Abstract
Background and Aims
Pollinator-mediated selection and evolution of floral traits have long fascinated evolutionary ecologists. No other plant family shows as wide a range of pollinator-linked floral forms as Orchidaceae. In spite of the large size of this model family and a long history of orchid pollination biology, the identity and specificity of most orchid pollinators remains inadequately studied, especially in the tropics where the family has undergone extensive diversification. Angraecum (Vandeae, Epidendroideae), a large genus of tropical Old World orchids renowned for their floral morphology specialized for hawkmoth pollination, has been a model system since the time of Darwin.
Methods
The pollination biology of A. cadetii, an endemic species of the islands of Mauritius and Reunion (Mascarene Islands, Indian Ocean) displaying atypical flowers for the genus (white and medium-size, but short-spurred) was investigated. Natural pollinators were observed by means of hard-disk camcorders. Pollinator-linked floral traits, namely spur length, nectar volume and concentration and scent production were also investigated. Pollinator efficiency (pollen removal and deposition) and reproductive success (fruit set) were quantified in natural field conditions weekly during the 2003, 2004 and 2005 flowering seasons (January to March).
Key Results
Angraecum cadetii is self-compatible but requires a pollinator to achieve fruit set. Only one pollinator species was observed, an undescribed species of raspy cricket (Gryllacrididae, Orthoptera). These crickets, which are nocturnal foragers, reached flowers by climbing up leaves of the orchid or jumping across from neighbouring plants and probed the most ‘fresh-looking’ flowers on each plant. Visits to flowers were relatively long (if compared with the behaviour of birds or hawkmoths), averaging 16·5 s with a maximum of 41·0 s. At the study site of La Plaine des Palmistes (Pandanus forest), 46·5 % of flowers had pollen removed and 27·5 % had pollinia deposited on stigmas. The proportion of flowers that set fruit ranged from 11·9 % to 43·4 %, depending of the sites sampled across the island.
Conclusions
Although orthopterans are well known for herbivory, this represents the first clearly supported case of orthopteran-mediated pollination in flowering plants.
Keywords: Angraecum, Mascarene Archipelago, oceanic islands, Orchidaceae, Orthoptera, plant–pollinator interactions, pollinator shifts
INTRODUCTION
Evolutionary biologists have long recognized the importance of interspecific interactions between plants and their pollinators in the extraordinary diversification of angiosperms (e.g. Darwin, 1862; Eriksson and Bremer, 1992). The idea that particular pollinators could cause convergent selective pressures on floral traits found strong support in early evolutionary literature (e.g. Darwin, 1862; Faegri and van der Pijl, 1979; Wyatt, 1983; Proctor et al., 1996), but understanding how pollinator-mediated selection acts on floral traits (i.e. ‘pollination syndromes’) and results in specific adaptations has challenged biologists for over two centuries (e.g. Darwin, 1862; Nilsson, 1988; Thompson, 1994; Waser et al., 1996; Johnson et al., 1998; Johnson and Steiner, 2000; Pellmyr and Krenn, 2002; Bradshaw and Schemske, 2003; Schiestl et al., 2003; Fenster et al., 2004; Wilson et al., 2006; Whittall and Hodges, 2007; Barrett, 2008).
No other plant family shows as wide a range of pollinator-linked floral forms as Orchidaceae, which exhibit pollination systems among the most diverse, specialized and complex of all angiosperms (Darwin, 1862; van der Pijl and Dodson, 1966; Tremblay, 1992; Johnson and Steiner, 2000; van der Cingel, 2001; Tremblay et al., 2005; Micheneau et al., 2009). Orchid pollination mechanisms have primarily involved the insect orders Hymenoptera (bees, wasps and ants; these pollinate roughly 60 % of orchid species), Diptera (flies and mosquitoes), Lepidoptera (moths, hawkmoths and butterflies) and Coleoptera (beetles; van der Pijl and Dodson, 1966; van der Cingel, 2001). Approximately 3 % of orchid species are estimated to be pollinated by birds (van der Pijl and Dodson, 1966), and 5–20 % of species are thought to be self-pollinating (Catling, 1990). However, in spite of the large size of this model family and a long history of orchid pollination biology (Darwin, 1862), the identity and specificity of most orchid pollinators remains inadequately studied, especially in the tropics where the family has undergone extensive diversification.
Since Darwin's observations on the genus Angraecum, these epiphytic orchids (approx. 200 species found in Africa, Madagascar, and nearby islands) have been much celebrated for providing the most extreme adaptations to large-moth pollination (e.g. A. sesquipedale with a nectar spur >30 cm long; Darwin, 1862; Wallace, 1867, 1871; Nilsson, 1988), which involves highly specialized pollination systems (Darwin, 1862; Nilsson et al., 1987; Wasserthal, 1997). These clear adaptations to hawkmoth pollination include floral traits involved in pollinator attraction (i.e. white flowers and strong nocturnal scent), pollinator fidelity (i.e. large sugar-rich nectar reward, situated in deep spurs only accessible to long-tongued moths) and structural modifications of floral organs (i.e. rostellar extensions, twisted spurs) that have all contributed to ensure contact between the sphingids and the orchid column, thus maximizing both pollination efficiency and pollinator specificity (Darwin, 1862; Nilsson et al., 1985, 1987; Nilsson and Rabakonandrianina, 1988; Martins and Johnson, 2007). It is then difficult to imagine how such extreme specializations, which can be viewed as a key innovation of this group, could preadapt these orchid species to be pollinated by any other animal. However, some species of Angraecum revealed surprises when it was recently discovered that two species on Reunion (Mascarene Islands, Indian Ocean) are pollinated by small songbirds in the genus Zosterops (Micheneau et al., 2006, 2008c). These two orchid species are members of an endemic Mascarene group, Angraecum section Hadrangis, that is involved in a rare case (for orchids) of an intra-archipelago radiation, albeit a small one (three species; Micheneau et al., 2008a).
In this article, a further highly unexpected pollinator shift in this same group of orchids to raspy cricket pollination (Glomeremus sp., Gryllacrididae, Orthoptera) is reported – the first clear case of orthopteran-mediated pollination in the angiosperms.
