Abstract
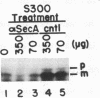
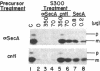
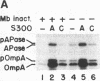
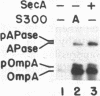
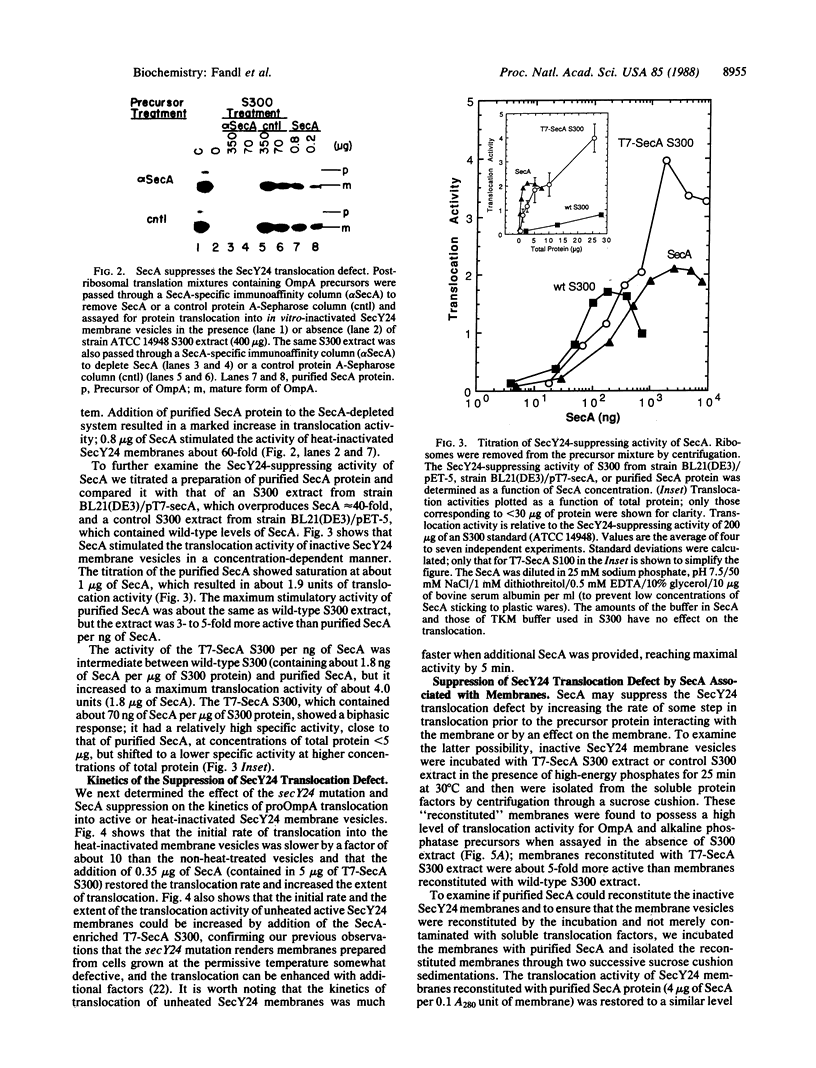
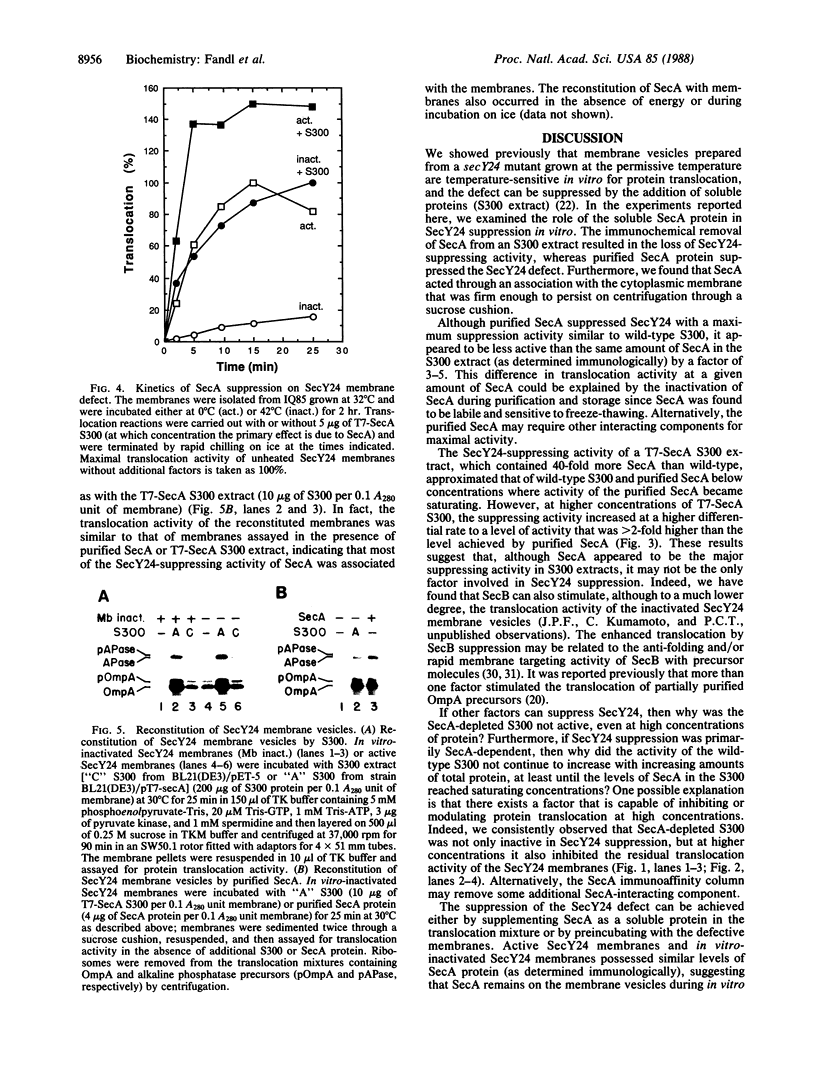
Genetic analysis of protein secretion in Escherichia coli has identified secY/prlA and secA as components of the secretory apparatus. We have examined the roles of the secY(prlA) gene product (an integral membrane protein) and the soluble secA gene product in translocation of OmpA and alkaline phosphatase precursors in an in vitro system. The protein translocation defect of the secY24 mutation was recently demonstrated in vitro as was its suppression by an S300 extract. We show here that the extract was essentially inactive in SecY24 suppression when SecA protein was removed from it by immunoaffinity chromatography. Furthermore, purified SecA protein suppressed the SecY24 defect. Preincubation of the inactivated SecY24 membrane vesicles either with S300 containing SecA or with purified SecA protein reconstituted the membranes and restored the translocation activity when assayed in the absence of additional soluble proteins. These results suggest that the SecY24 translocation defect is suppressed by SecA interacting, directly or indirectly, with SecY24 on the cytoplasmic membrane.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bacallao R., Crooke E., Shiba K., Wickner W., Ito K. The secY protein can act post-translationally to promote bacterial protein export. J Biol Chem. 1986 Sep 25;261(27):12907–12910. [PubMed] [Google Scholar]
- Bankaitis V. A., Bassford P. J., Jr Proper interaction between at least two components is required for efficient export of proteins to the Escherichia coli cell envelope. J Bacteriol. 1985 Jan;161(1):169–178. doi: 10.1128/jb.161.1.169-178.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckwith J., Ferro-Novick S. Genetic studies on protein export in bacteria. Curr Top Microbiol Immunol. 1986;125:5–27. doi: 10.1007/978-3-642-71251-7_2. [DOI] [PubMed] [Google Scholar]
- Brickman E. R., Oliver D. B., Garwin J. L., Kumamoto C., Beckwith J. The use of extragenic suppressors to define genes involved in protein export in Escherichia coli. Mol Gen Genet. 1984;196(1):24–27. doi: 10.1007/BF00334087. [DOI] [PubMed] [Google Scholar]
- Chen L., Rhoads D., Tai P. C. Alkaline phosphatase and OmpA protein can be translocated posttranslationally into membrane vesicles of Escherichia coli. J Bacteriol. 1985 Mar;161(3):973–980. doi: 10.1128/jb.161.3.973-980.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Tai P. C. ATP is essential for protein translocation into Escherichia coli membrane vesicles. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4384–4388. doi: 10.1073/pnas.82.13.4384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier D. N., Bankaitis V. A., Weiss J. B., Bassford P. J., Jr The antifolding activity of SecB promotes the export of the E. coli maltose-binding protein. Cell. 1988 Apr 22;53(2):273–283. doi: 10.1016/0092-8674(88)90389-3. [DOI] [PubMed] [Google Scholar]
