Abstract
Background and Aims
In flax hypocotyls, cadmium-induced reorientation of growth coincides with marked changes in homogalacturonan (HGA) epitopes that were recognized by JIM7 and JIM5 antibodies in the external tangential wall of the epidermis. In the present study, LM7 and 2F4 monoclonal antibodies were used, in addition to JIM5 and JIM7, to extend the investigation on the methyl-esterification pattern of HGA within various domains of the cortical tissues, including the cortical parenchyma where cell cohesion is crucial.
Methods
The PATAg (periodic acid thiocarbohydrazide–silver proteinate) test was applied to ultrathin sections so that the polysaccharides could be visualized and the ultrastructure studied. The monoclonal LM7, JIM5 and JIM7 antibodies that recognize differently methyl-esterified HGA were used. The monoclonal 2F4 antibody that is specific to a particular polygalacturonic acid conformation induced by a given calcium to sodium ratio was also applied. After immunogold labelling, the grids were stained with uranyl-acetate, the samples were observed using a transmission electron microscope and the gold particles were counted.
Key Results
In the presence of cadmium, the increase of LM7 labelling in external tangential wall of the epidermis, together with a decrease of JIM7 labelling, suggested a specific role for randomly partially de-esterified HGA to counteract the radial swelling stress. Enhanced JIM5 and 2F4 labelling in the junctions of the inner tissues indicated that the presence of blockwise de-esterified HGA might oppose cell separation.
Conclusions
The response of the hypocotyl to cadmium stress was to adapt the structure of the wall of cortical tissues by differently modulating the methyl-esterification pattern of HGA in various domains.
Keywords: Cadmium, cell wall, hypocotyl, homogalacturonan, JIM5, JIM7, Linum usitatissimum, LM7, 2F4
INTRODUCTION
To grow, plant cells must expand their cell walls (CWs) and at the same time preserve the mechanical integrity of the wall to resist high turgor pressure (Cosgrove, 1997). Thus, the CW is an important structural and functional component that contributes greatly to the diversity of plant form. Pectins are major components of the CW matrix in all dicotyledon plants and are generally present as multiblock copolysaccharides of which the simplest and most abundant are homogalacturonans (HGA; see Table 1 for list of abbreviations used in the text). HGA is a linear chain of (1 → 4)-linked α-d-galacturonic acid (GalA) residues, which can be methyl-esterified in diverse patterns along its length (e.g. review in Willats et al., 2006). Several monoclonal antibodies have been characterized that recognize differently methyl-esterified HGA epitopes. JIM7 binds to methyl-esterified oligo-uronide epitopes in HGA whose degree of methyl-esterification ranges between 35 and 81 % (Knox et al., 1990). LM7 antibody recognizes non-blockwise de-esterified HGA antigen and is specific to four un-esterified GalA residues flanked by methyl-ester groups (Clausen et al., 2003). JIM5 recognizes several epitopes containing from three to nine fully un-esterified oligo-galacturonates (Willats et al., 2001; Clausen et al., 2003) within HGA with up to a 40 % degree of methyl-esterification (VandenBosch et al., 1989). 2F4 antibody recognizes a conformational dimeric HGA epitope induced by a given ionic fraction between calcium and sodium (Liners et al., 1989). The dimer consists of two sequences of at least nine un-esterified GalA cross-linked with calcium ions.
Table 1.
Abbreviations used in the text
| Cd | Cadmium |
| CJ | Cell junction |
| CTJ | Cortical tricellular junction |
| CW | Cell wall |
| Ep | Epidermis |
| ETW | External tangential wall |
| GalA | Galacturonic acid |
| HGA | Homogalacturonan |
| ITW | Internal tangential wall |
| PATAg | Periodic acid–thiocarbohydrazide-silver proteinate |
| RW | Radial wall |
| SEL | Sub-epidermal layer |
| WLIS | Wall lining the intercellular spaces |
Many studies have shown that HGA methyl-esterification varies spatially within the CW and between tissues (e.g. Knox et al., 1990). Willats et al. (2001) have shown that, in pith parenchyma of pea stem where most cell junctions are expanded, JIM5 antibody bound throughout the primary wall including that surrounding the intercellular spaces, while LM7 antibody was restricted to the wall lining the intercellular spaces and bound particularly to the corners of the intercellular spaces. In cortical parenchyma, LM7 bound to all developing spaces, but in larger air-filled junctions the LM7 labelling was most abundant in regions of the expanded middle lamellae at the point of separation of CWs. In suspension-cultured carrot cells, Liners and Van Custem (1992) showed that 2F4 was essentially located in the middle lamellae expanded at three-way junctions or lining intercellular spaces, but was absent in primary wall.
Other reports have indicated that the methyl-esterification and fine structure of HGA are developmentally regulated. For example, the relationship between cell growth and the degree of methyl-esterification of HGA has been highlighted in many species (e.g. Goldberg et al., 1986; Knox et al., 1990; Kim and Carpita, 1992; Femenia et al., 1998) and was well demonstrated in Arabidopsis hypocotyl (Derbyshire et al., 2007). Hypocotyls of various species are commonly used as models to study cell elongation (Goldberg et al., 1986; Gendreau et al., 1997; Cosgrove, 2000; Takahashi et al., 2006), including flax (Roach and Deyholos, 2008). Studies on the development of flax hypocotyls in various light conditions have shown that the growth rate is related not only to the degree of methyl-esterification of HGA but also to their linkages within different CW domains (Morvan et al., 1991; Jauneau et al., 1994, 1997; Rihouey et al., 1995; Andème-Onzighi et al., 2000).
As a whole, pectins are subject to structural modulation during responses to the environment and abiotic stress. The habituation of cell culture to cellulose biosynthesis inhibitors showed reduced amounts of cellulose and increased levels of HGA (Manfield et al., 2004). Adaptation of tobacco cells (McCann et al., 1994) and cotton roots (Zhong and Läuchli, 1993) to growth on NaCl resulted in increased pectin content. Aluminium treatment led to an increase in root pectic content in sensitive maize (Schmohl and Horst, 2000; Schildknecht and Vidal, 2002) and rice (Yang et al., 2008). In both species, pectins of sensitive cultivars were characterized by a lower degree of methyl-esterification (higher JIM5 epi-fluorescence); conversely, the resistant-cultivar CW exhibited high level of JIM7 epi-fluorescence, indicating the presence of highly methyl-esterified HGA (Eticha et al., 2005; Yang et al., 2008). The binding of aluminium to pectins was reported to cause a decrease in extensibility and an increase in rigidity of the CW leading to organ elongation inhibition (Tabuchi and Matsumoto, 2001). A disorganized distribution of HGA epitopes in the CW was discussed as a possible mechanism for aluminium-induced root growth inhibition in maize (Li et al., 2009). Schildknecht and Vidal (2002) described an increase in the wall crystallinity resulting from pectin cross-linking in an aluminium-sensitive root.