MATERIALS AND METHODS
Study species
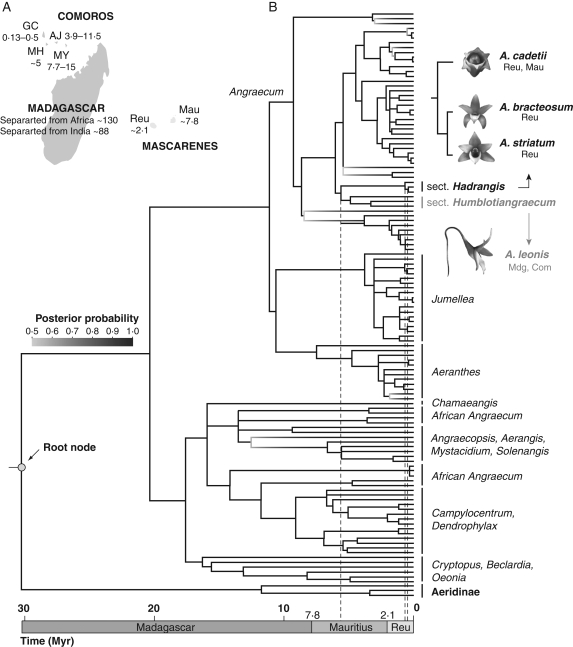
Angraecum cadetii is a monopodial epiphytic orchid endemic to Mauritius and Reunion, belonging to the Mascarene endemic Angraecum section Hadrangis, with A. bracteosum (Reunion) and A. striatum (Reunion; Bosser, 1987). The phylogenetic position of A. section Hadrangis within the angraecoid orchids is presented in Fig. 1, as well as age estimates of the section via penalized likelihood (see Supplementary methods and Table S1 in Supplementary data, available online). In Reunion, A. cadetii is relatively common in primary lowland wet forests (mainly from 0 to 1000 m a.s.l.; see Fig. 2), but the species is rare in Mauritius. Plants usually produce one to four erect racemes of one to five fleshy white-cream flowers (Fig. 3A), and their flowering time occurs during the austral summer, from January to March depending of the locality.
Fig. 1.
Phylogenetic position and age estimates of Angraecum section Hadrangis. (A) The geology of the western Indian Ocean: GC, Grande Comore; MH, Moheli; AJ, Anjouan; MY, Mayotte; Reu, La Reunion; and Mau, Mauritius. Dates correspond to island age in millions of years, after Warren et al. (2003). (B) Molecular clock chronogram of the subtribe Angraecinae estimated via penalized likelihood (for calibration points, see Supplementary methods in Supplementary data, available online). Mdg, Madagascar; Com, Comoros. Angraecum leonis has been chosen to illustrate flower morphology of A. section Humblotiangraecum, the closest relative of A. section Hadrangis.
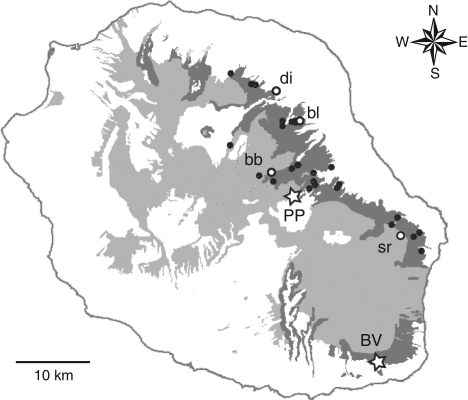
Fig. 2.
Distribution of Angraecum cadetii on Reunion (dark zones) among remaining preserved habitats (light zones; from Strasberg et al., 2005). Black dots represent precise localities where the species was encountered among 121 recorded sites; see Jacquemyn et al. (2005). White stars represent both study sites where pollinator observations have been undertaken: PP, Plaine des Palmistes, Pandanus wet thickets, 800 m; BV, Basse Vallée, mid-elevation rainforest, 700 m. White circles represent additional localities where fruit set has been estimated in 2008: di, Dioré, Pandanus wet thickets, 900–950 m; bl, Bras des Lianes, mid-elevation rainforest, 700 m; bb, Bébour, cloud forest, 1150 m; sr, Sainte Rose, mid-elevation forest, 900 m.
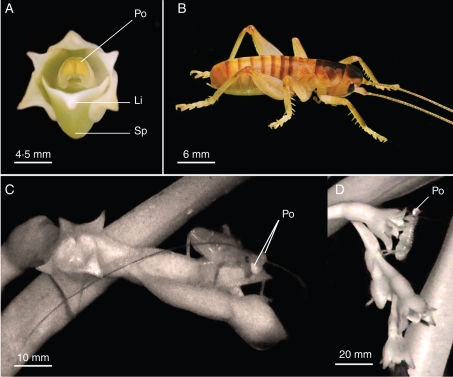
Fig. 3.
Angraecum cadetii and its pollinator Glomeremus sp. (A) Flower of A. cadetii with pollinia exposed (anther cap removed). A pollinarium (Po) consists of two hard, pale-yellow pollinia, both of unusual size and shape relative to those of related species; they are larger, triangular, and flat; both pollinia are removed simultaneously by pollinators. The fleshy lip (Li) is reduced relative to related species and forms a short conical spur (Sp) that has wide entrance with a large amount of nectar. (B) Glomeremus sp. Note the absence of wings. (C and D) Glomeremus sp. foraging on A. cadetii flowers and carrying pollinaria (Po) on its head (pictures from video captured with the night-shot option).
Pollinator-linked floral traits
Floral measurements were made on flowers collected at La Plaine des Palmistes and preserved in 70 % ethanol. Spur measurements were made in the field or in the laboratory on fully opened flowers, randomly selected from the population. Spur openings were measured from the pollinarium to the lip. All measurements were made to the nearest 0·01 mm using a digital caliper.
Nectar volume was measured on flowers from which pollinators were excluded using 5-μL capillary tubes. Per cent sucrose equivalents in nectar (grams of sucrose per 100 g of solution) were quantified by directly transferring nectar from capillary tubes to a hand-held refractometer (R5000; Atago Inc., Bellevue, WA, USA).
