Abstract
Background and Aims
The success of C4 plants lies in their ability to attain greater efficiencies of light, water and nitrogen use under high temperature, providing an advantage in arid, hot environments. However, C4 grasses are not necessarily less sensitive to drought than C3 grasses and are proposed to respond with greater metabolic limitations, while the C3 response is predominantly stomatal. The aims of this study were to compare the drought and recovery responses of co-occurring C3 and C4 NADP-ME grasses from the subfamily Panicoideae and to determine stomatal and metabolic contributions to the observed response.
Methods
Six species of locally co-occurring grasses, C3 species Alloteropsis semialata subsp. eckloniana, Panicum aequinerve and Panicum ecklonii, and C4 (NADP-ME) species Heteropogon contortus, Themeda triandra and Tristachya leucothrix, were established in pots then subjected to a controlled drought followed by re-watering. Water potentials, leaf gas exchange and the response of photosynthetic rate to internal CO2 concentrations were determined on selected occasions during the drought and re-watering treatments and compared between species and photosynthetic types.
Key Results
Leaves of C4 species of grasses maintained their photosynthetic advantage until water deficits became severe, but lost their water-use advantage even under conditions of mild drought. Declining C4 photosynthesis with water deficit was mainly a consequence of metabolic limitations to CO2 assimilation, whereas, in the C3 species, stomatal limitations had a prevailing role in the drought-induced decrease in photosynthesis. The drought-sensitive metabolism of the C4 plants could explain the observed slower recovery of photosynthesis on re-watering, in comparison with C3 plants which recovered a greater proportion of photosynthesis through increased stomatal conductance.
Conclusions
Within the Panicoid grasses, C4 (NADP-ME) species are metabolically more sensitive to drought than C3 species and recover more slowly from drought.
Keywords: C3 and C4 Panicoid grasses, NADP-ME subtype, drought response, stomatal and metabolic limitations, drought recovery
INTRODUCTION
C4 photosynthesis is the term used to describe the many combinations of anatomical, biochemical and physiological modifications that concentrate CO2 in the bundle sheath, effectively saturating Rubisco at ambient CO2 concentrations. This almost eliminates photorespiration and enables C4 plants to reduce stomatal aperture while fixing CO2 at rates equal to or greater than C3 plants (Pearcy and Ehleringer, 1984). These C4 characteristics and the resultant increased water-use efficiency led to the general view that C4 photosynthesis was insensitive to drought and was advantageous in arid environments (Barbour et al., 1987; Taiz and Zeiger, 1991; Haxeltine and Prentice, 1996).
Support for this tolerance comes both from the correlation between the increasing number of C4 species with decreasing annual rainfall (e.g. Ellis et al., 1980; Hattersley, 1992; Taub, 2000; Cabido et al., 2008) and from the competitive success of C4 species during periods of natural drought (Tilman and Downing, 1994). However, a general case for C4 drought tolerance is questionable because of several lines of evidence.
First, the combined C4 species relationship with rainfall masks the response of individual photosynthetic subtypes and unlike the overall C4 correlation the numbers of NADP-ME species declines with aridity (Ellis et al., 1980; Taub, 2000), suggesting that this subtype is drought-sensitive. This pattern is further complicated because the majority of NADP-ME species belong to the Panicoideae and hence drought sensitivity may be a characteristic of phylogenetic grouping rather than photosynthetic subtype (Taub, 2000; Osborne, 2008).
Secondly, physiological evidence indicates that C4 photosynthesis is sensitive to drought. C4 plants, like C3 species, initially respond to drought by decreasing stomatal and mesophyll conductance to CO2 (see reviews by Lawlor, 2002; Medrano et al., 2002; Flexas et al., 2004), although the quantification of the latter remains problematic (Lawlor and Tezara, 2009). A more severe water deficit further increases conductance limitations, but metabolic (biochemical) limitations become more important in decreasing photosynthetic potential (Ghannoum et al., 2003; Marques da Silva and Arrabaça, 2004; Ripley et al., 2007). The greater metabolic limitation in C4 species is probably associated with drought effects on the CO2-concentrating mechanism and may include impaired C4 biochemistry and plasmodesmatal function. Additionally, drought is proposed to limit ATP synthesis, which would decrease the regeneration of substrates for both the C3 and the C4 cycle (Weiner et al., 1988; Ghannoum, 2009; Lawlor and Tezara, 2009).
