Abstract
Background and Aims
Wild Sorghum species provide novel traits for both biotic and abiotic stress resistance and yield for the improvement of cultivated sorghum. A better understanding of the phylogeny in the genus Sorghum will enhance use of the valuable agronomic traits found in wild sorghum.
Methods
Four regions of chloroplast DNA (cpDNA; psbZ-trnG, trnY-trnD, trnY-psbM and trnT-trnL) and the internal transcribed spacer (ITS) of nuclear ribosomal DNA were used to analyse the phylogeny of sorghum based on maximum-parsimony analyses.
Key Results
Parsimony analyses of the ITS and cpDNA regions as separate or combined sequence datasets formed trees with strong bootstrap support with two lineages: the Eu-sorghum species S. laxiflorum and S. macrospermum in one and Stiposorghum and Para-sorghum in the other. Within Eu-sorghum, S. bicolor-3, -11 and -14 originating from southern Africa form a distinct clade. S. bicolor-2, originally from Yemen, is distantly related to other S. bicolor accessions.
Conclusions
Eu-sorghum species are more closely related to S. macrospermum and S. laxiflorum than to any other Australian wild Sorghum species. S. macrospermum and S. laxiflorum are so closely related that it is inappropriate to classify them in separate sections. S. almum is closely associated with S. bicolor, suggesting that the latter is the maternal parent of the former given that cpDNA is maternally inherited in angiosperms. S. bicolor-3, -11 and -14, from southern Africa, are closely related to each other but distantly related to S. bicolor-2.
Keywords: Molecular phylogeny, Sorghum, Eu-sorghum, Zea mays, non-coding regions, cpDNA, ITS
INTRODUCTION
Sorghum Moench is highly heterogeneous and with Cleistachne Bentham form Sorghastrae (Garber, 1950), one of the 16 subtribes belonging to tribe Andropogoneae. Species of the genus Sorghum have chromosome numbers of 2n = 10, 20, 30 or 40 (Garber, 1950; Lazarides et al., 1991). There are five recognized sections and 25 species within Sorghum. The sections are Eu-sorghum, Chaetosorghum, Heterosorghum, Para-sorghum and Stiposorghum (Garber, 1950; Lazarides et al., 1991). Eu-sorghum includes cultivated sorghums and their closest wild relatives (De Wet and Huckay, 1967). According to De Wet (1978) three species were recognized in section Eu-sorghum, including two perennial species, S. halepense and S. propinquum, and an annual, S. bicolor. However, in the earlier classification by Snowden (1935), Eu-sorghum is considered to comprise two subsections, Arundinacea and Halepensia. The subsection Arundinacea, commonly found in tropical Africa and India, consists of S. bicolor (L.) Moench, S. arundinaceum (Desv.) Stapf and S. drummondii (Steud.) Millsp. S. propinquum (Kunth) Hitchcock, S. halepense (L.) Pers and S. almum Parodi form subsection Halepensia, and are found in the Mediterranean region and Southeast Asia.
The wild Australian Sorghum species constitute over two-thirds of the recognized Sorghum species, of which one species each belongs to Chaetosorghum and Heterosorghum. The section Para-sorghum comprises seven species. Of these, five are native to northern monsoonal Australia, Africa and Asia (Garber, 1950; Lazarides et al., 1991). Stiposorghum consists of ten species that are endemic to northern Australia (Garber, 1950; Lazarides et al., 1991). The wild and weedy Sorghum species present a valuable source of agronomic traits such as pest and disease resistance (Sharma and Franzmann, 2001; Kamala et al., 2002; Komolong et al., 2002) for introgression into S. bicolor. Exploitation of these valuable traits requires a thorough understanding of the phylogenetic relationships between cultivated sorghum and the wild sorghum gene pool.
The chloroplast genome is useful in providing information on the inference of the evolutionary patterns and processes in plants (Raubeson and Jansen, 2005). The genome has, either solely or combined with other genomes, been widely used for inferring phylogenetic relationships of different taxa, including Hordeum, Triticum and Aegilops (Gielly and Taberlet, 1994), Guizotia (Geleta, 2007), Solanaceae (Melotto-Passarin et al., 2008) and Sorghum (Dillon et al., 2007). The non-coding chloroplast regions are phylogenetically more informative than the coding regions at lower taxonomic levels because they are under less functional constraints and evolve rapidly (Gielly and Taberlet, 1994). One of the chloroplast DNA (cpDNA) regions, trnT-trnL, used in this study was reported to possess sufficient phylogenetic signal for studies at lower taxonomic levels (Shaw et al., 2005).
The internal transcribed spacer (ITS) region of the 18S–5·8S–26S nuclear ribosomal DNA (nrDNA) has been commonly used for phylogenetic inference at the generic and infrageneric level in plants. The ITS loci properties of biparental inheritance, universality of primers, intragenomic uniformity and intergenomic variability merit their utility for phylogenetic reconstruction (Baldwin et al., 1995). Two ITS regions, ITS1 and ITS2, generally evolve more rapidly than coding regions and have shown to be equally informative, being able to differentiate between closely related species (Baldwin, 1992) and more specifically to resolve phylogenetic relationships of sorghum and related species (Sun et al., 1994; Dillon et al., 2001; Guo et al., 2006).
This study sought to resolve the phylogenetic relationships between species of the genus Sorghum based on four regions of the cpDNA: trnY-trnD, psbZ- trnG, trnY-psbM and trnT-trnL and the ITS of nrDNA and also to evaluate the usefulness of the five non-coding regions of cpDNA in resolving relationships among the closely related species within section Eu-sorghum.
MATERIALS AND METHODS
Plant material
Details of twenty-two Sorghum species along with GenBank germplasm and GenBank sequence accession numbers used in this study are given in Table 1. The germplasm accessions included wild sorghum and several cultivated sorghum obtained from the Australian Tropical Crops Genetic Resource Centre, Biloela, Queensland, Australia. In addition, five accessions of S. bicolor and one of S. arundinaceum were obtained from the Zambian National Plant Genetic Resources Centre.
Table 1.