- Crooke E., Wickner W. Trigger factor: a soluble protein that folds pro-OmpA into a membrane-assembly-competent form. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5216–5220. doi: 10.1073/pnas.84.15.5216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emr S. D., Hanley-Way S., Silhavy T. J. Suppressor mutations that restore export of a protein with a defective signal sequence. Cell. 1981 Jan;23(1):79–88. doi: 10.1016/0092-8674(81)90272-5. [DOI] [PubMed] [Google Scholar]
- Emr S. D., Silhavy T. J. Importance of secondary structure in the signal sequence for protein secretion. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4599–4603. doi: 10.1073/pnas.80.15.4599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fandl J. P., Tai P. C. Biochemical evidence for the secY24 defect in Escherichia coli protein translocation and its suppression by soluble cytoplasmic factors. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7448–7452. doi: 10.1073/pnas.84.21.7448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardel C., Benson S., Hunt J., Michaelis S., Beckwith J. secD, a new gene involved in protein export in Escherichia coli. J Bacteriol. 1987 Mar;169(3):1286–1290. doi: 10.1128/jb.169.3.1286-1290.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geller B. L., Movva N. R., Wickner W. Both ATP and the electrochemical potential are required for optimal assembly of pro-OmpA into Escherichia coli inner membrane vesicles. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4219–4222. doi: 10.1073/pnas.83.12.4219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye H., Michaelis S., Wright A., Beckwith J. Cloning and restriction mapping of the alkaline phosphatase structural gene (phoA) of Escherichia coli and generation of deletion mutants in vitro. J Bacteriol. 1981 May;146(2):668–675. doi: 10.1128/jb.146.2.668-675.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumamoto C. A., Beckwith J. Mutations in a new gene, secB, cause defective protein localization in Escherichia coli. J Bacteriol. 1983 Apr;154(1):253–260. doi: 10.1128/jb.154.1.253-260.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumamoto C. A., Gannon P. M. Effects of Escherichia coli secB mutations on pre-maltose binding protein conformation and export kinetics. J Biol Chem. 1988 Aug 15;263(23):11554–11558. [PubMed] [Google Scholar]
- Lee C. A., Beckwith J. Suppression of growth and protein secretion defects in Escherichia coli secA mutants by decreasing protein synthesis. J Bacteriol. 1986 Jun;166(3):878–883. doi: 10.1128/jb.166.3.878-883.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller M., Blobel G. Protein export in Escherichia coli requires a soluble activity. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7737–7741. doi: 10.1073/pnas.81.24.7737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver D. B., Beckwith J. E. coli mutant pleiotropically defective in the export of secreted proteins. Cell. 1981 Sep;25(3):765–772. doi: 10.1016/0092-8674(81)90184-7. [DOI] [PubMed] [Google Scholar]
