Abstract
Background and Aims
In spite of the abundance of archaeological, bio-archaeological, historical and genetic data, the origins, historical biogeography, identity of ancient grapevine cultivars and mechanisms of domestication are still largely unknown. Here, analysis of variation in seed morphology aims to provide accurate criteria for the discrimination between wild grapes and modern cultivars and to understand changes in functional traits in relation to the domestication process. This approach is also used to quantify the phenotypic diversity in the wild and cultivated compartments and to provide a starting point for comparing well-preserved archaeological material, in order to elucidate the history of grapevine varieties.
Methods
Geometrical analysis (elliptic Fourier transform method) was applied to grapevine seed outlines from modern wild individuals, cultivars and well-preserved archaeological material from southern France, dating back to the first to second centuries.
Key Results and Conclusions
Significant relationships between seed shape and taxonomic status, geographical origin (country or region) of accessions and parentage of varieties are highlighted, as previously noted based on genetic approaches. The combination of the analysis of modern reference material and well-preserved archaeological seeds provides original data about the history of ancient cultivated forms, some of them morphologically close to the current ‘Clairette’ and ‘Mondeuse blanche’ cultivars. Archaeobiological records seem to confirm the complexity of human contact, exchanges and migrations which spread grapevine cultivation in Europe and in Mediterranean areas, and argue in favour of the existence of local domestication in the Languedoc (southern France) region during Antiquity.
Keywords: Domestication syndrome, origin of cultivars, Vitis vinifera, seed, elliptic Fourier transforms
INTRODUCTION
The grapevine (Vitis vinifera) belongs to the family Vitaceae, which comprises about 60 inter-fertile wild Vitis species distributed in Asia, North America and Europe under subtropical, Mediterranean and continental–temperate climatic conditions. It is the single Vitis species that acquired significant economic interest over time; some other species, for example the North American V. rupestris, V. riparia or V. berlandieri, are used as breeding rootstock due to their resistance against grapevine pathogens, such as Phylloxera, Oidium and mildews. Indeed, a great majority of cultivars widely cultivated for fruit, juice and mainly for wine, classified as Vitis vinifera L. subsp. vinifera (or sativa), derive from wild forms [Vitis vinifera L. subsp. sylvestris (Gmelin) Hegi] (Rossetto et al., 2002; Sefc et al., 2003; Crespan, 2004; This et al., 2004).
The wild grapevine is a heliophilous liana growing generally along river banks, and in alluvial and colluvial deciduous and semi-deciduous forest (Levadoux, 1956; Arnold et al., 1998). It is distributed in a wide area from western Europe to the Trans-Caucasian zone and around the Mediterranean Basin, except the most southern infra-Mediterranean and non-Mediterranean zones (Arnold et al., 1998). The present distribution of the wild grapevine is highly fragmented, in disjoint micro-populations or metapopulations, with few individuals, at least in the western part of the Mediterranean Basin. Anthropogenic pressure on their natural habitats and pathogens introduced from North America during the second part of the 19th century, may explain the progressive decline of wild grape populations (Arnold et al., 1998). The ‘Phylloxera crisis’ that affected European vineyards had a considerable impact on both cultivated varieties and wild grapes. As a result, modern wild grapevines are endangered and threatened with extinction (Arnold et al., 2005). The future of Vitis vinifera subsp. sylvestris represents a major stake in biodiversity conservation.
The cultivation and domestication of the grapevine appears to have occurred between the seventh and the fourth millennia BC, in a geographical area between the Black Sea and Iran (Châtaignier, 1995; McGovern et al., 1996; McGovern and Rudolph, 1996; Zohary, 1996; Zohary and Hopf, 2000). From this area, cultivated forms would have been spread by humans in the Near East, Middle East and Central Europe. As a result, these areas may have constituted secondary domestication centres (Grassi et al., 2003; Arroyo-Garcia et al., 2006).
Indirect evidence of ancient winemaking (McGovern et al., 1996) is provided by the discovery of significant quantities of vinification residues (tartaric acid) with terebinth resin in clay jars, dating back to the end of the seventh millennium BC. In the Near East, numerous archaeological grape seeds attributed to the cultivated grapevine were found in Chalcolithic and mid Bronze Age archaeological levels (Hopf, 1983; Zohary, 1995). From the eastern Mediterranean areas, grape cultivation seems to have spread gradually westwards. In Greece and Crete, the beginnings of viticulture would have started during the fifth millennium BC (Valamoti et al., 2007). In Italy, the most ancient testimonies of grapevine cultivation date back to the ninth century BC (Di Vora and Castelletti, 1995). In Spain and in the Maghreb, the Phoenician influence during the first part of the last millennium BC appears to have played a significant part in the establishment and development of viticulture and viniculture (Rivera Nuñez and Walker, 1989; Buxó, 2008).
Finally, it is currently thought that the emergence of viticulture in France was concomitant with the foundation of Marseille (600 BC) by the Greek Phocaeans (Brun and Laubenheimer, 2001). Viticulture seems to have extended rapidly in southern France from the fifth century BC. Important coastal exchanges and trading centres such as Lattes (Hérault) would have also played an important role in this process. After the Roman conquest, viticulture was well established in the Languedoc region, reaching its height between the end of the first century and the second century AD. By way of the ‘Narbonnaise’ route, viticulture extended into Aquitaine (western France) during the first century AD. During this same period it also spread northwards, in the Rhône, Loire and Seine valleys (Bouby and Marinval, 2001). When the wine production of the Narbonnaise area started threatening the hegemony of Italian wines, the Emperor Domitian ordered the destruction of half of the region's vineyards. However, it appears that the Emperor's edict (92 AD) was not applied (Laubenheimer and Brun, 2001). Archaeological investigations have revealed a considerable development of viticulture and viniculture between the first and the end of the second century AD. It is only approx. 300 AD that Emperor Probus withdraws Domitien's prescriptions. From the fourth century onwards, while the Christian faith spread its influence throughout Europe, viticulture and viniculture again experienced a geographical expansion.
Although this model of development, expansion and diffusion of viniculture and viticulture in France (and more generally for the north-western Mediterranean areas) is well documented from archaeological and historical points of view, the ancestral cultivars and the varietal diversification process through time and space are not well known. In spite of the important corpus of bio-archaeological, morphological, historical and genetic data available, the identity of former cultivars, history, biogeography and mechanisms of grapevine domestication remain obscure.
Morphological criteria for the identification of archaeological remains (seeds and wood) attributed to Vitis vinifera are highly incomplete, and those ancient texts mentioning various cultivated grapevines are not exploitable to characterize ancient varieties (André, 1952). In the present state of research, in France and elsewhere, studies on seeds are still too limited to allow a precise discrimination between wild and cultivated grapes (Stummer, 1911; Kislev, 1988; Di Vora and Castelletti, 1995; Mangafa and Kotsakis, 1996; Marinval, 1997; Jacquat and Martinoli, 1999; Bouby and Marinval, 2001; Terral, 2002). Although informative, the main limits of these former studies concern the absence or the inadequacy of modern reference collections on which they are based. In every case, they represent local or regional studies and concern a reduced number of cultivars and wild specimens.
The present study is based on the geometrical analysis of grape seed structure. It aims to (1) test shape criteria in order to discriminate between wild forms and modern cultivars, (2) interpret changes that have occurred during domestication, (3) quantify the phenotypic diversity in the wild and the cultivated compartments and (4) interpret shape diversity in relation to the supposed geographical origin (country or region) and parentage evidenced by genetic approaches.
Although the analysis of genetic diversity based on living material is very successful (for a review see This et al., 2006), the first analyses based on ancient DNA, using a few microsatellite markers, though encouraging, have not yet provided significant results for the identification of ancient grapes (Manen et al., 2003).
For the modern grapevine, analysis of genetic diversity in the wild (This et al., 2000; Aradhya et al., 2003; Grassi et al., 2003; Snoussi et al., 2004) and in the cultivated compartment (Bowers et al., 1999; Sefc et al., 2000; Aradhya et al., 2003; This et al., 2004) allows us to propose hypotheses based on historical biogeography of domestication and dispersal routes, in relation to human migrations and exchanges (This et al., 2006; Vouillamoz and Grando, 2006) although we cannot date these events.