Accession identity and geographical origin of each accession of Sorghum species used in the study
| DNA sequence accession no. |
|||||||
|---|---|---|---|---|---|---|---|
| Species | Section | Germplasm accession no.* | trnY-trnD | psbZ-trnG | trnY-psbM | trnT-trnL | ITS |
| S. almum | Eu-sorghum | AusTRCF302386A | GQ121828 | GQ121769 | GQ121810 | GQ121791 | GQ121750 |
| S. amplum-1 | Stiposorghum | AusTRCF302455A | N/A | N/A | N/A | N/A | N/A |
| S. amplum-2 | Stiposorghum | AusTRCF302623A | GQ121822 | GQ121755 | GQ121799 | GQ121783 | GQ121727 |
| S. angustum-1 | Stiposorghum | AusTRCF302588A | GQ121824 | N/A | GQ121793 | GQ121775 | GQ121737 |
| S. angustum-2 | Stiposorghum | AusTRCF302606A | N/A | GQ121761 | N/A | N/A | N/A |
| S. arundinaceum | Eu-sorghum | ZMB 7203Zm | GQ121832 | GQ121766 | GQ121806 | GQ121790 | GQ121746 |
| S. bicolor-1 | Eu-sorghum | AusTRCF304111TA | N/A | N/A | N/A | N/A | N/A |
| S. bicolor-2 | Eu-sorghum | AusTRCF304113YA | N/A | N/A | N/A | N/A | GQ121748 |
| S. bicolor-3 | Eu-sorghum | AusTRCF304114ZwA | N/A | N/A | N/A | N/A | N/A |
| S. bicolor-4 | Eu-sorghum | AusTRCF304115BA | N/A | N/A | N/A | N/A | GQ121745 |
| S. bicolor-5 | Eu-sorghum | AusTRCF312813ZmA | N/A | N/A | N/A | N/A | N/A |
| S. bicolor-14 | Eu-sorghum | ZMB 5395Zm | N/A | N/A | N/A | N/A | N/A |
| S. bicolor-12 | Eu-sorghum | ZMB 5757Zm | GQ121829 | GQ121770 | GQ121813 | GQ121792 | GQ121743 |
| S. bicolor-15 | Eu-sorghum | ZMB 6665Zm | N/A | N/A | N/A | N/A | N/A |
| S. bicolor-10 | Eu-sorghum | ZMB 7016Zm | N/A | N/A | N/A | N/A | GQ121744 |
| S. bicolor-11 | Eu-sorghum | ZMB 7034Zm | N/A | N/A | N/A | N/A | N/A |
| S. bicolor-13 | Eu-sorghum | ZMB 7112Zm | N/A | N/A | N/A | N/A | N/A |
| S. brachypodum-1 | Stiposorghum | AusTRCF302480A | GQ121818 | GQ121756 | GQ121802 | GQ121774 | GQ121736 |
| S. brachypodum-2 | Stiposorghum | AusTRCF302481A | N/A | N/A | N/A | N/A | N/A |
| S. bulbosum-1 | Stiposorghum | AusTRCF302418A | N/A | N/A | N/A | N/A | N/A |
| S. bulbosum-2 | Stiposorghum | AusTRCF302646A | GQ121823 | GQ121758 | QG121803 | GQ121781 | GQ121732 |
| S. drummondii-1 | Eu-sorghum | AusTRCF300263EA | N/A | N/A | N/A | N/A | N/A |
| S. drummondii-2 | Eu-sorghum | AusTRCF300264KA | GQ121831 | GQ121765 | GQ121809 | GQ121789 | GQ121747 |
| S. ecarinatum-1 | Stiposorghum | AusTRCF302450A | GQ121821 | GQ121754 | GQ121800 | GQ121784 | GQ121730 |
| S. ecarinatum-2 | Stiposorghum | AusTRCF302662A | N/A | N/A | N/A | N/A | N/A |
| S. exstans-1 | Stiposorghum | AusTRCF302401A | N/A | N/A | N/A | N/A | N/A |
| S. exstans-2 | Stiposorghum | AusTRCF302473A | GQ121816 | GQ121759 | GQ121796 | GQ121782 | GQ121735 |
| S. halepense-1 | Eu-sorghum | AusTRCF300167A | GQ121830 | GQ121768 | GQ121808 | GQ121788 | N/A |
| S. halepense-2 | Eu-sorghum | AusTRCF300188A | N/A | N/A | N/A | N/A | GQ121749 |
| S. interjectum-1 | Stiposorghum | AusTRCF302396A | GQ121817 | GQ121753 | GQ121797 | GQ121772 | GQ121738 |
| S. interjectum-2 | Stiposorghum | AusTRCF302433A | N/A | N/A | N/A | N/A | N/A |
| S. intrans | Stiposorghum | AusTRCF302390A | GQ121825 | GQ121752 | GQ121795 | GQ121780 | GQ121733 |
| S. laxiflorum-1 | Heterosorghum | AusTRCF302503A | GQ121833 | GQ121771 | GQ1218011 | GQ121786 | GQ121741 |
| S. laxiflorum-2 | Heterosorghum | AusTRCF302607A | N/A | N/A | N/A | N/A | N/A |
| S. leiocladum-1 | Para-sorghum | AusTRCF300148A | GQ121814 | N/A | GQ121805 | N/A | N/A |
| S. leiocladum-2 | Para-sorghum | AusTRCF300170A | N/A | GQ121763 | N/A | GQ121778 | GQ121739 |
| S. macrospermum | Chaetosorghum | AusTRCF302367A | GQ121834 | GQ121767 | GQ121812 | GQ121787 | GQ121742 |
| S. matarankense-1 | Para-sorghum | AusTRCF302521A | GQ121826 | GQ121757 | GQ121804 | GQ121776 | GQ121731 |
| S. matarankense-2 | Para-sorghum | AusTRCF302636A | N/A | N/A | N/A | N/A | N/A |
| S. nitidum-1 | Para-sorghum | AusTRCF302539A | N/A | N/A | N/A | GQ121785 | N/A |
| S. nitidum-2 | Para-sorghum | AusTRCF302558A | GQ121815 | GQ121764 | GQ121807 | N/A | GQ121740 |
| S. plumosum-1 | Stiposorghum | AusTRCF302399A | GQ121819 | GQ121762 | GQ121798 | N/A | N/A |
| S. plumosum-2 | Stiposorghum | AusTRCF302489A | N/A | N/A | N/A | GQ121773 | GQ121729 |
| S. plumosum-3 | Stiposorghum | AusTRCF302635A | N/A | N/A | N/A | N/A | N/A |
| S. stipoideum-1 | Stiposorghum | AusTRCF302393A | GQ121827 | GQ121751 | GQ121794 | N/A | GQ121734 |
| S. stipoideum-2 | Stiposorghum | AusTRCF302669A | N/A | N/A | N/A | GQ121779 | N/A |
| S. timorense-1 | Para-sorghum | AusTRCF302381A | GQ121820 | GQ121760 | GQ121801 | GQ121777 | GQ121727 |
| S. timorense-2 | Para-sorghum | AusTRCF302459A | N/A | N/A | N/A | N/A | N/A |
N/A, not applicable.