Floral volatiles were analysed using a solid-phase micro-extraction technique (SPME; Zhang and Pawliszyn, 1993), employing a grey StableFlex divinylbenzene/carboxen/polydimethylsiloxane fibre (length 2 cm, film thickness 50/30 µm; Supelco Co., Bellefonte, PA, USA). Fibres were conditioned prior to use according to supplier's instructions for 1 h at 270 °C. Flowers of A. cadetii, A. bracteosum and A. striatum were obtained from wild specimens (two to six plants per species were collected). Angraecum bracteosum and A. striatum (ornithophyly, Reunion) were sampled because they belong to the endemic section Hadrangis, whereas A. leonis (sphingophyly, Madagascar and Comoros) was sampled because it belongs to section Humblotiangraecum, the closest Madagascan relative of section Hadrangis (Micheneau et al., 2008a). Plants were cultivated in the laboratory during the flowering period and put back in the field after experiments. Flowers of A. leonis were obtained from a cultivated specimen. Flowers were placed in a glass-bell, sealed at the large end with cotton wool and at the other by the SPME-fibre. Diurnal and nocturnal fragrance production was compared by collecting odour from the same flower during 9–10 h of daylight versus dark on two consecutive days to evaluate reproducibility. Analyses were performed using a Hewlett Packard 6890N gas chromatograph (GC), coupled directly to a Hewlett Packard 5973N mass spectrometer. Compounds were desorbed from the fibre in the GC injector (splitless injection mode) at 250 °C and separated on a capillary SPB-5 non-polar column (60 m × 32 mm; phase thickness 0·25 µm) with helium as the carrier gas (0·7 mL min−1). The GC oven was programmed to increase temperature from 60 °C to 230 °C at 4 °C min−1, followed by a stabilization at 230 °C for 40 min. Mass spectra were produced with a current ionization of 70 eV, in a scan range of m/z 30–550. Retention indices of constituents were determined by the method of Kovats using n-alkanes (C8–C22) as standards (Kovats, 1965). Compounds were identified by comparing their retention indices and mass spectral fragmentation with those reported in the literature (Adams, 2001) and stored on the mass spectrometer ‘Nist 2002’ and ‘Wiley 7’ libraries.
Breeding system and compatibility
Hand-pollination experiments were set up both in situ (in 2008) and ex situ (in 2003) to investigate the breeding system of A. cadetii, following the protocol described by Micheneau et al. (2006): in situ experiments were carried out on 11 individuals at the study site of Basse Vallée (11 plants, 83 flowers). Prior to anthesis, plants were first enclosed by fine-mesh size nylon (i.e. strands per half millimetre) to exclude pollinaria removal and deposition by potential pollinators. Three treatments were performed: (1) no pollination, to detect for repeatability of the ability of this species to set fruit in the absence of pollinators (auto-pollination sensu Catling, 1990) (32 flowers, four plants); (2) self-pollination to quantify self-compatibility (25 flowers, three plants); and (3) cross-pollination (26 flowers, four plants). Self-pollinations were carried out by hand, pollinating flowers with both their pollinia. Cross-pollinations were performed by hand, pollinating flowers with two pollinia from a conspecific plant ≥2 m away. Bags were maintained up to the end of the fruiting period to prevent predation. The same sets of experiments were performed ex situ on nine plants from the study site at La Plaine des Palmistes (35 flowers) cultivated in an open air greenhouse (where plants were misted with water for 3 min every 6 h) as follows: 13 flowers (four plants) were left untouched, 12 flowers (nine plants) were self-pollinated and 10 flowers (eight plants) were cross-pollinated.
In situ, each inflorescence received the same treatment, and pollinations were performed on all the flowers of an inflorescence. Ex situ treatments, however, were assigned randomly on a few flowers per inflorescence to avoid resource bias. Fruit set was recorded for each treatment four weeks after pollination, when capsules had reached maximum size.
Pollinator observations
Pollinator observations were performed using hard-disk camcorders with a night-shot option (Sony DCR-SR90E; and Sony DCR-SR72E) fixed on a tripod, with power supplied by long-duration rechargeable batteries (NP-FP90, NP-NH100 InfoLithium® P and H series rechargeable battery), and protected with a waterproof casing (Sony SPK-HCB marine sport pack). Observations were performed on Mauritius (Pétrin, in 2008) and Reunion (at two study sites, Plaine des Palmistes and Basse Vallée, in 2005, 2007 and 2008, represented by white stars in Fig. 2). Before and after each 12-h videotape session (i.e. either night or day), each flower of the target individual was examined for pollen removal and/or deposition. Pollinator observations were conducted during consecutive days; it was then possible to record within the sampled population if pollination occurred by night when video sessions were carried out by day and vice versa. The purpose of these observations on pollinia removal/deposition was to determine if pollinations other than those observed were being made by any sort of vector.
Pollination success and fruit set
Pollination and fruiting success were recorded during three flowering seasons (from 2003 to 2005) at La Plaine des Palmistes (Fig. 2). Individuals were randomly chosen within the population (21/99, 20/74, 20/83 individuals/flowers per year, respectively; one plant died in 2004), permanently tagged, and examined once a week for purposes of determining (a) male and female pollination success (pollen removal and deposition, respectively) and (b) reproductive success (fruit development). In addition, six supplementary sites were studied in 2008 in order to quantify fruit set under natural conditions (represented by white circles and white stars in Fig. 2). In these additional sites, a total of 30 plants were sampled, except at Bébour, where only eight individuals were found, probably due to fact that this forest corresponds to the elevational limit for A. cadetii.
Orthopteran head measurements
Orthopteran measurements were made on male and female individuals collected on Reunion. Head height was measured from the vertex to the apex of the labrum, and width was measured below the eyes (Fig. 4). All measurements were made to the nearest 0·1 mm using a digital calliper.
Fig. 4.

Scanning electron micrograph of Glomeremus sp. head, illustrating the characters that were measured. The vertical bar represents head height; the horizontal bar represents width.
RESULTS
Pollinator-linked floral traits
Floral features of A. cadetii are reported in Table 1. For this species, spurs are conical, averaging 6·3 mm in length, 5·2 mm in height and 8·4 mm in width at the opening. Nectar volume averaged 14·5 µL per flower with a concentration of 12·3 % sugar in sucrose equivalents.
Table 1.
