Abstract
Background and Aims
Asexual organisms are more widespread in previously glaciated areas than their sexual relatives (‘geographical parthenogenesis’). In plants, this pattern is probably dependent on reproductive isolation and stability of cytotypes within their respective distribution areas. Both partial apomixis and introgressive hybridization potentially destabilize the spatial separation of sexual and apomictic populations. The wide distribution of apomicts may be further enhanced by uniparental reproduction which is advantageous for colonization. These factors are studied in the alpine species Ranunculus kuepferi.
Methods
Geographical distribution, diversity and mode of reproduction of cytotypes were assessed using flow cytometry and flow cytometric seed screening on samples from 59 natural populations of Ranunculus kuepferi. Seed set of cytotypes was compared in the wild.
Key Results
Diploid sexuals are confined to the south-western parts of the Alps, while tetraploid apomicts dominate in previously glaciated and in geographically isolated areas despite a significantly lower fertility. Other cytotypes (3x, 5x and 6x) occur mainly in the sympatric zone, but without establishing populations. The tetraploids are predominantly apomictic, but also show a partial apomixis via an uncoupling of apomeiosis and parthenogenesis in the seed material. Both pseudogamy and autonomous endosperm formation are observed which may enhance uniparental reproduction.
Conclusions
Diploids occupy a glacial relic area and resist introgression of apomixis, probably because of a significantly higher seed set. Among the polyploids, only apomictic tetraploids form stable populations; the other cytotypes arising from partial apomixis fail to establish, probably because of minority cytotype disadvantages. Tetraploid apomicts colonize previously devastated and also distant areas via long-distance dispersal, confirming Baker's law of an advantage of uniparental reproduction. It is concluded that stability of cytotypes and of modes of reproduction are important factors for establishing a pattern of geographical parthenogenesis.
Keywords: Apomixis, flow cytometry, geographical parthenogenesis, glaciations, polyploidy, Ranunculus kuepferi
INTRODUCTION
Vandel (1928) presented the term ‘geographical parthenogenesis’ for the phenomenon that related sexual and asexual taxa have different distribution areas. Later, several authors discussed the causality of the pattern for animals and plants by addressing a number of new aspects related to the wider distributions of asexuals (Bell, 1982; Bierzychudek, 1985; Asker and Jerling, 1992; Law and Crespi, 2002; Van Dijk, 2003; Kearney, 2005, 2006; Hörandl, 2006; Lundmark, 2006). Plants reproducing via apomixis, i.e. via asexually formed seed, tend to grow at higher altitudes and latitudes, and colonize more frequently previously glaciated areas than their sexual relatives (Bierzychudek, 1985; Van Dijk, 2003; Hörandl et al., 2008).
Asexual reproduction is in general frequently connected to polyploidy, and almost all apomictic plants are polyploid (Asker and Jerling, 1992). The tight connection of polyploidy and apomixis usually leads to differentiation and reproductive isolation of cytotypes (Van Dijk, 2007; Kao, 2007; Mráz et al., 2008). However, facultative apomixis can increase considerably the cytotype diversity within apomictic populations. Apomixis usually requires the co-ordination of embryo sac development without meiosis (apomeiosis) and the development of the egg cell without fertilization (parthenogenesis). The uncoupling of these processes leads to shifts in ploidy levels (Nogler, 1984a); meiosis plus parthenogenesis results in a dihaploid offspring (n + 0), while fertilization of an apomeiotic egg cell results in an increase of ploidy level (2n + n, Biii hybrids). Dihaploids and Biii hybrids are expected to undergo a decrease in fitness (Van Dijk and Vijverberg, 2005); continued reduction of ploidy levels results in the expression of previously masked recessive disadvantageous alleles, while continued increase in ploidy levels is limited by cellular constraints and functional disturbances of regulation mechanisms (Comai, 2005). A newly arisen, partially sexual cytotype may further suffer from minority cytotype disadvantages in the population (Levin, 1975), because it will mostly receive pollen of the wrong ploidy level. This may not only have negative effects on the fitness of the embryo, but also on endosperm formation. In the endosperm of flowering plants, a ratio of two maternal and one paternal copies of the genome are optimal for development, probably because of genomic imprinting; deviations from this ratio are sometimes tolerated, but often result in disturbances in seed formation (Vinkenoog et al., 2003, Spielmann et al., 2003; Talent, 2009). Since the majority of apomictic plants are pseudogamous and need pollen for endosperm fertilization, interploidal crosses pose a problem because they cause endosperm imbalance and potentially seed abortion. For these reasons, stability of cytotypes is probably an important factor for fitness and the distributional success of apomictic lineages. In fact, patterns of geographical parthenogenesis have so far not been reported for those taxa with a highly facultative and unstable apomixis (reviewed in Hörandl et al., 2008).
Most apomicts produce fertile, meiotically reduced pollen, and the genetic factors controlling apomixis can be inherited via the pollen (e.g. Asker and Jerling, 1992; Mogie, 1992). In mixed populations, an apomictic pollen donor can fertilize a sexual plant, thereby transferring apomixis to the offspring of the sexual. Under a simple model of dominant, single locus control for apomixis and an apomictic pollen donor heterozygous for the apomixis factor, some of the offspring of the sexual will become apomictic. In turn, the pollen of the sexual does not fertilize an apomictic plant, because the egg cell develops parthenogenetically. This unidirectional hybridization would result in introgression of apomixis from the apomicts into the sexual populations, but not vice versa. Theoretically, sexuality should disappear from the population after a few generations (Mogie, 1992; Mogie et al., 2007). Among other factors, this process could contribute to geographical parthenogenesis by replacing sexuals by apomicts in sympatric areas. However, Mogie (1992) has already pointed out that the amount of actual introgression also depends on female fertility, and a significantly higher fertility of sexuals prevents their replacement by apomictic cytotypes (Hörandl and Temsch, 2009). The fertility of cytotypes is therefore an important factor for geographical parthenogenesis.
Other theories explain geographical parthenogenesis by superior colonizing abilities. The capacity to found a new population from a single individual or seed is a big advantage for colonization, especially after long-distance dispersal (Baker's law; Baker, 1967; Hörandl, 2008, Hörandl et al., 2008). Here apomixis with autonomous endosperm formation provides an advantage over pseudogamy which still needs pollination, unless the plants can use self-pollen for endosperm fertilization (Dickinson et al., 2007; Hörandl, 2008). Sexuals, in contrast, are probably more efficient in habitats with regular pollinator frequencies and benefit from the advantage of genetic diversity. Since sexual species of apomictic complexes are usually self-sterile (Dickinson et al., 2007; Hörandl, 2009b), their ability to found populations in geographically distant areas is limited by the need of mating partners and pollinators.
Other theories explaining patterns of geographical parthenogenesis rely rather on genetic diversity of populations and a different response of sexual and apomictic populations to variable environments [e.g. host–parasite interactions (Van Valen, 1973; Vorburger, 2006); the benefit of general purpose genotypes (Lynch, 1984); niche differentiation of clones (Vrijenhoek, 1984, 1994)]. However, genetic diversity and the response to selection can be altered by polyploidy (Levin, 2002) and by clonal diversity (e.g. Van Dijk, 2003). Therefore, the assessment of distribution and stability of cytotypes and of modes of reproduction is indirectly of crucial importance for these models.
The alpine species Ranunculus kuepferi is an interesting model system for studying patterns and processes of geographical parthenogenesis in previously glaciated areas. Küpfer (1974) first recognized it as a separate species with diploid (2n = 16) and tetraploid (2n = 32) cytotypes. He found diploids only in the south-western parts of the Alps that have remained ice-free during the last glacial maximum of Würm glaciation (approx. 10 000 years ago). This area has long been known to be a glacial refugium for many plant species (Merxmüller, 1952, 1953, 1954; Schönswetter et al., 2005). The tetraploids, in contrast, were observed in the previously glaciated central western Alps. Later, Huber (1988) and Burnier et al. (2009) refined the distribution and reported the tetraploid cytotype eastwards to Eastern Tyrol (Austria). They assessed the presence of triploids (2n = 24) in the sympatric area of the diploid and the tetraploid cytotype, suggesting a hybrid zone. Outside the Alps, tetraploid cytotypes have been detected in Corsica and in the Apennines (Küpfer, 1974; Huber, 1989; Burnier et al., 2009).
Küpfer (1974) had already observed reduced pollen fertility (50–80 % aborted pollen grains), and reduced fertility (10–100 % of achenes aborted) in the tetraploid cytotype, while the diploid cytotypes had good pollen (0–20 % aborted) and achenes (0–10 % aborted). Information on other cytotypes and statistical evaluations of differences, however, were missing so far. An embryological observation by Burnier et al. (2009) on a single tetraploid specimen suggested an embryo-sac formation similar to the well-studied apomictic model system R. auricomus (Nogler, 1984a, b, 1995). Here meiosis takes place, but the megaspore tetrad aborts during the later development. Instead, a somatic cell of the nucellus develops into an unreduced, 8-nucleate embryo sac of the Polygonum type. This apomeiotic (aposporous) embryo sac development is coupled to a parthenogenetic development of the egg cell. These two processes are facultative and can be uncoupled, resulting in shifts of ploidy levels in the offspring (Nogler, 1984a, b, 1995).
In R. kuepferi, mode of reproduction and cytotype diversity within populations has not yet been assessed on population samples throughout the distributional range. Furthermore, the ploidy level and mode of reproduction of some geographically isolated outposts in the North Apennines (Mt Cusna) and in the eastern central Alps (populations around Turracherhöhe) were so far unknown. These areas potentially could represent glacial refugia for sexual diploid populations. The easternmost populations (Fig. 1, no. 59) are located very near to the last glacial maximum eastern refugia, a spot well known for its endemic flora (Tribsch, 2004; Schönswetter et al., 2005). Disjunct peripheral distributions of diploid species with related polyploid cytotypes in the centre of the Alps have been observed, for example, in the R. auricomus complex (Paun et al., 2006; Hörandl, 2009a) and in sexual Biscutella laevigata (Parisod and Besnard, 2007). In North America, diploid sexual cytotypes of Townsendia hookeri have a strongly disjunct distribution in peripheral refugia of the Wisconsin glaciation, while polyploid apomicts occur in the central previously glaciated area (Thompson and Whitton, 2006). Alternatively, these outposts in R. kuepferi could have been founded after long-distance dispersal by tetraploid apomicts. This scenario would support an idea of superior founder abilities of tetraploid apomicts according to Baker's law.