Studies involving cadmium (Cd) have documented its toxicity on plants (reviewed, for example, in Sanitá Di Toppi and Gabrielli, 1999; Benavides et al., 2005), and several papers have reported Cd-related inhibition of growth, especially of roots (Sandalio et al., 2001; Wojcik and Tukiendorf, 2005). However, there are only a few reports on the impact of Cd on plant CW structure (Barceló et al., 1988; Maruthi et al., 2005). Interestingly, Xiong et al. (2009) reported that the addition of exogenous nitric oxide enhanced Cd tolerance by increasing pectin and hemicellulose in root CWs of rice.
Treating flax seedlings with Cd over 18 d, it was noted (a) marked changes in length and diameter of the hypocotyl, (b) cell-wall thickening and (c) significant alteration of the pectin structure (Douchiche et al., 2007). Using JIM5 and JIM7 monoclonal antibodies, two major events were observed in the epidermis of Cd-treated hypocotyls. The first one occurred in the external tangential wall. A sharp decrease of highly methyl-esterified HGA (labelled with JIM7) was observed in the outer part of the wall concomitant with a decrease in low methyl-esterified HGA (labelled with JIM5) in the inner part, indicating a differential impact of Cd on the fine structure of HGA. The second event was maintenance of cell cohesion despite the Cd-induced swelling.
The present study was undertaken to complete a previous electron microscopy investigation by analysing the variation in HGA structure, especially the methyl-esterification pattern, using two additional antibodies, LM7 and 2F4. Although the LM7 epitope was reported to be fragile when plant material is submitted to resin embedding (Willats et al., 2001), significant LM7 gold-labelling was found in cell junctions of flax hypocotyl. Such localization has also been reported for other plants when using immunofluorescence assays (Willats et al., 2001), which validated the immunogold labelling data. On the other hand, the 2F4 gold-labelling has been largely used in electron microscopy and was mainly localized in junction zones (e.g. Liners and Van Custem 1992). It was also localized in the tangential CW of the epidermis of maize coleoptiles (Schindler et al., 1995). Thus, the impact of Cd on the pattern of methyl-esterification of HGA was investigated within various domains of the cortical tissues, including the cortical parenchyma where cell cohesion is crucial.
MATERIALS AND METHODS
Plant material
Seeds of flax (Linum usitatissimum L. ‘Hermes’) were a gift from the ‘Cooperative Terre de Lin’ (Normandy, France). They were surface-sterilized and allowed to germinate in the dark at 25 °C for 3 d. Plants were then transferred to continuous light for 15 d in test tubes on sterilized medium containing phytagel. Cadmium was added as Cd(NO3)2 at a concentration of 0·5 mm. Seedlings were in good health with a minimal hypocotyl length of 15 mm, with no apparent sign of senescence.
Specimen preparation for microscopy
Small fragments were excised from the middle part of the hypocotyl. The method used for fixation and embedding was based on that described in Douchiche et al. (2007). Specimens were fixed in a mixture of paraformaldehyde, glutaraldehyde and cacodylate buffer and post-fixed in osmium tetroxide. After dehydration in an ethanol series, the samples were embedded in LR white resin.
PATAg staining
The PATAg (periodic acid thiocarbohydrazide–silver proteinate) test for the visualization of polysaccharides was applied in ultrathin sections according to the procedure of Thiery (1967).
Immunolabelling procedures
The monoclonal antibodies: LM7, JIM5 and JIM7 used in this study are all specific to partially methyl-esterified HGA (VandenBosch et al., 1989; Knox et al., 1990; Willats et al., 2001). JIM5 antibody is able to recognize several epitopes containing from three to nine fully un-esterified GalA residues adjacent to or flanked by residues with methyl-esterified ester group (Willats et al., 2000). JIM7 antibody binds to methyl-esterified HGA with a degree of methyl-esterification ranging between 35 and 81 % (Knox et al., 1990). LM7 recognizes non-blockwise partially methyl-esterified HGA (Willats et al., 2001), with a specific epitope consisting of four un-esterified GalAs with flanking methyl-ester groups (Clausen et al., 2003). The monoclonal 2F4 antibody is specific to a particular polygalacturonic acid conformation induced by calcium (Liners et al., 1989).
Non-specific binding of JIM5, JIM7, LM7 and 2F4 antibodies was tested by the omission of the primary antibody, and as previously described (e.g. Liners and Van Custem, 1992; Jauneau et al., 1997), no labelling was found.
Immunogold labelling
Ultrathin sections (80–90 nm) from LR white resin-embedded samples, mounted on gold grids, were submitted to two different protocols of immunogold labelling.
(1) For JIM5, JIM7 and LM7 immunogold labelling, sections were treated in a mixture of PBS/BSA-C (0·2 %) solution for 5 min. They were then incubated in a blocking solution of 3 % non-fat dried milk in 0·1 % PBS for 30 min. Grids were washed in 0·1 % PBS/0·2 % BSA-C and incubated for 3 h at 25 °C in droplets of JIM5 or JIM7 antibodies (diluted 1 : 5 in 0·1 % PBS/0·2 % BSA-C) or LM7 antibody (dilution 1 : 2 in the same buffer). After washing, the grids were treated for 2 h at 25 °C with the secondary antibody conjugated to 10 nm colloidal gold solution (anti-rat IgG) diluted 1 : 25 in 0·1 % PBS/0·2 % BSA-C. Sections were rinsed with 0·1 % PBS/0·2 % BSA-C then with distilled water.
(2) For 2F4 immunogold labelling, sections were treated as described by Liners and Van Custem (1992). Briefly, after a 1-h blocking step with 5 % low-fat dried milk in TCaS buffer, slides were incubated for one night at 4 °C with 2F4 supernatant, diluted 1 : 20 in TCaS buffer supplemented with 0·75 % Tween 20 and 1 % low-fat dried milk. After four successive washes of 5 min each with TCaS buffer, the grids were transferred for 1 h into the secondary antibody – goat anti-mouse IgG–IgM 10 nm gold diluted 1 : 20 in TCaS. The grids were washed for 5 min with TCaS, incubated 3 min in 2·5 % glutaraldehyde in TCaS buffer to fix the labelling then rinsed with distilled water.
After immunolabelling, all the grids were stained with uranyl-acetate 2 % (w/v) in ethanol 50 %. The samples were observed using a transmission electron microscope (Philips Tecnai 12 at 80 kV).