The severity of the water-stress influences the relative contributions of diffusional and metabolic limitations to photosynthesis and hence has important implications for the recovery of photosynthetic physiology (Ignace et al., 2007). In C3 species the initial limitations by stomatal and mesophyll conductance are rapidly and completely reversed by re-watering, but more severe metabolic limitations are slowly reversed and plants may therefore take more time to recover (Galle et al., 2007). Hence, if C4 species do suffer from increased metabolic limitation relative to C3 species, this is likely to affect the rate at which they can recover from drought. This response would have important ecological implications influencing competitive interactions and plant distribution.
Data for direct comparisons of the drought responses of C3 and C4 species and of the differences between C4 photosynthetic subtypes are limited, and experiments have largely not controlled for phylogenetic effects. This is despite the ecological relevance of such comparisons justified by the co-occurrence of C3 and C4 grasses (Gibbs Russell et al., 1991) and because numbers of NADP-ME and/or Panicoid species decline with aridity. To begin to address this shortfall, the present study compares three C4 (NADP-ME) species and three C3 species, this comparison being limited to Panicoid grasses. All species grow in close proximity (in an area <10 m2) in South Africa, and have similar perennial habits and growth phenologies characterized by spring and summer growth, and winter dormancy. Therefore, this experimental system represents a unique opportunity to remove the confounding effects that adaptations to different habitats may introduce and to control for phylogeny. The selected species were transferred to pots, subjected to a controlled drought and subsequently watered and used to investigate: (1) the relative contributions of stomatal and metabolic limitations to the decline of C3 and C4 (NADP-ME) photosynthesis under conditions of increasing drought; (2) the C4 photosynthetic and water-use advantage under well-watered and drought conditions; and (3) the recovery rate of photosynthesis when plants were re-watered.
MATERIALS AND METHODS
Plant collection and growth conditions
Six grass species (Poaceae) were selected as C3 and C4 (NADP-ME) representatives within the subfamily Panicoideae. C3 species were Alloteropsis semialata (R. Br.) Hitchc. subsp. eckloniana (Nees) Gibbs Russell, Panicum aequinerve Nees and Panicum ecklonii Nees. C4 (NADP-ME) species were Heteropogon contortus (L.) Roem. & Schult., Themeda triandra Forssk. and Tristachya leucothrix Nees. Gibbs Russell et al. (1991) and Clayton et al. (2006) provide full descriptions of these species. They represent the dominant Panicoid grasses that co-occur naturally in grasslands around Grahamstown, South Africa, and can be collected from within a small area (10 m2). All species share a common summer growing season and winter dormancy period. Fourteen plants of each species were carefully excavated at Faraway Farm, 8 km from Grahamstown (33°19′S, 26°28′E) on 25 June, 2006, thinned to five tillers, and planted into 10-L pots with 6·7 kg of topsoil similar to that of the field site. The potted plants were maintained in a naturally lit, clear polyethylene tunnel where average daily temperatures ranged between approx. 16 and 30 °C, with an average of 25 °C. The plants were kept well-watered for the month leading up to the experiment and once a week were watered with 0·1 % (v/v) hydroponic fertilizer (Chemicult Products, Cape Town, South Africa). Seven plants of each species were maintained well-watered for the duration of the experiment and seven were subjected to drought.