Summary of floral features and reproductive success related to orthopteran pollination
| Variable | Mean ± s.d. (n) |
|---|---|
| Floral features | |
| Flower colour | White/white-cream |
| Spur length | 6·26 ± 0·91 mm (24) |
| Spur opening | |
| Height (between lip and pollinarium) | 5·17 ± 0·67 mm (60) |
| Width | 8·36 ± 0·80 mm (60) |
| Nectar volume | 14·5 ± 13·7 µL (25) |
| Sugar content | 12·3 ± 3·6 % sugar (21) |
| Floral scent* | Nocturnal emission only |
| Breeding system | |
| In situ | |
| Self-pollinations | 92 % (25) |
| Cross-pollinations | 100 % (26) |
| Ex situ | |
| Self-pollinations | 100 % (12) |
| Cross-pollinations | 100 % (10) |
| Pollination success | |
| Pollinia removal rate | |
| 2003 (21 plants, 99 flowers) | 51·1 ± 36·1 % (21) |
| 2004 (17 plants, 74 flowers) | 46·1 ± 42·9 % (17) |
| 2005 (17 plants, 83 flowers) | 42·3 ± 35·4 % (17) |
| Pollinia deposition rate | |
| 2003 (21 plants, 99 flowers) | 36·7 ± 34·3 % (21) |
| 2004 (17 plants, 74 flowers) | 23·6 ± 19·1 % (17) |
| 2005 (17 plants, 83 flowers) | 25·2 ± 33·3 % (17) |
| Fruit set | |
| Natural conditions | |
| 2003 (21 plants, 99 flowers) | 24·7 ± 33·6 % (21) |
| 2004 (17 plants, 74 flowers) | 22·5 ± 18·0 % (17) |
| 2005 (17 plants, 83 flowers) | 18·7 ± 33·6 % (17) |
| Pollinator excluded (in situ and ex situ) | 0 % (45) |
n = sample size.
* Further details are given in Table 2.
No compounds detected, day or night, from the two bird-pollinated species, A. striatum and A. bracteosum, but emissions from the two other species, A. leonis (sphingophily) and A. cadetii (raspy cricket pollination), were clearly higher during the night than the day. Nocturnally emitted fragrances gave well-resolved GC chromatograms with peaks of high intensity, indicating that A. cadetii and A. leonis emit fragrance on a nocturnal rhythm. For this reason, only volatile compounds detected during the night are reported in Table 2.
Table 2.
Nocturnal headspace composition of A. cadetii (sect. Hadrangis, orthopteran pollination) and A. leonis (sect. Humblotiangraecum, sphingophily)
| Composition (%)† |
|||
|---|---|---|---|
| Compounds | RI* | A. cadetii | A. leonis |
| Aldehydes | |||
| Nonanal | 1116 | 2·0 | 0·2 |
| Decanal | 1218 | tr | 0·3 |
| Esters | |||
| 2-Hydroxy-3-methyl methylbutanoate‡ | 908 | 8·9 | – |
| 2-Hydroxy-3-methyl methylpentanoate | 1008 | 1·6 | – |
| Ketones | |||
| 6-Methyl-5-hepten-2-one | 999 | tr | – |
| Monoterpene hydrocarbons | |||
| Myrcene | 1004 | 1·1 | – |
| Limonene | 1045 | 0·9 | – |
| (E)-β-Ocimene | 1061 | 52·6 | – |
| γ-Terpinene | 1070 | tr | – |
| 2,6-Dimethyl-1,3,5,7-octatetraene | 1146 | tr | – |
| Neo-allo-ocimene | 1157 | tr | – |
| Oxygenated monoterpenes | |||
| 1,8-Cineole | 1051 | 7·1 | – |
| α-Terpineol | 1211 | 4·7 | – |
| Geraniol | 1268 | 18·3 | – |
| Sesquiterpene hydrocarbons | |||
| γ-Muurolene | 1508 | tr | – |
| Germacrene D | 1514 | 2·8 | – |
| γ-Cadinene | 1545 | tr | – |
| δ-Cadinene | 1552 | tr | – |
| Aromatic compounds | |||
| Benzaldehyde | 978 | – | tr |
| Benzyl alcohol | 1048 | – | 0·4 |
| p-Cresol | 1085 | – | 0·2 |
| Methyl benzoate | 1113 | – | tr |
| Benzyl acetate | 1179 | – | tr |
| Methyl salicylate | 1215 | – | tr |
| Chavicol | 1269 | – | 72·4 |
| Chavicol isomer | 1325 | – | tr |
| Chavicol isomer | 1356 | – | 3·6 |
| Benzyl butanoate | 1363 | – | tr |
| 4-Hydroxybenzaldehyde | 1375 | – | 1·9 |
| Eugenol | 1377 | tr | – |
| α-Ethylbenzenemethanol‡ | 1386 | – | 0·8 |
| Vanillin | 1421 | – | tr |
| (E)-Cinnamyl acetate | 1463 | – | 0·4 |
| 2-Butylphenol‡ | 1466 | – | tr |
| (E)-Isoeugenol | 1471 | – | tr |
| Benzyl tiglate | 1519 | – | 0·3 |
| Benzyl benzoate | 1797 | – | 5·5 |
| Benzyl salicylate | 1905 | – | 7·3 |
| Nitrogenous aromatic compounds | |||
| Methyl nicotinate | 1155 | – | 5·3 |
| 4-Quinolinecarboxaldehyde | 1513 | – | 0·6 |
| Unidentified compounds | |||
| Unidentified 1 | 1666 | – | 0·5 |
| Unidentified 2 | 1701 | – | 0·3 |
* RI: Retention indices relative to C5–C22 n-alkanes on SPB-5 non-polar capillary column.
† Relative percentage obtained from peak area: tr, trace (< 0·1 %); –, absent.
‡ Tentatively identified.
The fragrance of A. cadetii is largely dominated by monoterpenes, of which (E)-β-ocimene (52·6 %) and geraniol (18·3 %) are the two dominant compounds. These major volatiles are accompanied by a lower content of aldehydes, esters and other mono- and sesquiterpenes (Table 2). The nocturnal floral bouquet of A. leonis is largely dominated by aromatic compounds, of which chavicol is the dominant compound (72·4 %; Table 2).