Quantification of immunogold labelling
The cross-sections of the hypocotyl were divided into anatomical regions. In epidermis, the quantification of the number of gold particles was performed in the external tangential wall (ETW) that was divided into two domains – the outer (ETWo) and the inner (ETWi) parts. Gold particles were also counted in the cell junctions (CJ), radial wall (RW) and internal tangential wall of the epidermis (ITW). In the sub-epidermal layer (SEL), labelling was counted in the radial wall (SEL RW), the tangential wall (SEL TW) and in the junctions (SEL CJ). In cortical parenchyma, the observations were restricted to the primary walls lining the intercellullar spaces (WLIS) and the core of tricellular junctions (CTJ). The density of immunogold labelling was estimated as the mean number of gold particles per μm2. More than ten observations were done per CW domain from at least two or three different hypocotyls.
RESULTS
Cadmium-induced ultrastructural alterations were distinct in tangential and radial walls of cortical tissues
PATAg reactivity allows contrasted CW domains, which contain a large amount of hydrosoluble polysaccharides including pectins, to be discriminated from non-contrasted regions, which are mainly composed of crystalline cellulose (where the vic-glycol functions are engaged into hydrogen bonds; Roland 1978).
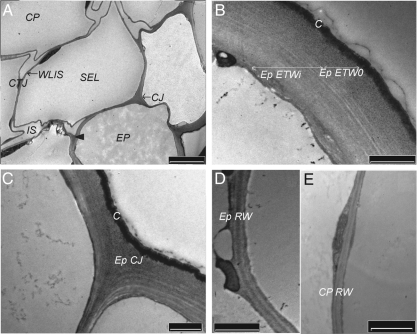
In all cortical tissues of control seedlings, the cell junctions (CJ) including the tricellular ones (CTJ) appeared as the most reactive domains to PATAg staining (Fig. 1A). The CW region close to the plasma membrane was generally weakly reactive to PATAg staining (Fig. 1B). The outer part of the epidermis (ETWo), the middle zone of the tangential walls between the epidermis and the sub-epidermal layer (SEL), as well as the centre of their junctions were heavily stained, showing a granular texture (Fig. 1A–C). Thin layers with more and less reactivity were observed in the internal half of the epidermis tangential wall (ETWi; Fig. 1B). The cuticle appeared regular, closely adhered to ETWo and penetrated by PATAg-reactive polysaccharides. The radial walls were relatively homogeneously stained with some higher contrast within the middle lamella (Fig. 1D and E). In the thin primary wall of the cortical parenchyma cells, polysaccharides were weakly stained whereas the middle lamella, the wall lining the intercellular spaces (WLIS) and the core of tricellular junctions (CTJ) were particularly contrasted (Fig. 1A).
Fig. 1.
Ultrastructure of cell-wall domains within the cortical tissues of control hypocotyl; PATAg staining. (A) General view of the cortical tissues. Note the heavily stained centre of the cell junctions (arrow head). (B) External tangential wall of the epidermis (Ep). Note the differently stained outer (ETWo) and inner (ETWi) regions; statistical observations indicated a respective thickness of 0·35 ± 0·06 and 0·84 ± 0·14 µm. (C) Cell junction (CJ) of the epidermis. (D, E) Radial walls (RW) of epidermis and cortical parenchyma (CP). The walls were very thin compared with ETW (see B) and contrasted at the level of the middle lamella. C, Cuticle; CTJ, cortical tricellular junction; IS, intercellular space; SEL, sub-epidermal layer; WLIS, wall lining the intercellular spaces. Scale bars: (A) = 5 µm; (B–E) = 1 µm.
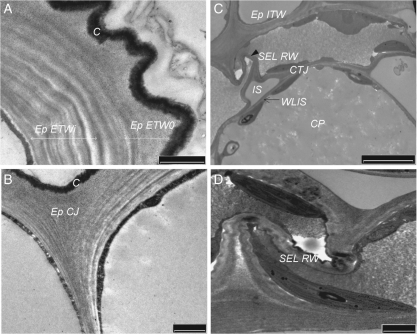
In Cd-treated hypocotyls, the most prominent effect of Cd was well illustrated by the PATAg reactivity within the epidermis (Fig. 2). A number of changes in CW architecture were observed in the external tangential wall, with polysaccharides being deposited in an irregular fashion. Contrasted layers alternating with non-contrasted layers were arranged in large multilamellate waves in the innermost domain (ETWi; Fig. 2A). The thickness of the internal fibrillar zone increased from 0·84 ± 0·14 to 1·55 ± 0·15 µm, while that of the outermost zone increased relatively less from 0·35 ± 0·06 to 0·50 ± 0·10 µm. Together with the cuticle, this latter zone appeared winding and curving. Cd-induced multilayer structures also increased in the other tangential walls (ITW and SEL), so as a granular contrasted zone could hardly be observed in the middle lamella. The junction structure of epidermis and SEL was globally unaffected by the presence of Cd although the fibrillar zones appeared extended (Fig. 2B).
Fig. 2.
Cadmium-induced alterations of the ultrastructure of cell-wall domains within the cortical tissues; PATAg staining. (A) External tangential wall of the epidermis (Ep). Note the tortuous aspect of the tangential wall (ETWo) and of the cuticle (C) and the multilamellate structure of the internal region close to the plasma membrane (ETWi). This figure was observed in 60 % of the sections compared with 10 % slight waving noted in the control sections. (B) Cell junction of the epidermis (Ep CJ). (C) Sub-epidermal layer (SEL) and cortical parenchyma (CP). In cortical parenchyma, note the well-formed intercellular spaces (IS) and the PATAg-contrasted cortical tricellular junction (CTJ). In SEL, the radial walls (RW) appeared highly compressed and Z-shaped (arrow; 100 % sections). (D) Detail of radial wall of sub-epidermal layer (SEL RW). ITW, Internal tangential wall between Ep and SEL; WLIS, wall lining the intercellular spaces. Scale bars: (A, B, D) = 1 µm; (C) = 5 µm.
In the radial walls of the epidermis and SEL, the PATAg staining appeared more or less homogeneous. Importantly, these walls displayed a Z-shaped fold, the most obvious being observed in the SEL (Fig. 2C, D).
In the cortical parenchyma where the cell swelling was the most prominent, the cells remained cohesive and the PATAg feature looked unchanged, with reinforced contrast in CTJ and in the wall lining the intercellular spaces (WLIS; Fig. 2C).
In control seedlings, the distribution of HGA epitopes varied within the wall domains
In the previous paper, the study was focused on the distribution of JIM5 and JIM7 antibodies in the epidermis. In this paper, observations were extended to all the cortical tissues and the gold labellings of two additional LM7 (Fig. 3) and 2F4 antibodies (Fig. 4) were quantified, comparing them with JIM5 and JIM7 ones (Table 2).