Soil water content and controlled pot drought
To subject plants to drought conditions representative of those encountered in the field where these species co-occur, the soil water content (SWC) at Faraway Farm was monitored over a 6-month period from August, 2006 to January, 2007. A soil moisture probe (ECH20, Decagon Devices Inc., Pullman, WA, USA) automatically recorded hourly SWC over a 20-cm soil profile and on nine occasions during the period ten randomly positioned SWC measurements were made at a depth of 6 cm with a dielectric probe (ThetaProbe, ML2X, Delta-T Devices, Cambridge, UK). These data were correlated to the gravimetric SWC of samples collected at depths >6 cm and this relationship was used to calculated the actual gravimetric SWC.
Low rainfall from 16 December to 24 January decreased SWC at Faraway farm from approx. 25 to 5 % and the magnitude and duration of this drought was replicated in the pot experiment as follows. To prevent evaporation from the soil surface, a 4-cm-deep layer of pre-weighed fine stone (0·5–1 cm in diameter) was spread across the soil surface. ThetaProbe measurements of SWC, in conjunction with pot weighing, were used as a guide either to maintain pots at constant SWC or to allow them to dehydrate in a controlled fashion. Well-watered pots were maintained at 15–20 % gravimetric SWC for the duration of the experiment, while drought-treated pots were initially maintained at this level for 4 weeks, and then allowed to dry by approx. 0·4 % d−1 for a period of 48 d. At the end of the 48-d drought plants were re-watered and SWC was maintained above 15 % for a further 26 d. To attain the standard rate of dehydration, SWC of pots was measured every second day and water was added as required. Pot weights were recorded each time the probe readings were taken and were used calculate the actual gravimetric SWC. This calculation was only possible after the final dry soil masses were measured at the end of the experiment and the pots were oven dried at 60 °C. This approach allowed all plants, regardless of total leaf area, to dry down at similar rates and to have SWCs that were not different between species.
Midday leaf water potentials
Prior to and after 36 and 48 d of drought, the leaf previously used for gas-exchange measurements was immediately excised and the leaf water potential (Ψleaf) was determined using a Scholander pressure chamber. This procedure was followed during the drought but not re-watering phase of the experiment. To assess the relevance of the pot-imposed drought, Ψleaf of ten randomly selected individuals of each species of grass was measured at Faraway Farm on 24 January when SWC was approx. 5 %.
Leaf gas exchange
Gas exchange measurements were made on the control and treatment plants prior to imposing the drought treatment on 10 April, 2007, and after 20, 36 and 48 d of drought. Plants were then re-watered and recovery was measured after 3, 4, 20 and 24 d. Gas exchange was measured between 1100 and 1500 h on fully expanded leaves using a Li-6400 photosynthesis system (Li-Cor Inc., Lincoln, NE, USA). Cuvette conditions were: photosynthetic photon flux density (PPFD) of 2000 µmol m−2 s−1, air temperature of 25 °C and vapour pressure deficit (VPD) of 1–2·5 kPa. On repeated occasions during the measurement period the empty Li-Cor leaf chamber was held open to prevailing ambient conditions to record VPD and air temperature. Gas exchange parameters were calculated according to von Caemmerer and Farquhar (1981) and instantaneous water-use efficiency was calculated as the ratio between net photosynthetic rate and stomatal conductance to water vapour (A/gST).
Photosynthetic recovery after re-watering
The photosynthetic rates (A) of individual control and drought-stressed plants were measured on five occasions during a 27-d recovery period after re-watering. The length of time required for drought-stressed plants to recover their pre-drought photosynthetic rates involved fitting the data from each replicate water-stressed plant with the following function: A = a × [1 – exp (b – c × number of days of recovery)] and all fits had r2 > 0·92. Photosynthetic rates of control plants were combined for each species and fitted with a linear equation to produce a single average response over the five measurement occasions. The intercept of the individual plant drought recovery curve with the average linear control response was used to define the number of days required for each plant to recover. Some individuals of Th. triandra and Tr. leucothrix did not recover and were assigned a conservative recovery period of 27 d, the duration of the recovery experiment.