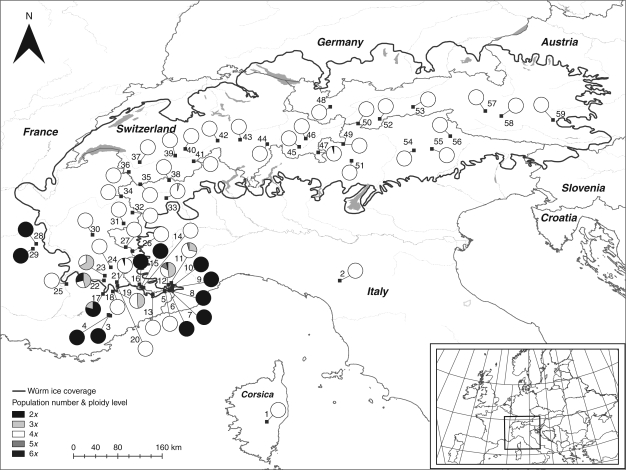
Fig. 1.
Map of the distribution of Ranunculus kuepferi. Small black squares connected to the pie diagrams indicate the location, population numbers correspond to Table 1. Pie diagrams present the proportions of cytotypes for each population, as indicated. The black line indicates the extension of the last glacial maximum of the Würm glaciation.
For R. kuepferi, a detailed study on cytotype diversity and mode of reproduction throughout the range of the species has been missing so far, i.e. it is uncertain whether the species shows a pattern of geographical polyploidy or geographical parthenogenesis. In the light of the reduced fertility of tetraploid cytotypes, their distributional success appears to be a paradox; advantages of apomixis may explain the observed pattern. Therefore, it is desirable to study stability and modes of apomixis of the species throughout its distributional range and to test stability of cytotypes and modes of reproduction within the respective areas. For the latter, possible introgression of apomixis into sexual species within the sympatric area, and the female fertility of cytotypes are of interest. It is followed by testing whether geographically isolated outposts outside the ice-shield of the Würm glaciation represent diploid sexual refugia or colonies of tetraploid apomicts. For these investigations into cytotype diversity, involving large sample sizes, the use of flow cytometric methods is a powerful approach. To assess modes of reproduction and pathways of embryo formation, flow cytometric seed screening (FCSS) is a highly efficient method (Matzk et al., 2000). The focus here is on intrinsic features of apomixis as factors of geographical parthenogenesis; patterns of genetic diversity within and among populations, and hypotheses on origins of apomictic cytotypes will be presented in forthcoming papers.
The following specific questions were addressed in the study. Does this distribution pattern represent a geographic distribution of sexual polyploidy or geographical parthenogenesis? How constant is the ploidy level within populations over all the distribution range, and how can new cytotypes be formed? In the hybrid zone, is there an indication of introgression of apomixis into the sexual populations, and do apomicts have a potential to replace the sexual lineages? Are the areas outside or near the margin of the previous ice-shield refugias of diploid sexual populations or is a colonization scenario by tetraploid apomicts more likely?
MATERIALS AND METHODS
Plant material
The plants of the species used in this research, Ranunculus kuepferi Greuter et Burdet, were collected in the wild during spring and summer 2004–2007; for details, see Table 1 and Fig. 1. About one-third of the individuals were cultivated in the experimental fields of the botanical garden of the University of Vienna. The rest of the samples were dried in silica gel for further molecular analysis. Mature achenes were collected in the wild and dried with silica gel, or taken from the plants in the experimental garden directly after collection (which means that embryo-sac formation and fertilization already has happened before on the natural site) and stored in the fridge at 4 °C.
Table 1.
Provenance of materials used in this study
| Population no.* | Country† | Province | Locality‡ | Altitude | Latitude | Longitude | Collector§ | Date |
|---|---|---|---|---|---|---|---|---|
| 1 | F | Corse-du-Sud | Corsica | 1541 m | 42°01′46·4″ | 9°12′34·5″ | ACC | 28 May 2005 |
| 2 | I | Emilia-Romagna | Mt Cusna | 1594 m | 44°18′06·7″ | 10°22′26·6″ | ACC–AC | 26 May 2006 |
| 3 | F | Var | La Chens I | 1610 m | 43°44′59·3″ | 6°39′25·5″ | PK | 4 June 2004 |
| 4 | F | Var | La Chens II | 1607 m | 43°44′58·5″ | 6°39°29·1″ | ACC–AC | 28 May 2006 |
| 5 | F | Alpes Maritimes | Col de Tende | 1888 m | 44°09′03·0″ | 7°33′56·3″ | ACC | 9 June 2004 |
| 6 | I | Piemonte | Valle di Pesio I 9589 | 1700 m | 44°11′43·68″ | 7°39′33·85″ | EH | 9 July 2007 |
| 7 | I | Piemonte | Valle di Pesio II 9592 | 1700 m | 44°11′43·68″ | 7°39′33·85″ | EH | 10 July 2007 |
| 8 | I | Piemonte | Passo del Duca 9525/9534 | 1700 m | 44°11′43·68″ | 7°39′33·85″ | EH | 14 July 2004 |
| 9 | I | Piemonte | Valle di Pesio III 9593 | 1925 m | 44°11′43·68″ | 7°39′33·85″ | EH | 10 July 2007 |
| 10 | I | Piemonte | Vallone Cravina 9595 | 1960 m | 44°13′24·72″ | 7°37′11·16″ | EH | 12 July 2007 |
| 11 | I | Piemonte | Col della Perla I 9596 | 2080 m | 44°9′11·05″ | 7°37′21·14″ | EH | 13 July 2007 |
| 12 | I | Piemonte | Col della Perla II 9597 | 2200 m | 44°9′11·05″ | 7°37′21·14″ | EH | 14 July 2007 |
| 13 | F | Alpes Maritimes | Notre Dame de la Fenestre | 1885 m | 44°05′45·3″ | 7°21′34·2″ | ACC | 11 June 2004 |
| 14 | F | Alpes Maritimes | Col d′Isola | 2210 m | 44°11′43·7″ | 7°09′19·7″ | ACC | 11 June 2004 |
| 15 | I | Piemonte | Colle della Lombarde I 9601 | 2260 m | 44°12′25·68″ | 7°8′51·98″ | EH | 15 July 2007 |
| 16 | I | Piemonte | Colle della Lombarde II 9602 | 2477 m | 44°13′17·60″ | 7°9′9·25″ | EH | 16 July 2007 |
| 17 | F | Alpes de Haute Provence | Vallette | 1820 m | 44°07·5′ | 6°33·5′ | PK | 4 June 2004 |
| 18 | F | Alpes de Haute Provence | Champs I | 1925 m | 44°09′59·5″ | 6°42′28·0″ | ACC | 13 June 2004 |
| 19 | F | Alpes Maritimes | Champs II | 2080 m | 44°10′33·8″ | 6°41′53·0″ | ACC–AC | 29 May 2006 |
| 20 | F | Alpes Maritimes | Cayolle I | 2193 m | 44°15′13·6″ | 6°44′52·1″ | ACC | 13 June 2004 |
| 21 | F | Alpes de Haute Provence | Cayolle II | 2325 m | 44°15′34·4″ | 6°44′41·3″ | ACC–AC | 30 May 2006 |
| 22 | F | Alpes de Haute Provence | Allos I | 2247 m | 44°22″′ | 6°37″′ | PK | 4 June 2004 |
| 23 | F | Alpes de Haute Provence | Allos II | 2080 m | 44°18′03·4″ | 6°35′06·0″ | ACC–AC | 29 May 2006 |
| 24 | F | Hautes Alpes | Vars | 2134 m | 44°32′21·3″ | 6°42′05·6″ | ACC | 14 June 2004 |
| 25 | F | Hautes Alpes | Raboux | 1859 m | 44°38′38·4″ | 5°58′43·1″ | ACC | 15 June 2004 |
| 26 | F | Hautes Alpes | Haute Queyras, Col de La Croix | 2000 m | 44°46′0″ | 7°01″′ | MH | 22 May 2004 |
| 27 | F | Hautes Alpes | Haute Queyras, Montette | 2000 m | 44°50′0″ | 6°55′0″ | MH | 23 May 2004 |
| 28 | F | Drôme | Vercors I | 1470 m | 44°54′07″ | 5°28′039″ | PK | 3 June 2004 |
| 29 | F | Drôme | Vercors II | 1325 m | 44°50′26·1″ | 5°25′23·1″ | ACC–AC | 30 May 2006 |
| 30 | F | Hautes Alpes | Col de Lautaret | 2060 m | 45°02′40·6″ | 6°24′04·1″ | ACC | 15 June 2004 |
| 31 | F | Savoie | Mt Cenis | 2025 m | 45°13′55·4″ | 6°53′53·6″ | ACC–AC | 3 July 2007 |
| 32 | F | Savoie | Col d′Iseran | 2768 m | 45°25′09·2″ | 7°01′52·9″ | ACC–AC | 3 July 2007 |
| 33 | I | Valle d′Aoste | Gran Paradiso | 2079 m | 45°37′0·5″ | 7°33′12·2″ | ACC–AC | 4 July 2007 |
| 34 | F | Savoie | Petit St Bernard | 2215 m | 45°40′40·4″ | 6°52′55·2″ | ACC–AC | 2 July 2007 |
| 35 | CH | Valais | Grand St Bernard | 2380 m | 45°52′09·9″ | 7°09′33·3″ | ACC–AC | 2 July 2007 |
| 36 | CH | Valais | Arpilles | 1830 m | 46°04·94′ | 7°.78′ | AC | 18 June 2006 |
| 37 | CH | Valais | Ovronnaz | 1840 m | 46°13·03′ | 7°09·52′ | AC | 18 June 2006 |
| 38 | I | Valle d′Aoste | Cervinia | 2200 m | 45°55′54·6″ | 7°38′18·1″ | ACC–AC | 4 July 2007 |
| 39 | CH | Valais | Jeizinen | 2020 m | 46°20′07″ | 7°43′85″ | ACC | 5 July 2004 |
| 40 | CH | Valais | Lötschental | 1773 m | 46°26′05·5″ | 7°51′48·0″ | ACC | 29 June 2006 |
| 41 | CH | Valais | Simplon Pass | 2019 m | 46°15′03·2″ | 8°01′48·2″ | ACC | 29 June 2006 |
| 42 | CH | Valais | Furka Pass | 2162 m | 46°34′45·8″ | 8°25′30·9″ | ACC | 28 June 2006 |
| 43 | CH | Ticino | Lukmanier Pass | 1946 m | 46°33′49·2″ | 8°47′54·7″ | ACC | 28 June 2006 |
| 44 | CH | Graubunden | Rheinwald 9603 | 2100 m | 46°33′16·05″ | 9°14′52·32″ | EH | 18 July 2007 |
| 45 | CH | Graubunden | Julier Pass | 2277 m | 46°28′20·9″ | 9°44′01·4″ | ACC | 27 June 2006 |
| 46 | CH | Graubunden | Albula Pass | 2312 m | 46·58333° | 9·83333° | ACC | 26 June 2006 |
| 47 | CH | Graubunden | Bernina Pass | 2301 m | 46°24′47·3″ | 010°01′17·3″ | ACC | 27 June 2006 |
| 48 | A | Vorarlberg | Arlberg Pass | 2269 m | 47°08′49·1″ | 10°14′55·3″ | ACC | 25 June 2006 |
| 49 | CH | Graubunden | Umbrail Pass | 2463 m | 46°32′51·3″ | 10°26′06·3″ | ACC | 26 June 2006 |
| 50 | A | Tirol | Kaunertal | 2525 m | 46°52′21·8″ | 10°42′37·5″ | ACC | 25 June 2006 |
| 51 | I | Trento | Tonale Pass | 2400 m | 46°16′21·27″ | 10°34′40·88″ | EH | 14 July 2006 |
| 52 | A | Tirol | Timmelsjoch | 2105 m | 46°55′13·5″ | 11°03′10·6″ | ACC | 24 June 2006 |
| 53 | A | Tirol | Tuxer Alps | 2315 m | 47°07′ | 11°34′ | CS | 6 July 2006 |
| 54 | I | Trento | Rosengarten | 2500 m | 46°27′19·56″ | 11°37′56·51″ | EH | 11 July 2006 |
| 55 | I | Trento | Padon Pass | 2350 m | 46°27′47·80″ | 11°53′42·88″ | EH | 9 July 2006 |
| 56 | I | Veneto | Mt Dürrenstein | 2400 m | 46°39′39·24″ | 12°10′57·86″ | EH | 18 July 2006 |
| 57 | A | Carinthia | Mt Großglockner | 2220 m | 47°4′47·63″ | 12°45′50·37″ | EH | 12 August 2005 |
| 58 | A | Carinthia | Mt Sadnig | 2200 m | 46°57′42″ | 13°′35″ | PS–GS | 7 July 2006 |
| 59 | A | Carinthia | Turracherhöhe | 2220 m | 46°55′20·47″ | 13°52′44·35″ | EH | 14 August 2005 |
* The numbers correspond to those used in Fig. 1.