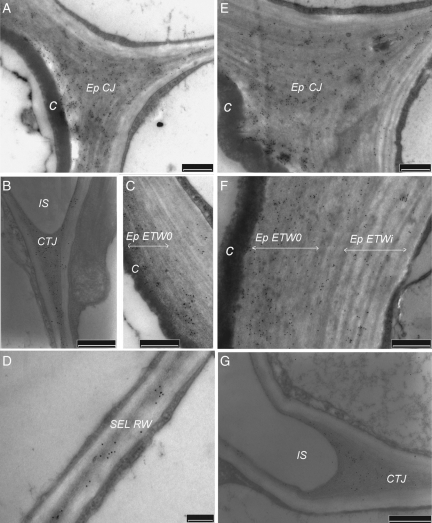
Fig. 3.
Effect of cadmium on the LM7 immunogold labelling in the cortical tissues: (A–D) untreated samples; (E–G) samples treated with Cd. In control seedlings, LM7 labelling was maximal in cell junctions (Ep CJ) of the epidermis (A) and in tricellular junctions (CTJ) of the cortical parenchyma (B). The labelling was minimal in the inner part of the external tangential wall (Ep ETWi; C) and in all radial walls (RW; D). In the presence of Cd, the gold particle number remained about the same in Ep CJ (E) and CTJ (G) and increased significantly in the outer part of Ep ETW (compare C and F; see Table 2). More than ten observations were done per CW domain on two or three independent sections. C, Cuticle; ETW, external tangential wall,; IS, intercellular space; SEL, sub-epidermal layer. Scale bars: (A–C, E–G) = 500 nm; (D) = 200 nm.
Fig. 4.
Effect of cadmium on the 2F4 immunogold labelling in the cortical tissues: (A–D) untreated samples; (E–G) samples treated with Cd. In control seedlings, 2F4 labelling was intense in the core of cell junctions (Ep CJ) of the epidermis (A) and the tricellular junctions (CTJ) of cortical parenchyma (B). In external tangential wall, the gold particles were localized in the outer half of the wall (Ep ETWo; C). In radial walls (RW), only a few scattered gold granules were detected (D). In the presence of Cd, the labelling decreased significantly in the junction of the epidermis (Ep CJ; E) and sub-epidermal layer (SEL CJ; G) but slightly in CTJ (F). More than ten observations were done per CW domain on two or three independent sections. C, Cuticle. Scale bars: (A–D) = 50 nm; (E–G) = 500 nm.
Table 2.
Effect of cadmium on the distribution of LM7, JIM5, 2F4 and JIM7 epitopes in the cell-wall domains of the cortical tissues
| Mean number of gold particles per square micrometre |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Antibody | Treatment | Ep ETWo | Ep ETWi | Ep ITW | Ep RW | Ep CJ | SEL RW | SEL TW | SEL CJ | CTJ | WLIS |
| LM7 | –Cd | 39 ± 9 | 10 ± 3 | 35 ± 13 | 21 ± 5 | 138 ± 20 | 41 ± 13 | 32 ± 14 | 175 ± 21 | 162 ± 55 | 108 ± 36 |
| +Cd | 96 ± 21* | 15 ± 5 | 16 ± 5* | 15 ± 3* | 101 ± 29 | 29 ± 14 | 29 ±14 | 159 ± 39 | 163 ± 25 | 125 ± 34 | |
| JIM5 | –Cd | 653 ± 70 | 273 ± 37 | 330 ± 86 | 327 ± 74 | 1033 ±216 | 312 ± 82 | 317 ± 58 | 659 ± 73 | 610 ± 67 | 555 ± 80 |
| +Cd | 585 ± 72 | 42 ± 27* | 162 ± 28* | 199 ± 31* | 938 ± 170 | 116 ± 51* | 117 ± 53* | 885 ± 51* | 606 ± 102 | 664 ± 140 | |
| 2F4 | –Cd | 85 ± 20 | 5 ± 3 | 15 ± 5 | 25 ± 7 | 220 ± 35 | 15 ± 5 | – | 125 ± 25 | 145 ± 40 | 40 ± 25 |
| +Cd | 40 ± 15* | 3 ± 2 | 6 ± 3* | 10 ± 5* | 60 ± 20* | 11 ± 5 | – | 20 ± 10* | 85 ± 30* | 30 ± 25 | |
| JIM7 | –Cd | 257 ± 61 | 238 ± 77 | 276 ± 80 | 210 ± 76 | 184 ± 51 | 210 ± 60 | 220 ± 87 | 170 ± 34 | 201 ± 35 | 184 ± 41 |
| +Cd | 41 ± 21* | 223 ±46 | 104 ± 38* | 86 ± 26* | 19 ± 20* | 63 ± 28* | 55 ± 36* | 30 ± 21* | 68 ± 15* | 65 ± 21* | |
The density of immunogold labelling was estimated as the mean number of gold particles per square micrometre. Means ± standard deviation were calculated from a minimum of ten observations per cell-wall domain from at least two different hypocotyls. Cd, Cadmium; CJ, junction; CTJ, cortical tricellular junction; Ep, epidermis; ETWo and ETWi, outer and inner part of the external tangential wall, respectively; ITW, internal tangential wall; RW, radial wall; SEL, sub-epidermal layer; WLIS, wall lining the intercellular spaces; –, not reported.
* Significantly different from control (Student's t-test; P < 0·05).
In order to validate the LM7 data at the level of the electron microscopy, the LM7 labellings were compared with those of the three other antibodies in junction zones of the cortical tissues of control seedlings. In these domains, the LM7 distribution was homogeneous apart from the area adjacent to the plasmalemma, where no labelling was observed (Fig. 3A, B). In the epidermis junctions, it was noted that gold particles were also associated with the wall area adjacent to the cuticle. In general, the LM7 labelled significantly all the junctions and more particularly SEL CJ and CTJ (Table 2). The number of gold particles in these domains was of the same order as that counted after JIM7 labelling. As expected, the LM7-recognized junction-cores were also strongly labelled with JIM5 and 2F4 antibodies (Table 2). The latter antibody was essentially localized in Ep CJ (Fig. 4A) compared with SEL CJ and CTJ (Fig. 4B). Thus, the comparison of the localization of the four antibodies in the junctions validated the LM7 data at the level of electron microscopy. Interestingly, the number of LM7 gold particles counted in the wall lining the intercellular spaces was also significant.
In Ep ETW, the LM7 labelling was rather low compared with JIM5 and JIM7 ones (Table 2), and was essentially localized in ETWo (Fig. 3C). Also the 2F4 labelling appeared relatively high (Fig. 4C) which indicates the presence of blockwise de-esterified HGA, beside highly methyl-esterified HGA (labelled with JIM7).