* Superscripts at the end of the accession number denote the country of origin and the donor of that particular accession; if only a single country code is present then that country is both a donor and the origin of the accession. A, Australia; B, Burundi; E, Ethiopia; K, Kenya; T, Tanzania; Y, Yemen; Zm, Zambia; Zw, Zimbabwe.
DNA extraction, PCR and sequencing
Each Sorghum species was represented by 1–2 accessions, except for S. bicolor for which 11 accessions were used. Genomic DNA was extracted from fresh leaf tissues of seedlings raised in the greenhouse at approx. 2 weeks of age using a modified CTAB extraction method (Doyle and Doyle, 1987). The quality of the DNA was analysed by agarose gel electrophoresis and DNA concentration was determined using a Nanodrop® ND-1000 spectrophotometer (Saveen Werner, Malmö, Sweden).
Primers for amplification and sequencing of the trnS-trnfM, trnY-psbM and trnT-trnD regions were designed for this study while the trnT-trnL region was amplified and sequenced using the universal primers designed by Taberlet et al. (1991). A primer pair was used for each of the cpDNA regions. However, two primer pairs were designed for amplification of the trnY-psbM region. Universal primers ITS4 and ITS5 (White et al., 1990) were used for amplification and sequencing of the ITS region.
The sequences of the primers and information on specific primers supplied by Eurofins MWG GmbH (Ebersberg, Germany) used in this study are given in Table 2. A GeneAMP PCR system 9700 thermocycler was used for amplification with the following temperature regime: denaturation at 94 °C, followed by 30 cycles of 1 min denaturing at 94 °C, 1 min primer annealing at 51 °C and primer extension for 2 min at 72 °C, and a final 7-min extension at 72 °C. Successfully amplified samples were purified using the QIAquick PCR purification kit (Qiagen GmbH, Hilden, Germany) and microcentrifuge according to the manufacturer's instructions. Nine microlitres of purified PCR products was mixed with 1 µL of sequencing primers and sent to the sequencing facility in the University of Oslo, Norway (http://www.bio.uio.no/ABI-lab/), where DNA sequencing was done. The quality of the sequences was evaluated using Sequence Scanner version 1.0 (Applied Biosystems, www.appliedbiosystems.com/) and only high-quality sequences were used for the analysis. All regions were sequenced using both forward and reverse primers. The sequences from the forward and reverse primers were aligned for each sample in order to generate a consensus sequence. As the sequences were of high quality, the forward and reverse sequences are identical, except in a few cases. These few discrepancies were resolved by repeated PCR and sequencing.
Table 2.
Primers used to amplify and sequence the five non-coding regions of cpDNA and the ITS of nrDNA
| Region of cpDNA | Primer name | Primer sequence (5′ → 3′) | Source of primer sequences |
|---|---|---|---|
| psbZ-trnG | tnSM –fw† | TGCTTCTCCTGATGGTTGGT | This study |
| tnSM – rv† | GCTCGCTACATTGAACTACGC | ||
| trnY-psbM | psBD – fw* | CTGTCAAGGCGGAAGCTG | This study |
| psBD – rv† | GGGTCACATAGACATCCCAAT | ||
| trYB – fw† | GGTTAATGGGGACGGACT | ||
| trYB – rv† | AGGAAGTTAAGATGAGGGTGG | ||
| trnY-trnD | trTD – fw† | TGACGATATGTCTACGCTGGT | This study |
| trTD – rv* | AATCCCTGCGGGGTGTAT | ||
| trnT-trnL | trTL – fw† | CATTACAAATGCGATGCTCT | Taberlet et al. (1991) |
| trTL – rv† | TCTACCGATTTCGCCATATC | ||
| ITS | ITS5 –fw† | GGAAGTAAAAGTCGTAACAAGG | White et al. (1990) |
| ITS4 – rv† | TCCTCCGCTTATTGATATGC |
* Primer was used for amplification only.
† Primer used for both PCR amplification and sequencing.
Sequence alignment and data analyses
The quality of the sequences was visually inspected using Sequence Scanner version 1.0 (Applied Biosystems). Multiple sequence alignment was performed using ClustalX version 2.1.10 (Larkin et al., 2007). The sequences were edited using BioEdit version 7.0.9 (Hall, 1999) and PAUP* 4·0 Beta 10 was used for phylogenetic analyses. The phylogenetic analyses were approached in three ways. In the first approach, the ITS sequences of the nrDNA were analysed separately. In the second approach, the sequences of the four non-coding regions of the cpDNA were also analysed separately. In the final approach, a combined analysis of the cpDNA regions and the ITS was carried out. In all the cases indel positions were treated as missing data. Zea mays (GenBank accession no. U04796) was used as an out-group species.
RESULTS
Sequence characteristics of the Sorghum species
The sequence characteristics and parsimony-based tree statistics of four non-coding regions of cpDNA and the ITS are summarized in Table 3. The aligned sequences derived from all the cpDNA regions and the ITS revealed differences in sequence length between the Sorghum species. The longest sequences were obtained from the trnY-psbM spacer and ranged from 1028 nt (S. drummondii) to 1053 nt (S. exstans). The eight S. bicolor sequences from this spacer exhibited 2–3 nt differences between them. By contrast, the psbZ-trnG spacer provided the shortest sequences, which ranged between 286 nt (Eu-sorghum species) and 291 nt (S. intrans). The similarity in sequence length between the Eu-sorghum species could be attributed to the occurrence of 5-nt indels within the psbZ-trnG intergenic spacer. Indels of similar size at corresponding positions were also observed in S. laxiflorum and S. macrospermum. Sequence length variations were also observed between Sorghum species in the trnT-trnL spacer, ranging from 684 nt (S. arundinaceum) to 693 nt (S. leiocladum and S. laxiflorum). Low sequence length differences of 2 nt in the trnT-trnL spacer were observed among the S. bicolor accessions. Significant sequence variations arising from transitions and transversions were observed at eight positions, which resulted in the discrimination of S. bicolor-12, -13 and -14 from the rest of the S. bicolor accessions. The sequences obtained from the trnY-trnD spacer were between 318 nt (S. amplum, S. angustum) and 329 nt (S. exstans). The sequences obtained from the ITS showed narrow length differences of 528–534 nt between the Sorghum species; base substitutions in the ITS1 accounted for most of this variation. The S. bicolor accessions exhibited sequence length differences arising from a single nucleotide indel in the ITS1 region.