A/Ci curves
The response of photosynthesis (A) to intercellular concentrations of CO2 (Ci) were measured using the Li-6400 photosynthesis system on well-watered plants with SWC of 15–20 % and after 36 d of drought when SWCs were decreased to 4 %. Plants were transferred to the laboratory and gas exchange measures were made on a fully expanded, first non-apical leaf after it had adjusted to the environment of the cuvette (TL = 25 °C, PPFD = 2000 µmol m−2 s−1, VPD = approx. 1·3 kPa). External concentrations of CO2 (Ca) were supplied in the sequence 37, 25, 15, 10, 5, 3·5, 37, 50, 75, 100, 130 and 160 Pa and photosynthetic parameters were calculated. CO2 response curves for the C3 grasses were analysed using the models of von Caemmerer (2000) and temperature corrections were performed using the equations from Bernacchi et al. (2001, 2003). C4 curves were modelled according to Collatz et al. (1992). Further measurements were made after 48 d of drought when SWC was reduced to 3 %, but rather than constructing full A/Ci curves, measures were made at ambient Ca and then Ca was increased so that Ci equalled 38 Pa; this allowed stomatal and metabolic limitations to be calculated.
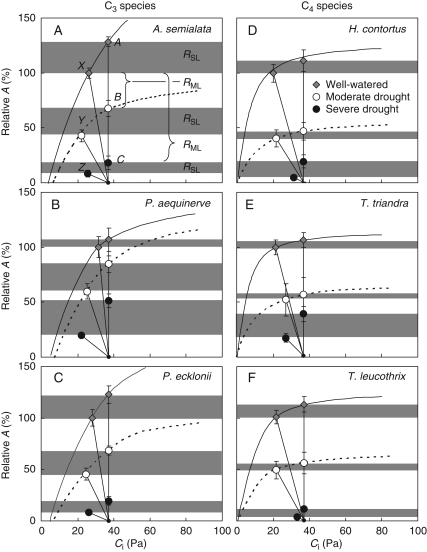
Relative stomatal limitation (RSL) and relative metabolic limitation (RML) were calculated to explain how drought reduced A relative to the average value for well-watered plants at an ambient CO2 concentration of 38 Pa (X), according to:
A, B and C are photosynthetic rates at an atmospheric CO2 concentration of 38 Pa, with no stomatal limitation, at 0, 36 and 48 d of drought, respectively. X, Y and Z are photosynthetic rates at an atmospheric CO2 concentration of 38 Pa, with prevailing stomatal limitations, at 0, 36 and 48 d of drought, respectively (see Fig. 4).
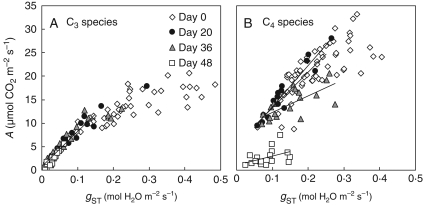
Fig. 4.
Response of net leaf photosynthesis (A) to stomatal conductance (gST) of C3 (A) and C4 (B) plants subjected to controlled drought. Species data for each photosynthetic type were pooled and the different symbols indicate the number days for which water was withheld. For the C4 species individual lines were fitted to the initial response of A to gST measured on each date because the SMA test (at P < 0·05) indicated significant heterogeneity among slopes (Wald test, P < 0·01). Individual linear regression statistics are as follows: day 0, r2 = 0·55, F1,39 = 50, P < 0·0001; day 20, r2 = 0·9, F1,13 = 133, P < 0·0001; day 36, r2 = 0·5, F1,17 = 18, P < 0·001; day 48, r2 = 0·2, F1,16 = 5, P = 0·05. A single slope described this response for the C3 species (Wald test, P > 0·09) and regression statistics are: r2 = 0·86, F1,91 = 592, P < 0·0001.