In the other tangential walls as well as in RW, the LM7 (Fig. 3D) and 2F4 (Fig. 4D) labellings were low (10–40 particles μm−2). The value of labelling with JIM5 was about half its value in ETWo, while JIM7 labelling was high (Table 2) and evenly distributed over the entire width of the walls. Consequently, these CWs appeared enriched in partially methyl-esterified blocks of HGA (DME > 31 %).
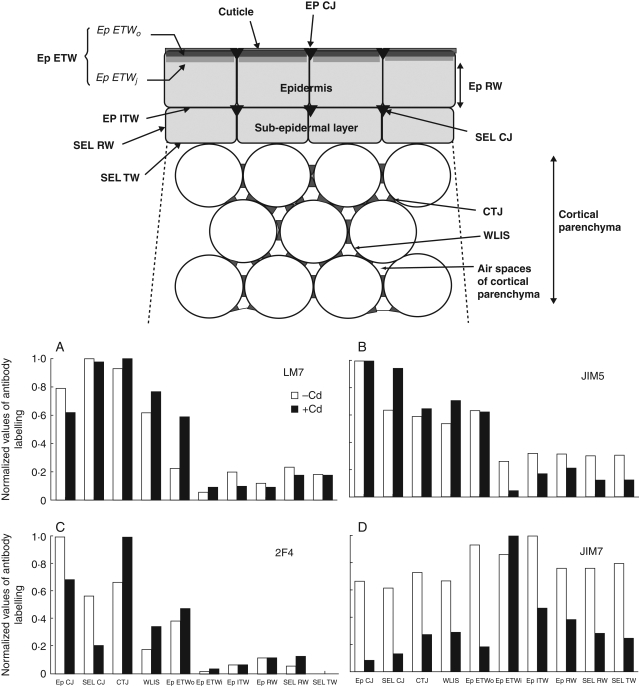
Altogether, the comparison of the numbers of gold particles made it possible to distinguish specific localization of the respective antibodies in elongated hypocotyl; the LM7 antibody was at its highest level in the most internal junctions (SEL CJ and CTJ; Fig. 5A) while JIM5 and 2F4 antibodies were more specifically localized in the epidermis junctions (Fig. 5B, C). The JIM7 labelling did not vary significantly whatever the tissue with the highest values occurring in tangential walls of the epidermis (Fig. 5D).
Fig. 5.
Comparison of the labelling distribution of the four antibodies within the cell-wall domains of cortical tissues. The data were normalized, taken the maximal value per antibody from Table 2 as being equal to 1. A schematic figure indicates the different domains under study and their acronyms. (A) LM7; (B) JIM5; (C) 2F4; (D) JIM7 labelling. Note, in the presence of Cd, the maximal increase of LM7 in the outer part of the external tangential wall of the epidermis (Ep ETWo), the increase of JIM5 labelling in the cell junction of the sub-epidermal layer (SEL CJ), the increase of 2F4 in the tricellular junctions (CTJ) of the cortical parenchyma, while JIM7 labelling became maximal in the inner part of the epidermis external tangential wall (Ep ETWi). ITW, Internal tangential wall of the epidermis; RW, radial wall; WLIS, wall lining the intercellular space.
Impact of Cd on the distribution of HGA epitopes in cortical tissues
When Cd was applied, LM7 labelling appeared high in the epidermis outer domains close to the cuticle (Fig. 3E and F), especially in ETWo (compare F and C in Fig. 3). On the other hand, the 2F4 labelling appeared to decrease in these outer areas (Fig. 4E). Concerning the slight decrease of JIM5 labelling in ETWo (Table 2), opposite effects had to be considered: the decrease in the common epitopes recognized by JIM5 and JIM7 (DME > 35 %), together with those that might be common with 2F4 (un-esterified nonagalacturonates), would be partly compensated by the increase in the common LM7/JIM5 epitopes. In ETWi, the number of LM7 gold particles would increase slightly while that of JIM5 and 2F4 decreased significantly, and JIM7 labelling remained constant.
In the junctions, different patterns were observed with each antibody (Table 2). The density of LM7 labelling was about as high as in the control and remaind maximal in the most internal junctions (Fig. 3G). The JIM5 labelling maximum extended from the epidermis to the SEL junctions. A significant decrease of 2F4 labelling occurred in all junctions (Table 2 and compare E and F with A and B in Fig. 4), the most important shift being observed in the core of SEL CJ (Fig. 4G). Besides, the JIM7 labelling was greatly reduced in all the junctions apart from the area close to the plasmalemma. Interestingly, the number of LM7 and more particularly of JIM5 gold particles increased in the wall lining the intercellular spaces (Table 2).
In the tangential (apart of ETW) and radial walls, the labelling of the four antibodies was significantly reduced. This reduction was the most severe for JIM7 and JIM5, especially in the sub-epidermal layer (Table 2).
As illustrated in Fig. 5, the impact of Cd on the HGA acidification arose differently in the CW domains of cortical tissues, with a strong effect in the outermost zones of the epidermis. The highest increase of LM7 labelling in ETWo (Fig. 5A), together with the reduction of JIM7 labelling, indicated an occurrence of random partial de-esterification. On the other hand, the normalized values indicated a net increase of JIM5 and 2F4 labelling in the junctions of the sub-epidermal layer and CTJ, respectively (Fig. 5B, C). One hypothesis is that there were two types of acidification in CTJ, one being random and the other being blockwise and more specifically Cd-induced.
DISCUSSION
This report represents one of the first quantifications of gold-labelling of the four main anti-HGA antibodies in all cell-wall domains of hypocotyl cortical tissues. These data allow an analysis of the redistribution of the methyl-esterification pattern of HGAs in response to Cd stress.
In elongated cells of dicotyledon species, the acidic HGA epitopes (recognized by JIM5, LM7 or 2F4) were generally localized in cell junctions (Liners and Van Custem, 1992; Rihouey et al., 1995; Bush et al., 2001), as well as in the expanded regions of the middle lamellae and areas lining the intercellular spaces (Jauneau et al., 1997; Bush et al., 2001; Willats et al., 2001). In maize coleoptile epidermis, a tissue known to control the growth rate of the organ (Savaldi-Goldstein et al., 2007), JIM5 and 2F4 labelling was clearly observed in the outer part of the tangential wall (Schindler et al., 1995). On the other hand, methyl-esterified pectins seem characteristic of meristems (Sobry et al., 2005; Peaucelle et al., 2008) and elongating cells (Goldberg et al., 1986).