Table 3.
Sequence characteristics and tree statistics of the cpDNA and ITS regions from maximum-parsimony (MP) analysis
| cpDNA regions |
|||||||
|---|---|---|---|---|---|---|---|
| psbZ-trnG | trnY-trnD | trnY-psbM | trnT-trnL | ITS | Combined cpDNA regions | Combined cpDNA regions and ITS | |
| LAS | 286–291 | 318–329 | 1028–1053 | 684–693 | 528–534 | 2316–2366 | 2844–3111 |
| PICs* | 8 (2·7 %) | 12 (3·6 %) | 32 (3·9 %) | 19 (2·7 %) | 69 (12·8 %) | 71 (3·0 %) | 140 (4·5 %) |
| TL | 16 | 48 | 101 | 57 | 190 | 536 | 743 |
| CI | 0·9375 | 0·8958 | 0·6931 | 0·8947 | 0·8737 | 0·6250 | 0·6743 |
| HI | 0·0625 | 0·1048 | 0·31 | 0·1053 | 0·1263 | 0·3750 | 0·3257 |
| RI | 0·9846 | 0·9734 | 0·93 | 0·9757 | 0·9764 | 0·8463 | 0·8938 |
| RC | 0·9231 | 0·8720 | 0·6489 | 0·8730 | 0·8531 | 0·5252 | 0·6027 |
* Inclusive of the out-group.
LAS, length of aligned sequences; PICs, parsimony-informative characters (number and per cent); TL, tree length; CI, consistency index; HI, homoplasy index; RI, retention index; RC, rescaling consistency index.
Parsimony analysis of the ITS sequences
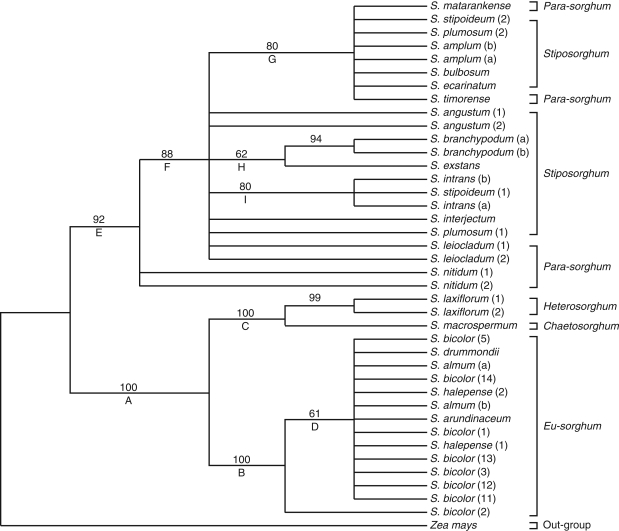
The aligned sequences of the ITS of the nrDNA provided the highest number of parsimony-informative characters (69; 12·8 %) of the regions used in this study, which could be attributed to an overall faster rate of base substitutions in the ITS than in the non-coding regions of the cpDNA. The ITS provided consistency and retention indices of 0·87 and 0·97, respectively (Table 3). The 50 % majority rule consensus tree from the phylogenetic analysis of DNA sequences of the ITS of 21 Sorghum species and Zea mays as an out-group species is shown in Fig. 1. Two lineages, A and E, were resolved. Lineage A was resolved with strong bootstrap support (100 %) and contained the Eu-sorghum species (clade B, 100 % bootstrap) and clade C with similar bootstrap support containing S. laxiflorum and S. macrospermum. The moderately supported internal clade D (61 %) contains unresolved relationships of S. bicolor accessions with other Eu-sorghum species but excludes S. bicolor-2 originally from Yemen. The other lineage, E, with 92 % bootstrap support contained the remaining native Australian Sorghum species which, except for S. nitidum, are contained in clade F with moderate bootstrap support (88 %; Fig. 1).
Fig. 1.
Maximum-parsimony 50 % majority rule consensus tree (1000 bootstrap replicates with 100 random additions; MaxTrees = 100) generated from a phylogenetic analysis of DNA sequence data from the internal transcribed spacers of the nrDNA of 21 Sorghum species and Zea mays as an out-group species. Indels are treated as missing data. Clades are indicated by letters below the branch. Bootstrap values of >50 % are indicated above the branches.
Analysis of the non-coding regions of cpDNA sequence data
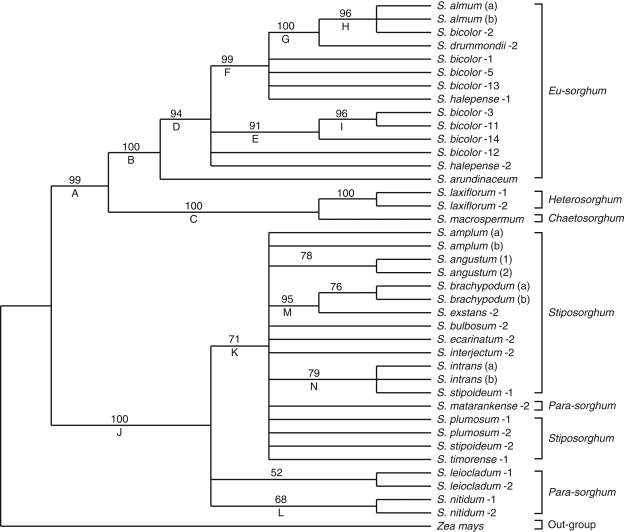
The cpDNA regions, psbZ-trnG, trnY-psbM, trnY-trnD and trnT-trnL, revealed differences in the number of parsimony-informative characters, consistency and retention indices (Table 3). The cpDNA data show less homoplasy than the ITS data (Table 3), resulting in more fully resolved 50 % majority rule consensus trees and generally greater bootstrap values for various nodes. The trnY-psbM spacer provided the highest number of parsimony-informative characters (32; 3·9 %). The psbZ-trnG region provided the lowest number of parsimony-informative characters (eight; 2·7 %). The trnT-trnL and trnY-trnD intergenic spacers generated sequences that had 19 (2·7 %) and 12 (3·6 %) parsimony-informative characters, respectively. As measures of accuracy for the topologies obtained, consistency and retention indices were highest (0·94 and 0·98, respectively) for psbZ-trnG among the cpDNA regions used. The trnY-psbM spacer had the lowest consistency index (0·69) and retention index (0·93). The 50 % majority rule consensus of 100 most-parsimonious trees is shown in Fig. 2. Lineage A is resolved and includes all the Eu-sorghum species, clade B with strong support (100 %), and S. laxiforum and S. macrospermum (clade C) with equal bootstrap support. The strongly supported (94 %) clade D includes all Eu-sorghum species but excludes S. arundinaceum. The strongly supported (96 %) internal clade H containing S. almum and S. bicolor-2 from Yemen excludes S. drummondii-2. All wild Sorghum species from Australian except S. laxiflorum and S. macrospermum form the second lineage (lineage J), which has very strong bootstrap support (100 %; Fig. 2). Clade K, with moderate bootstrap support (71 %), includes all Stiposorghum species and some Parasorghum species except S. leiocladum and S. nitidum. The internal relationships within clade K are either moderately to strongly supported by the bootstrap data (76–95 %) or remain unresolved (Fig. 2).