Shape characterization of cultivated varieties combined with genetic data should allow a better understanding of the changes that have occurred during domestication and finally identification of ancestral forms of current cultivars, based on the analysis of archaeological grape seeds.
MATERIALS AND METHODS
Modern reference material
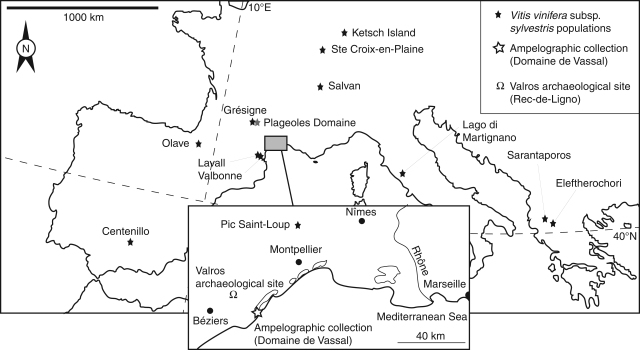
Wild Vitis vinifera L. grapevines, mainly distinguishable from cultivated varieties and feral forms because they are dioecious whereas cultivars are mainly hermaphroditic, were collected in 12 locations (Fig. 1, Table 1), some of them mentioned and described previously [Spain (Ocete et al., 1997, 2008), France (Arnold et al., 1998; This et al., 2001; Lacombe et al., 2004), Switzerland (Perret, 1997), Germany (Perret, 1997), Italy (Anzani et al., 1990)]. However, despite the precautions taken during sampling, it is impossible to guarantee that all the specimens are wild. Introgressed individuals from cultivated grapes growing in the area could have been accidentally included in our reference collection, as was demonstrated by Di Vecchi et al. (2008).
Fig. 1.
Geographical location of wild, cultivated and archaeological material collected for this study.
Table 1.
Vitis vinifera subsp. sylvestris material included in the present study
| Population | Origin | No. of individuals; no. of pips | Accession number | Ecological context |
|---|---|---|---|---|
| El Centenillo | Spain | 3; 30 | Cent-2, Cent-5, Cent-9 | Riparian Mediterranean forest |
| Olave | Spain | 3; 30 | Olav-1, Olav-2, Olav-3 | Riparian, Atlantic – collinean vegetation |
| Lavall | France | 3; 30 | Lava-1, Lava-3, Lava-4 | Riparian Mediterranean forest – Mesomediterranean bioclimatic conditions |
| Valbonne | France | 3; 30 | Valb-1, Valb-3, Valb-7 | Riparian Mediterranean forest –Mesomediterranean bioclimatic conditions |
| Grésigne | France | 3; 30 | Gres-1, Gres-2, Gres-B | Deciduous oak forest border under Atlantic bioclimatic conditions |
| 2; 30 | GreV-1, GreV-9 | Originated from the Vassal grape collection | ||
| Pic Saint-Loup | France | 2; 40 | Psl-12, Psl-13 | Rocky slope – Mediterranean evergreen oak vegetation (Quercus ilex) under Mesomediterranean bioclimatic conditions |
| Sainte-Croix-en-Plaine | France | 1; 10 | Stcr-1 | Deciduous oak forest border in alluvial context and under continental conditions |
| Salvan | Switzerland | 3; 30 | Salv-1, Salv-2, Salv-4 | Rocky slope – continental bioclimatic conditions |
| Ketsch Island | Germany | 3; 30 | Kets-3, Kets-8, Kets-11 | Alluvial context under continental climatic conditions |
| Lago di Martignano | Italy | 2; 40 | Lgma-1, Lgma-2 | Alluvial context under Mesomediterranean bioclimatic conditions |
| Sarantaporos | Greece | 2; 40 | Sara-1, Sara-2 | Riparian Mesomediterranean vegetation |
| Eleftherochori | Greece | 3; 30 | Elef-1, Elef-2, Elef-11* | Riparian Mesomediterranean vegetation |
* Produces white berries.
A total of 370 seeds from 13 female individuals were sampled (Table 1). With regard to cultivated accessions, sampled material comprised 1350 seeds (30 seeds per cultivar) from 45 cultivars (including different mutants based on berry skin colour), preserved in the INRA Domaine de Vassal Grape Germplasm Repository (Marseillan, France, www.montpellier.inra.fr/vassal), mainly of French origin (Table 2, Fig. 1). Thirty seeds from wild grape individuals collected in natural conditions and introduced some years ago in a vineyard of the Vassal collection were also incorporated in the study (Table 1), in order to test the influence of cultivation on seed shape. Moreover, the influence of environmental conditions on seed shape was evaluated through comparison of material from two different origins. For the ‘Syrah’ and the ‘Len del Lel’ cultivars, specimens from the Plageoles Domaine vineyard (Cahuzac-sur-Vere, Tarn, France) situated a few kilometres from the Grésigne population (Table 2, Fig. 1) were compared with specimens from the Vassal collection.
Table 2.
Vitis vinifera subsp. vinifera cultivars analysed
| Cultivar name | Supposed origin (country, region) | Berry skin colour* | Use |
|---|---|---|---|
| ‘Arvine’ | Switzerland, Valais | B | Wine |
| ‘Aspiran noir’ | France, Languedoc | N | Wine |
| ‘Aspiran blanc’ | France, Languedoc | B | Wine |
| ‘Colombaud’ | France, Provence | B | Wine |
| ‘Cabernet franc’ | France, Aquitaine | N | Wine |
| ‘Cabernet-Sauvignon’ | France, Aquitaine | N | Wine |
| ‘Chardonnay’† | France, Burgundy | B | Wine |
| ‘Chasselas’ | Burgundy or Switzerland | B | Wine/table |
| ‘Chenin’ | France, Pays-de-Loire | B | Wine |
| ‘Cinsaut’ | France, Provence | N | Wine |
| ‘Clairette’ | France, Languedoc | B | Wine/table |
| ‘Cot’ | France, south-west | N | Wine |
| ‘Duras’ | France, south-west | N | Wine |
| ‘Dureza’ | France, Rhône-Alpes | N | Wine |
| ‘Gamay’‡ | France, Burgundy | N | Wine |
| ‘Gouais’ | unknown | B | Wine |
| ‘Henab’ | Turkey | B | Table |
| ‘Humagne’ | Switzerland, Valais | B | Wine |
| ‘Len de L'el’§ | France, Midi-Pyrénées | B | Wine |
| ‘Mauzac’ | France, Midi-Pyrénées | B | Wine |
| ‘Melon’ | France, Burgundy | B | Wine |
| ‘Merlot’ | France, Aquitaine | N | Wine |
| ‘Mondeuse blanche’ | France, Rhône-Alpes | B | Wine |
| ‘Mourvèdre’ | Spain | N | Wine |
| ‘Muscat à petits grains’ | Greece | B | Wine |
| ‘Negrette’ | France, south-west | N | Wine |
| ‘Petit Verdot’ | France, Aquitaine | N | Wine |
| ‘Pinot noir’† | France, Burgundy | N | Wine |
| ‘Pinot blanc’ | France, Burgundy | B | Wine |
| ‘Pinot gris’ | France, Burgundy | G | Wine |
| ‘Piquepoul noir’ | France, Languedoc | N | Wine |
| ‘Piquepoul blanc’ | France, Languedoc | B | Wine |
| ‘Riesling’ | France or Germany, Rhine Valley | B | Wine |
| ‘Roussanne’ | France, Rhône-Alpes | B | Wine |
| ‘Sauvignon’ | France, centre or south-west | B | Wine |
| ‘Savagnin blanc’ (syn. ‘Traminer weiss’) | France, Franche-Comté or Germany, Palatinat | B | Wine |
| ‘Savagnin rose’ | France, Franche-Comté or Germany, Palatinat | Rs | Wine |
| ‘Syrah’†§ | France, Rhône-Alpes | N | Wine |
| ‘Tempranillo’† | Spain, Rioja | N | Wine |
| ‘Terret’ | France, Languedoc | N | Wine |
| ‘Tibouren’ | France, Provence | N | Wine |
| ‘Ugni blanc’ (syn. ‘Trebbiano Toscano’) | Italy, Toscana | B | Wine |
| ‘Vermentino’ | Italy | B | Wine |
| ‘Viognier’ | France, Rhône-Alpes | B | Wine |
* Berry skin colour: B, white; N, black; Rs, rose; G, grey.