† F, France; I, Italy; A, Austria; CH, Switzerland.
‡ A roman number indicates repeated sampling on the same population in different years; arabic numbers are herbaria numbers of E. Hörandl (vouchers were deposited in the herbarium of the University of Vienna, WU).
§ ACC, Anne-Caroline Cosendai; ACC–AC, Anne-Caroline Cosendai and André Cosendai; CS, Christoph Seger; EH, Elvira Hörandl; MH, Marc Hämmerli; PK, Philippe Küpfer; PS–GS, Peter Schönswetter and Gerald Schneeweiss.
Ploidy-level determination
Previous chromosome counts of root tip squashes (Cosendai, 2005) were used to fix genome size measures to ploidy levels. Samples were measured via flow cytometry (FC) on fresh and silica-dried material of leaves. The difference in genome size between dried and fresh material was never >10 % of variation, comparable to results obtained by Suda and Trávnícek (2006) for silica-dried material. It is noted that the genome size of the fresh material is, in this case, almost always slightly smaller than the silica-gel material; some of the smallest DNA content values are due to old silica gel-dried material (>4 years old) and look very degraded (yellow brownish material). These samples are indicated with an asterisk in Table 3 and may be unreliable for absolute genome-size estimates, but nevertheless, allow for a reliable assessment of ploidy levels. Nuclei extraction was prepared following the procedure presented in Galbraith et al. (1983) and Doležel et al. (2007a). Leaf material was chopped in Otto buffer I modified (with 1·26 g citric acid and 6 mL Triton X-100 for 60 mL final volume) (Doležel et al., 2007b; Temsch et al., 2008) with Pisum sativum (Ps) line ‘Ctirad’ but mostly with Zea mays (Zm) line ‘CE-777’ seedlings (4·38 pg and 2·73 pg DNA/2C, respectively; Doležel et al., 1998; Greilhuber, 2008) as standard; then RNase (3 mg mL–1; Sigma) was added to the extract and incubated at 37 °C for 0·5 h in a water bath. Otto II buffer with propidium iodide (VWR international; final concentration 50 µg mL) (on the basis of Baranyi and Greilhuber, 1996; Temsch et al., 2009) was added to the suspension of nuclei and stored at 4 °C for about 1 h. Samples were analysed on a CyFlow ML flow cytometer (Partec, Muenster, Germany) equipped with a green laser (100 mW, 532 nm, Cobolt Samba; Cobolt AB, Stockholm, Sweden). Five thousand particles were measured per run and mostly three runs were conducted per sample. Analyses of the runs and peak detection were made with the FloMax® software 2·0·0·1 (Partec, Muenster, Germany). The Cx value in the sense of Greilhuber et al. (2005) was calculated based on a linear relationship between the standard and the sample fluorescence intensity. The mean values are calculated on the basis of measuring 25 samples per populations. Preparations were mostly repeated three times for statistical stability. Several measures were done by pooling the samples of several individuals. Appearance of a single peak indicated that the pooled samples were of the same genome size and consequently of the same ploidy level. The picogram and Cx values were calculated per population (Table 2).
Table 3.
Summary of seed flow cytometric data with the observed ploidy levels in embryo and endosperm, and the inferred mode of reproduction
| Mode of reproduction |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Population no. | Population name | Individual no. | Embryo | Second peak/G2 phase | Endosperm | Origin of embryo sac | Egg cell | Endosperm development* | Mother plant† |
| 8 | Passo del Duca 9534 | 04 | 3x | 8x | Disturbed meiotic | Parthenogenetic | Pseudogamous I | NA | |
| 08 | 3x | 8x | Disturbed meiotic | Parthenogenetic | Pseudogamous I | NA | |||
| 11 | Col della Perla I 9596 | 13 | 5x | 10x | 8x | Meiotic | Fertilized | Sexual | 5x |
| 12 | Col della Perla II 9597 | 07 | 3x | 6x | 8x | Disturbed meiotic | Parthenogenetic | Pseudogamous I | 4x |
| 01 | 4x | 6x | Meiotic | Fertilized | Sexual | 4x | |||
| 03 | 4x | 8x | 6x | Meiotic | Fertilized | Sexual | 4x | ||
| 03 (new) | 4x | 5x | 6x | Meiotic | Fertilized | Sexual | 4x | ||
| 08 | 4x | 10x | Disturbed meiotic | Parthenogenetic | Pseudogamous I | 5x | |||
| 15 | 4x | 10x | Apomeiotic | Parthenogenetic | Pseudogamous I | 4x | |||
| 26 | 4x | 8x | Apomeiotic | Parthenogenetic | Autonomus | 4x | |||
| 13 | Notre Dame de la Fenestre | 05 | 3x | 10x | Disturbed meiotic | Parthenogenetic | Pseudogamous I | 4x | |
| 02 | 4x | 12x | Apomeiotic | Parthenogenetic | Pseudogamous II | 4x | |||
| 15 | Colle della Lombarde I 9601 | 01 | 2x | 3x | Meiotic | Fertilized | Sexual | 2x | |
| 21 | Cayolle II | 09·1 | 4x | 12x | Apomeiotic | Parthenogenetic | Pseudogamous II | 4x | |
| 09·2 | 4x | 10x | Apomeiotic | Parthenogenetic | Pseudogamous I | 4x | |||
| 15 | 4x | 8x | Apomeiotic | Parthenogenetic | Autonomous | 4x | |||
| 23 | Allos II | 09 | 3x | 8x | Apomeiotic | Parthenogenetic | Pseudogamous I | 3x | |
| 24 | Vars | 06 | 3x | NA | Disturbed meiotic | Parthenogenetic | Aborted | 4x | |
| 15 | 3x | NA | Disturbed meiotic | Parthenogenetic | Aborted | 4x | |||
| 16 | 3x | NA | Disturbed meiotic | Parthenogenetic | Aborted | 4x | |||
| 29 | Vercors II | 05·1 | 2x | 3x | Meiotic | Fertilized | Sexual | 2x | |
| 05·2 | 2x | 3x | Meiotic | Fertilized | Sexual | 2x | |||
| 06 | 2x | 3x | Meiotic | Fertilized | Sexual | 2x | |||
| 07 | 2x | 3x | Meiotic | Fertilized | Sexual | 2x | |||
| 19 | 2x | 3x | Meiotic | Fertilized | Sexual | 2x | |||
| 23 | 2x | 3x | Meiotic | Fertilized | Sexual | 2x | |||
| 05 | 2x | 3x | Meiotic | Fertilized | Sexual | 2x | |||
| 31 | Mt Cenis | 06 | 4x | NA | Apomeiotic | Parthenogenetic | Aborted | 4x | |
| 32 | Col d'Iseran | 01 | 4x | NA | Apomeiotic | Parthenogenetic | Aborted | 4x | |
| 02 | 4x | NA | Apomeiotic | Parthenogenetic | Aborted | 4x | |||
| 33 | Grand St Bernard | 11 | 3x | 5x | 8x | Disturbed meiotic | Parthenogenetic | Pseudogamous I | 4x |
| 14 | 3x | NA | Disturbed meiotic | Parthenogenetic | Aborted | 4x | |||
| 15 | 3x | 14x | Disturbed meiotic | Parthenogenetic | Pseudogamous II | 4x | |||
| 11 + 12 | 4x | 6x | 12x | Apomeiotic | Parthenogenetic | Pseudogamous II and sexual | 4x | ||
| 12 | 4x | 10x | Apomeiotic | Parthenogenetic | Pseudogamous I | 4x | |||
| 13 (new) | 4x | 10x | Apomeiotic | Parthenogenetic | Pseudogamous I | 4x | |||
| 21 | 4x | 10x | Apomeiotic | Parthenogenetic | Pseudogamous I | 4x | |||
| 21 | 4x | 10x | Apomeiotic | Parthenogenetic | Pseudogamous I | 4x | |||
| 26 | 4x | NA | Apomeiotic | Parthenogenetic | Aborted | 4x | |||
| 13 | 6x | 20x | Apomeiotic | Fertilized | Pseudogamous II | 4x | |||
| 37 | Ovronnaz | 19 | 4x | 12x | Apomeiotic | Parthenogenetic | Pseudogamous II | 4x | |
| 40 | Lötschental | 26 | 3x | 8x | Disturbed meiotic | Parthenogenetic | Pseudogamous I | 4x | |
| 41 | Simplon Pass | 21 | 3x | 10x | Disturbed meiotic | Parthenogenetic | Pseudogamous I | 4x | |
| 25 | 3x | 6x | 9x | Disturbed meiotic | Parthenogenetic | Pseudogamous I | 4x | ||
| 08 | 4x | 12x | Apomeiotic | Parthenogenetic | Pseudogamous II | 4x | |||
| 18 | 4x | NA | Apomeiotic | Parthenogenetic | Aborted | 4x | |||
| 23 | 4x | 12x | Apomeiotic | Parthenogenetic | Pseudogamous II | 4x | |||
| 43 | Lukmanier Pass | 12 | 3x | 8x | Disturbed meiotic | Parthenogenetic | Pseudogamous I | 4x | |
| 26 | 3x | 12x | Disturbed meiotic | Parthenogenetic | Pseudogamous II | 4x | |||
| 44 | Rheinwald | 03 | 3x | 10x | Disturbed meiotic | Parthenogenetic | Pseudogamous I | 4x | |
| 01 | 3x | 10x | Disturbed meiotic | Parthenogenetic | Pseudogamous I | 4x | |||
| 47 | Bernina Pass | 22 | 3x | 8x | Disturbed meiotic | Parthenogenetic | Pseudogamous I | 4x | |
| 57 | Mt Großglockner | 02 | 3x | 9x | Disturbed meiotic | Parthenogenetic | Pseudogamous I | 4x | |
| Herbar | 3x | 10x | Disturbed meiotic | Parthenogenetic | Pseudogamous II | 4x | |||
| 58 | Mt Sadnig | Herbar | 3x | 8x | Disturbed meiotic | Parthenogenetic | Pseudogamous I | 4x | |
| 59 | Turracherhöhe | 01 | 4x | 10x | Apomeiotic | Parthenogenetic | Pseudogamous I | 4x | |
| 02 | 4x | 10x | Apomeiotic | Parthenogenetic | Pseudogamous I | 4x | |||
| 03 | 4x | 10x | Apomeiotic | Parthenogenetic | Pseudogamous I | 4x | |||
NA, Not applicable.
