rate, the highest P-uptake rate and, consequently, very high accumulation of P in plant tissues (about 20 mg g−1 d. wt at 10 µm P) was observed (Tables 1 and 2, Fig. 4). A concentration of 1 µm P in the nutrient solution did not induce P deficiency symptoms, suggesting this species is adapted to an environment where P supply is always limiting. However, the reduction in percentage of cluster-root dry mass at 1–10 µm P at the final harvest was 68 % (Table 2, Fig. 3). Also, at least part of this reduction in cluster roots at 10 µm P was related to P toxicity, due to greater sensitivity to P toxicity in this species, suggesting poorer regulation of P uptake. Sensitivity to shoot P status in L. micranthus is even less than that of L. albus; hence L. micranthus responded even less to increasing P supply with respect to reducing allocation to cluster roots. Slow growth and thus little dilution of the internal P concentration may be one explanation for this phenomenon (Lambers and Poorter, 1992). On the other hand, the formation of cluster roots suggests that L. micranthus is able to mobilize sparingly soluble P forms in soils. However, in a hydroponic culture system, where P supply is not limited by diffusion or solubility, the formation of cluster roots has limited advantage and P acquisition may rather depend on the properties of P-uptake systems than on root morphological characteristics. Consequently, a threshold P concentration in leaves for partial inactivation of cluster-root formation could not be identified.
A complex series of signalling cascades is emerging that control transcriptional cascades and initiate plant responses to P starvation. Several investigators have reported that P may act directly as a signal and regulate some aspects of plant P-starvation responses, such as root-meristem activity, root-hair development and cluster-root formation (López-Bucio et al., 2003; Zhang et al., 2003; Pearse et al., 2006). Also, it is well known that the balance of plant growth regulators plays a role in the final stage(s) in the signal-transduction pathway (Gilbert et al., 2000). The fine balance between auxin, ethylene and local cytokinin concentrations, their transport from the shoot to the root or changes in the sensitivity of target tissues to these plant regulators may be involved in the control of systemic responses to P starvation (Vance et al., 2003; Hammond and White, 2008). In addition, many recent studies have implicated the involvement of shoot-derived carbohydrate signals (sucrose, glucose and fructose) in control of plant P-starvation responses (Liu et al., 2005; Müller et al., 2007; Zhou et al., 2008). A role of the microRNA family as an intermediate component of this signalling cascade regulating P-starvation-induced genes has recently been identified. Members of this family are specifically and rapidly up-regulated by P starvation, but are not detectable under P-replete conditions (Fujii et al., 2005; Bari et al., 2006). Present knowledge suggests that achieving an internal threshold P concentration (Li et al., 2008) is a first step of signalling cascades necessary to stimulate other signals and trigger plant responses (Hammond and White, 2008). The absence of a P-induced suppression of cluster-root formation in L. micranthus may therefore also reflect a suppression of an existing control mechanism for cluster-root formation due to interference of P toxicity with the factors mentioned above, induced by an artificially high P uptake under the investigated culture conditions.
Taken together, it is the shoot P status that stimulates downstream signals and regulates cluster-root formation in L. albus and L. atlanticus, while inhibition of cluster-root production is less responsive to P status in L. micranthus. The exact mechanism accounting for this difference needs further elucidation. In addition, the presence of cluster roots does not necessarily reflect a high activity in terms of release of P-mobilizing root exudates; this also warrants further investigation.
ACKNOWLEDGMENTS
We are grateful to Michael Shane and Perry Swanborough for helpful advice with analyses and to Stuart Pearse for critical review of an earlier version of this paper.