† Used for testing intra-cultivar variations. For these three varieties: 80 seeds per cultivar, 40 seeds per clone, 20 seeds per individual and 10 seeds per bunch.
‡ Used for measurement errors and reproducibility.
§ Used to test for the effect of environmental conditions on seed shape.
Finally, ten seeds from three distinct bunches were sampled for three cultivars (Table 2) in order to test intra-cultivar variation in shape, which is thought to increase from bunch to the individual level.
Archaeological material
Rescue archaeological excavations carried out prior to the construction of the A 75 motorway, between Béziers and Pézenas (Languedoc, France) allowed the identification of the remains of former vineyards and orchards, delimited by pits, hedges and roads. Ancient Vitis plantation pits are easily recognizable by their shape and distribution (rows of narrow rectangular pits) (Boissinot, 2001). At Rec-de-Ligno, near Valros (Fig. 1), grape plantations surrounding an agricultural building containing dolia (earthenware vases) and connected with a funerary edifice were planted approx. 75 AD. They seem to have been cultivated until the second half of second century AD (C. Jung and V. Bel, unpubl. res.). Also, the identification of orchard areas with rectangular and equidistant plantation pits is particularly interesting but their establishment is most probably later (end of the second to beginning of the third century AD). It is most probable that these pits were dug to plant fruit trees. A stone-lined well was discovered in the agricultural building situated in the close vicinity of these plantation pits and contained, in its fill, well-preserved waterlogged grape seeds dated to approx. 125–150 AD (C. Jung and Bel, unpubl. res.). Fifty undistorted seeds were analysed and compared with the modern reference material.
Quantification of seed shape
Seed shape was studied using outline analysis based on the elliptic Fourier transform (EFT) method. Each seed is photographed in dorsal and lateral view. Using an image analysis system, 64 points equally spaced are semi-automatically positioned along the outline of each seed. From the seed apex, defined as the starting point of the outline, the x and y coordinates of points are extracted and then analysed by the EFT method. This method is based on the separate Fourier decompositions of the incremental changes of the x and y coordinates as a function of the cumulative length along the outline (Kuhl and Giardina, 1982; Renaud et al., 1996, 1999a, b; Renaud and Michaud, 2003). Each harmonic corresponds to four coefficients (An, Bn for x and Cn, Dn for y) defining the ellipse in the xy-plane. The coefficients of the first harmonic (H1) describing the best fitting ellipse of outlines are used to standardize size (surface area) and to orientate seeds.
Number of harmonics for an optimal description of seed outlines
In order to determine the number of harmonics to be retained for a suitable and rigorous description of outlines, measurement errors and information content provided by harmonics are estimated. Measurement errors are evaluated based on ten repeated measurements of three seeds of the ‘Gamay’ cultivar and expressed as the coefficient of variation of the harmonic amplitude (Renaud and Millien, 2001). The information content added by each harmonic is evaluated using the cumulative power corresponding to the deviation of reconstructed outlines for an increasing number of harmonics from the reconstruction based on the maximum number of harmonics (optimal outline) (H32 in a case of an outline defined by 64 points) (Crampton, 1995).
Furthermore, with the aim of testing the reproducibility of measurements taken by various observers, a set of 30 seeds of the ‘Gamay’ cultivar was measured by three different operators. The possible influence of calibration on shape quantification and of environmental conditions on seed development were considered.
For ‘Chardonnay’, ‘Pinot noir’ and ‘Syrah’ (Table 2), a study of shape variation at different levels (from the variety to the bunch) was also performed. This aimed to determine how morphological diversity may be accurately analysed, with particular attention given to inter-population and inter-cultivar differences.
Statistical analyses
Measurement errors and measurement reproducibility were tested using different MANOVAs. Shape variation at different levels (from the variety to the bunch) was tested using a principal components analysis (PCA) carried out on 240 seeds (80 per cultivar) according to 40 quantitative parameters. For each cultivar, coordinates of seed point cloud centroid in the multivariate space of the PCA were determined. Shape variation was also evaluated, at each level of variation, via computation of the dispersion of points around the cultivar centroid, calculated as the mean distance separating each point and its own centroid.
After these analyses, data relating to shape were treated by canonical variate analyses (CVAs) in order to provide evidence concerning the discrimination between wild grape populations and cultivars. Moreover, the Mahalanobis distance matrix between each wild population and cultivar centroids (also called consensus population individual) was used to expresses dissimilarities in shape.
In order to test the influence of environment on seed development, results of morphometric analyses carried out on the ‘Syrah’ and ‘Len de Lel’ cultivars from the Plageoles Domaine (Fig. 1, Table 2) were compared with the CVA previously performed. Dissimilarity in shape between seeds from the Vassal collection and seeds from the Plageoles Domaine was evaluated in terms of morphological deviation (Mahalanobis distance) compared with other cultivars in the multivariate space of the CVA.
The Mahalanobis distance matrix between wild populations and cultivar centroids was used as the basis for an UPGMA (unweighted pair group method with arithmetic mean) clustering analysis performed to interpret interrelationships between populations and cultivars based on morphological relationships among samples and distinct morphological groups.
Archaeological seed specimens were compared with reference samples as additional individuals. Shape descriptors were brought into the CVA to be assigned to a precise group. Assignment of archaeological seeds may help to identify ancient cultivated forms and this information is included in an historical context.
RESULTS
Number of harmonics for an optimal description of seed outlines
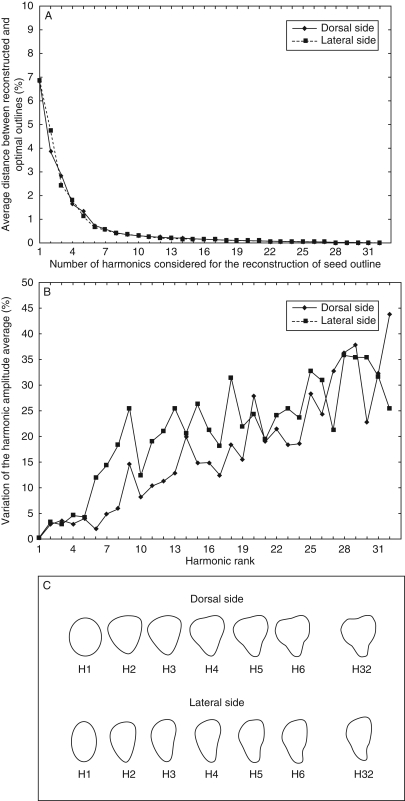
Distance or deviation between outlines reconstituted from a sum of harmonics and the optimal outline (H32) evolves similarly for dorsal and lateral sides of the three ‘Gamay’ seeds analysed (Fig. 2). Taking into account the first harmonic, the distance is less than 7 % and decreases sharply with addition of harmonics of superior rank. After H6, this distance is less than 1 % (Fig. 2A).
Fig. 2.
Assessment of the number of harmonics needed for an optimal description of seed outlines based on the analysis of three seeds of the ‘Gamay’ cultivar. (A) Average distance (deviation) between reconstructed and optimal outlines in relation to the number of harmonics considered. (B) Measurement errors on harmonics, from H1 to H32, evaluated based on the coefficient of variation of the same seed photographed ten times. (C) Seed outline reconstructed by addition of successive harmonics (optimal description of seed outline is reached by H32).
As observed from repeat measurements, the increase in variation of harmonic amplitude is very low (<5 %) for the first harmonics (Fig. 2B). This variation increases significantly from harmonics H5 and H7 for the lateral and the dorsal sides, respectively. Thus, consideration of harmonics up to H6 for both the lateral and the dorsal sides allows the minimization of measurement errors and optimizes the efficiency of shape reconstruction (Fig. 2C). Finally, after the exclusion of H1, used for size standardization, five harmonics (H2, H3, H4, H5 and H6) are considered.
Errors and reproducibility of measurements tested for the ‘Gamay’ cultivar
MANOVA carried out on the amplitudes of H2–H6 revealed no shape differences between distinct measurement sessions (Wilks' Lambda = 0·99, F = 0·008, P = 0·99). Measurement errors were not responsible for the shape differences. Similarly, the non-significant influence of the operator on shape results indicated that the measurements were reproducible (Wilks' Lambda = 0·99, F = 0·001, P = 1·00).