* Pseudogamous I and II refer to a fertilization by one or two pollen nuclei, respectively (see also Hörandl et al., 2008).
† Data are taken from leaf measurements.
Table 2.
Summary of the population's genome size and ploidy level
| Populations names* | Mean values of population (pg) | Standard deviation | Coefficient of variation (%) | No. of individuals | Percentage of ploidy level in the population |
|---|---|---|---|---|---|
| 2x cytotypes | |||||
| Vallone Cravina 9595 (s) | 4·433 | 0·040 | 0·911 | 17 | 100·0 |
| Lachens II (s) | 4·144 | 0·069 | 1·675 | 22 | 100·0 |
| Colle della Lombarde I 9601 (s) | 4·459 | 0·091 | 2·037 | 24 | 100·0 |
| Colle della Lombarde II 9602 (s) | 4·405 | 0·027 | 0·602 | 11 | 100·0 |
| Valle di Pesio I 9589 (s) | 4·429 | 0·036 | 0·805 | 25 | 100·0 |
| Valle di Pesio II 9592 (s) | 4·462 | 0·042 | 0·946 | 4 | 100·0 |
| Valle di Pesio III 9593 (s) | 4·419 | 0·045 | 1·015 | 14 | 100·0 |
| Passo del Duca 9525 (s)† | 3·820 | 0·024 | 0·639 | 5 | 100·0 |
| Valette (s)† | 3·941 | 0·164 | 4·149 | 24 | 79·2 |
| Vercors II (s) | 4·058 | 0·050 | 1·220 | 18 | 100·0 |
| Mean | 4·257 | 0·244 | |||
| 3x cytotypes | |||||
| Allos I (f) | 6·322 | 0·057 | 0·909 | 3 | 66·7 |
| Allos I (s) | 6·487 | 0·058 | 0·893 | 12 | 91·7 |
| Allos II (s) | 6·487 | 0·058 | 0·891 | 24 | 45·8 |
| Champs I (s) | 6·431 | 0·015 | 0·228 | 1 | 100·0 |
| Champs II (f) | 6·269 | 0·246 | 3·920 | 23 | 47·8 |
| Champs II (s) | 6·450 | 0·040 | 0·624 | 7 | 28·6 |
| Gran Paradiso (s) | 5·519 | 24 | 4·2 | ||
| Col della Perla I 9596 (s) | 6·599 | 0·079 | 1·195 | 23 | 47·8 |
| Col della Perla II 9597 (s) | 6·652 | 0·072 | 1·078 | 22 | 27·3 |
| Valette (s)† | 5·964 | 0·318 | 5·325 | 24 | 20·8 |
| Mean | 6·318 | 0·340 | |||
| 4x populations | |||||
| Arpilles (s) | 8·372 | 0·223 | 2·666 | 19 | 100·0 |
| Albula (s) | 8·704 | 0·095 | 1·087 | 25 | 100·0 |
| Allos I (s) | 8·559 | 0·021 | 0·246 | 12 | 8·3 |
| Allos II (s) | 8·597 | 0·128 | 1·485 | 24 | 25·0 |
| Allos I (f) | 8·015 | 0·000 | 0·000 | 3 | 33·3 |
| Allos II (f) | 8·341 | 0·025 | 0·299 | 2 | 50·0 |
| Bernina Pass (s) | 8·707 | 0·226 | 2·599 | 24 | 95·8 |
| Cayolle II (f) | 8·468 | 0·135 | 1·596 | 20 | 95·0 |
| Cayolle II (s) | 8·543 | 0·142 | 1·662 | 9 | 88·9 |
| Cervinia (s) | 8·578 | 0·038 | 0·438 | 25 | 100·0 |
| Champs II (f) | 8·419 | 0·082 | 0·973 | 23 | 52·2 |
| Champs II (s) | 8·631 | 0·032 | 0·376 | 7 | 71·4 |
| Corsica (f) | 8·196 | 0·277 | 3·385 | 2 | 100·0 |
| Mt Cusna (f) | 8·552 | 0·017 | 0·201 | 4 | 100·0 |
| Dürrenstein (s) | 8·514 | 0·093 | 1·097 | 10 | 100·0 |
| Furka Pass (s) | 8·524 | 0·071 | 0·831 | 25 | 100·0 |
| Gd Paradiso (s) | 8·671 | 0·361 | 4·163 | 24 | 95·8 |
| Grand St Bernard (s) | 8·716 | 0·091 | 1·048 | 24 | 100·0 |
| Col d'Iseran (s) | 8·603 | 0·049 | 0·572 | 25 | 100·0 |
| Julier Pass (s) | 8·251 | 0·051 | 0·621 | 25 | 100·0 |
| Kaunertal (s) | 8·603 | 0·097 | 1·132 | 25 | 100·0 |
| Lötschental (s) | 8·136 | 0·103 | 0·025 | 25 | 100·0 |
| Lukmanier (s) | 8·158 | 0·059 | 0·720 | 25 | 100·0 |
| Mt Cenis (s) | 8·596 | 0·094 | 1·098 | 24 | 100·0 |
| Ovronnaz (s) | 8·552 | 0·008 | 0·096 | 8 | 100·0 |
| Padon (s) | 8·835 | 0·092 | 1·044 | 25 | 100·0 |
| Col della Perla I 9596 (s) | 8·658 | 0·125 | 1·438 | 23 | 34·8 |
| Col della Perla II 9597 (s) | 8·643 | 0·089 | 1·027 | 22 | 68·2 |
| Pt St Bernard (s) | 8·702 | 0·068 | 0·776 | 24 | 100·0 |
| Rheinwald 9603 (s) | 8·547 | 0·067 | 0·781 | 21 | 100·0 |
| Rosengarten (s) | 8·584 | 0·067 | 0·780 | 20 | 100·0 |
| Mt Sadnig (s) | 8·749 | 0·067 | 0·771 | 15 | 100·0 |
| St Anton (s) | 8·834 | 0·094 | 1·068 | 25 | 100·0 |
| Simplon (s) | 8·808 | 0·247 | 2·803 | 25 | 100·0 |
| Timmelsjoch (f) | 8·314 | 0·077 | 0·931 | 1 | 100·0 |
| Timmelsjoch (s) | 8·559 | 0·146 | 1·702 | 25 | 100·0 |
| Tonale Pass (f) | 8·462 | 0·014 | 0·160 | 1 | 100·0 |
| Turracherhöhe (s) | 8·617 | 0·065 | 0·753 | 26 | 100·0 |
| Tuxer Alps (s) | 8·608 | 0·062 | 0·724 | 25 | 100·0 |
| Umbrail Pass (s) | 8·731 | 0·295 | 3·375 | 25 | 100·0 |
| Mean | 8·552 | 0·184 | |||
| 5x populations | |||||
| Allos II (f) | 10·337 | 0·134 | 1·292 | 2 | 50·0 |
| Allos II (s) | 10·649 | 0·163 | 1·533 | 24 | 29·2 |
| Col della Perla I 9596 (s) | 10·633 | 0·121 | 1·134 | 23 | 13·10 |
| Col della Perla II 9597 (s) | 10·755 | 0·052 | 0·484 | 22 | 4·50 |
| Mean | 10·540 | 0·175 | |||
| 6x populations | |||||
| Bernina (s) | 12·685 | 0·220 | 1·731 | 24 | 4·20 |
| Cayolle II (f) | 12·671 | 0·114 | 0·900 | 20 | 5·0 |
| Cayolle II (s) | 13·024 | 0·118 | 0·905 | 9 | 11·10 |
| Perla I 9596 (s) | 12·916 | 0·062 | 0·482 | 23 | 4·30 |
| Mean | 12·793 | 0·199 | |||
| Summary of ploidy level | No. of individuals | Percentage of cytotype | |||
| 2x | 159 | 15·7 | |||
| 3x | 61 | 6·02 | |||
| 4x | 778 | 76·8 | |||
| 5x | 12 | 1·18 | |||
| 6x | 3 | 0·3 | |||
| Total | 1013 | ||||
* (s) or (f) at the end of the population name indicates silica-dried or fresh material, respectively.
† Indicates old material (collected in 2004).