The present data in elongated hypocotyl are in general agreement with the literature, especially the data concerning LM7 that was found to be abundantly localized in CTJ. A striking finding was a clear zonation which occurred in Ep ETW whose outer domain was weakly labelled with LM7 antibody compared with the 2F4 one. In the presence of Cd, a massive acidification of HGA was observed in all the cell junctions of cortical tissues as well as in the most external wall domains of the epidermis. Specific redistribution of the different epitopes occurred in various CW domains of the expanded hypocotyl. In the following sections, their localization is discussed in relation to their possible roles in CW ultrastructure and in counteracting the radial stress imposed by Cd-induced swelling of the hypocotyl.
Is the role of HGA enriched in the LM7 epitope to adapt the external tangential wall of the epidermis to radial stress?
The PATAg-stained sections showed remarkable ultrastructural alterations to Ep ETW during growth in the presence of Cd, illustrating a significant radial stress that expanded to the cuticle. In parallel, ETWo became significantly labelled with LM7 antibody, compensating the marked decrease of JIM7, while JIM5 labelling remained about constant and the 2F4 one shifted down. These data pointed to a possible role of a pectin methylesterase whose mechanism of action would lead to a random de-esterification of HGA (Paynel et al., 2009). In vitro, partially methyl-esterified HGA gel, enriched in LM7 epitope, was reported to retain water and to exhibit a pronounced elastic strain under compression stress (Willats et al., 2001). Hence, the observation that LM7 epitope was increased in the presence of Cd is consistent with an increase in partially methyl-esterified HGA which might facilitate transmission of the radial swelling stress from the inside to the outside part of ETW limited by the cuticle.
Which HGA epitopes might be related to the maintenance of cell cohesion in the innermost junctions of cortical tissues?
During normal growth of most dicotyledon plants (Willats et al., 2001), including flax (this paper), the innermost junctions of cortical tissues are specifically enriched in partially randomly methyl-esterified HGA, recognized by LM7 antibody. In the presence of Cd, JIM7 labelling decreased dramatically in all the junctions, LM7 labelling remained about constant while JIM5 labelling specifically increased in the junctions of the sub-epidermal layer and in the wall lining the intercellular spaces of the cortical parenchyma. The fact that JIM5 epitopes increased more than LM7 epitopes suggests a specific blockwise de-esterification. Indeed, JIM5 has been reported to recognize up to nine successive GalAs (Willats et al., 2000). We hypothesized that, in these domains, a blockwise pattern would complete the acidification process initiated by a sporadic de-esterification (as indicated by LM7 labelling in the same domains). Pectin methylesterases have been previously localized in these domains, in elongated hypocotyl (Morvan et al., 1998) and a particular isoform was shown to be expressed specifically in the presence of Cd (Paynel et al., 2009). Using 2F4 antibody, it was observed that the relative value of labelling significantly decreased in Ep and especially in SEL junctions, but became maximal in CTJ. This means that the impact of Cd on the HGA structure had led to the presence of more than nine blockwise un-esterified GalAs (Liners and Van Custem, 1992), which have a good probability of interacting and forming a gel with calcium ions (Jarvis, 1984). Due to the affinity of some peroxidases for such HGA structure (Ferrer et al., 1991; Penel and Greppin, 1996), cross-linking might be enhanced in these junctions, that possibly involved the formation of dimeric feruloyl esters (Fry, 1986; Waldron et al., 1997). As previously observed in S. alba apices (Sobry et al., 2005), regions with calcium-binding sites enriched in 2F4 epitopes correspond to sites of maximum mechanical stresses due to cell ‘turgor’. The localization of 2F4-binding sites in CTJ when their abundance was greatly decreased elsewhere suggests a specific developmental change to combat cell separation stress by reinforcing zones at low-cohesion junctions.
Thus, several HGA de-esterification patterns led to HGA of increasing acidity in the innermost junctions during Cd-provoked expansion. As indicated in previous papers, the formation of calcium bridges in CTJ would be key-factors in the maintenance of cell cohesion at the point of cell separation (Liners and Van Custem, 1992; Jauneau et al., 1994; Jarvis et al., 2003).
Impact of Cd on the HGA structure in primary wall
When Cd was applied, JIM7 epitopes and also the acidic HGA epitopes recognized by LM7, JIM5 and 2F4 were detected less abundantly in all the primary wall of cortical tissues, while the thickness of these tissues increased. This resembles a pectin/cellulose compensatory phenomenon often observed in mutant CWs (e.g. Nicol et al., 1998). Indeed, chemical data showed a significant increase in the cellulosic residue after Cd-treatment of the seedlings (Douchiche, 2007).
Ralet et al. (2008) reported that, in arabidopsis mutants (e.g. QUA2), the inhibition/repression of a pectin methyltransferase might be accompanied by an enrichment of rhamnogalacturonan RG-I. The authors also indicated that the HGA/RG-I balance might be related to the overall stiffness of pectic molecules. Previous data have shown some increase in the labelling with anti-PGA/RG-I antibody in the epidermis radial walls (Douchiche et al., 2007). Thus, in the radial wall, an increased flexibility of the pectic matrix would allow a Z-shape structure but no CW failure/disruption, despite the high compression stress provoked by the cortical cell swelling.
Conclusions
During Cd-induced expansion of flax hypocotyl, the cells engaged in a differential acidification of HGA whose intensity depended on cell-wall domains. For the first time, a particular role for randomly de-esterified HGA, recognized by LM7 antibody, is suggested to facilitate transmission of the radial swelling stress in epidermis. In the low tightened CTJ, the blockwise distribution of un-esterified GalAs can lead to the formation of calcium pectate gels. Thus the cell/tissue separation strengths could be counteracted by calcium cross-linkages.
ACKNOWLEDGMENT
We acknowledge Michael Deyholos, University of Alberta (Canada) for critically reading the manuscript.