Shape variation at different levels of organization (from the grape to the variety)
Shape variation (evaluated by calculating a dispersion index) appeared to increase from the bunch to the clone and cultivar levels, except in some cases such as the ‘Chardonnay’ cultivar (clone 1, individual 1, bunch 2); these unexpected results may be related to the atypical morphology of a single seed (Table 3). In consequence, the morphological diversity in Vitis vinifera seeds may be accurately analysed, at the cultivar level, for the cultivated compartment. For the wild compartment, we favour investigation at the inter-individual level because, unlike cultivated individuals, wild individuals are essentially sexually propagated at more or less long distance, thus increasing the potential for morphological variation.
Table 3.
Shape variation at different levels (from the cultivar to the bunch) evaluated for ‘Chardonnay’, ‘Pinot noir’ and ‘Syrah’ cultivars
| Cultivar | Clone (C) | Individual (I) | Bunch (B) | Index of shape variation |
|---|---|---|---|---|
| ‘Chardonnay’ | 1·14 | |||
| C1 | 1·02 | |||
| I1 | 0·73 | |||
| B1 | 0·65 | |||
| B2 | 0·77 | |||
| I2 | 0·94 | |||
| B1 | 0·82 | |||
| B2 | 0·85 | |||
| C2 | 1·09 | |||
| I1 | 0·95 | |||
| B1 | 0·94 | |||
| B2 | 0·66 | |||
| I2 | 0·69 | |||
| B1 | 0·51 | |||
| B2 | 0·77 | |||
| ‘Pinot noir’ | 1·10 | |||
| C1 | 1·08 | |||
| I1 | 0·91 | |||
| B1 | 0·66 | |||
| B2 | 0·99 | |||
| I2 | 1·07 | |||
| B1 | 0·85 | |||
| B2 | 0·98 | |||
| C2 | 1·04 | |||
| I1 | 0·96 | |||
| B1 | 1·04 | |||
| B2 | 0·81 | |||
| I2 | 0·93 | |||
| B1 | 0·97 | |||
| B2 | 0·79 | |||
| ‘Syrah’ | 0·78 | |||
| C1 | 0·77 | |||
| I1 | 0·71 | |||
| B1 | 0·51 | |||
| B2 | 0·56 | |||
| I2 | 0·54 | |||
| B1 | 0·56 | |||
| B2 | 0·43 | |||
| C2 | 0·51 | |||
| I1 | 0·52 | |||
| B1 | 0·47 | |||
| B2 | 0·45 | |||
| I2 | 0·69 | |||
| B1 | 0·42 | |||
| B2 | 0·34 |
Influence of environmental parameters on shape
For the two varieties considered, Mahalanobis distances between centroids of the two sets of seeds (Table 2) showed that the ‘Syrah’ and ‘Len de Lel’ varieties from the Plageoles Domaine were significantly closer morphologically to their corresponding Vassal variety than to other studied cultivars. These results indicate that environmental conditions (soil, climate and ecological parameters) are not confounding factors.
Seed shape diversity in Vitis vinifera
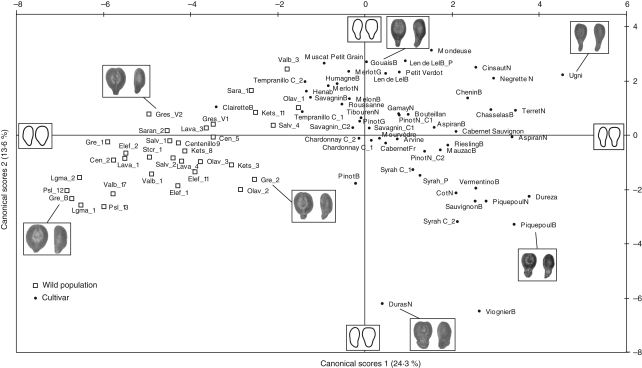
The CVA carried out on 1750 seeds, for 41 variables [40 quantitative, corresponding to the harmonic parameters (20 for both the lateral and the dorsal outlines) and one qualitative, expressing 78 classes (33 wild individuals and 45 cultivated varieties)], showed in the projection of the samples on the first two axes of the CVA (explaining 37·9 % of the total variance) an obvious discrimination between wild individuals and varieties (Fig. 3). The discriminant power (wild vs. cultivars) computed by the CVA, in which wild individuals growing in the Vassal collection are affiliated to the wild compartment, was 87·6 %. However, the a posteriori probabilities of affiliations calculated using Mahalanobis distance between seeds and each centroid group in the CVA space showed that wild individuals such as Ketsch11, Olave1, Sarantaporos1, Salvan4 and Valbonne3 possess a real morphological affinity with the cultivated compartment.
Fig. 3.
Canonical variate analysis biplot 1–2 (37·9 % of the total variance) showing that wild grape accessions and cultivated varieties are discriminated according to the EFT method. For additional clarity, photographs of distinctive seeds and centroid of groups are represented.
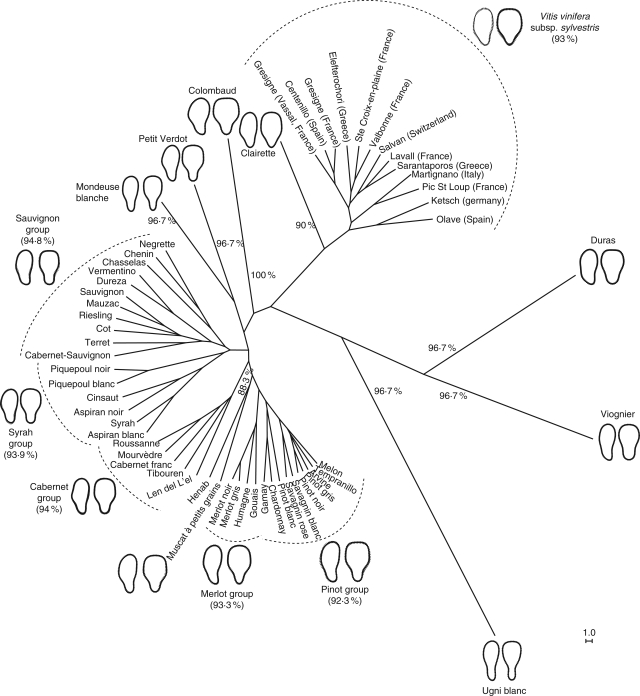
These individuals were removed before proceeding to a UPGMA clustering analysis, as their status (feral forms or introgressed from local cultivars) could be questioned. This analysis was based on the Mahalanobis distance dissimilarity matrix from a second CVA with 13 wild populations and 45 cultivars (Fig. 4). Furthermore, to take into account shape variability at the varietal level, random drawing of groups of 30 seeds per cultivar among the different clones analysed was carried out.
Fig. 4.
UPGMA dendrogram based on the minimum Mahalanobis distance among wild grape populations and cultivars. Discriminant rate (%) and reconstructed seed outlines in dorsal and lateral view of morphoclades identified are also presented.
Cluster analysis distinguished several groups or morphoclades (Fig. 4). Compared with the grape groups defined a priori for the second CVA, the overall posterior discriminant ratio corresponding to the global percentage of well-classification seeds was 85·8 %. At the level of aggregation highlighted in Fig. 5, the morphoclades identified had a reliable differentiation rate (DR ≥ 90 %).
Fig. 5.
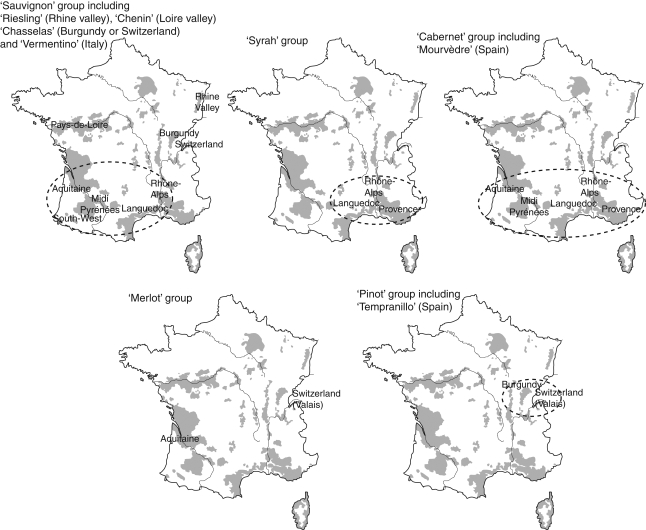
Geographical structure of morphological diversity related to the distribution of French vineyards (shaded).