Fig. 2.
Maximum-parsimony 50 % majority rule consensus tree (1000 bootstrap replicates with 100 random additions; MaxTrees = 100) from a phylogenetic analysis of DNA sequence data from the four regions of cpDNA of 21 Sorghum species and Zea mays as an out-group species. Indels are treated as missing data. Clades are indicated by letters below the branch. Bootstrap values of >50 % are indicated above the branches.
Combined analysis of cpDNA and ITS sequence data
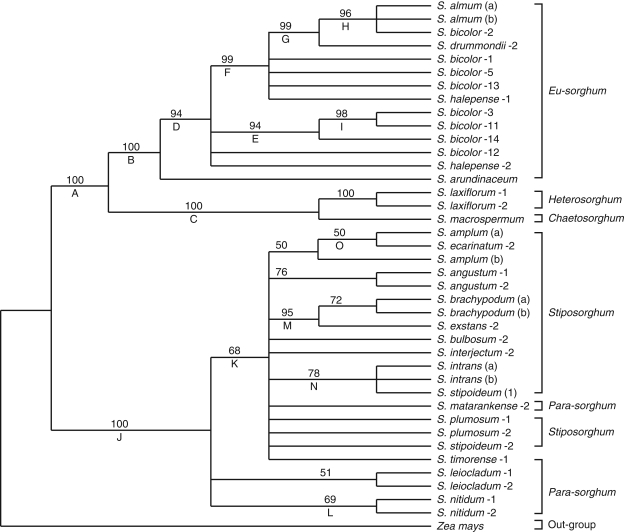
The combined cpDNA and ITS sequences generated a total of 3096 characters, 140 of which (4·5 %) were parsimony-informative (Table 3). The maximum-parsimony (MP) analysis involving the combined data from the cpDNA regions and the ITS sequence data, with gaps either considered as missing values (Fig. 3) or scored as presence or absence characters (data not shown), produced two main lineages. Lineage A contains all the Eu-sorghum species (clade B), which includes all S. bicolor and their immediate wild relatives, S. × almum, S. halepense, S. drummondii and S. arundinaceum with 100 % bootstrap support. The other lineage, lineage J, consists of all Australian wild Sorghum species except S. laxiflorum and S. macrospermum with high bootstrap support (Fig. 3). S. laxiflorum and S. macrospermum not only form a single clade (C) with strong bootstrap support but are also more closely related to the Eu-sorghum species with 100 % bootstrap support than to other Australian wild Sorghum species. Within the Eu-sorghum section, clade D excludes S. arundinaceum from the rest of the species, but a subgroup comprising S. halepense-1, S. drummondii, S. almum, and S. bicolor-1, -2, -5 and -13 is formed as clade F with 99 % bootstrap support (Fig. 3). The strongly supported (94 %) clade E consists of three accessions of S. bicolor (-3, -11 and -14). The S. bicolor accessions in this clade originated from southern Africa, one from Zimbabwe (S. bicolor-3) and the other two from Zambia. S. bicolor-2, an accession from Yemen, seems to be distantly related to S. bicolor accessions from southern Africa but has a stronger association (clade H) with S. almum with strong bootstrap support (Fig. 3).
Fig. 3.
Maximum-parsimony 50 % majority rule consensus tree (1000 bootstrap replicates with 100 random additions; MaxTrees = 100) from a phylogenetic analysis of DNA sequence data from the four regions of cpDNA and the internal transcribed spacers of the nrDNA of 21 Sorghum species and Zea mays as an out-group species. Indels are treated as missing data. Clades are indicated by letters below the branch. Bootstrap values of >50 % are indicated above the branches.
Stiposorghum and Para-sorghum form clade J with 100 % bootstrap support (Fig. 3). The internal nodes of this particular clade, however, lack strong bootstrap support. Most of the Para-sorghum and all of the Stiposorghum species form clade K with moderate bootstrap support and the two accessions of S. nitidum form a single clade (L) with equally moderate bootstrap support (Fig. 3). Clade M consists of S. brachypodum and S. exstans with 95 % bootstrap support. S. intrans and S. stipoideum-1 form clade N, and S. amplum and S. ecarinatum form clade O but with only moderate bootstrap support (78 %; Fig. 3).
DISCUSSION
Comparative DNA sequencing has become a widespread tool for inferring phylogenetic relationships and in systematic studies as it is relatively fast and convenient. Phylogenetic inference and elucidation of the evolutionary processes that generate biological diversity have been accomplished even at lower taxonomic levels using non-coding regions of the chloroplast genome and the ITSs of the nrDNA (Mort et al., 2007; Kårehed et al., 2008). In the present study, all the five cpDNA primers used successfully amplified the target regions in the Sorghum species. Mort et al. (2007) assessed the phylogenetic utility of the ITS and nine rapidly evolving cpDNA loci (including trnS-trnfM, trnD-trnT, psbM-trnD and trnT-trnL) involving six taxa sets of 13–23 taxa using published primer sequences (Shaw et al., 2005). Failure of PCR amplification was reported in Tolpis (Asteraceae) and Chrysosplenium (Saxifragaceae) with the primer pair trnD-trnT. Attempts to amplify the trnT-trnL region was not successful in all the taxa used. This implies that successful amplification using published primers for some cpDNA regions of one taxon may not have universal application across taxa. In this study, trnY-psbM provided the highest number of parsimony-informative characters followed by trnT-trnL and trnY-trnD. Based on the potentially informative characters generated, trnT-trnL and psbM-trnD were identified as suitable for low taxonomic level phylogenetic studies (Shaw et al., 2005). Of the cpDNA regions used in this study, trnY-psbM, trnT-trnL and trnY-trnD intergenic spacers were useful in the inference of phylogenetics at low taxonomic level in general and in the genus Sorghum in particular.