Statistical analysis
Nested General Linear Models (GLM) were used to detect the effects of drought, date, photosynthetic type, species and their interactions on SWC, Ψleaf, A, gST and A/gST. Species were treated as nested within photosynthetic type and separate analyses were conducted for the drought (days 0–48) and re-watering (days 48–75) phases of the experiment. RSL and RML were compared between drought-treated plants at 0, 36 and 48 d, photosynthetic type, species and their interactions. A/Ci parameters were similarly compared, but were analysed separately for C3 and C4 photosynthetic types, and the number of days required for the recovery of photosynthetic rates were compared between species and photosynthetic type. Homogeneity of variance for all models was determined with Levene's test and data transformations were performed as needed. Statistical differences between means were determined by Fisher-LSD post-hoc tests (at P < 0·05) if the GLM effect was significant.
A comparison of the initial linear relationship of A to gST measured on different days of drought was made using the standardized major axis (SMA) technique and tested for heterogeneity from a common slope. Where a common slope was found across all days, axis scores were used to determine shifts along this common axis due to drought using the WALD test (Warton et al., 2006). Common slope or individual slopes were fitted with linear regressions.
RESULTS
Field soil water content and pot drought experiment
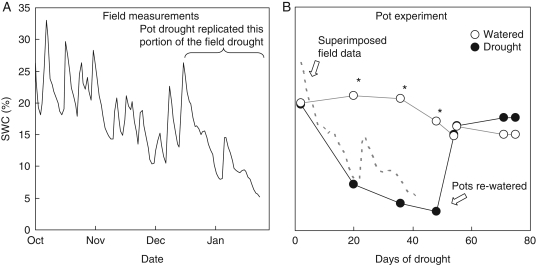
Field SWC declined from October to February with episodes of rain temporarily increasing values. During the period 16 December to 22 January the field site SWC showed an average decline of 0·4 % d−1 (Fig. 1A); these drought conditions were approximately replicated in the pot experiment by decreasing SWC by a similar rate and extent (Fig. 1B). The pot drought decreased SWC from 20 to 3 % over the 48-d period and then subsequent re-watering rapidly returned SWC to above 15 %, the level that was maintained for the rest of the experiment.
Fig. 1.
(A) Soil water contents (SWC) at the field site (solid line). (B) SWC of pot-cultivated plants that were watered or subjected to drought, as indicated. For the pot experiment the data presented are for measurements made prior to the adjustment of pot water content (see Methods for details). The pot dehydration lasted 48 d and SWC was decreased at a similar rate to that observed for field measures over the period 16 December to 24 January. This period of field data (—) has been superimposed on the pot SWC response to demonstrate this similarity. The pots were re-watered on the 28 May and an asterisk indicates significant differences in SWC between well-watered and drought-treated pots at P < 0·05 (Fisher LSD test).
Comparisons of control and drought-treated plants
On day 0, control and drought-treated plants had similar values of Ψleaf, A, gST and A/gST, but values for control plants showed fluctuations over the subsequent 75-d period (Fig. 2 and Supplementary Data, available online). Hence, all further comparisons of these parameters were made between control and drought-treated plants at each measurement occasion. Similar analysis was not possible for all parameters as A/Ci curves, RSL and RML were measured only on drought-treated plants at 0, 36 and 48 d. Hence, the response of these parameters includes the effects of drought and to a lesser extent those related to time.
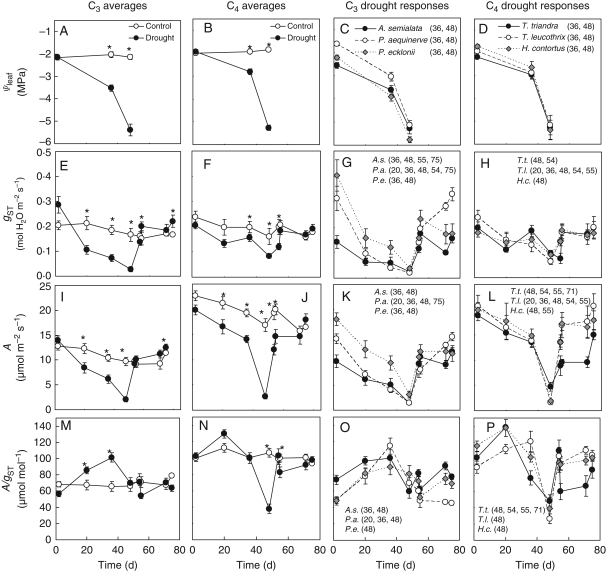
Fig. 2.