- Oliver D. B., Beckwith J. Regulation of a membrane component required for protein secretion in Escherichia coli. Cell. 1982 Aug;30(1):311–319. doi: 10.1016/0092-8674(82)90037-x. [DOI] [PubMed] [Google Scholar]
- Oliver D. B., Liss L. R. prlA-mediated suppression of signal sequence mutations is modulated by the secA gene product of Escherichia coli K-12. J Bacteriol. 1985 Feb;161(2):817–819. doi: 10.1128/jb.161.2.817-819.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall L. L., Hardy S. J., Thom J. R. Export of protein: a biochemical view. Annu Rev Microbiol. 1987;41:507–541. doi: 10.1146/annurev.mi.41.100187.002451. [DOI] [PubMed] [Google Scholar]
- Rhoads D. B., Tai P. C., Davis B. D. Energy-requiring translocation of the OmpA protein and alkaline phosphatase of Escherichia coli into inner membrane vesicles. J Bacteriol. 1984 Jul;159(1):63–70. doi: 10.1128/jb.159.1.63-70.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggs P. D., Derman A. I., Beckwith J. A mutation affecting the regulation of a secA-lacZ fusion defines a new sec gene. Genetics. 1988 Apr;118(4):571–579. doi: 10.1093/genetics/118.4.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg A. H., Lade B. N., Chui D. S., Lin S. W., Dunn J. J., Studier F. W. Vectors for selective expression of cloned DNAs by T7 RNA polymerase. Gene. 1987;56(1):125–135. doi: 10.1016/0378-1119(87)90165-x. [DOI] [PubMed] [Google Scholar]
- Schmidt M. G., Rollo E. E., Grodberg J., Oliver D. B. Nucleotide sequence of the secA gene and secA(Ts) mutations preventing protein export in Escherichia coli. J Bacteriol. 1988 Aug;170(8):3404–3414. doi: 10.1128/jb.170.8.3404-3414.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiba K., Ito K., Nakamura Y., Dondon J., Grunberg-Manago M. Altered translation initiation factor 2 in the cold-sensitive ssyG mutant affects protein export in Escherichia coli. EMBO J. 1986 Nov;5(11):3001–3006. doi: 10.1002/j.1460-2075.1986.tb04598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiba K., Ito K., Yura T., Cerretti D. P. A defined mutation in the protein export gene within the spc ribosomal protein operon of Escherichia coli: isolation and characterization of a new temperature-sensitive secY mutant. EMBO J. 1984 Mar;3(3):631–635. doi: 10.1002/j.1460-2075.1984.tb01859.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stader J., Benson S. A., Silhavy T. J. Kinetic analysis of lamB mutants suggests the signal sequence plays multiple roles in protein export. J Biol Chem. 1986 Nov 15;261(32):15075–15080. [PubMed] [Google Scholar]
- Studier F. W., Moffatt B. A. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J Mol Biol. 1986 May 5;189(1):113–130. doi: 10.1016/0022-2836(86)90385-2. [DOI] [PubMed] [Google Scholar]
- Tai P. C. Biochemical studies of bacterial protein export. Curr Top Microbiol Immunol. 1986;125:43–58. doi: 10.1007/978-3-642-71251-7_5. [DOI] [PubMed] [Google Scholar]
- Weng Q. P., Chen L. L., Tai P. C. Requirement of heat-labile cytoplasmic protein factors for posttranslational translocation of OmpA protein precursors into Escherichia coli membrane vesicles. J Bacteriol. 1988 Jan;170(1):126–131. doi: 10.1128/jb.170.1.126-131.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]