In the ITS analysis, all Stiposorghum and Para-sorghum species were resolved into a lineage separate from Eu-sorghum, Heterosorghum and Chaetosorghum species with strong bootstrap support (92 %). These results are consistent with findings based on the analysis of the ITS sequences (Sun et al., 1994; Dillon et al., 2001). However, in general the internal relationships between species within section are unresolved (Fig. 1). As implied and based on its utility in numerous studies, the ITS is a useful marker for resolving phylogenetic relationships at various taxonomic levels, in particular the infrageneric level. However, caution needs to be taken when analysing ITS sequence data to avoid problems resulting from concerted evolution on the rDNA arrays. Concerted evolution may homogenize different paralogous gene copies in a genome leading to the loss of all but one of the copies, i.e. different copies may be present in different organisms by chance and consequently this will create disagreement between the gene trees and species trees (Álvarez and Wendel, 2003). A fundamental requirement for historical inference based on nucleic acid or protein sequences is that the genes compared are orthologous as opposed to paralogous. However, there are inherent risks in relying exclusively on rDNA sequences for phylogenetic inferences given the ‘nomadic’ nature of the rDNA loci between inclusion of paralogous genes and exclusion of orthologous comparisons (Álvarez and Wendel, 2003).
The combined analysis of the cpDNA and ribosomal ITS sequence data, as when only the combined cpDNA dataset was used, resolved two major lineages (Figs 2 and 3). In one lineage, A, the Eu-sorghum species form a clade B with 100 % bootstrap support. These results indicate a close association between species within the section Eu-sorghum. The present results are in agreement with the findings from an assessment of phylogenetic relationships among Sorghum taxa based on 30 allozyme loci (Morden et al., 1990), which could not show clear delimitation between the Eu-sorghum taxa. Weedy forms of sorghum (e.g. S. drummondii) occur wherever cultivated sorghum and S. arundinaceum grow sympatrically (De Wet, 1978). Sympatric speciation, one of the theoretical models for the phenomenon of speciation, is the genetic divergence of various populations from a single parent species inhabiting the same geographical region, such that these populations become different species. However, the present study has shown emergence of two subgroups within Eu-sorghum with strong bootstrap support (Fig. 2). A strong phylogenetic affinity was obtained between S. bicolor- 3, an accession from Zimbabwe, three other S. bicolor accessions (-11, -12 and -14) from Zambia and S. halepense-1, as shown in clade E. The other subgroup, clade F, contains all other S. bicolor accessions (-1, -2, -5 and -13; Fig. 2). Within this clade, S. almum is closely associated with S. bicolor-2, an accession from Yemen. S. almum is believed to be a recent fertile hybrid between S. halepense and S. bicolor (Doggett, 1970). As the chloroplast genomes are believed to display maternal inheritance in the majority of angiosperms (Mogensen, 1996; Keeling, 2004; Udall and Wendel, 2006), the present phylogenetic results suggest that S. bicolor could be the maternal parent of S. almum.
S. drummondii, commonly known as Sudan grass, is believed to be a segregate from a natural hybrid between S. bicolor and S. arundinaceum and is thought to have originated in the region from southern Egypt to the Sudan (Hacker, 1992). The cultivated species, S. bicolor, is allied to S. arundinaceum, its assumed wild progenitor (Lazarides et al., 1991). This is consistent with the present results, which place S. arundinaceum in close relationship with S. bicolor with 100 % support (Fig. 3).
Various models of the origin of S. halepense have been suggested. Generally, the species is believed to have arisen as a segmental allotetraploid derived from the cross of two diploids (n = 10) species. Doggett (1970) suggested that S. halepense was derived from the rhizomatous perennial S. propinquum and the annual S. arundinaceum. In the allozyme variation study involving Eu-sorghum, S. halepense could not be differentiated from S. bicolor, suggesting that the latter was one of the parental species of S. halepense (Morden et al., 1990). The present results (Figs 1 and 2) support the suggestion that S. bicolor is one of the parents of S. halepense.
Eu-sorghum species are closely related to S. macrospermum and S. laxiflorum with strong bootstrap support (Fig. 3), consistent with previous reports based on combined ITS1/ndhF/adh1 (Dillon et al., 2007) and ITS sequence data (Sun et al., 1994). This study has also revealed a very close relationship between S. macrospermum and S. laxiflorum with 100 % support (Figs 2 and 3), which suggests these species should not be classified under different sections. The close association between these two species has prompted the suggestion that Chaetosorghum and Heterosorghum be combined in a single section (Sun et al., 1994; Dillon et al., 2004), which is strongly supported by the present data. The ancestry of cultivated sorghum is not well resolved. Based on the ease of formation of crosses (Doggett, 1970) and chromosome morphological similarities (Gu et al., 1984) within Eu-sorghum, it has been assumed that no other sections except Eu-sorghum provided the ancestral material for cultivated sorghum (van Oosterhout, 1992). However, the close association of S. macrospermum and S. laxiflorum with section Eu-sorghum indicates that there is strong sequence homology among them, suggesting that these species are phylogenetically closely related.
The phylogenetic relationships among the Australian wild Sorghum species have been described in detail (Sun et al., 1994; Spangler et al., 1999; Dillon et al., 2001, 2004, 2007; Spangler, 2003; Price et al., 2005). The internal relationships among the Australian wild sorghums are moderately well supported. S. intrans and S. stipoideum belonging to section Stiposorghum form a clade N with moderate support (Figs 2 and 3). These species have also been reported to be comparable in morphology and distribution (Lazarides et al., 1991).