Breeding system and compatibility
None of the flowers tested for autonomous self-pollination produced fruits, either in situ or ex situ, indicating that A. cadetii requires a pollinating agent to achieve fruit set (Table 1). However, the species is fully self-compatible, as the fruit set in cases of self-pollination was ≥92 % (Table 1).
Pollinator identity and specificity
The present observations represented a total of 48 days (577 h 40 min; 508 flowers) and 14 nights (171 h 35 min; 74 flowers), during which raspy crickets probed a total of 75 flowers in 15 visits. Pollen transfer by raspy crickets (i.e. pollinarium removal and deposition) was clearly observed (Table S2 and video in Supplementary data, available online), indicating that this as yet unnamed species of Glomeremus (Gryllacrididae, Orthoptera) is an effective pollinator of A. cadetii on Reunion (Fig. 3). Flowers of A. cadetii were, however, also frequently visited during the day by birds (Zosterops borbonicus borbonicus and Z. olivaceus olivaceus, Zosteropidae) and at night by a host of small arthropods, including spiders, centipedes and several insect groups (cockroaches, moths, mosquitoes and crickets), none of which removed pollinia. A single diurnal visit by a gecko (Phelsuma borbonica, Gekkonidae) was also observed (Table S2 in Supplementary data). In contrast, the species of Glomeremus regularly visited flowers at night from 1950 h to 0440 h; the average duration of a single flower visit was 16·5 ± 8·7 s (min. 3·1; max. 41·0; n = 75 flowers). Typically, these raspy crickets reached flowers by climbing up leaves of the orchid or jumping across from neighbouring plants. In all successful pollination events, the raspy cricket positioned itself on the fleshy lip of the flower with its dorsal side orientated towards the orchid column and probed deep within the spur. Pollinaria of the orchid became stuck to the head of the crickets as they retreated from flowers (video in Supplementary data). At the peak flowering time, raspy crickets visited the same patch of plants with high fidelity night after night, adopting the same behaviour each time, i.e. probing deeply into the orchid spur, the head totally hidden in the centre of the flower, and visiting the majority of ‘fresh-looking’ flowers within reach.
Pollination success and fruit set
At La Plaine des Palmistes, flowering time was regular each year, occurring between the end of January to the middle of March, with a flowering peak around mid-February. On the 21 plants permanently tagged in 2003, one died in 2004, and three other individuals did not produce flowers in 2004 and 2005. This 3-year study of pollination and reproductive success in natural populations indicates that the rate of pollinarium removal averages 46·5 %, pollen deposition reaches approx. 27·5 %, and fruit set averages 21·9 % (results per year are given in Table 1). Fruit set ranged between 11·9 % (Bébour) and 43·3 % (Bras des Lianes) in additional sites that were sampled in 2008 (Table 3).
Table 3.
Fruit set (%) recorded in 2008 in different localities across the distribution of A. cadetii on Réunion
| Localities | Altitude (m a.s.l.) | % Fruit set ± s.d. (n) |
|---|---|---|
| Bébour | 1150 | 11·90 ± 24·28 (8) |
| Plaine des Palmistes | 900 | 23·42 ± 29·90 (30) |
| Dioré | 900–950 | 24·39 ± 27·69 (30) |
| Basse Vallée | 700 | 29·53 ± 31·65 (30) |
| Sainte Rose | 900 | 35·23 ± 30·69 (30) |
| Bras des Lianes | 700 | 43·33 ± 33·46 (30) |
n = sample size.
Orthopteran head measurements
Heads of raspy cricket males averaged 6·3 × 4·5 mm (n = 12), whereas the same measurements made on female specimens were slightly larger, averaging 6·7 × 4·8 mm (n = 9; Table 4).
Table 4.
Measurements of Glomeremus sp. head in millimetres
| Glomeremus sp. | Mean ± s.d. | Minimum | Maximum | n |
|---|---|---|---|---|
| Male | ||||
| Face height | 6·3 ± 0·3 | 5·8 | 6·7 | 12 |
| Face width | 4·5 ± 0·2 | 4·2 | 4·7 | 12 |
| Female | ||||
| Face height | 6·7 ± 0·4 | 6·3 | 7·3 | 9 |
| Face width | 4·8 ± 0·2 | 4·5 | 5·2 | 9 |
n = sample size.
DISCUSSION
Pollinator identity and specificity
Orthopterans are well known for herbivory, and this insect order is not normally considered to be capable of regular pollination (e.g. Darwin, 1862; Knuth, 1909; van der Pijl and Dodson, 1966; Proctor et al., 1996; van der Cingel, 2001). Although orthopterans have been recorded as floral visitors in a few studies, occasionally carrying pollen after having consumed it, no evidence of regular pollination has ever been clearly documented (Table S3 in Supplementary data). Although casual cases of pollination by orthopterans have always been linked with herbivory and pollinivory, the present observations revealed that A. cadetii seems to rely on the unique services of an orthopteran to achieve fruit set, at least in the Pandanus forest of La Plaine des Palmistes. In the south of the island (Basse Vallée), observations were conducted by day and failed to record any pollination events; only the two species of white-eyes probed flowers without removal of pollinia. However, at the end of the flowering time at this study site, it was observed that some pollen transfers occurred at night, which suggests that raspy crickets were successfully pollinating A. cadetii over its geographical range on the island, although more studies are needed to document this fully. Nevertheless, the preliminary results indicate that (a) orthopterans regularly visit orchid flowers, presumably for the nectar they contain (food reward), and (b) the role of these insects in the pollination of A. cadetii does not appear to be occasional or incidental. Along with lack of pollination by all other recorded flower visitors (by day or night), it appears that A. cadetti is dependent on its orthopteran pollinator on Reunion.