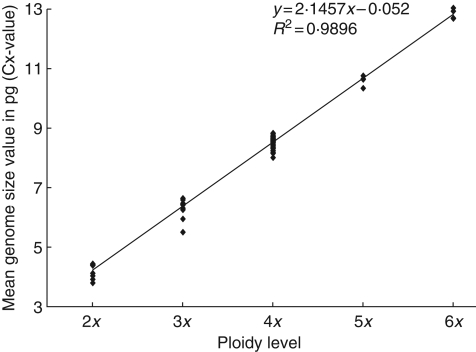
Low differences in standard deviations and low coefficients of variation (mostly below 3 %; Table 2) indicate a rather stable genome size within populations, falling clearly into distinct classes corresponding to cytotypes. A regression analysis confirmed that the increase in genome size with ploidy levels is almost perfectly linear, so that ploidy levels are almost exactly double or 1·5-fold the previous ploidy level (Fig. 2). This analysis confirms the reliability of the measurements for the ploidy level assessments.
Fig. 2.
Linear regression of the relative ploidy level Cx value and the genome size in picograms based on leaf material. The slope of the regression is indicated.
FCSS
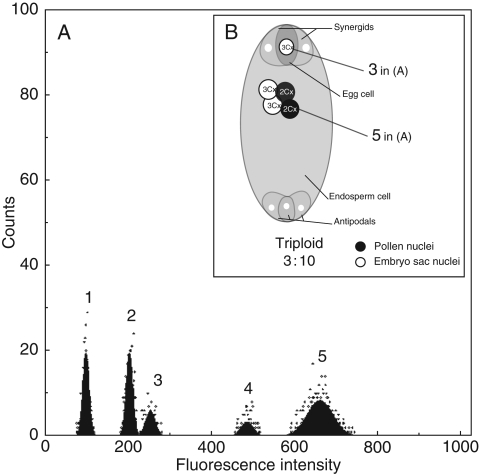
This part of the research is based on mature seeds and the mode of reproduction on the basis of Polygonum embryo sac type, as found by Vuille and Küpfer (1985) in the species Ranunculus parnassifolius and by Nogler (1984b, 1995) in Ranunculus auricomus. Furthermore, some recent embryological observations (Burnier et al., 2009; J. Wagner, pers. comm.) confirm that Ranunculus kuepferi has an embryo sac of the Polygonum type. The principle here is to measure the quantity of the genome in the embryo and the endosperm, which appear in two different peaks in the histogram file. With this information, one can determine the ploidy level of the embryo and endosperm, and reconstruct the pathway of seed formation (Matzk et al., 2000). Achenes were prepared from 58 individuals according to the same protocol as for leaves. The achenes were softened in Otto I buffer for about 5 min on ice and then chopped following the same procedure as in FC with Zea mays (Zm) or Pisum sativum (Ps) standard. Per sample, four to seven achenes were used, depending on the quality and the quantity of fruits produced by the plant. Figure 3 illustrates an example of the interpretation of an FCSS histogram. Beside the peaks of the standard (Zm), three peaks for the seeds were observed: the first peak (Rk) is the embryo corresponding to a triploid Cx value; the second peak is interpreted as the G2 of the embryo as it has double the amount of DNA of the embryo peak. The third peak has a ratio/picogram value of 10 Cx, corresponding to an endosperm that has arisen from two polar nuclei (3x + 3x) plus fertilization of two pollen nuclei (2x + 2x). Based on these data, it was possible to determine the formation of seeds and the assumed mode of reproduction.
Fig. 3.
(A) Histogram of FCSS with five peaks: 1 and 2, standard Zea mays in the G1 and G2 phase, respectively; 3–5, Ranunculus kuepferi (Rk): 3, 3 Cx, embryo G1 phase; 4, embryo G2 phase; 5, 10 Cx endosperm (peak G1 phase). (B) Scheme of the respective embryo sac after fertilization, illustrating a triploid unfertilized embryo corresponding to peak 3, and the 10 Cx endosperm corresponding to peak 5. Since the mother plant was tetraploid, the embryo sac must have developed via a disturbed meiosis. The 3x em developed without fertilization, the endosperm via pseudogamy.
Fertility of cytotypes
Female fecundity of cytotypes was assessed on 116 individuals from 21 populations, by using achenes collected in the wild or directly after transfer to the experimental garden. The number of well-developed and aborted achenes was assessed as in Hörandl (2008). Percentages of well-developed achenes per collective fruit were calculated, pooled for cytotypes. After arcsin transformation of percentages, the significance of differences between cytotypes was tested via one-way ANOVA; for this test, the single sample from a hexaploid plant was pooled with the values of pentaploids. Since Levene's statistic revealed unequal variances among groups, Tanhame's test for pairwise multiple comparisons (not assuming equal variances) was used to test differences among cytotypes. SPSS for Windows vs. 12 was used for all calculations.
RESULTS
Spatial distribution and frequencies of cytotypes
The geographical distribution of cytotypes largely confirms a spatial separation of the two most frequent ploidy levels, the diploids and tetraploids (Fig. 1). The distribution of diploids is confined to the south-western borders of the Alps outside the range of the last previous glacial maximum. Tetraploids are distributed all over the Alps in the previously glaciated area, including the population near the eastern margin of the former ice-shield. Also the geographically isolated populations at Mt Cusna and Corsica are tetraploid. A more diverse zone occurs at the overlapping distribution area of diploids and tetraploids in the south-western Alps, where diploids, triploids, tetraploids, pentaploids and hexaploids are sympatric, and co-occur sometimes in the same population. Notably, triploids and hexaploids also do occur occasionally outside the hybrid zone, in Gran Paradiso (no. 33) and in the Bernina massif (no. 47). Only diploid and tetraploid cytotypes are predominant within their respective populations (100 % frequencies; Fig. 1). Triploid, pentaploid and hexaploid cytotypes are less stable, because they occur either only in mixed populations, or as low-frequency cytotypes within predominantly diploid or tetraploid populations. Within mixed populations, frequencies of triploids are highest among all the other rare cytotypes.
Mode of reproduction
FCSS revealed an unexpected high diversity of reproductive pathways (Table 3). FCSS confirms that diploids are sexual with a 2x embryo and a 3x endosperm. There was only one seed sample from a triploid mother plant (Allos, no. 23), which appeared to be apomictic and pseudogamous. Tetraploid mother plants showed a broader range of variation in seed formation, indicating that the species has not a fixed apomixis. The majority of tetraploid plants, are apomeiotic and pseudogamous with the use of one or two pollen nuclei for fertilization of the endosperm (4x embryos and 10x to 12x endosperm); two cases of autonomous endosperm formation (8x) are also indicated. Three individuals from the Col della Perla population (no. 12) had a fully sexual reproduction with 4x embryos and 6x endosperms. One tetraploid plant from Grand St Bernard (no. 35) had seeds with a hexaploid embryo, and a highly ploid, 20x endosperm. Here fertilization of an unreduced 4x egg cell by 2x pollen, and an endopolyploid endosperm is likely. Surprisingly, several samples showed triploid embryos as in Fig. 3. As they came from tetraploid parents, the embryo sac must have been formed via an unbalanced meiosis to produce triploid egg cells and polar nuclei. The egg cell developed parthenogenetically, while the endosperm was probably fertilized by one or two diploid sperm nuclei. Only this way can the ratio of 3x : 8x or 3x : 10x be explained. These seeds occur in a couple of populations with predominantly 4x adult plants outside the putative hybrid zone. Several tetraploid plants formed 3x or 4x embryos, but no endosperm peak was detected. Here either abortion or a rapid consumption of the endosperm by the growing embryo could have happened, as it is known from Asteraceae (Krahulcova and Suda, 2006). Pentaploid individuals from the Col della Perla population (no. 11) seem to form reduced embryo sacs and fertilized egg cells. No seeds were available for analysis from hexaploid plants.
Fertility of cytotypes
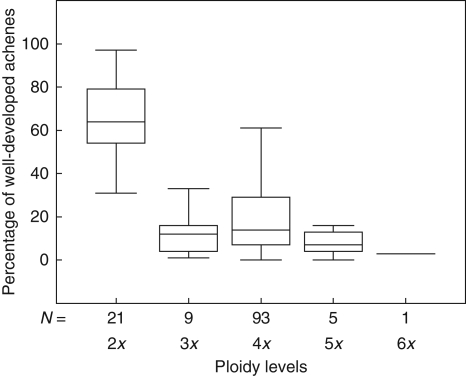
The diploid cytotype has the highest fertility, with 30·8–97·1 % of achenes within collective fruits well developed, while all the polyploids ranged from 0 to 60·9 % (Table 4 and Fig. 4). The tetraploid cytotype falls with its maximum within the range of the diploids, but the interquartile range remains below the minimum of the diploids (Fig. 4). However, several samples (15 individuals) had no well-developed achenes at all, including the population from Mt Cusna. This explains that also the mean values of tetraploids remain low. ANOVA revealed a significant difference between percentages of well-developed achenes among the cytotypes (5x and 6x pooled; d.f. among groups = 3; d.f. within groups = 125; F = 68·055; P < 0·001). The pairwise multiple comparisons using Tanhame's post hoc tests revealed highly significant differences between the diploids and all polyploid cytotypes (P < 0·001 for all pairs). Between the 4x and the pooled 5x–6x cytotype, the difference is significant (P = 0·017), but there is neither a significant difference between 4x and 3x nor between 3x and 5x–6x cytotypes (P > 0·5).
Table 4.
Descriptive statistics of the percentages of well-developed achenes per collective fruit
| Cytotypes | N | Mean | Minimum | Maximum | s.d. |
|---|---|---|---|---|---|
| 2x | 21 | 67·79 | 30·77 | 97·14 | 17·96 |
| 3x | 9 | 11·73 | 1·25 | 33·33 | 9·96 |
| 4x | 93 | 17·96 | 0·00 | 60·87 | 15·51 |
| 5x | 5 | 7·83 | 0·00 | 15·56 | 6·24 |
| 6x | 1 | 3·17 | 3·17 | 3·17 |
N = no. of collective fruits analysed.
Fig. 4.
Boxplots of the variation of percentages of well-developed achenes per collective fruit for each cytotype. The box shows the 25th and 75th percentile range and the median value. N = number of collective fruits.
DISCUSSION
Stability of tetraploid cytotypes enhances geographical parthenogenesis
The present data confirm that the diploid sexual populations are confined to a relic area in the south-western Alps outside the range of the ice cover of the last glacial maximum. On the whole distribution range in the previously glaciated area, tetraploids predominate; they are stable in the sense that only occasionally do other cytotypes occur within the tetraploid populations. Since tetraploids are confirmed to be predominantly apomictic or at least facultatively apomictic, these results confirm geographical parthenogenesis in this species.