The analysis of the combined data set involving ITS and cpDNA resulted in a tree that is identical to that inferred from cpDNA alone. Similar results were obtained using the two loci on Crassula (Mort et al., 2007). In contrast to a cpDNA-based approach, phylogenetic studies using nuclear DNA sequences have traditionally been hampered by difficulties in distinguishing between orthologous and paralogous sequences (Small et al., 2004). The practice of obtaining sequence data from two or more loci that can reasonably provide independent tests of phylogeny is a proven means of avoiding well-supported but incorrect phylogenies that do not track organismal phylogeny (Mort et al., 2007). Chloroplast DNA loci, which are often assumed to be uniparentally inherited and non-recombining, have been extensively used for systematics and phylogenetics. However, the rate of evolution of the cpDNA genome is slower than that of the nuclear genome. Correspondingly, the cpDNA regions that have been used for phylogenetic studies are less variable than the most extensively used nuclear loci, internal transcribed spacers of nrDNA (ITS) (Small et al., 2004; Mort et al., 2007). It is often difficult to obtain adequate resolution of any phylogeny of closely related taxa using few cpDNA loci due to the low number of phylogenetically informative characters (Rokas et al., 2003). Hence, the practice of acquiring sequence data from several loci is a proven means of acquiring a better resolved phylogeny (Rokas and Carroll, 2005; Mort et al., 2007). In the present study, the phylogeny of the genus Sorghum is well resolved when the combined data from ITS and four cpDNA regions were used.
Conclusions
The cpDNA regions used in this study have provided phylogenetic relationships even at low taxonomic level. The trnY-psbM, trnT-trnL and trnY-trnD intergenic spacers have specifically been identified to be more useful in inferring phylogenetics even at infraspecies level. The close relationship between S. macrospermum and S. laxiflorum suggest that they should not be classified under different sections and support the proposal that sections Chaetosorghum and Heterosorghum be merged. The results also indicated that the Eu-sorghum species are more closely related to S. macrospermum and S. laxiflorum than to any other Australian wild Sorghum species. S. almum is more closely associated with S. bicolor than with S. halepense, its known parents. As the chloroplast genome is maternally inherited, the results suggest that S. bicolor is the most probable maternal parent of S. almum. The S. bicolor accessions (-3, -11 and -14) from southern Africa form a distinct and well-supported clade. S. bicolor-2, originally from Yemen, is distantly related to other S. bicolor accessions used in this study. These results may provide opportunities to use sorghum gene pools outside section Eu-sorghum for development and improvement of cultivated sorghum.
ACKNOWLEDGEMENTS
We thank the Nordic Genebank (now Nordgen) for financial support. We are indebted to Dr Sally Dillon of the Australian Tropical Crops and Forage Genetic Resource Centre, Biloela, Queensland, Australia, for the provision of sorghum germplasm. We would also like to thank Ms Ann-Charlotte Strömdahl at SLU, Alnarp, Sweden, for her assistance in the laboratory.
LITERATURE CITED
- Álvarez I, Wendel JF. Ribosomal ITS sequences and plant phylogenetic inference. Molecular Phylogenetics and Evolution. 2003;29:417–434. doi: 10.1016/s1055-7903(03)00208-2. [DOI] [PubMed] [Google Scholar]
- Baldwin BG. Phylogenetic utility of the internal transcribed spacers of the nuclear ribosomal DNA in plants. An example from the Compositae. Molecular Phylogenetics and Evolution. 1992;1:3–16. doi: 10.1016/1055-7903(92)90030-k. [DOI] [PubMed] [Google Scholar]
- Baldwin BG, Sanderson MJ, Porter JJ, Wojciechowski MF, Campbell CS, Donoghue MJ. The ITS region of the nuclear ribosomal DNA. A valuable source of evidence on angiosperm phylogeny. Annals of the Missouri Botanic Gardens. 1995;85:247–277. [Google Scholar]
- De Wet JMJ. Systematics and evolution of Sorghum sect (Gramineae) American Journal of Botany. 1978;65:477–484. [Google Scholar]
- De Wet JMJ, Huckay JP. The origin of Sorghum bicolor. II. Distribution and domestication. Evolution. 1967;21:787–802. doi: 10.1111/j.1558-5646.1967.tb03434.x. [DOI] [PubMed] [Google Scholar]
- Dillon SL, Lawrence PK, Henry RJ. The use of ribosomal ITS to determine phylogenetic relationships within Sorghum. Plant Systematics and Evolution. 2001;230:97–110. [Google Scholar]
- Dillon SL, Lawrence PK, Henry RJ, Ross L, Price HJ, Johnston JS. Sorghum laxiflorum and S-macrospermum, the Australian native species most closely related to the cultivated S-bicolor based on ITS1 and ndhF sequence analysis of 25 Sorghum species. Plant Systematics and Evolution. 2004;249:233–246. [Google Scholar]
- Dillon SL, Lawrence PK, Henry RJ, Price HJ. Sorghum resolved as a distinct genus based on combined ITS1, ndhF and Adh1 analyses. Plant Systematics and Evolution. 2007;268:29–43. [Google Scholar]
- Doggett J. Sorghum. London: Longmans, Green and Co; 1970. [Google Scholar]
- Doyle JJ, Doyle JL. A rapid DNA isolation procedure for small quantities of leaf tissue. Phytochemical Bulletin. 1987;19:11–15. [Google Scholar]
- Garber ED. Cytotaxonomic studies in the genus Sorghum. 1950;23:283–362. University of California Publications in Botany. [Google Scholar]
- Geleta M. Genetic diversity, phylogenetics and molecular systematics of Guizotia Cass. (Asteraceae). Doctoral dissertation. 2007 Plant Protection Biology, Alnarp, Swedish University of Agricultural Sciences. [Google Scholar]
- Gielly L, Taberlet P. The use of chloroplast DNA to resolve plant phylogenies – noncoding versus RBCL sequences. Molecular Biology and Evolution. 1994;11:769–777. doi: 10.1093/oxfordjournals.molbev.a040157. [DOI] [PubMed] [Google Scholar]