Morphoclades consist of the group of wild grape populations (DR = 93 %), groups formed by a single variety (for example ‘Clairette’) or morphologically related varieties such as the Sauvignon group (Fig. 4).
Allocation of archaeological seeds
Table 4 shows the allocation of archaeological seeds to morphoclades defined on the basis of CVA and UPGMA. Most of the samples are allocated to cultivated forms even if the morphoclade ‘wild grapes’ is also well represented (Fig. 4). Unfortunately, 16 seeds could not be classified, possibly due to morphological damage that was undetected during analysis or because no analogues existed in the 45 cultivars studied.
Table 4.
Allocation of archaeological grape seeds to modern morphoclades (or group of cultivars) defined by UPGMA
| Morphoclade | N* | Probability of allocation |
|---|---|---|
| Wild grapes | 10 (8) | 0·76 ≤ P ≤ 1 (P ≥ 0·97) |
| Merlot group | 8 (7) | 0·88 ≤ P ≤ 1 (P ≥ 0·98) |
| Clairette | 6 (5) | 0·87 ≤ P ≤ 1 (P ≥ 0·97) |
| Mondeuse blanche | 6 (4) | 0·86 ≤ P ≤ 1 (P ≥ 0·97) |
| Cabernet Franc group | 2 | P = 0·91; P = 0·94 |
| Henab – Muscat à petits grains | 2 | P = 0·98; P = 0·99 |
| Unclassified | 16 |
* Number of seeds allocated to a morphoclade at high probability in parentheses.
DISCUSSION
Results have shown that shape variability increases from bunch at the intra-individual level, distinct bunches to different individuals to clones and grape varieties (Table 3). These variations, explained by either environmental, developmental, genetic or epigenetic parameters (or a combination of these factors), may cause physiological, phenological and morphological variations (Franks et al., 2002) which could also affect the shape of seeds.
In spite of this pattern of shape variability, distinction between wild grapes and varieties, as well as the study of diversity in the cultivated compartment can be achieved accurately.
Discrimination between wild and cultivated compartments
Shape analysis reveals a morphological differentiation between wild grapes and varieties (Figs 3 and 4). The existence of spherical seeds with a small beak, characterizing Vitis vinifera subsp. sylvestris, and those more pyriform-shaped with a well-developed beak in the case of cultivated vines (Vitis vinifera subsp. vinifera), is a result that has previously been found based on size morphometrics analyses (for recent reviews see Bouby and Marinval, 2001; Bouby et al., 2005–2006). However, the ‘Clairette’ cultivar appears to be an exception in the cultivated sample studied. Although different in seed body morphology (rather elliptic), ‘Clairette’ seeds are characterized by their small beak, comparable with that of wild individuals.
In addition, wild grapes cultivated in the Vassal collection (accessions Gres-V1 and Gres-V9) do not show significant differences from other Grésigne wild individuals collected in natural conditions. Consequently, if cultivation practices such as pruning, training, fertilization, elimination of competitors or treatment against pathogens generally lead to a greater development of berries and thus to an increase in seed size, through hormonal mechanisms (Champagnol, 1984), they do not have a significant influence on shape. A similar pattern was recorded for varieties cultivated under distinct ecological conditions. Thus, seed shape seems to be at least partially independent of environmental factors.
Differences quantified between wild and cultivated grapes could be connected to the domestication process. Whereas size, shape and colour of berries are phenotypic traits, which might have been traditionally selected by humans, it appears that seed shape was probably not a target of selective pressures.
However, a pleiotropic effect may connect seed shape to one or several characters selected artificially. Viala and Péchoutre (1910) have shown that ablation of the beak enhances ability and speed of germination. An increase in beak size of varieties from the wild to the domesticated forms could thus be correlated with a decrease in germination ability. This trait allows us to define the domestication syndrome (Harlan, 1975) such as change occurring from dioecious wild individuals to hermaphroditic cultivated plants. The inward curve of seeds on the lateral side could support this hypothesis given that this character links the existence of a less-developed embryo and the presence of reduced reserves. A reduction in the selective value of individuals is frequently observed in other plants, such as rice (Lu et al., 2006). In asexually multiplied plants, successive cloning, interrupted by rare recombination events, allows the process of selection to continue towards economically interesting phenotypes. An accumulation of deleterious alleles leading to a decrease in selective value of individuals may result from this process (Lu et al., 2006).
Distinction between wild grapes and cultivars evidenced via quantification of seed shape seems to confirm results from microsatellites (simple sequence repeats) (Perret, 1997; This et al., 2001; Aradhya et al., 2003; Grassi et al., 2003; Lacombe et al., 2004; Snoussi et al., 2004; Arroyo-Garcia et al., 2006). As observed in Figs 3 and 4, an absence of morphological or genetic differentiation according to ecological and geographical parameters was noticed (Perret, 1997; Lacombe et al., 2004). Such a result appears surprising. Indeed, modern populations of wild grapes are of very small size and relatively isolated, making pollen dispersal improbable (Turner and Brown, 2004). The absence of differentiation among populations may be explained by population fragmentation occurring most probably recently. The introduction in Europe (19th century) of American pathogens such as Phylloxera supports this suggestion.
It can be hypothesized that low morphological disparity (or morphological diversity) exists, as a result of introgression processes evidenced by Di Vecchi-Staraz et al. (2008) for wild individuals of French populations. It would imply long-term intensive trade and exchange of grapevines around the Mediterranean Basin, and massive gene flow from the cultivated to the wild compartment, so that repeated sexual crosses during several generations allowed a return to the wild morphotype (Levadoux, 1956; Zohary, 2004). However, beyond the fact that some varieties are cultivated in restricted geographical zones, gene exchange among compartments appears to be reduced due probably to a phenological gap (This et al., 2001; Lacombe et al., 2004). On the other hand, as fruits are mature during the migration period of birds such as Turdidae, the homogeneity of the wild compartment could be explained by bird seed-dispersal.
Morphological diversity in the cultivated compartment
With regard to berry skin colour, seeds from mutants are all associated within the same groups (Fig. 4), consistent with studies showing no genetic differentiation between white and black varietal mutants (Sefc et al., 2000). Considered as different cultivars on the basis of the properties and characters of fruit and wine produced, mutants such as ‘Pinot noir’ or ‘Pinot blanc’ contribute to the diversity in grapevines. The determination of skin colour is dependent essentially on variation present in the VvmybA1 gene, a transcriptional regulator of anthocyanin biosynthesis (This et al., 2007). Neither random amplification of polymorphic DNA markers (Ye et al., 1998) nor microsatellite markers (Bowers et al., 1996; Regner et al., 2000) make it possible to distinguish various clones of ‘Pinot noir’ and ‘Pinot gris’.
The morphological groups (or morphoclades) revealed by UPGMA (Fig. 4) appear to be structured according to the supposed geographical origin and parentage relationships among varieties (Fig. 5). However, as the identity of the male relative is unknown, the parental contribution in the development of seed was not investigated. Structure in shape diversity presented below was interpreted as the result of strong human selection pressures.
The ‘Sauvignon’ group includes varieties originating from south and south-western France, to which ‘Chenin’ (Loire valley), ‘Riesling’ (Rhine valley) and ‘Chasselas’ (Burgundy or Switzerland) are affiliated, along with ‘Cabernet-Sauvignon’, a single hybrid from cross-pollination between ‘Cabernet Franc’ and ‘Sauvignon’ (Bowers and Meredith, 1997; Bowers et al., 1999). The ‘Syrah’ and ‘Cabernet Franc’ groups connect varieties from southern France, particularly the first group, comprising varieties from regions bordering the Mediterranean Sea. In these two linked morphoclades, of note is the presence of the ‘Dureza’ variety (of Rhône-Alps origin, France), which is one parent of the ‘Syrah’ variety (Bowers et al., 2000). In the present study, the second ‘Syrah’ parent, the ‘Mondeuse blanche’ cultivar (Rhône-Alpes, France) (Bowers et al., 2000) itself constitutes as a distinct group, such as ‘Duras’, ‘Viognier’ and ‘Ugni blanc’ (Trebbiano Toscano). The three last groups, comprising a single variety, may be considered as outgroups characterized by a very different seed (extreme development of a beak).