Pollinator-linked floral traits and reproductive success
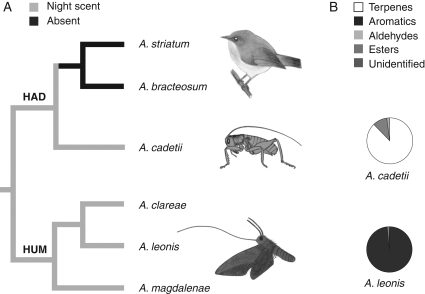
Floral morphology of A. cadetii appears to be adapted for efficient pollination by raspy crickets; there is a close match in size between the head of the insect and the flower opening. The size of the mouth of the nectar spur (4·3–7·9 mm) is slightly less than that of the head of the orthopteran (5·8–7·3 mm), leading to good contact with the pollinia (Tables 1 and 4). Although orthopterans are generally destructive, no herbivory was observed on the fleshy flowers of A. cadetii (Fig. 3A). The peculiar bouquet of A. cadetii (i.e. nocturnal emission of a monoterpene-dominated scent; Table 2) may attract and perhaps guide raspy crickets to orchid flowers, but this needs further study. Nevertheless, qualitative floral scent composition appears to be under pollinator-mediated selection within these orchids (Fig. 5): (a) the closest relative of section Hadrangis, A. leonis (Madagascar and Comoros) typically matches the hawkmoth pollination syndrome (well represented among Angraecum species) and at dusk emits a strong and sweet scent, highly dominated by aromatic volatiles (i.e. containing benzene rings), a chemical class known to attract nocturnal lepidopterans (e.g. Huber et al., 2005); (b) A. cadetii flowers emit a fragrance dominated by monoterpenes throughout the night (barely perceptible to the human nose); and (c) ornithophilous species of section Hadrangis, namely A. bracteosum and A. striatum, are scentless, a typical feature of bird-pollinated plants (Knudsen et al., 2004; Table 2). In addition, pollination in this orchid species is unusually efficient and further produces high rates of pollination and fruit set (Tables 1 and 3). Reproductive success, which can reach >43 % in some insular populations (e.g. Plaine des Lianes), is even higher than those recorded in the bird-pollinated sister-species at the study site of La Plaine des Palmistes: fruit set averaged 6·0 % in 2003, 3·5 % in 2004 and 5·7 % in 2005 for A. bracteosum, and 10·7 %, 16·8 % and 12·1 %, respectively, for A. striatum (Micheneau et al., 2006, 2008c).
Fig. 5.
Fragrance chemistry of sections Hadrangis and Humblotiangraecum in relation to pollination syndromes: (A) emission rhythms; (B) nocturnal scent composition of A. cadetii (orthopteran–pollination) and A. leonis (sphingophily) according to relative percentages (>0·1 %) obtained from GC chromatograms and volatile chemical classes (Table 2). No compounds were detected for A. bracteosum and A. striatum (ornithophily), day or night. Terpenes include mono- and sesquiterpenes; aromatics include nitrogenous aromatic compounds. A., Angraecum; HAD, section Hadrangis; HUM, section Humblotiangraecum.
Orthopteran behaviour
Gryllacridinae have a high level of endemicity in the Mascarenes; eight species are known in the Archipelago, all endemic (five species in Mauritius, three in Reunion; S. Hugel, unpubl. data). On Reunion, Glomeremus sp. populations are found in the same habitats as A. cadetii (i.e. wet forests, mainly 0–1000 m a.s.l.). Gryllacridinae are nocturnal foragers, climbing on branches and foliage while exploring the surroundings with their tremendously long antennas. Gryllacridinae return each night after foraging to the same silk nest (Hale and Rentz, 2001), and such fidelity depends on gryllacridid ability to use spatial landmarks and measure translational displacements and their capacity to recognize these parameters and their sequence (Hale and Bailey, 2004). The capacity of Gryllacridinae to relocate their nest might also be used to help them re-locate food sources. Although mainland species of Gryllacridinae are typically omnivorous and eat plant material (seeds, fruits, flowers) and other arthropods, depending on the species (Hale and Rentz, 2001), unidentified plant parts including pollen and seeds and only a few insect parts have been found in the stomach of these raspy crickets (five specimens). Consumption of nectar may have evolved within Mascarene Gryllacridinae to compensate for paucity of other food resources; it is not rare that insular arthropod-feeding species often include nectar, seeds and/or fruits in their diet to compensate for the general scarcity of arthropods on volcanic islands (e.g. Barrett, 1996; Olesen and Valido, 2003). Consequently, selective pressures exerted by these opportunist flower visitors are expected to be more significant on insular plants compared with mainland floras (Olesen and Valido, 2003).
Concluding remarks
Whether the present finding represents a unique case of orthopteran pollination or a more common but as yet undocumented phenomenon (especially in the tropics) remains to be investigated. In addition, more observations are needed throughout island populations of orchids to determine the level of specialization of the surprising orchid–orthopteran pollination system described here. Nevertheless, distinguishing characteristics of this first record of regular pollination by an orthopteran are (a) a species in a family renowned for high rates of floral evolution (Orchidaceae); (b) a young insular environment without many hawkmoths that elsewhere pollinate this group of orchids; and (c) likely preadaptations of both the plant and orthopteran colonists.
High rates of floral evolution may not only be intrinsic to Orchidaceae, but may also have been promoted in the small island populations of the Mascarene Archipelago where drift and selective pressures would have been higher (McArthur and Wilson, 1967). Also related to the insular environment, the original pollinator of the orchid colonist was probably absent on its arrival in the Archipelago, and therefore selective pressure may have favoured a pollinator shift (e.g. Barrett, 1996). In support of this inference, the two long-tongued moth genera known to pollinate Angraecum species in Madagascar, which is where this lineage of orchids originated (Micheneau et al., 2008a), are absent from the Mascarenes (Guillermet, 2006). Shifts observed in other plant lineages following their arrival in the Mascarenes [hermaphrodism to dioecy (Pailler et al., 1998); outcrossing to selfing (Micheneau et al., 2008b)] are consistent with the historical absence of long-tongued pollinator diversity in the Mascarenes.
Further favouring this shift, the tight interaction between plant and pollinator need not be the exclusive result of adaptation. That the ancestral Angraecum colonist was ‘preadapted’ to orthopteran pollination is supported by the fact that the Madagascan relatives (Micheneau et al., 2008a) are night-scented (e.g. A. leonis; Table 2) and white, which makes them easily targeted by nocturnal insects. Madagascan relatives also display a wide spur opening: nectar could have been more accessible for ‘proboscis-less’ pollinators in these flowers with a larger entrance. Glomeremus may have been further ‘preadapted’ to orchid pollination since it belongs to Gryllacridinae, an orthopteran subfamily with many opportunistic omnivorous species, in which the building of silk nests is widespread. This behaviour obliges the orthopteran to return to the same nest every day involving memory and navigational abilities that may have favoured repeated nectar-feeding from Angraecum flowers, which form a highly localized food resource.