- Gu MH, Ma TH, Liang GH. Karyotype analysis of seven species in the genus Sorghum. Journal of Heredity. 1984;75:196–202. [Google Scholar]
- Guo Q, Huang K, Yu Y, Huang Z, Wu Z. Phylogenetic relationships of Sorghum and related species inferred from sequence analysis of the nrDNA ITS region. Agricultural Sciences in China. 2006;5:250–256. [Google Scholar]
- Hacker JB. Sorghum × drummondii (Steud) Millsp. & Chase. In: Mannetje LT, Jones RM, editors. Plant resources of South-East Asia. 4. Forages. Wageningen: Pudoc Scientific Publishers; 1992. pp. 206–208. [Google Scholar]
- Hall TA. BioEdit: a user-friendly biological sequence alignment editor and analysis programme of Windows 95/98/NT. Nucleic Acids Symposium Series. 1999;41:95–98. [Google Scholar]
- Kamala V, Singh SD, Bramel PJ, Rao DM. Sources of resistance to downy mildew in wild and weedy Sorghums. Crop Science. 2002;42:1357–1360. [Google Scholar]
- Keeling PJ. Diversity and evolutionary history of plastids and their hosts. American Journal of Botany. 2004;91:1481–1493. doi: 10.3732/ajb.91.10.1481. [DOI] [PubMed] [Google Scholar]
- Komolong B, Chakraborty S, Ryley M, Yates D. Identity and genetic diversity of the sorghum ergot pathogen in Australia. Australian Journal of Agricultural Research. 2002;53:621–628. [Google Scholar]
- Kårehed J, Groeninckx I, Dessein S, Motley TJ, Bremer B. The phylogenetic utility of chloroplast and nuclear DNA markers and the phylogeny of the Rubiaceae tribe Spermacoceae. Molecular Phylogenetics and Evolution. 2008;49:843–866. doi: 10.1016/j.ympev.2008.09.025. [DOI] [PubMed] [Google Scholar]
- Larkin MA, Blackshields G, Brown NP, et al. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- Lazarides M, Hacker JB, Andrew MH. Taxonomy cytology and ecology of indigenous Australian sorghums, Sorghum Moench, Adropogoneae, Poaceae. Australian Systematic Botany. 1991;4:591–636. [Google Scholar]
- Melotto-Passarin DM, Berger IJ, Dressano K, et al. Phylogenetic relationships in Solanaceae and related species based on cpDNA sequence from plastid trnE-trnT region. Crop Breeding and Applied Biotechnology. 2008;8:85–95. [Google Scholar]
- Mogensen HL. The hows and whys of cytoplasmic inheritance in seed plants. American Journal of Botany. 1996;83:383–404. [Google Scholar]
- Morden CW, Doebley J, Schertz KF. Allozyme variation among the spontaneous species of Sorghum section Sorghum (Poaceae) Theoretical and Applied Genetics. 1990;80:296–304. doi: 10.1007/BF00210063. [DOI] [PubMed] [Google Scholar]
- Mort ME, Archibald JK, Randle CP, et al. Inferring phylogeny at low taxonomic levels: utility of rapidly evolving cpDNA and nuclear ITS loci. American Journal of Botany. 2007;94:173–183. doi: 10.3732/ajb.94.2.173. [DOI] [PubMed] [Google Scholar]
- van Oosterhout SAM. The biosystems and ethnobotany of Sorghum bicolor in Zimbabwe. 1992 D. phil thesis, University of Zimbabwe, Harare. [Google Scholar]
- Price HJ, Dillon SL, Hodnett G, Rooney WL, Ross L, Johnston JS. Genome evolution in the genus Sorghum (Poaceae) Annals of Botany. 2005;95:219–227. doi: 10.1093/aob/mci015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raubeson LA, Jansen RK. Chloroplast genomes of plants. In: Henry RJ, editor. Plant diversity and evolution: genotypic and phenotypic variation in higher plants. Wallingford, UK: CABI Publishing; 2005. pp. 45–68. [Google Scholar]
- Rokas A, Carroll SB. More genes or more taxa? The relative contribution of gene number and taxon number to phylogenetic accuracy. Molecular Biology and Evolution. 2005;22:1337–1344. doi: 10.1093/molbev/msi121. [DOI] [PubMed] [Google Scholar]
- Rokas A, Williams BL, King N, Carroll SB. Genome-scale approaches to resolving incongruence in molecular phylogenies. Nature. 2003;425:798–804. doi: 10.1038/nature02053. [DOI] [PubMed] [Google Scholar]
- Sharma HC, Franzmann BA. Host-plant preference and oviposition responses of the sorghum midge, Stenodiplosis sorghicola (Coquillett) (Dipt., Cecidomyiidae) towards wild relatives of sorghum. Journal of Applied Ethomology. 2001;125:109–114. [Google Scholar]
- Shaw J, Lickey EB, Beck JT, et al. The tortoise and the hare II: relative utility of 21 noncoding chloroplast DNA sequences for phylogenetic analysis. American Journal of Botany. 2005;92:142–166. doi: 10.3732/ajb.92.1.142. [DOI] [PubMed] [Google Scholar]
- Simmons MP, Ochoterena H. Gaps as characters in sequence-based phylogenetic analyses. Systematic Biology. 2000;49:369–381. [PubMed] [Google Scholar]
- Small RL, Cronn RC, Wendel JF. Use of nuclear genes for phylogeny reconstruction in plants. Australian Systematic Botany. 2004;17:145–170. [Google Scholar]
- Snowden JD. A classification of cultivated sorghum. Kew Bulletin. 1935;5:221–255. [Google Scholar]
- Spangler RE. Taxonomy of Sarga, Sorghum and Vacoparis (Poaceae: Andropogoneae) Australian Systematic Botany. 2003;16:279–299. [Google Scholar]
- Spangler RE, Zaitchik B, Russo E, Kellogg E. Andropogoneae evolution and generic limits in Sorghum (Poaceae) using ndhF sequences. Systematic Botany. 1999;24:267–281. [Google Scholar]
- Sun Y, Skinner DZ, Liang GH, Hulbert SH. Phylogenetic analysis of Sorghum and related taxa using internal transcribed spacers of nuclear ribosomal DNA. Theoretical and Applied Genetics. 1994;89:26–32. doi: 10.1007/BF00226978. [DOI] [PubMed] [Google Scholar]
- Taberlet PL, Gielly L, Pautou G, Bouvet J. Universal primers for amplification of three non-coding regions of chloroplast DNA. Plant Molecular Biology. 1991;17:1105–1109. doi: 10.1007/BF00037152. [DOI] [PubMed] [Google Scholar]
- Udall JA, Wendel JF. Polyploidy and crop improvement. Crop Science. 2006;46:S-3–S-14. [Google Scholar]
- White TJ, Bruns T, Lee S, Taylor J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis DGM, Sninsky J, White T, editors. PCR protocols: a guide to methods and applications. San Diego: Academic Press; 1990. pp. 315–322. [Google Scholar]