LITERATURE CITED
- Andème-Onzighi C, Girault R, His I, Morvan C, Driouich A. Immunocytochemical characterization of early-developing flax fiber cell walls. Protoplasma. 2000;213:235–245. [Google Scholar]
- Barceló J, Vasquez MD, Poschenrieder C. Cadmium induced structural and ultrastructural changes in the vascular system of bush bean stems. Botanica Acta. 1988;101:254–261. [Google Scholar]
- Benavides MP, Gallego SM, Tomaro ML. Cadmium toxicity in plants. Journal of Plant Physiology. 2005;17:21–34. [Google Scholar]
- Bush MS, Marry M, Huxham IM, Jarvis MC, McCann MC. Developmental regulation of pectic epitopes during potato tuberisation. Planta. 2001;213:869–880. doi: 10.1007/s004250100570. [DOI] [PubMed] [Google Scholar]
- Clausen MH, Willats WGT, Knox JP. Synthetic methyl hexagalacturonate hapten inhibitors of anti-homogalacturonan monoclonal antibodies LM7, JIM5 and JIM7. Carbohydrate Research. 2003;338:1797–1800. doi: 10.1016/s0008-6215(03)00272-6. [DOI] [PubMed] [Google Scholar]
- Cosgrove DJ. Assembly and enlargement of the primary cell wall in plants. Annual Review of Cell Developmental Biology. 1997;13:171–201. doi: 10.1146/annurev.cellbio.13.1.171. [DOI] [PubMed] [Google Scholar]
- Cosgrove DJ. Expansive growth of plant cell walls. Plant Physiology and Biochemistry. 2000;38:109–124. doi: 10.1016/s0981-9428(00)00164-9. [DOI] [PubMed] [Google Scholar]
- Derbyshire P, McCann M, Roberts K. Restricted cell elongation in Arabidopsis hypocotyls is associated with a reduced average pectin esterification level. BMC Plant Biology. 2007;7:31–42. doi: 10.1186/1471-2229-7-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douchiche O. 2007. Implication de la paroi de l'hypocotyle de lin (Linum usitatissimum) dans la réponse au cadmium:rôle des pectines dans la restructuration des parois. PhD Thesis, University of Rouen, France. [Google Scholar]
- Douchiche O, Rihouey C, Schaumann A, Driouich A, Morvan C. Cadmium-induced alterations of the structural features of pectins in flax hypocotyl. Planta. 2007;225:1301–1312. doi: 10.1007/s00425-006-0425-7. [DOI] [PubMed] [Google Scholar]
- Eticha D, Stass A, Horst W. Cell-wall pectin and its degree of methylation in the maize root-apex: significance for genotypic differences in aluminium resistance. Plant, Cell & Environment. 2005;28:1410–1420. [Google Scholar]
- Femenia A, Garosi P, Roberts K, Waldron KW, Selvendran RR, Robertson JA. Tissue-related changes in methyl-esterification of pectic polysaccharides in cauliflower (Brassica oleracea L. var. botrytis) stems. Planta. 1998;205:438–444. doi: 10.1007/s004250050341. [DOI] [PubMed] [Google Scholar]
- Ferrer MA, Muñoz R, Barcelö AR. A biochemical and cytochemical study of cuticle-associated peroxidases in Lupinus. Annals of Botany. 1991;67:561–568. [Google Scholar]
- Fry SC. Cross-linking of matrix polymers in the growing cell walls of angiosperms. Annual Review of Plant Physiology. 1986;37:165–186. [Google Scholar]
- Gendreau E, Traas J, Desnos T, Grandjean O, Caboche M, Höfte H. Cellular basis of hypocotyl growth in Arabidopsis thaliana. Plant Physiology. 1997;114:295–305. doi: 10.1104/pp.114.1.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg R, Morvan C, Roland JC. Composition, properties and localization of pectins in young and mature cells of themung bean hypocotyl. Plant and Cell Physiology. 1986;27:417–429. [Google Scholar]
- Jarvis MC. Structure and properties of pectin gels in plant cell walls. Plant, Cell & Environment. 1984;7:153–164. [Google Scholar]
- Jarvis MC, Briggs SPH, Knox JP. Intercellular adhesion and cell separation in plants. Plant, Cell & Environment. 2003;26:977–989. [Google Scholar]
- Jauneau A, Cabin-Flaman A, Verdus MC, Ripoll C, Thellier M. Involvement of calcium in the inhibition of endopolygalacturonase activity in epidermis cell wall of flax (Linum usitatissimum) Plant Physiology and Biochemistry. 1994;6:839–846. [Google Scholar]
- Jauneau A, Quentin M, Driouich A. Micro-heterogeneity of pectins and calcium distribution in the epidermal and cortical parenchyma cell walls of flax hypocotyl. Protoplasma. 1997;198:9–19. [Google Scholar]
- Kim JB, Carpita NC. Changes in esterification of the uronic acid groups of cell wall polysaccharides during elongation of maize coleoptiles. Plant Physiology. 1992;98:646–653. doi: 10.1104/pp.98.2.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox JP, Linstead PJ, King J, Cooper C, Roberts K. Pectin esterification is spatially regulated both within cell walls and between developing tissues of root apices. Planta. 1990;181:512–521. doi: 10.1007/BF00193004. [DOI] [PubMed] [Google Scholar]
- Li YY, Yang JL, Zhang YJ, Zheng SJ. Disorganized distribution of homogalacturonan epitopes in cell wall as one possible mechanism for aluminium-induced root growth inhibition in maize. Annals of Botany. 2009;104:235–241. doi: 10.1093/aob/mcp123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liners F, Van Custem P. Distribution of pectic polysaccharides throughout walls of suspension-cultured carrot cells: an immunocytochemical study. Protoplasma. 1992;170:10–21. [Google Scholar]
- Liners F, Letesson J-J, Didembourg C, Van Custem P. Monoclonal antibodies against pectin: recognition of a conformation induced by calcium. Plant Physiology. 1989;91:1419–1424. doi: 10.1104/pp.91.4.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann MC, Shi J, Roberts K, Carpita NC. Changes in pectin structure and localization during the growth and NaCl- adapted tobacco cells. The Plant Journal. 1994;5:773–785. [Google Scholar]
- Manfield IW, Orfila C, McCartney L, et al. Novel cell wall architecture of isoxaben-habituated Arabidopsis suspension-cultured cells: global transcript profiling and cellular analysis. The Plant Journal. 2004;40:260–275. doi: 10.1111/j.1365-313X.2004.02208.x. [DOI] [PubMed] [Google Scholar]
- Maruthi Srihar BB, Diehl SV, Han FX, Monts DL, Su Y. Anatomical changes due to uptake and accumulation of Zn and Cd in indian mustard (Brassica juncea) Environmental and Experimental Botany. 2005;54:131–141. [Google Scholar]
- Morvan C, Abdul-Hafez A, Jauneau A, Thoiron B, Demarty M. Incorporation of D-[U-14C]glucose in the cell wall of Linum plantlets during the first steps of growth. Plant and Cell Physiology. 1991;32:609–621. [Google Scholar]
- Morvan O, Quentin M, Jauneau A, Mareck A, Morvan C. Immunogold localization of pectin methylesterases in the cortical tissues of flax hypocotyl. Protoplasma. 1998;202:175–184. [Google Scholar]