The ‘Merlot’ group shows geographical heterogeneity. It comprises two varieties from the Aquitaine region and Switzerland and Gouais of unknown origin (Table 2). Finally, ‘Savagnin’ (syn. Traminer Weiss), ‘Pinot’, ‘Gamay’, ‘Chardonnay’, ‘Melon’ and ‘Tempranillo’ appear to be grouped (Pinot group). The origins of ‘Savagnin’ and ‘Pinot’, presumably ancient, are poorly known (Regner et al., 2000). They could have arisen from hybridization between Roman grapes and local wild populations or from secondary domestication of the latter (Bouquet, 1982). The ancient origin of ‘Pinot’ could explain the fact that it was identified as one of the parents of 46 modern varieties (Boursiquot et al., 2004), including ‘Gamay’, ‘Chardonnay’ and ‘Melon’, which are offspring of crosses between ‘Pinot’ and ‘Gouais’ (Merlot group). Cultivation of this last variety been abandoned due to the lower quality of its production, but it was widely cultivated during the Middle Ages in north-east France (Bowers et al., 1999). Morphological convergence between ‘Tempranillo’ and ‘Pinot’ is surprising because their parentage is not supported by any scientific data (Rubio and Yuste, 2004).
The position of the ‘Henab’ (Turkey) and ‘Muscat à petits grains’ (Greece) group within a cluster consisting of the ‘Cabernet franc’, ‘Merlot’ and ‘Pinot’ groups could suggest relationships between European and Eastern varieties. This might reflect the scale of trade, which spread grapevine by vegetative propagation (cutting) through the Mediterranean Basin.
As there was overall congruence between results regarding shape differentiation and genetic molecular data based on supposed geographical origin of varieties and parentage relationships, we consider that shape quantification of seeds is an efficient tool to characterize grapevine diversity and to identify groups of cultivars. Shape comparison between current forms and archaeological material may elucidate the temporal scale of domestication events, origins of cultivars, exchange and cultural interactions.
Allocation of archaeological seeds
The importance of the allocation of wild grape seeds (n = 8–10 based on level of probability) may be surprising in a Roman archaeological context associated with cultivation areas and structures of wine production and storage (Fig. 6). During this period, wild grapes were probably more numerous than today (i.e. before the Phylloxera crisis and other cryptogamic invaders) (Arnold et al., 1998). They might have been continuously and significantly exploited through fruit gathering or integration of individuals within cultivation systems. Therefore, some wild grape individuals might have been used as rootstock. As use of this technique was recommended and described by Columella, a famous Roman agronomist of the mid first century AD (Columella, de Re Rustica, IX in du Bois, 1844), imported varieties from other regions or more distant areas could have been grafted in order to favour their acclimatization, such as the ‘Mondeuse blanche’ considered a mountain variety and to which 4–6 seeds were attributed. Some morphological variants, interesting from an agronomic perspective, and/or hermaphrodite individuals, rare but present in ecosystems (Anzani et al., 1990), might have been selected and integrated in the agricultural system to increase productivity and guarantee fruit production by each individual.
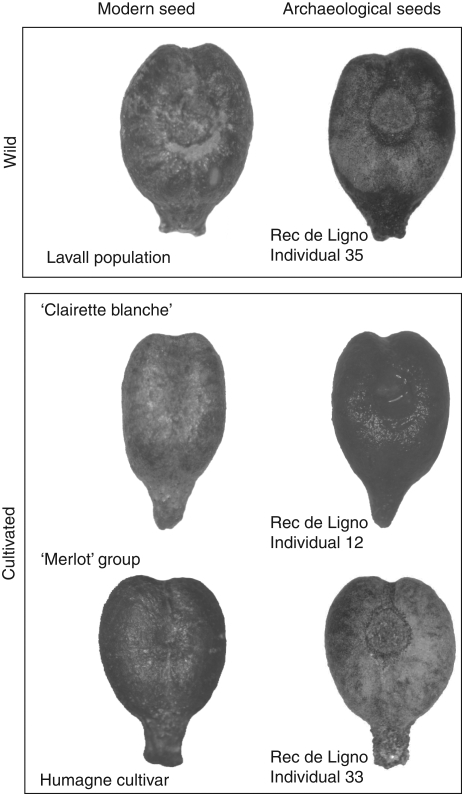
Fig. 6.
Photographs of archaeological seeds allocated to modern grapevine forms.
In this regard, archaeological data reveal the emergence since the first century AD of novel organization of agricultural territory, including buildings dedicated to wine production and storage (Fig. 6). This process appears to have increased during the second century AD (Brun, 2003).
The allocation of seeds morphologically close to wild forms and attributed to ‘Clairette’ (n = 5–6), a variety attested in Languedoc since the beginning of the 12th century (Martin, 2003) and today widely cultivated some kilometres north of the Valros area, suggests an autochthonous and ancient origin. If the existence of this variety is confirmed by new data, such as from current archaeogenetic investigations, ‘Clairette’ would become one of the oldest authenticated varieties.
Finally, the allocation of 7–8 seeds (Table 4) to the ‘Merlot’ morpho-clade pooling varieties from distinct regions (Fig. 5) may reflect the scale of human contact, exchange and migrations which spread grapevine cultivation through Europe. Although anecdotal, the record of seeds classified in the ‘Henab’/‘Muscat à petits grains’ group (n = 2) and in the ‘Cabernet’ group (n = 2) could be related to east–west communication channels at the scale of the Mediterranean Basin and between Italy and Spain via the Via Domitia.
The EFT method applied to grape seeds from wild individuals and cultivars allow the quantification of patterns of morphological differentiation and changes in seed functional traits interpreted as domestication syndrome occurring during the domestication process. Seeds from wild grapes may be distinguished from varieties based on shape analysis. In the cultivated compartment, results have shown that shape diversity is partially structured according to parentage relationship and geographical origin of cultivars.
On the basis of archaeological and historical data, the present comparison between current samples and archaeological specimens suggests that the Languedoc region might have constituted a domestication centre, where putative morphological variants might have been selected in the wild and in progenies from crosses between allochthonous forms and local grapes. Although not exhaustive, morphological diversity in the reference collection as well as in the archaeological material testifies to the complexity of exchange among classical populations, which spread viticulture and viniculture in the Mediterranean Basin and in other European areas. Finally, these results constitute a new contribution to the search of the identity of ancestral cultivars such as ‘Clairette’ and ‘Mondeuse blanche’, which are at the origin of the world grapevine patrimony.
ACKNOWLEDGMENTS
We thank Claire Arnold and Annik Schnitzler for their geographical help in our search for wild grape populations, Patrick Ortigosa and Robert Plageoles for their help during sampling in the INRA Vassal collections and the Cahuzac-sur-Vère vineyard, respectively. We are also grateful to Sabrina Renaud and Julien Claude for their support and pedagogy concerning EFT, Catherine Breton for assistance with clustering analysis and Jean-Pierre Brun for constructive comments about viticulture history. Laurent Caussignac and Laurent Pullara are gratefully acknowledged for their logistic support. We thank the two anonymous referees for their constructive comments. This work was supported by the CNRS ATIP project ‘ARCHEO-VITIS’, the ANR program ‘FRUCTIMEDHIS’, the GDR 2474 CNRS ‘Morphométrie et Evolution des Formes’ and the INRAP – PAS ‘Lodévois’.