An important question is whether all characteristics of the Mascarene situation were critical in establishing the orchid–orthopteran interaction or if one component has been of overriding importance. Further study of the diverse array of poorly known tropical orthopteran species and their interactions with plants is required.
SUPPLEMENTARY DATA
Supplementary Material
ACKNOWLEDGEMENTS
We thank the National Parks and Conservation Service of Mauritius (NPCS), the National Park of Reunion and the National Office of Forests of Reunion (ONF) for material support in the field, and the project ANR BIOTAS and the Region Reunion for financial support. We also warmly thank Urs Ziegler (University of Zürich) for the use of the scanning electron microscope, and Laurence Humeau (University of La Réunion) for her help concerning access to bibliographic data on plant–orthopteran interactions.
LITERATURE CITED
- Adams RP. Identification of essential oil components by gas chromatography/quadrupole mass spectroscopy. Carol Stream: Allured Publishing Corporation; 2001. [Google Scholar]
- Barrett SCH. The reproductive biology and genetics of island plants. Philosophical Transactions of the Royal Society of London, Series B. 1996;351:725–733. [Google Scholar]
- Barrett SCH. Major evolutionary transitions in flowering plant reproduction: an overview. International Journal of Plant Sciences. 2008;169:1–5. [Google Scholar]
- Bosser J. Contribution à l'étude des Orchidaceae de Madagascar et des Mascareignes. XXII. Adansonia. 1987;3:249–254. [Google Scholar]
- Bradshaw HD, Schemske DW. Allele substitution at a flower colour locus produces a pollinator shift in monkeyflowers. Nature. 2003;426:176–178. doi: 10.1038/nature02106. [DOI] [PubMed] [Google Scholar]
- Catling PM. Auto-pollination in the Orchidaceae. In: Arditti J, editor. Orchid biology, reviews and perspectives. Vol. V. Portland, OR: Timber Press; 1990. pp. 121–158. [Google Scholar]
- van der Cingel NA. An atlas of orchid pollination: America, Africa, Asia and Australia. Rotterdam: A. A. Balkema Publishers; 2001. [Google Scholar]
- Darwin C. On the various contrivances by which British and foreign orchids are fertilised by insects, and on the good effect of intercrossing. London: John Murray; 1862. [PMC free article] [PubMed] [Google Scholar]
- Eriksson O, Bremer B. Pollination systems, dispersal modes, life forms, and diversification rates in angiosperm families. Evolution. 1992;46:258–266. doi: 10.1111/j.1558-5646.1992.tb02000.x. [DOI] [PubMed] [Google Scholar]
- Faegri K, van der Pijl L. The principles of pollination ecology. Oxford: Pergamon; 1979. [Google Scholar]
- Fenster CB, Armbruster WS, Wilson P, Dudash MR, Thompson JD. Pollination syndromes and floral specialization. Annual Review of Ecology, Evolution and Systematics. 2004;35:375–403. [Google Scholar]
- Guillermet C. Contribution à l'étude des papillons Hétérocères de l'île de La Réunion. Vol. 2. Saint-Gilles-les-Bains: Association Nature, Découverte et Partage; 2006. [Google Scholar]
- Hale RJ, Bailey WJ. Homing behaviour of juvenile Australian raspy crickets (Orthoptera: Gryllacrididae) Physiological Entomology. 2004;29:426–435. [Google Scholar]
- Hale RJ, Rentz DCF. The Gryllacrididae: an overview of the world fauna with emphasis on Australian examples. In: Field LH, editor. The biology of wetas, king crickets and their allies. Wallingford: CABI Publishing; 2001. pp. 95–110. [Google Scholar]
- Huber FK, Kaiser R, Sauter W, Schiestl FP. Floral scent emission and pollinator attraction in two species of Gymnadenia (Orchidaceae) Oecologia. 2005;142:564–575. doi: 10.1007/s00442-004-1750-9. [DOI] [PubMed] [Google Scholar]
- Jacquemyn H, Micheneau C, Roberts DL, Pailler T. Elevation gradients of species diversity, breeding system and floral traits of orchid species on Réunion Island. Journal of Biogeography. 2005;32:1751–1761. [Google Scholar]
- Johnson SD, Steiner KE. Generalization versus specialization in plant pollination systems. Trends in Ecology and Evolution. 2000;15:140–143. doi: 10.1016/s0169-5347(99)01811-x. [DOI] [PubMed] [Google Scholar]
- Johnson SD, Linder HP, Steiner KE. Phylogeny and radiation of pollination systems in Disa (Orchidaceae) American Journal of Botany. 1998;85:402–411. [PubMed] [Google Scholar]
- Knudsen JT, Tollsten L, Groth I, Bergström G, Raguso RA. Trends in floral scent chemistry in pollination syndromes: floral scent composition in hummingbird-pollinated taxa. Botanical Journal of the Linnean Society. 2004;146:191–199. [Google Scholar]
- Knuth P. Handbook of flower pollination. Vol. III. Oxford: Clarendon Press; 1909. [Google Scholar]
- Kovats E. Gas chromatographic characterization of organic substances in the Retention Index System. In: Giddings JC, Keller RA, editors. Advances in chromatography. Vol. 1. New York, NY: Marcel Dekker; 1965. pp. 229–247. [Google Scholar]
- McArthur RH, Wilson EO. The theory of island biogeography. Princeton, NJ: Princeton University Press; 1967. [Google Scholar]
- Martins DJ, Johnson SD. Hawkmoth pollination of aerangoid orchids in Kenya, with special reference to nectar sugar concentration gradients in the floral spurs. American Journal of Botany. 2007;94:650–659. doi: 10.3732/ajb.94.4.650. [DOI] [PubMed] [Google Scholar]