(A–D) Midday leaf water potential (Ψleaf), (E–H) net leaf photosynthesis (A), (I–L) stomatal conductance (gST) and (M–P) instantaneous water-use efficiency (A/gST) of C3 and C4 Panicoid grasses subjected to controlled drought. Data are presented for individual C3 and C4 species or averaged by photosynthetic type and include the average photosynthetic type responses of the well-watered controls. The individual species control responses are available as Supplementary Data, available online. Plants were subjected to a 48-d period of drought after which pots were re-watered and maintained at an SWC above 16 %. Values are means ± s.e. and n > 5 for each species. For the average C3 and C4 responses, an asterisk indicates significant differences between means for control and drought-stressed plants. Likewise, in the individual species responses these differences are indicated by the numbers included after the species legends indicating for which days these differences were significant. Differences are considered significant at P < 0·05 (Fisher LSD test).
Midday leaf water potential and gas exchange response to drought
Drought reduced SWC relative to controls leading to values of midday Ψleaf that were significantly different between treatments (Fig. 2A–D, Table 1). The rate of decrease was initially different between photosynthetic types and was less in the C4 than C3 species (Fig. 2A, B). On days 0 and 36, the C4 species had less negative Ψleaf than C3 species, with the exception of P. aequinerve (Fig. 2C, D), but by day 48 values were similar for all species.
Table 1.
General Linear Model (GLM) results of a comparison of midday leaf water potential (Ψleaf), stomatal conductance (gST), photosynthetic rate (A) and water-use efficiency (A/gST) between C3 and C4 Panicoid species (represented as species nested in photosynthetic type) in response to conditions of adequate water supply, decreasing SWC and after re-watering
| Treatment | Treatment × date | Treatment × date × type | Treatment × date × species | |
|---|---|---|---|---|
| Plants subject to drought (Drought phase, days 0–48) | ||||
| Ψleaf | F1,239 = 505·0*** | F4,239 = 207·9*** | F6,239 = 5·4*** | F24,239 = 6·8*** |
| gST | F1,254 = 115·8*** | F6,254 = 46·0*** | F8,254 = 9·5*** | F32,254 = 5·5*** |
| A | F1,254 = 235·7*** | F6,254 = 108·6*** | F8,254 = 40·4*** | F32,254 = 3·9*** |
| A/gST | F1,254 = 1·0 n.s. | F6,254 = 34·6*** | F8,254 = 34·2*** | F32,254 = 5·0*** |
| Plants re-watered subsequent to drought (Re-watering phase, days 48–75) | ||||
| gST | F1,265 = 21·6*** | F8,265 = 34·9*** | F10,265 = 4·5*** | F40,265 = 5·2*** |
| A | F1,265 = 98·4*** | F8,265 = 84·1*** | F10,265 = 26·0*** | F40,265 = 4·2*** |
| A/gST | F1,265 = 20·7*** | F8,265 = 11·1*** | F10,265 = 19·7*** | F40,265 = 4·6*** |
All parameters were compared between well-watered controls and drought-treated plants and the analyses tested for the interacting effects of treatment, date, photosynthetic type and species. Separate analyses were conducted for the drought and re-watering phases of the experiment and levels of significance are indicated as: n.s. (not significant) P > 0·05; *P< 0·05; **P < 0·01; ***P < 0·001.