LITERATURE CITED
- André J. Contribution au vocabulaire de la viticulture: les noms de cépages. Revue d'Etudes Latines. 1952;30:126–156. [Google Scholar]
- Anzani R, Failla O, Scienza A, Campostrani F. Wild grapevine (Vitis vinifera var silvestris) in Italy: diffusion, characteristics and germplasm preservation, 1989 report. Proceedings of the 5th International Symposium on Grape Breeding. Vitis. 1990:97–113. Bundesforschungsanstalt fur Rebenzuchtung, Siebeldingen, Germany, ed. special issue. [Google Scholar]
- Aradhya MK, Dangl GS, Prins BH, et al. Genetic structure and differentiation in cultivated grape, Vitis vinifera L. Genetics Research. 2003;81:179–192. doi: 10.1017/s0016672303006177. [DOI] [PubMed] [Google Scholar]
- Arnold C, Gillet F, Gobat JM. Situation de la vigne sauvage Vitis vinifera subsp. silvestris en Europe. Vitis. 1998;37:159–170. [Google Scholar]
- Arnold C, Schnitzler A, Douard A, Peter R, Gillet F. Is there a future for wild grapevine (Vitis vinifera subsp. silvestris) in the Rhine Valley? Biodiversity and Conservation. 2005;14:1507–1523. [Google Scholar]
- Arroyo-Garcia R, Ruiz-Garcia L, Bolling L, et al. Multiple origins of cultivated grapevine (Vitis vinifera L. ssp. sativa) based on chloroplast DNA polymorphisms. Molecular Ecology. 2006;15:3707–3714. doi: 10.1111/j.1365-294X.2006.03049.x. [DOI] [PubMed] [Google Scholar]
- Boissinot P. Archéologie des vignobles antiques du sud de la Gaule. Gallia. 2001;58:45–58. [Google Scholar]
- Bouby L, Marinval P. La vigne et les débuts de la viticulture en France: apports de l'archéobotanique. Gallia. 2001;58:13–28. [Google Scholar]
- Bouby L, Terral JF, Ivorra S, Marinval P, Pradat B, Ruas MP. Vers une approche bio-archéologique de l'histoire de la vigne cultivée et de la viticulture : problématique, choix méthodologiques et premiers résultats. Archéologie du Midi Médiéval. 2005–2006;23–24:61–74. [Google Scholar]
- Bouquet A. Origine et évolution de l'encépagement français à travers les siècles. Progrès Agricole et Viticole. 1982;5:110–121. [Google Scholar]
- Boursiquot JM, Lacombe T, Bowers J, Meredith C. Le Gouais, un cépage clé du patrimoine viticole européen. Bulletin de l'OIV. 2004;77:799–809. [Google Scholar]
- Bowers JE, Meredith CP. The parentage of a classic wine grape, Cabernet Sauvignon. Nature Genetics. 1997;16:84–87. doi: 10.1038/ng0597-84. [DOI] [PubMed] [Google Scholar]
- Bowers JE, Dangl GS, Vignani R, Meredith CP. Isolation and characterization of new polymorphic simple sequence repeat loci in grape (Vitis vinifera L.) Genome. 1996;39:628–633. doi: 10.1139/g96-080. [DOI] [PubMed] [Google Scholar]
- Bowers JE, Boursiquot JM, This P, Chu K, Johansson H, Meredith C. Historical genetics: the parentage of Chardonnay, Gamay, and other wine grapes of north eastern France. Science. 1999;289:1562–1565. doi: 10.1126/science.285.5433.1562. [DOI] [PubMed] [Google Scholar]
- Bowers JE, Siret R, Meredith CP, This P, Boursiquot JM. Bouquet A, Boursiquot JM, editors. A single pair of parents proposed for a group of grapevine varieties in Northeastern France. Proceedings of the 7th International Symposium on Grapevine Genetics and Breeding. Acta Horticulturae. 2000;528:129–134. [Google Scholar]
- Brun JP. Le vin et l'huile dans la Méditerranée antique. Viticulture, oléiculture et procédés de fabrication. Collection des Hespérides Paris: Errance; 2003. [Google Scholar]
- Buxó R. The agricultural consequences of colonial contacts on the Iberian Peninsula in the first millennium BC. Vegetation History and Archaeobotany. 2008;17:145–154. [Google Scholar]
- Champagnol F. Eléments de Physiologie de la Vigne et de Viticulture Générale. Montpellier: Imprimerie Dehan; 1984. [Google Scholar]
- Châtaignier C. La Transcaucasie au Néolithique et au Chalcolithique. British Archaeological Series. 1995;624:1–240. [Google Scholar]
- Crampton JS. Elliptic Fourier shape analysis of fossil bivalves: some practical considerations. Lethaia. 1995;28:179–186. [Google Scholar]
- Crespan M. Evidence on the evolution of polymorphism of microsatellite markers in varieties of Vitis vinifera L. Theoretical and Applied Genetics. 2004;108:231–237. doi: 10.1007/s00122-003-1419-5. [DOI] [PubMed] [Google Scholar]
- du Bois L. Paris: Panckoucke édition; 1844. French translated edition of De agricultura from Columella. Bibliothèque latine-française, Seconde série. [Google Scholar]
- Brun JP, Laubenheimer F. La viticulture en Gaule. Gallia. 2001;58:203–219. [Google Scholar]
- Di Vecchi-Staraz M, Laucou V, Bruno G, et al. Low level of pollen-mediated gene flow from cultivated to wild grapevine: consequences for the evolution of the endangered subspecies Vitis vinifera L. subsp. silvestris. Journal of Heredity. 2008;99:45–55. doi: 10.1093/jhered/esn084. [DOI] [PubMed] [Google Scholar]
- Di Vora A, Castelletti L. Indagine preliminare sull'archeologia della vite (Vitis vinifera L.) in base ai caratteri diagnostici del vinacciolo. Rivista Archeologica dell'Antica Provincia e Diocesi di Como. 1995;176:333–358. [Google Scholar]
- Franks T, Botta R, Thomas MR. Chimerism in grapevines: implications for cultivars identity, ancestry and genetic improvement. Theoretical and Applied Genetics. 2002;104:192–199. doi: 10.1007/s001220100683. [DOI] [PubMed] [Google Scholar]
- Grassi F, Labra M, Imazio S, Spada A, Sgorbati S, Scienza A, Sala F. Evidence of a secondary grapevine domestication centre detected by SSR analysis. Theoretical and Applied Genetics. 2003;107:1315–1320. doi: 10.1007/s00122-003-1321-1. [DOI] [PubMed] [Google Scholar]
- Harlan JR. Crops and man. Madison, WI: American Society for Agronomy; 1975. American Society of Agronomy, ed. [Google Scholar]
- Hopf M. Jericho plant remains. In: Kenyon KM, Holland TA, editors. Excavations at Jericho. The pottery phases of the Tell and other finds. London: British School of Archaeology in Jerusalem; 1983. pp. 576–621. [Google Scholar]
- Jacquat C, Martinoli D. Vitis vinifera L.: wild or cultivated? Study of the grape pips found at Petra (Jordan; 150bc-400ad) Vegetation History and Archaeobotany. 1999;8:25–30. [Google Scholar]
- Kislev ME. Fruit remains. In: Rothenberg B, editor. The Egyptian mining temple at Timna. London: Institute of Archaeology, University College; 1988. pp. 236–242. [Google Scholar]
- Kuhl FP, Giardina CR. Elliptic fourier features of a closed contour. Computer Graphics and Image Processing. 1982;18:259–278. [Google Scholar]
- Lacombe T, Di Vecchi M, Laucou V, Dechesne F, Varès D, This P. Les populations de vignes sauvages du massif de l'Albera. Actes del Colloqui l'Albera i el patrimoni en l'espai transfronterer. Colloqui internacional, Figueres (Spain), 1–2 Abril de 2004. 2004:313–322. Consell Comarcal de l'Alt Empordà. [Google Scholar]
- Laubenheimer F, Brun JP, editors. La viticulture en Gaule. Gallia. 2001;58:1–260. [Google Scholar]
- Levadoux L. Les populations sauvages et cultivées de Vitis vinifera L. Annales de l'Amélioration des Plantes. 1956;6:59–117. [Google Scholar]
- Lu J, Tang T, Tang H, Huang J, Shi S, Wu CI. The accumulation of deleterious mutations in rice genomes: a hypothesis on the cost of domestication. Trends in Genetics. 2006;22:126–131. doi: 10.1016/j.tig.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Mangafa M, Kostakis K. A new method for the identification of wild and cultivated charred grape seeds. Journal of Archaeological Science. 1996;23:409–418. [Google Scholar]
- Manen JF, Bouby L, Dalnoki O, Marinval P, Turgay M, Schlumbaum A. Microsatellites from archaeological Vitis vinifera seeds allow a tentative assignment of the geographical origin of the ancient cultivars. Journal of Archaeological Science. 2003;30:721–729. [Google Scholar]