LITERAURE CITED
- Bari R, Pant BD, Stitt M, Scheible WR. PHO2, micro-RNA399, and PHR1 define a phosphate-signaling pathway in plants. Plant Physiology. 2006;141:988–999. doi: 10.1104/pp.106.079707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolland MDA, Siddique KHM, Loss SP, Baker MJ. Comparing responses of grain legumes, wheat and canola to applications of superphosphate. Nutrient Cycling in Agroecosystems. 1999;53:157–175. [Google Scholar]
- Cordell D, Drangert J-O, White S. The story of phosphorus: global food security and food for thought. Global Environmental Change. 2009;19:292–305. [Google Scholar]
- Curtin D, Syers JK, Bolan NS. Phosphate sorption by soil in relation to exchangeable cation composition and pH. Australian Journal of Soil Research. 1993;31:137–149. [Google Scholar]
- Fujii H, Chiou T, Lin S, Aung K, Zhu J. A miRNA involved in phosphate-starvation response in Arabidopsis. Current Biology. 2005;15:2038–2043. doi: 10.1016/j.cub.2005.10.016. [DOI] [PubMed] [Google Scholar]
- Gahoonia TS, Nielsen NE. Variation in root hairs of barley cultivars doubled soil phosphorus uptake. Euphytica. 1997;98:177–182. [Google Scholar]
- Gilbert GA, Knight JD, Vance CP, Allan DL. Proteoid root development of phosphorus deficient white lupin is mimicked by auxin and phosphonate. Annals of Botany. 2000;85:921–928. [Google Scholar]
- Hammond JP, White PJ. Sucrose transport in the phloem: integrating root responses to phosphorus starvation. Journal of Experimental Botany. 2008;59:93–109. doi: 10.1093/jxb/erm221. [DOI] [PubMed] [Google Scholar]
- Handreck KA. Interactions between iron and phosphorus in the nutrition of Banksia ericifolia L. f. var. ericifolia (Proteaceae) in soil-less potting media. Australian Journal of Botany. 1991;39:373–384. [Google Scholar]
- Hinsinger P. Bioavailability of soil inorganic P in the rhizosphere as affected by root-induced chemical changes: a review. Plant and Soil. 2001;237:173–195. [Google Scholar]
- Holford ICR. Soil phosphorus: its measurement and its uptake by plants. Australian Journal of Soil Research. 1997;35:227–239. [Google Scholar]
- Irving GCJ, McLaughlin MJ. A rapid and simple test for phosphorus in Olsen and Bray No. 1 extracts of soil. Communications in Soil Science and Plant Analysis. 1990;21:2245–2255. [Google Scholar]
- Käss E, Wink M. Molecular phylogeny and phylogeography of Lupinus (Leguminosae) inferred from nucleotide sequences of the rbcL gene and ITS 1 + 2 regions of rDNA. Plant Systematics and Evolution. 1997;208:139–167. [Google Scholar]
- Keerthisinghe G, Hocking PJ, Ryan PR, Delhaize E. Effect of phosphorus supply on the formation and function of proteoid roots of white lupin (Lupinus albus L.) Plant Cell and Environment. 1998;21:467–478. [Google Scholar]
- Lambers H, Poorter H. Inherent variation in growth rate between higher plants: a search for physiological causes and ecological consequences. Advances in Ecological Research. 1992;23:187–261. [Google Scholar]
- Lambers H, Juniper D, Cawthray GR, Veneklaas EJ, Martinez E. The pattern of carboxylate exudation in Banksia grandis (Proteaceae) is affected by the form of phosphate added to the soil. Plant and Soil. 2002;238:111–122. [Google Scholar]
- Lambers H, Cramer MD, Shane MW, Wouterlood M, Poot P, Veneklaas EJ. Structure and functioning of cluster roots and plant responses to phosphate deficiency. Plant and Soil. 2003;248:ix–xix. [Google Scholar]
- Li C, Liang R. Root cluster formation and citrate exudation of white lupin (Lupinus albus L.) as related to phosphorus availability. 2005;47:172–177. Journal of Integrative Plant Biology. [Google Scholar]
- Li H, Shen J, Zhang F, Tang C, Lambers H. Is there a critical level of shoot phosphorus concentration for cluster-root formation in Lupinus albus? Functional Plant Biology. 2008;35:328–336. doi: 10.1071/FP07222. [DOI] [PubMed] [Google Scholar]
- Li YF, Luo AC, Wei XH, Yao XG. Genotypic variation of rice in phosphorus acquisition from iron phosphate: contributions of root morphology and phosphorus uptake kinetics. 2007;54:230–236. Russian Journal of Plant Physiology. [Google Scholar]
- Liu J, Samac DA, Bucciarelli B, Allan DL, Vance CP. Signaling of phosphorus deficiency-induced gene expression in white lupin requires sugar and phloem transport. Plant Journal. 2005;41:257–268. doi: 10.1111/j.1365-313X.2004.02289.x. [DOI] [PubMed] [Google Scholar]