At high SWC, before the drought treatment, the average stomatal conductance (gST) of C3 and C4 plants was not different (Fig. 2E, F), although this was largely due to the low value for A. semialata that reduced the average for the C3 species (Fig. 2G). gST declined with drought more in C3 than C4 species and at the advanced stages of drought (days 36–48), the C4 species maintained higher gST than the C3 species (Fig. 2E, F, Table 1). The gST of individual species within a photosynthetic type did not respond uniformly to drought, with certain species responding earlier than others, as is evident from the day on which species became different to well-watered controls (Fig. 2G, H).
Photosynthetic rates of the C4 species were significantly higher than those of the C3 species and this difference was maintained on all but the severest day of drought (Fig. 2I, J, Table 1). Photosynthesis declined at a similar rate in both photosynthetic types until day 36; thereafter, A declined more abruptly in the C4 than C3 species (Fig. 2I, J). All species within a photosynthetic type responded uniformly to drought, although P. ecklonii had photosynthetic rates that were higher than those of the other two C3 species.
Among the well-watered plants, the average A/gST values for C4 species were higher than those of the C3 species (Fig. 2M, N). Initially drought increased A/gST in both photosynthetic types and this was sustained until day 36 in the C3 species, and only decreased on the most severe day of drought to values comparable with those of the well-watered controls (Fig. 2M). In contrast, the C4 plants showed a decrease in A/gST after day 20, and by day 48 these values were lower than those of the controls (Fig. 2N). Not all the species within each photosynthetic type responded uniformly to drought, with Tr. leucothrix and P. ecklonii responding more slowly than the other species within their type (Fig. 2O, P).
Photosynthetic recovery
After re-watering, the gST of drought-treated C3 species increased by more than that of C4 species (Fig. 2E–H), but individual species took different numbers of days to recover irrespective of photosynthetic type. Two of the C3 species, A. semialata and P. aequinerve, recovered such that gST values exceeded those of the controls by day 75 (Fig. 2K).
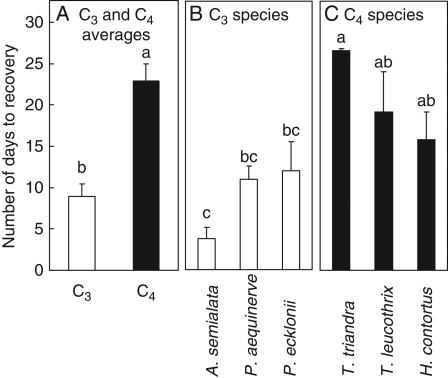
Irrespective of gST recovery, the photosynthetic rates of all the C3 species recovered by day 54 (Fig. 2I, K), within 6 d of re-watering. In contrast, the average photosynthetic rate of the C4 species had not recovered by day 55 (Fig. 2J) and Th. triandra did not recover by day 71 (Fig. 2L). The slow C4 recovery, and particularly that of Th. triandra, meant that the average C4 photosynthetic superiority was only attained by day 75 (Fig 2I, J). However, comparing averages in this way limits resolving recovery times because of the sampling intervals used, which can be avoided by comparing the intersection of curves fitted to control and recovery data (see Methods). This approach estimated that the C4 species took on average 23 d to recover (Fig. 3A) and individual C4 species recoveries ranged from 16 d for H. contortus to recoveries that were incomplete within the duration of the experiment (Fig. 3C). In contrast, the average C3 species recovered within 9 d (Fig. 3A), while individual species recoveries ranged from 4 to 12 d (Fig. 3B).
Fig. 3.
The number of days required for re-watered C3 or C4 Panicoid grasses to recover photosynthetic rates. Data are averaged by photosynthetic type (A) or are presented for individual species (B, C). Values are means ± s.e. and n ≥ 5 for each species. Different lower-case letters indicate significant differences between means for individual species or differences between averages for photosynthetic types at P < 0·05 (Fisher LSD test).