Within the tetraploid populations, only a few rare hexaploid and triploid individuals occur far away from the putative hybrid zone where they are common. It seems that new cytotypes arise occasionally from partial apomixis (i.e. by uncoupling of apomeiosis and parthenogenesis), but they do have some difficulty in becoming established. They probably encounter few mating partners of the same ploidy level, resulting in meiotic disturbances and a lower fitness in the offspring. These factors may block the establishment of a critical number of individuals for survival of the new cytotype, as Levin (1975, 2002) described under the term ‘minority cytotype disadvantage’. The apomictic tetraploid cytotype, in contrast, avoids negative effects of meiotic disturbances and does not need a mating partner. Therefore, apomicts do not suffer from being a minority in the population (Hörandl, 2006), and can readily establish even aneuploid populations, as shown, for example, by Mraz et al. (2008) in Pilosella. For endosperm fertilization, apomictic R. kuepferi can use either self-pollen (Huber, 1988; E. Hörandl, unpubl. res.) or pollen of another cytotype in the population. The great variation in ploidy levels observed in the endosperm of well-developed seed suggests that seed formation in R. kuepferi is not highly sensitive against endosperm imbalance, similar to some pseudogamous Rosaceae (Talent and Dickinson, 2007). Therefore, pollination by another cytotype would not confer a minority cytotype disadvantage for endosperm formation in this species. Autonomous endosperm formation, as also observed occasionally in R. kuepferi, is completely pollen-independent and may enhance uniparental reproduction. Nevertheless, strong endosperm imbalance could potentially contribute to the high amount of seed abortion in polyploid cytotypes.
A different pathway may lead to the formation of pentaploids in the contact zone: one seed sample from a 5x mother plant indicates a fully sexual pathway, probably via a meiotic reduction of the embryo sac (3x) and fertilization by diploid pollen to form a 5x embryo and a 8x endosperm (Table 3). The origin of these pentaploids from other cytotypes, however, may also involve apomeiotic and parthenogenetic pathways.
Outside of the contact zone, no diploids occur which would explain the formation of triploids. But, triploid embryos seem to arise quite often in the seed of tetraploid populations (Table 3 and Fig. 3), but almost never appear in the leaf material of adult plants. The reduction in ploidy level in the embryo can only be explained by a meiotically reduced embryo sac. It is supposed that meiosis was disturbed in the mother plant, resulting in megaspores with unbalanced chromosome numbers. Molecular markers (microsatellites, AFLPs) do not indicate the contribution of another parental species to the genome of the tetraploid cytotype (A.-C. Cosendai and E. Hörandl, unpubl. res.). Autopolyploid origin and multivalent formation during meiosis may cause such unbalanced chromosome numbers. The ratio obtained in the endosperm seems to confirm this. Effectively, with ratio 3x (embryo) : 8x; 9x; 10x (endosperm), the endosperm nuclei would be 6x, plus 2x, 3x and 4x derived from the pollen. Since the 3x embryo must have developed parthenogenetically, again either one or both pollen nuclei (2x + 2x) could have been used for endosperm fertilization. In some of the samples with 3x embryos (populations 12 and 41), an additional, but smaller, 6x peak was observed, which represents the G2 peak of the growing embryo (e.g. Fig. 3). Moreover, disturbances in microsporogenesis and formation of unbalanced pollen (Izmailow, 1965, 1973, 1976; Jankun, 1965) can contribute to variation in the endosperm ploidy levels. Most importantly, these tetraploid plants obviously show only a partial apomixis by keeping a disturbed meiosis, but developing the egg cell parthenogenetically. In the next generations, further reduction of ploidy levels would finally lead to haploid offspring, in which recessive deleterious mutations would be fully expressed. These processes may strongly reduce fitness of such lineages with only a partial apomixis (e.g. Van Dijk and Vijverberg 2005). The present data show only a single triploid adult plant in the whole area (Fig. 1 and Table 2), but 17 triploid embryos in the seeds from tetraploid adults. These plants come from 11 populations outside the area of diploid influence, suggesting a rather frequent phenomenon (Table 3). However, triploids that have been formed via this partial apomixis probably have difficulty in becoming established. In contrast, the fully apomictic pathway with a combination of apomeiosis and parthenogenesis maintains the tetraploid level and is obviously more successful in the establishment of this cytotype, as seen in the frequency of adult plants. In two of the populations analysed (Grand St Bernard and Simplon Pass), both 3x and 4x embryos occur in the seeds of tetraploids, but both populations had 100 % adult tetraploid plants. In populations with both pathways, selection obviously favours individuals expressing full apomixis, which stabilizes the ploidy level, over parthenogenesis alone which forms new cytotypes.
Hexaploids can be formed in a tetraploid population from a cross between an unreduced embryo and reduced pollen. In this case, the endosperm would be 10x (8x + 2x). Only one seed sample with a 6x embryo and an 20x endosperm were found. Here endopolyploidy could explain the double DNA content of the expected 10x. Endopolyploidy in the endosperm is known, for example, from Zea mays and other species (Kowles et al., 1988; Barow and Meister, 2003; Barow, 2006). Such hexaploid Biii hybrids may also give rise to triploids via meiosis and parthenogenetic development of the egg cell, but are obviously not stable.
These aspects would also explain why coexistence of cytotypes is rare in R. kuepferi, in contrast to what Kao (2007) showed for apomictic Arnica cordifolia. In Arnica, apomictic reproduction is predominant and probably stable enough to keep cytotypes reproductively isolated. Additionally, differences in phenology maintain a stable coexistence of cytotypes. Stable triploidy is also widespread in dandelions (Taraxacum officinale group; Van Dijk, 2003). In Asteraceae, apomixis may actually arise at the triploid level because of their mode of embryo sac development and endosperm formation (Talent, 2009). In Ranunculus kuepferi, the present results rather suggest that apomixis originated in tetraploid cytotypes, because of the existence of rare sexual tetraploids and a frequent uncoupling of apomeiosis and parthenogenesis on this ploidy level.
The variety of pathways indicates again the broad flexibility of modes of reproduction within this species. It appears to be able to express all possible solutions of embryo formation between fully sexual to complete apomixis. The coupling of apomeiosis and parthenogenetic development of the egg cell seems not yet fully established. Furthermore, R. kuepferi can undergo all kinds of endosperm development from the pseudogamous to the autonomous type. These phenomena suggest a very young, postglacial or even recurrent evolutionary origin of apomixis. Further support for this hypothesis comes from linear correlations of genome size to ploidy levels (Fig. 2), which infers that the frequently observed genome downsizing in polyploids has not yet occurred (see Leitch and Bennett, 2004).
Stability of the diploid sexual populations
Diploids maintain themselves via sexual reproduction and high seed set, but they can accept pollen from tetraploids in the sympatric zone to form triploid or pentaploid offspring. In the hybrid zone, triploids and pentaploids may be recurrently formed but still they are quite rare and do not form populations on their own. The relatively frequent triploids can arise from different pathways: a crossing between a haploid meiotic egg cell (x) and tetraploid meiotic pollen (2x) gives a triploid embryo, while the endosperm (2x) plus one pollen nucleus (2x) would be tetraploid; this 3x : 4x ratio was not actually observed in the wild seeds, but could account in for triploids being in the majority in the present data. The tetraploid pollen donor could be either sexual or apomictic. Alternatively, within a diploid population, occasionally unreduced pollen (2x) can be formed, which, when it fertilizes a haploid egg cell, results in the same embryo : endosperm ratio as above. The reciprocal cross would retrieve a 3x : 5x ratio. These processes could be a spontaneous step towards autopolyploidy via the triploid bridge (Ramsey and Schemske, 1998) and would not involve a cross between cytotypes. The present limited sampling of achenes in diploid populations may account for the lack of evidence on these pathways.
The hypothesis that apomixis can be transferred to sexual populations via the pollen of apomicts would suggest a replacement of sexual by apomictic cytotypes (Mogie, 1992; Mogie et al., 2007). There is little evidence for this pathway in the present dataset, partly because of low seed set in triploids. A triploid plant from the Allos population (no. 23), that shows fully apomictic reproduction (Table 3), may have indeed originated from an apomictic pollen donor. In the seed material, the number of triploid embryos in the hybrid zone is very low (three), but these embryos have originated from a tetraploid mother plant. Therefore, introgression of apomixis into diploid sexuals cannot be proven directly with the present seed dataset. Leaf material, however, shows that almost all triploid adult plants come from the hybrid zone, suggesting that crossings between diploid and tetraploid cytotypes probably do occur recurrently. This does not necessarily indicate an introgression process, because the tetraploid parents in this area could also be fully sexual, as shown in three individuals from the Col della Perla population (no. 12 in Table 3). If triploids in the hybrid zones could be sexual or partly sexual, then disturbances of meiosis, reduced fitness and minority cytotype disadvantages may strongly limit the establishment of triploid lineages. Triploid apomicts, in contrast, should be able to establish purely triploid populations, but such populations have not been observed. It is concluded that triploids originated mainly from sexual events rather than from triploid apomictic parents.
The present results indicate that introgression of apomixis into sexual populations in the 2x–4x hybrid zone may occur, but frequencies need to be studied further. It remains questionable whether this process plays a major role for the observed pattern of geographical parthenogenesis. Moreover, the diploid sexual individuals of R. kuepferi do have a significantly higher fertility than all the polyploid cytotypes (Fig. 4). In such cases, it is unlikely that apomixis can replace sexuality in mixed populations (see Mogie, 1992; Hörandl and Temsch, 2009). This is in accordance with results from R. auricomus, where findings of low crossability between different cytotypes, and a significantly higher fertility of sexuals rejected the introgression hypothesis (Hörandl and Temsch, 2009). Low frequencies of introgression were also found in population studies on Taraxacum (Brock, 2004).