- Lynch J. Root architecture and plant productivity. Plant Physiology. 1995;109:7–13. doi: 10.1104/pp.109.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Bucio J, Cruz-Ramírez A, Herrera-Estrella L. The role of nutrient availability in regulating root architecture. Current Opinion in Plant Biology. 2003;6:280–287. doi: 10.1016/s1369-5266(03)00035-9. [DOI] [PubMed] [Google Scholar]
- Müller R, Morant M, Jarmer H, Nielsen TH. Genome-wide analysis of the Arabidopsis leaf transcriptome reveals interaction of phosphate and sugar metabolism. Plant Physiology. 2007;143:156–171. doi: 10.1104/pp.106.090167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozanne PG, Specht RL. Mineral nutrition of heathlands: phosphorus toxicity. In: Specht RL, editor. Ecosystems of the world, Vol 9A, heathlands and related shrublands. Descriptive studies. Amsterdam: Elsevier Scientific; 1981. pp. 277–289. [Google Scholar]
- Parks SE, Haigh AM, Creswell GC. Stem tissue phosphorus as an index of the phosphorus status of Banksia ericifolia L. Plant and Soil. 2000;227:59–65. [Google Scholar]
- Pearse SJ, Veneklaas EJ, Cawthray GR, Bolland MDA, Lambers H. Carboxylate release of wheat, canola and 11 grain legume species as affected by phosphorus status. Plant and Soil. 2006;288:127–139. [Google Scholar]
- Ricker WE. Growth rates and models. In: Hoar WS, Randall DJ, Brett JR, editors. Fish physiology. New York: Academic Press; 1979. pp. 678–742. [Google Scholar]
- Ryan PR, Delhaize E, Jones DL. Function and mechanism of organic anion exudation from plant roots. Annual Review of Plant Physiology and Plant Molecular Biology. 2001;52:527–560. doi: 10.1146/annurev.arplant.52.1.527. [DOI] [PubMed] [Google Scholar]
- Shane MW, Lambers H. Cluster roots: a curiosity in context. Plant and Soil. 2005;274:101–125. [Google Scholar]
- Shane MW, De Vos M, De Roock S, Lambers H. Shoot P status regulates cluster-root growth and citrate exudation in Lupinus albus grown with a divided root system. Plant, Cell and Environment. 2003;26:265–273. [Google Scholar]
- Shane MW, McCully ME, Lambers H. Tissue and cellular phosphorus storage during development of phosphorus toxicity in Hakea prostrata (Proteaceae) Journal of Experimental Botany. 2004;55:1033–1044. doi: 10.1093/jxb/erh111. [DOI] [PubMed] [Google Scholar]
- Shen J, Li H, Neumann G, Zhang F. Nutrient uptake, cluster root formation and exudation of protons and citrate in Lupinus albus as affected by localized supply of phosphorus in a split-root system. Plant Science. 2005;168:837–845. [Google Scholar]
- Smith FA, Jakobsen I, Smith SE. Spatial differences in acquisition of soil phosphate between two arbuscular mycorrhizal fungi in symbiosis with Medicago truncatula. New Phytologist. 2000;147:357–366. [Google Scholar]
- Soon YK. Nutrient uptake by barley roots under field conditions. Plant and Soil. 1988;109:171–179. [Google Scholar]
- Steen I. Phosphorus management in the 21st century. Management of a non-renewable resource. Phosphorus & Potassium. 1998;217:25–31. [Google Scholar]
- Vance CP, Uhde-Stone C, Allan DL. Phosphorus acquisition and use: critical adaptations by plants for securing a nonrenewable resource. New Phytologist. 2003;157:423–447. doi: 10.1046/j.1469-8137.2003.00695.x. [DOI] [PubMed] [Google Scholar]
- Watt M, Evans JR. Linking development and determinacy with organic acid efflux from proteoid roots of white lupin grown with low phosphorus and ambient or elevated atmospheric CO2 concentration. Plant Physiology. 1999;120:705–716. doi: 10.1104/pp.120.3.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson LC, Ribrioux S, Fitter AH, Leyser O. Phosphate availability regulates root system architecture in Arabidopsis. Plant Physiology. 2001;126:875–882. doi: 10.1104/pp.126.2.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YJ, Lynch JP, Brown KM. Ethylene and phosphorus availability have interacting yet distinct effects on root hair development. Journal of Experimental Botany. 2003;54:2351–2361. doi: 10.1093/jxb/erg250. [DOI] [PubMed] [Google Scholar]
- Zhou K, Yamagishi M, Osaki M, Masuda K. Sugar signalling mediates cluster root formation and phosphorus starvation-induced gene expression in white lupin. Journal of Experimental Botany. 2008;59:2749–2756. doi: 10.1093/jxb/ern130. [DOI] [PMC free article] [PubMed] [Google Scholar]