- Marinval P. Vigne sauvage et vigne cultivée dans le Bassin méditerranéen : émergence de la viticulture, contribution archéobotanique. L'histoire du vin, une histoire de rites. 1997:137–172. Paris: OIV edn. [Google Scholar]
- Martin JC. La Clairette du Languedoc: un cépage, un vin, une histoire. Le Progrès agricole et viticole. 2003;120:43–46. [Google Scholar]
- McGovern PE, Rudolph HM. The analytical and archaeological challenge of detecting ancient wine: two case studies from the ancient Near East. In: McGovern PE, Fleming SJ, Katz SH, editors. The origins and ancient history of wine. New York: Gordon and Breach; 1996. pp. 57–67. [Google Scholar]
- McGovern PE, Glusker DL, Exner LJ, Voigt MM. Neolithic resinated wine. Nature. 1996;381:480–481. [Google Scholar]
- Ocete R, López MA, Lara M, Del Tío R. The sanitary state of a phytogenetic resource: the Spanish wild grapevine, Vitis vinifera sylvestris Gmelin (Hegi), populations. Plant Genetic Resources Newsletter. 1997;110:5–12. [Google Scholar]
- Ocete R, Lopez MA, Gallardo A, Arnold C. Comparative analysis of wild and cultivated grapevine (Vitis vinifera) in the Basque region of Spain and France. Agriculture, Ecosystems and Environment. 2008;123:95–98. [Google Scholar]
- Perret M. Polymorphisme des génotypes sauvages et cultivés de Vitis vinifera L., détecté à l'aide de marqueurs RAPD. Bulletin de la société neuchâteloise des sciences naturelles. 1997;120:45–54. [Google Scholar]
- Regner F, Stadlbauer A, Eisenheld C, Kaserer H. Genetic relationships among Pinots and related cultivars. American Journal of Enology and Viticulture. 2000;51:7–14. [Google Scholar]
- Renaud S, Michaux JR. Adaptive latitudinal trends in the mandible shape of Apodemus wood mice. Journal of Biogeography. 2003;30:1617–1628. [Google Scholar]
- Renaud S, Millien V. Intra- and interspecific morphological variation in the field mouse species Apodemus argenteus and A. speciousus in the Japanese archipelago: the role of insular isolation and biogeographic gradients. Biological Journal of the Linnean Society. 2001;74:557–569. [Google Scholar]
- Renaud S, Michaux J, Jaeger JJ, Auffray JC. Fourier analysis applied to Stephanomys (Rodentia, Muridae) molars: non progressive evolutionary pattern in a gradual lineage. Paleobiology. 1996;22:255–265. [Google Scholar]
- Renaud S, Benammi M, Jaegger JJ. Morphological evolution of the murine rodent Paraethomys in response to climatic variations (Mio-Pleistocene of North Africa) Paleobiology. 1999a;25:369–382. [Google Scholar]
- Renaud S, Michaux J, Mein P, Aguilar JP, Auffray JC. Patterns of size and shape differentiation during the evolutionary radiation of the European Miocene murine rodents. Lethaia. 1999b;32:61–71. [Google Scholar]
- Rivera Nuñez D, Walker MJ. A review of palaeobotanical findings of early Vitis in the Mediterranean and the origins of cultivated grape-vines, with special reference to new pointers to prehistoric exploitation in the Western Mediterranean. Review of Palaeobotany and Palynology. 1989;61:205–237. [Google Scholar]
- Rossetto M, McNally J, Henry RJ. Evaluating the potential of SSR flanking regions for examining relationships in Vitaceae. Theoretical and Applied Genetics. 2002;104:61–66. doi: 10.1007/s001220200007. [DOI] [PubMed] [Google Scholar]
- Rubio JA, Yuste J. Ampelographic differentiation of Tempranillo clones from different area of origin, according to their synonyms. Acta Horticulturae. 2004;652:73–79. [Google Scholar]
- Sefc KM, Lopes MS, Lefort F, et al. Microsatellite variability in grapevine cultivars from different European regions and evaluation of assignment testing to assess the geographic origin of cultivars. Theoretical and Applied Genetics. 2000;100:498–505. [Google Scholar]
- Sefc KM, Steinkellner H, Lefort F, et al. Evaluation of the genetic contribution of local wild vines to European grapevine cultivars. American Journal of Enology and Viticulture. 2003;54:15–21. [Google Scholar]
- Snoussi H, Harbi Ben Slimane M, Ruiz-Garcίa L, Martίnez-Zapater JM, Arroyo-Garcίa R. Genetic relationship among cultivated and wild grapevine accessions from Tunisia. Genome. 2004;47:1211–1219. doi: 10.1139/g04-072. [DOI] [PubMed] [Google Scholar]
- Stummer A. Zur Urgeschichte der Rebe und des Weinbaues. Mitteilungen der Anthropologischen Gesellschaft in Wien. 1911;61:283–296. [Google Scholar]
- Terral JF. Quantitative anatomical criteria for discriminating wild grape vine (Vitis vinifera ssp. sylvestris) from cultivated vines (Vitis vinifera ssp. vinifera) British Archaeological Reports (International Series) 2002;1063:59–64. [Google Scholar]
- This P, Roux C, Parra P, et al. Caractérisation de la diversité d'une population de vignes sauvages du Pic Saint Loup (Hérault) et relations avec le compartiment cultivé. Genetics Selection Evolution. 2000;33:289–304. [Google Scholar]
- This P, Roux C, Parra P, et al. Caractérisation de la diversité d'une population de vignes sauvages du Pic Saint-Loup (Hérault) et relations avec le compartiment cultivé. Genetics Selection Evolution. 2001;33(suppl. 1):289–304. [Google Scholar]
- This P, Jung A, Boccacci P, et al. Development of a common set of standard varieties and standardized method of scoring microsatellites markers for the analysis of grapevine genetic resources. Theoretical and Applied Genetics. 2004;109:1448–1458. doi: 10.1007/s00122-004-1760-3. [DOI] [PubMed] [Google Scholar]
- This P, Lacombe T, Thomas MR. Historical origins and genetic diversity of wine grapes. Trends in Genetics. 2006;22:511–519. doi: 10.1016/j.tig.2006.07.008. [DOI] [PubMed] [Google Scholar]
- This P, Lacombe T, Cadle Davidson M, Owens CL. Wine grape (Vitis vinifera L.) colour associates with allelic variation in the domestication gene VvmybA1. Journal of Theoretical and Applied Genetics. 2007;114:723–730. doi: 10.1007/s00122-006-0472-2. [DOI] [PubMed] [Google Scholar]
- Turner SD, Brown AG. Vitis pollen dispersal in and from organic vineyards. I. pollen trap and soil pollen data. Review of Palaeobotany and Palynology. 2004;129:117–132. [Google Scholar]
- Valamoti SM, Mangafa M, Koukouli-Chrysanthaki C, Malamidou D. Grape-pressings from northern Greece: the earliest wine in the Aegean? Antiquity. 2007;81:54–61. [Google Scholar]
- Viala P, Péchoutre F. Origines de la vigne, in: Viala and Vermorel. Traité général de viticulture, ampélographie. 1910;1:497–504. [Google Scholar]
- Vouillamoz JF, Grando S. Genealogy of wine grape cultivars: ‘Pinot’ is related to ‘Syrah. Heredity. 2006;97:102–110. doi: 10.1038/sj.hdy.6800842. [DOI] [PubMed] [Google Scholar]
- Ye GN, Soylemezoglu G, Weeden NF, Lamboy WF, Pool RM, Reisch BI. Analysis of the relationship between grapevine cultivars, sports and clones via DNA fingerprinting. Vitis. 1998;37:33–38. [Google Scholar]
- Zohary D. Domestication of the grapevine Vitis vinifera L. in the Near East. In: McGovern PE, Fleming SJ, Katz SH, editors. The origins and ancient history of wine. New York: Gordon and Breach; 1995. pp. 23–30. [Google Scholar]
- Zohary D. The mode of domestication of the founder crops of the Southwest Asian agriculture In. In: Harris DR, editor. The origin and spread of agriculture and pastoralism in Eurasia. London: University College London Press; 1996. pp. 142–158. [Google Scholar]
- Zohary D. Unconscious selection and the evolution of domesticated plants. Economic Botany. 2004;58:5–10. [Google Scholar]
- Zohary D, Hopf M. Domestication of plants in the Old World. 3rd edn. New York: Oxford University Press; 2000. pp. 151–159. [Google Scholar]