Colonizing abilities of tetraploids
The easternmost population at Turracherhöhe and the outposts at Corsica and Mt Cusna are all tetraploid. For the population at Turracherhöhe, the FCSS data (Table 3) revealed fully apomictic reproduction. The samples from Mt Cusna had completely aborted achenes, as is typical for apomicts. There were no achenes in the populations in Corsica, but Huber (1989) also reported seed and pollen abortion. Apomictic reproduction in both the Corsica and Mt Cusna populations is further indicated by a clonal population genetic structure (Cosendai, 2005; unpubl. res.). These outposts have been most probably founded by efficiently colonizing tetraploid apomicts, probably via long-distance dispersal. Corsica has had no land bridge to the European continent since the Miocene (Loÿe-Pilot et al., 2004). The location of Mt Cusna in the northern Apennines may lead to a hypothesis that the species could have migrated from the Alps towards the Apennines, but there is no high mountain in Liguria to support this idea. As the diploid populations occupy a somewhat central position between Corsica and the major tetraploid area, it is likely that tetraploids originated in this area, but expanded their range rapidly via a centrifugal dispersal. Such distribution patterns of central diploid sexuals surrounded by expanding polyploid cytotype apomicts are common in other cases of geographical parthenogenesis [Antennaria (Bayer, 1990); Stevia (Soejima et al., 2001); Paspalum (Urbani, 2002); Taraxacum and Chondrilla (Van Dijk, 2003); Ranunculus auricomus (Hörandl, 2009)]. Long-distance dispersal, even between continents, is a frequent phenomenon in the genus Ranunculus (K. Emadzade and E. Hörandl, unpubl. res.). The achenes of R. kuepferi are small and have a cavity between the seed and the pericarp, aiding wind dispersal (Müller-Schneider, 1986). Otherwise, birds or mammals may have been other important dispersal vectors. The ability of apomicts to found populations with a single diaspore may have enhanced the success of rare dispersal events (Hörandl et al., 2008). Since the apomicts do not have a fitness advantage with respect to female fecundity, it is likely that superior colonizing abilities, as postulated by Baker's Law (Baker, 1965, 1967), are a main factor for the observed centrifugal distribution. For the diploid sexual cytotype, self-sterility (Huber, 1988) and pollinator-dependence may strongly limit range expansions.
Conclusions
The results on R. kuepferi overall confirm a high stability of the diploid and the tetraploid cytotype in their respective areas. Aneuploid cytotypes are probably recurrently formed, but fail to establish as long as remnants of the sexual pathway are maintained. The stability of the apomictic tetraploid cytotypes may consequently be important for establishing a large distribution area. In contrast, the significantly higher fertility of the diploids may stabilize the diploid cytotype in its relic area. Moreover, the present data tend to prove the difficulty for a new cytotype to establish, as long as partial sexuality is maintained; minority cytotype effects sensu Levin (1975), Husband (2000) and Husband et al. (2008) seem to play an important role in this pattern for reducing the presence of different cytotypes in the population, although the seeds are constantly newly produced.
ACKNOWLEDGEMENTS
Financial support from the Austrian Research Foundation (FWF, project P19006-B03) and the Austrian Academy of Sciences, Commission for Interdisciplinary Ecological studies (KIÖS, project P2009-03), both granted to E.H., is gratefully acknowledged. We thank Eva Maria Temsch and Johann Greilhuber (both at Vienna) for introducing us to flow cytometry and for helpful discussions, our colleagues for collection of materials, and two anonymous referees for valuable comments on the manuscript. Funding to pay the Open Access publication charges for this article was provided by the Austrian Research Foundation (FWF).
LITERATURE CITED
- Asker SE, Jerling L. Apomixis in plants. Boca Raton, FL: CRC Press; 1992. [Google Scholar]
- Baker HG. Characteristics and modes of origin of weeds. In: Baker HG, Stebbins GL, editors. The genetics of colonizing species. New York, NY: Academic Press; 1965. [Google Scholar]
- Baker HG. Support for Baker's law – as a rule. Evolution. 1967;21:853–856. doi: 10.1111/j.1558-5646.1967.tb03440.x. [DOI] [PubMed] [Google Scholar]
- Baranyi M, Greilhuber J. Flow cytometric and feulgen densitometric analysis of genome size variation in Pisum. Theoretical and Applied Genetics. 1996;92:297–307. doi: 10.1007/BF00223672. [DOI] [PubMed] [Google Scholar]
- Barow M. Endopolyploidy in seed plants. Bioessays. 2006;28:271–281. doi: 10.1002/bies.20371. [DOI] [PubMed] [Google Scholar]
- Barow M, Meister A. Endopolyploidy in seed plants is differently correlated to systematics, organ, life strategy and genome size. Plant, Cell & Environment. 2003;26:571–584. [Google Scholar]
- Bayer RJ. Investigations into the evolutionary history of the Antennaria rosea (Asteraceae: Inuleae) polyploid complex. Plant Systematics and Evolution. 1990;169:97–110. [Google Scholar]
- Bell G. The masterpiece of nature: the evolution and genetics of sexuality. Berkeley, CA: California Press; 1982. [Google Scholar]
- Bierzychudek P. Patterns in plant parthenogenesis. Experientia. 1985;41:1255–1264. doi: 10.1007/978-3-0348-6273-8_9. [DOI] [PubMed] [Google Scholar]
- Brock MT. The potential for genetic assimilation of a native dandelion species, Taraxacum ceratophorum (Asteraceae), by the exotic congener T. officinale. American Journal of Botany. 2004;91:656–663. doi: 10.3732/ajb.91.5.656. [DOI] [PubMed] [Google Scholar]
- Burnier J, Burki S, Arrigo N, Küpfer P, Alvarez N. Genetic structure and evolution of alpine polyploid complexes: Ranunculus kuepferi (Ranunculaceae) as a case study. Molecular Ecology. 2009;18:3720–3744. doi: 10.1111/j.1365-294X.2009.04281.x. [DOI] [PubMed] [Google Scholar]
- Comai L. The advantages and disadvantages of being polyploid. Nature Reviews Genetics. 2005;6:836–846. doi: 10.1038/nrg1711. [DOI] [PubMed] [Google Scholar]
- Cosendai A-C. 2005. Liens de parenté et origine de Ranunculus kuepferi en Corse et dans le sud de la France. MSc Thesis, University of Neuchâtel, Neuchâtel. [Google Scholar]
- Dickinson TA, Lo E, Talent N. Polyploidy, reproductive biology, and Rosaceae: understanding evolution and making classifications. Plant Systematics and Evolution. 2007;266:59–78. [Google Scholar]
- Doležel J, Greilhuber J, Lucretti S, et al. Plant genome size estimation by flow cytometry: inter-laboratory comparison. Annals of Botany. 1998;82(Suppl. A):17–26. [Google Scholar]
- Doležel J, Greilhuber J, Suda J. Estimation of nuclear DNA content in plants using flow cytometry. Nature Protocols. 2007a;2:2233–2244. doi: 10.1038/nprot.2007.310. [DOI] [PubMed] [Google Scholar]
- Doležel J, Greilhuber J, Suda J. Flow cytometry with plant cells: analysis of genes, chromosomes and genomes. Weinheim: Wiley-VCH; 2007b. [Google Scholar]
- Galbraith DW, Harkins KR, Maddox JM, Ayres NM, Sharma DP, Firoozabady E. Rapid flow cytometric analysis of the cell cycle in intact plant tissues. Science. 1983;220:1049–1051. doi: 10.1126/science.220.4601.1049. [DOI] [PubMed] [Google Scholar]
- Greilhuber J. Cytochemistry and C-values: the less-well-known world of nuclear DNA amounts. Annals of Botany. 2008;101:791–804. doi: 10.1093/aob/mcm250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greilhuber J, Lysák MA, Doležel J, Bennett MD. The origin, evolution and proposed stabilisation of the terms ‘genome size’ and ‘C-value’ to describe nuclear DNA contents. Annals of Botany. 2005;94:255–260. doi: 10.1093/aob/mci019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hörandl E. The complex causality of geographical parthenogenesis. New Phytologist. 2006;171:525–538. doi: 10.1111/j.1469-8137.2006.01769.x. [DOI] [PubMed] [Google Scholar]
- Hörandl E. Evolutionary implications of self-compatibility and reproductive fitness in the apomictic Ranunculus auricomus polyploid complex (Ranunculaceae) International Journal of Plant Sciences. 2008;169:1219–1228. doi: 10.1086/591980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hörandl E. Geographical parthenogenesis: opportunities for asexuality. In: Schoen I, Martens K, Van Dijk P, editors. Lost sex. Heidelberg: Springer; 2009a. pp. 161–186. [Google Scholar]
- Hörandl E. The evolution of self-fertility in apomictic plants. Sexual Plant Reproduction. 2009b doi: 10.1007/s00497-009-0122-3. DOI 10.1007/s00497-009-0122-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hörandl E, Temsch EM. Introgression of apomixis into sexual species is inhibited by mentor effects and ploidy barriers in the Ranunculus auricomus complex. Annals of Botany. 2009;104:81–89. doi: 10.1093/aob/mcp093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hörandl E, Cosendai AC, Temsch EM. Understanding the geographic distributions of apomictic plants: a case for a pluralistic approach. Plant Ecology and Diversity. 2008;1:309–320. doi: 10.1080/17550870802351175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber W. Natürliche Bastardierungen zwischen weißblühenden Ranunculus-Arten in den Alpen [Natural hybridizations between white-flowered species of Ranunculus in the Alps] [in German with English abstract] Veröffentlichungen des Geobotanischen Institutes der ETH Zürich. 1988;100:1–160. [Google Scholar]
- Huber W. Ranunculus kuepferi Greuter & Burdet in Korsica (Gruppe R. pyrenaeus L.) Candollea. 1989;44:630–637. [Google Scholar]
- Husband BC. Constraints on polyploid evolution: a test of the minority cytotype exclusion principle. Proceedings of the Royal Society of London Series B – Biological Sciences; 2000. pp. 217–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husband BC, Ozimec B, Martin SL, Pollock L. Mating consequences of polyploid evolution in flowering plants: current trends and insights from synthetic polyploids. International Journal of Plant Sciences. 2008;169:195–206. [Google Scholar]