- Nicol F, His I, Jauneau A, Vernhettes S, Canut H, Höfte H. A plasma membrane-bound putative endo-1,4-β-d-glucanase is required for normal wall assembly and cell elongation in Arabidopsis. EMBO Journal. 1998;17:5563–5576. doi: 10.1093/emboj/17.19.5563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paynel F, Schaumann A, Arkoun M, Douchiche O, Morvan C. Temporal regulation of cell-wall pectin methylesterase and peroxidase isoforms in cadmium-treated flax hypocotyl. Annals of Botany. 2009;104:1363–1372. doi: 10.1093/aob/mcp254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peaucelle A, Louvet R, Johansen JN, et al. Arabidopsis phyllotaxis is controlled by the methyl-esterification status of cell-wall pectins. Current Biology. 2008;18:1943–1948. doi: 10.1016/j.cub.2008.10.065. [DOI] [PubMed] [Google Scholar]
- Penel C, Greppin H. Pectin binding proteins: characterization of the binding and comparison with heparin. Plant Physiology and Biochemistry. 1996;34:479–488. [Google Scholar]
- Ralet MC, Crépeau MJ, Lefèbvre J, Mouille G, Höfte H, Thibault JF. Reduced number of homogalacturonan domains in pectins of an Arabidopsis mutant enhances the flexibility of the polymer. Macromolecules. 2008;9:1454–1460. doi: 10.1021/bm701321g. [DOI] [PubMed] [Google Scholar]
- Rihouey C, Jauneau A, Cabin-Flaman A, Demarty M, Lefèbvre F, Morvan C. Calcium and acidic pectin distribution in flax cell-wall-evidence for different kinds of linkages in the cell junction and middle lamella of the cortical parenchyma of flax hypocotyls. Plant Physiology and Biochemistry. 1995;33:497–508. [Google Scholar]
- Roach MJ, Deyholos MK. Microarray analysis of developing flax hypocotyls identifies novel transcripts correlated with specific stages of phloem fibre differentiation. Annals of Botany. 2008;102:317–330. doi: 10.1093/aob/mcn110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roland JC. Cell wall differentiation and stages involved in intercellular gas space opening. Journal of Cell Science. 1978;32:325–336. doi: 10.1242/jcs.32.1.325. [DOI] [PubMed] [Google Scholar]
- Sandalio LM, Dalurzo HC, Gomez M, Romero-Puertas MC, Del Rio LA. Cadmium induced changes in the growth and oxidative metabolism of pea plants. Journal of Experimental Botany. 2001;52:2115–2126. doi: 10.1093/jexbot/52.364.2115. [DOI] [PubMed] [Google Scholar]
- Sanitá Di Toppi L, Gabbrielli R. Response to cadmium in higher plants. Environmental and Experimental Botany. 1999;41:105–130. [Google Scholar]
- Savaldi-Goldstein S, Peto C, Chory J. The epidermis both drives and restricts plant shoot growth. Nature. 2007;446:199–202. doi: 10.1038/nature05618. [DOI] [PubMed] [Google Scholar]
- Schildknecht PHPA, Vidal BC. A role for the cell wall in Al3+ resistance and toxicity: cristallinity and availability of negative charges. International Archives of Bioscence. 2002;2002:1087–1095. [Google Scholar]
- Schindler TM, Bergfeld R, Van Cutsem P, Schopfer DV, Sengbusch A. Distribution of pectins in cell walls of maize coleoptiles and evidence against their involvement in auxin-induced extension growth. Protoplasma. 1995;188:213–224. [Google Scholar]
- Schmohl N, Horst WJ. Cell wall pectin content modulates aluminium sensitivity of Zea mays (L.) cells grown in suspension culture. Plant, Cell & Environment. 2000;23:735–742. [Google Scholar]
- Sobry S, Havelange A, Liners F, Van Cutsem P. Immunolocalization of homogalacturonans in the apex of the long-day plant Sinapis alba at floral transition: the pectin content drops dramatically in the first hour of this transition. Physiologia Plantarum. 2005;123:339–347. [Google Scholar]
- Tabuchi A, Matsumoto H. Changes in cell-wall properties of wheat (Triticum aestivum) roots during aluminium-induced growth inhbition. Physiologia Plantarum. 2001;112:353–358. doi: 10.1034/j.1399-3054.2001.1120308.x. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Hirata S, Kido N, Katou K. Wall-yielding properties of cell walls from elongating cucumber hypocotyls in relation to the action of expansin. Plant and Cell Physiology. 2006;47:1520–1529. doi: 10.1093/pcp/pcl017. [DOI] [PubMed] [Google Scholar]
- Thiery JP. Mise en évidence des polysaccharides sur coupes fines en microscopie électronique. Journal of Microscopy. 1967;6:927–1017. [Google Scholar]
- VandenBosch KA, Bradley DJ, Knox JP, Perotto S, Butcher GW, Brewin N. Common components of the infection thread matrix and the intercellular space identified by immunocytochemical analysis of pea nodules and uninfected roots. EMBO Journal. 1989;8:335–342. doi: 10.1002/j.1460-2075.1989.tb03382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldron KW, Ng A, Parker ML, Parr AJ. Ferulic acid dehydrodimers in the cell walls of Beta vulgaris and their possible role in texture. Journal of Science of Food and Agriculture. 1997;74:221–228. [Google Scholar]
- Willats WGT, Limberg G, Buchholt HC, et al. Analysis of pectic epitopes recognised by hybridoma and phage display monoclonal antibodies using defined oligosaccharides, polysaccharides and enzymatic degradation. Carbohydrate Research. 2000;327:309–320. doi: 10.1016/s0008-6215(00)00039-2. [DOI] [PubMed] [Google Scholar]
- Willats WGT, Orfila C, Limberg G, et al. Modulation of the degree and pattern of methyl-esterification of pectic homogalacturonan in plant cell walls: implication for pectin methylesterase action, matrix properties, and cell adhesion. Journal of Biology and Chemistry. 2001;276:19404–19413. doi: 10.1074/jbc.M011242200. [DOI] [PubMed] [Google Scholar]
- Willats WGT, Knox JP, Mikkelson JD. Pectin: new insights into an old polymer are starting to gel. Trends in Food Science and Technology. 2006;17:97–104. [Google Scholar]
- Wojcik M, Tukiendorf A. Cadmium uptake, localization and detoxification in Zea mays. Biologia Plantarum. 2005;49:237–245. [Google Scholar]
- Xiong J, An L, Lu H, Zhu C. Exogenous nitric oxide enhances cadmium tolerance of rice by increasing pectin and hemicellulose contents in root cell wall. Planta. 2009;230:755–765. doi: 10.1007/s00425-009-0984-5. [DOI] [PubMed] [Google Scholar]
- Yang JL, Li YY, Zhang YJ, et al. Cell wall polysaccharides are specifically involved in the exclusion of aluminium from the rice root apex. Plant Physiology. 2008;146:602–611. doi: 10.1104/pp.107.111989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong H, Laüchli A. Changes of cell wall composition and polymer size in primary roots of cotton seedlings under high salinity. Journal of Experimental Botany. 1993;44:773–778. [Google Scholar]