- Micheneau C, Fournel J, Pailler T. Bird pollination in an angraecoid orchid on Reunion Island (Mascarene Archipelago, Indian Ocean) Annals of Botany. 2006;97:965–974. doi: 10.1093/aob/mcl056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micheneau C, Carlsward BS, Fay MF, Bytebier B, Pailler T, Chase MW. Phylogenetics and biogeography of Mascarene angraecoid orchids (Vandeae, Orchidaceae) Molecular Phylogenetics and Evolution. 2008a;46:908–922. doi: 10.1016/j.ympev.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Micheneau C, Fournel J, Gauvin-Bialecki A, Pailler T. Auto-pollination in a long-spurred endemic orchid (Jumellea stenophylla) on Reunion Island (Mascarene Archipelago, Indian Ocean) Plant Systematics and Evolution. 2008b;272:11–22. [Google Scholar]
- Micheneau C, Fournel J, Humeau L, Pailler T. Orchid–bird interactions: a case study from Angraecum (Vandeae, Angraecinae) and Zosterops (white-eyes, Zosteropidae) on Reunion Island. Botany. 2008c;86:1143–1151. [Google Scholar]
- Micheneau C, Johnson SD, Fay MF. Orchid pollination: from Darwin to the present day. Botanical Journal of the Linnean Society. 2009;161:1–19. [Google Scholar]
- Nilsson LA. The evolution of flowers with deep corolla tubes. Nature. 1988;334:147–149. [Google Scholar]
- Nilsson LA, Rabakonandrianina E. Hawk-moth scale analysis and pollination specialization in the epilithic Malagasy endemic Aerangis ellisii (Reichenb. fil.) Schltr. (Orchidaceae) Botanical Journal of the Linnean Society. 1988;97:49–61. [Google Scholar]
- Nilsson LA, Jonsson L, Rason L, Randrianjohany E. Monophily and pollination mechanisms in Angraecum arachnites Schltr. (Orchidaceae) in a guild of long-tongued hawk-moths (Sphingidae) in Madagascar. Biological Journal of the Linnean Society. 1985;26:1–19. [Google Scholar]
- Nilsson LA, Jonsson L, Ralison L, Randrianjohany E. Angraecoid orchids and hawkmoths in central Madagascar: specialized pollination systems and generalist foragers. Biotropica. 1987;19:310–318. [Google Scholar]
- Olesen JM, Valido A. Lizards as pollinators and seed dispersers: an island phenomenon. Trends in Ecology and Evolution. 2003;18:177–181. [Google Scholar]
- Pailler T, Humeau L, Figier J, Thompson JD. Reproductive biology of the functionally dioecious and morphologically heterostylous island endemic Chassalia coralliodes (Rubiaceae) Biological Journal of the Linnean Society. 1998;64:297–313. [Google Scholar]
- Pellmyr O, Krenn HW. Origin of a complex key innovation in an obligate insect–plant mutualism. Proceedings of the National Academy of Sciences of the USA. 2002;99:5498–5502. doi: 10.1073/pnas.072588699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Pijl L, Dodson CH. Orchid flowers, their pollination and evolution. Coral Gables, FL: University of Miami Press; 1966. [Google Scholar]
- Proctor M, Yeo P, Lack A. The natural history of pollination. Portland, OR: Timber Press; 1996. [Google Scholar]
- Schiestl FP, Peakall R, Mant JG, et al. The chemistry of sexual deception in an orchid–wasp pollination system. Science. 2003;302:437–438. doi: 10.1126/science.1087835. [DOI] [PubMed] [Google Scholar]
- Strasberg D, Rouget M, Richardson DM, Baret S, Dupont J, Cowling RM. An assessment of habitat diversity and transformation on La Réunion Island (Mascarene Islands, Indian Ocean) as a basis for identifying broad-scale conservation priorities. Biodiversity and Conservation. 2005;14:3015–3032. [Google Scholar]
- Thompson JN. The coevolutionary process. Chicago, IL: Chicago University Press; 1994. [Google Scholar]
- Tremblay RL. Trends in the pollination ecology of the Orchidaceae: evolution and systematics. Botany. 1992;70:642–650. [Google Scholar]
- Tremblay RL, Ackerman JD, Zimmerman JK, Calvo RN. Variation in sexual reproduction in orchids and its evolutionary consequences: a spasmodic journey to diversification. Biological Journal of the Linnean Society. 2005;84:1–54. [Google Scholar]
- Wallace AR. Creation by law. Quarterly Journal of Science. 1867;4:470–488. [Google Scholar]
- Wallace AR. Contributions to the theory of natural selection. 2nd edn. London: Macmillan; 1871. [Google Scholar]
- Warren BH, Bermingham E, Bowie RCK, Prys-Jones RP, Thébaud C. Molecular phylogeography reveals island colonization history and diversification of western Indian Ocean sunbirds (Nectarinia: Nectariniidae) Molecular Phylogenetics and Evolution. 2003;29:67–85. doi: 10.1016/s1055-7903(03)00063-0. [DOI] [PubMed] [Google Scholar]
- Waser NM, Chittka L, Price MV, Williams NM, Ollerton J. Generalization in pollination systems, and why it matters. Ecology. 1996;77:1043–1060. [Google Scholar]
- Wasserthal LT. The pollinators of the Malagasy star orchids Angraecum sesquipedale, A. sororium and A. compactum and the evolution of extremely long spurs by pollinator shift. Botanica Acta. 1997;110:343–359. [Google Scholar]
- Whittall JB, Hodges SA. Pollinator shifts drive increasingly long nectar spurs in columbine flowers. Nature. 2007;447:706–709. doi: 10.1038/nature05857. [DOI] [PubMed] [Google Scholar]
- Wilson P, Castellanos MC, Wolfe AD, Thomson JD. Shifts between bee and bird pollination in penstemons. In: Waser NM, Ollerton J, editors. Plant–pollinator interactions: from specialization to generalization. Chicago, IL: University of Chicago Press; 2006. pp. 47–68. [Google Scholar]
- Wyatt R. Pollinator–plant interactions and the evolution of breeding systems. In: Real L, editor. Pollination biology. Orlando, FL: Academic Press; 1983. pp. 51–95. [Google Scholar]
- Zhang Z, Pawliszyn J. Headspace solid-phase micro-extraction. Analytical Chemistry. 1993;65:1843–1852. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.