- Izmailow R. Macrosporogenesis in apomictic species Ranunculus cassubicus. Acta Biologica Cracoviensia Series Botanica. 1965;8:183–195. [Google Scholar]
- Izmailow R. Cyto-embryological studies in experimental hybrids of apomictic species Ranunculus-cassubicus L. Acta Biologica Cracoviensia Series Botanica. 1973;16:99–120. [Google Scholar]
- Izmailow R. Problem of apomixis in Ranunculus-auricomus group. Acta Biologica Cracoviensia Series Botanica. 1976;19:15–28. [Google Scholar]
- Jankun A. Studies of meiosis in various chromosomic types of Ranunculus cassubicus L. Acta Biologica Cracoviensia Series Botanica. 1965;8:171–180. [Google Scholar]
- Kao RH. Asexuality and the coexistence of cytotypes. New Phytologist. 2007;175:764–772. doi: 10.1111/j.1469-8137.2007.02145.x. [DOI] [PubMed] [Google Scholar]
- Kearney M. Hybridization, glaciation and geographical parthenogenesis. Trends in Ecology & Evolution. 2005;20:495–502. doi: 10.1016/j.tree.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Kearney M. Response to Lundmark: polyploidization, hybridization and geographical parthenogenesis. Trends in Ecology & Evolution. 2006;21:10. doi: 10.1016/j.tree.2005.10.007. [DOI] [PubMed] [Google Scholar]
- Kowles RV, Phillips RL. Endosperm development in maize. International Review of Cytology. 1988;112:97–136. [Google Scholar]
- Krahulcova A, Suda J. A modified method of flow cytometric seed screen simplifies the quantification of progeny classes with different ploidy levels. Biologia Plantarum. 2006;50:457–460. [Google Scholar]
- Küpfer P. The affinities between orophyte flora of the Alps and that of the Pyrenees. Boissiera. 1974;23:1–322. [Google Scholar]
- Law JH, Crespi BJ. The evolution of geographic parthenogenesis in timema walking-sticks. Molecular Ecology. 2002;11:1471–1489. doi: 10.1046/j.1365-294x.2002.01547.x. [DOI] [PubMed] [Google Scholar]
- Leitch IJ, Bennett MD. Genome downsizing in polyploid plants. Biological Journal of the Linnean Society. 2004;82:651–663. [Google Scholar]
- Levin DA. Somatic cell hybridization application in plant systematics. Taxon. 1975;24:261–270. [Google Scholar]
- Levin DA. The role of chromosomal change in plant evolution. Oxford: Oxford University Press; 2002. [Google Scholar]
- Loye-Pilot MD, Durand-Delga M, Feinberg H, Gourinard Y, Magne J. The Burdigalian of eastern Corsica within its geodynamic setting. Comptes Rendus Geoscience. 2004;336:919–930. [Google Scholar]
- Lundmark M. Polyploidization, hybridization and geographical parthenogenesis. Trends in Ecology & Evolution. 2006;21:9. doi: 10.1016/j.tree.2005.10.007. [DOI] [PubMed] [Google Scholar]
- Lynch M. Destabilizing hybridization, general-purpose genotypes and geographic parthenogenesis. Quarterly Review of Biology. 1984;59:257–290. [Google Scholar]
- Matzk F, Meister A, Schubert I. An efficient screen for reproductive pathways using mature seeds of monocots and dicots. The Plant Journal. 2000;21:97–108. doi: 10.1046/j.1365-313x.2000.00647.x. [DOI] [PubMed] [Google Scholar]
- Merxmüller H. Untersuchungen zur Sippengliederung und Arealbildung in den Alpen. Teil 1. Jahrbuch des Vereins zum Schutze der Alpenpflanzen und -tiere. 1952;17:96–133. [Google Scholar]
- Merxmüller H. Untersuchungen zur Sippengliederung und Arealbildung in den Alpen. Teil 2. Jahrbuch des Vereins zum Schutze der Alpenpflanzen und -tiere. 1953;18:135–158. [Google Scholar]
- Merxmüller H. Untersuchungen zur Sippengliederung und Arealbildung in den Alpen. Teil 3. Jahrbuch des Vereins zum Schutze der Alpenpflanzen und -tiere. 1954;19:97–139. [Google Scholar]
- Mogie M. The evolution of asexual reproduction in plants. London: Chapman and Hall; 1992. [Google Scholar]
- Mogie M, Britton NF, Stewart-Cox JA. Asexuality, polyploidy and the male function. In: Hörandl E, Grossniklaus U, Van Dijk PJ, Sharbel T, editors. Apomixis: evolution, mechanisms and perspectives. Ruggell, Liechtenstein: ARG-Gantner; 2007. pp. 195–214. [Google Scholar]
- Mraz P, Singliarova B, Urfus T, Krahulec F. Cytogeography of Pilosella officinarum (Compositae): altitudinal and longitudinal differences in ploidy level distribution in the Czech republic and Slovakia and the general pattern in Europe. Annals of Botany. 2008;101:59–71. doi: 10.1093/aob/mcm282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller-Schneider P. Verbreitungsbiologie der Blütenpflanzen Graubündens [Diasporology of the spermatophytes of the Grisons (Switzerland)] Berichte des Geobotanischen Institutes ETH, Zürich. 1986;85:1–263. [Google Scholar]
- Nogler GA. Gametophytic apomixis. In: Johri BM, editor. Embryology of angiosperms. Berlin: Springer-Verlag; 1984a. pp. 475–566. [Google Scholar]
- Nogler GA. Genetics of apospory in apomictic Ranunculus auricomus. 5. Conclusion. Botanica Helvetica. 1984b;94:411–422. [Google Scholar]
- Nogler GA. Genetics of apomixis in Ranunculus auricomus. 6. Epilogue. Botanica Helvetica. 1995;105:111–115. [Google Scholar]
- Parisod C, Besnard G. Glacial in situ survival in the western Alps and polytopic autopolyploidy in Biscutella laevigata L. (Brassicaceae) Molecular Ecology. 2007;16:2755–2767. doi: 10.1111/j.1365-294X.2007.03315.x. [DOI] [PubMed] [Google Scholar]
- Paun O, Stuessy TF, Hörandl E. The role of hybridization, polyploidization and glaciation in the origin and evolution of the apomictic Ranunculus cassubicus complex. New Phytologist. 2006;171:223–236. doi: 10.1111/j.1469-8137.2006.01738.x. [DOI] [PubMed] [Google Scholar]
- Ramsey J, Schemske DW. Pathways, mechanisms, and rates of polyploid formation in flowering plants. Annual Review of Ecology and Systematics. 1998;29:467–501. [Google Scholar]
- Schönswetter P, Stehlik I, Holderegger R, Tribsch A. Molecular evidence for glacial refugia of mountain plants in the European Alps. Molecular Ecology. 2005;14:3547–3555. doi: 10.1111/j.1365-294X.2005.02683.x. [DOI] [PubMed] [Google Scholar]
- Soejima A, Yahara T, Watanabe K. Distribution and variation of sexual and agamospermous populations of Stevia (Asteraceae: Eupatorieae) in the lower latitudes, Mexico. Plant Species Biology. 2001;16:91–105. [Google Scholar]
- Spielman M, Vinkenoog R, Scott RJ. Genetic mechanisms of apomixis. Philosophical Transactions of the Royal Society of London Series B – Biological Sciences. 2003;358:1095–1103. doi: 10.1098/rstb.2003.1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suda J, Trávnicek P. Estimation of relative nuclear DNA content in dehydrated plant tissues by flow cytometry. Current Protocols in Cytometry. 2006 doi: 10.1002/0471142956.cy0730s38. Chapter 7: Unit 7·30. [DOI] [PubMed] [Google Scholar]
- Talent N. Evolution of gametophytic apomixis in flowering plants: an alternative model from maloid Rosaceae. Theory in Biosciences. 2009;128:121–138. doi: 10.1007/s12064-009-0061-4. [DOI] [PubMed] [Google Scholar]
- Talent N, Dickinson TA. Endosperm formation in aposporous Crataegus (Rosaceae, Spiraeoideae, tribe Pyreae): parallels to Ranunculaceae and Poaceae. New Phytologist. 2007;173:231–249. doi: 10.1111/j.1469-8137.2006.01918.x. [DOI] [PubMed] [Google Scholar]
- Temsch EM, Greilhuber J, Hammett KRW, Murray BG. Genome size in Dahlia cav. (Asteraceae-Coreopsideae) Plant Systematics and Evolution. 2008;276:157–166. [Google Scholar]
- Temsch EM, Greilhuber J, Krisai R. Genome size in liverworts. Preslia. 2009 in press. [Google Scholar]
- Thompson SL, Whitton J. Patterns of recurrent evolution and geographic parthenogenesis within apomictic polyploid Easter daises (Townsendia hookeri) Molecular Ecology. 2006;15:3389–3400. doi: 10.1111/j.1365-294X.2006.03020.x. [DOI] [PubMed] [Google Scholar]
- Tribsch A. Areas of endemism of vascular plants in the eastern Alps in relation to Pleistocene glaciation. Journal of Biogeography. 2004;31:747–760. [Google Scholar]
- Urbani MH, Quarin CL, Espinoza F, Penteado MIO, Rodrigues IF. Cytogeography and reproduction of the Paspalum simplex polyploid complex. Plant Systematics and Evolution. 2002;236:99–105. [Google Scholar]
- Van Dijk PJ. Ecological and evolutionary opportunities of apomixis: insights from Taraxacum and Chondrilla. Philosophical Transactions of the Royal Society of London Series B – Biological Sciences. 2003;358:1113–1120. doi: 10.1098/rstb.2003.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dijk PJ. Potential and realized costs of sex in dandelions, Taraxacum officinale s.l. In: Hörandl E, Grossniklaus U, Van Dijk PJ, Sharbel T, editors. Apomixis: evolution, mechanisms and perspectives. Ruggell, Liechtenstein: ARG-Gantner; 2007. pp. 215–233. [Google Scholar]
- Van Dijk PJ, Vijverberg K. The significance of apomixis in the evolution of the angiosperms: a reappraisal. In: Bakker F, Chatrou L, Gravendeel B, Pelser PB, editors. Plant species-level systematics: new perspectives on pattern and process. Ruggell, Liechtenstein: Gantner; 2005. pp. 101–116. [Google Scholar]
- Vandel A. La parthénogenese geographique. Contribution a l'étude biologique et cytologique de la parthénogenese naturelle. Bulletin Biologique de la France et de la Belgique. 1928;62:164–182. [Google Scholar]
- Van Valen L. A new evolutionary law. Evolutionary Theory. 1973;1:1–30. [Google Scholar]
- Vinkenoog R, Bushell C, Spielmann M, Adams S, Dickinson HG, Scott RJ. Genomic imprinting and endosperm development in flowering plants. Molecular Biotechnology. 2003;25:149–184. doi: 10.1385/MB:25:2:149. [DOI] [PubMed] [Google Scholar]
- Vorburger C. Geographic parthenogenesis: recurrent patterns down under. Current Biology. 2006;16:R641–R643. doi: 10.1016/j.cub.2006.07.027. [DOI] [PubMed] [Google Scholar]
- Vrijenhoek RC. Ecological differentiation among clones: the frozen niche variation model. In: Woermann K, Loeschcke V, editors. Population biology and evolution. Berlin: Springer; 1984. pp. 217–231. [Google Scholar]
- Vrijenhoek RC. Unisexual fish: Model systems for studying ecology and evolution. Annual Review of Ecology and Systematics. 1994;25:71–96. [Google Scholar]
- Vuille C, Küpfer P. Aposporie chez le Ranunculus parnassifolius. L. I. Etude cytoembryologique. Bulletin de la Societe Neuchateloise des Sciences Naturelles. 1985;108:123–134. [Google Scholar]