A/gST values for the C3 species during the recovery were not different from control values and this was consistent across all three species (Fig. 2M, O). This pattern contrasted with the response for C4 species, where values were greatly reduced at the severest drought, essentially as a result of a faster decline in A than in gST, but rapidly recovered on re-watering (Fig. 2N, P). Like photosynthetic rates, A/gST values for C4 species only became superior to those for C3 species by the final day of the experiment.
Responses of photosynthesis to stomatal conductance
A single straight-line relationship described the initial response of C3 A to gST for all species, across all levels of drought, at gST values below 0·15 mol H2O m−2 s−1 (Fig. 4A). As gST was decreased by drought, A was shifted significantly along this relationship (WALD test, P < 0·001). In contrast, the C4 species relationship of A to gST at values lower than 0·25 mol H2O m−2 s−1 could not be described by an identical relationship across all treatments. The relationship of A to gST was similar for plants measured under well-watered conditions and after 20 d of drought, but the slope was successively decreased after 36 and 48 d of drought (Fig. 4B).
CO2 response curves
CO2 response curves of well-watered pot plants demonstrated typical C3 and C4 variation. The C3 species had consistently higher CO2 compensation points and lower carboxylation efficiencies than the C4 species. C3 photosynthesis saturated at a Ci > 100 Pa and at ambient conditions had an operating Ci of 22·6–31·4 Pa (Fig. 5A–C). In contrast, the C4 species were saturated at a Ci of approx. 40 Pa and operated with a Ci of 20·5–21·5 Pa (Fig. 5D–F).
Fig. 5.
A/Ci responses for C3 (A–C) and C4 (D–F) Panicoid grasses during three watering treatments: well-watered, moderate drought and severe drought, as indicated. Photosynthetic rates are expressed as a percentage of the rates measured at Ca = 38 Pa for well-watered plants (X). During the severe drought treatment, photosynthetic rates were only measured at a Ca of 38 Pa, or at a Ca corresponding to a Ci of 38 Pa. Also shown are the average photosynthetic rates at an atmospheric CO2 concentration (Ca) of 38 Pa assuming no stomatal limitation (i.e. Ca = Ci = 38 Pa; A, B and C) and supply functions representing the limitation on A imposed by CO2 diffusion through the stomata (X, Y and Z) for plants treated with three levels of drought. Each of these functions has a slope, which is set by the stomatal conductance and intercepts the Ci axis at Ca. n ≥ 4 and vertical bars represent standard errors. Shading indicates relative stomatal limitations (RSL) and brackets the relative metabolic limitations (RML).
In all cases, drought significantly decreased both the estimated carboxylation efficiencies (k and Vcmax) and CO2-saturated photosynthetic rates (Vmax and Jmax; Table 2). After 36 d of drought, k and Vcmax decreased by on average 64 and 54 %, respectively, while Vmax and Jmax decreased by 55 and 46 %, respectively. The magnitude of these reductions was not always consistent across species for each photosynthetic type, resulting in a significant species-by-drought interaction (Table 2). These parameters could not be calculated for the plants subjected to 48 d of drought as complete CO2 response curves were not constructed for these plants. However, the selected points that were measured indicate further reductions in both carboxylation efficiencies and CO2-saturated photosynthetic rates (Fig. 5A–F).
Table 2.
A/Ci parameters and GLM results of a comparison between C3 and C4 Panicoid species in response to conditions of adequate water supply and decreasing SWC measured on days 0 and 36
| Model parameter |
Species |
Species | Drought | Species × Drought | |||
|---|---|---|---|---|---|---|---|
| C4 species | H. contortus | Th. triandra | Tr. leucothrix | ||||
| Control | k (mol m−2 s−1) | 0·39 ± 0·10 | 0·28 ± 0·01 | 0·24 ± 0·03 | F2,30 = 0·86 n.s. | F1,30 =21·6*** | F2,30 = 0·8 n.s. |
| Drought | 0·10 ± 0·04 | 0·12 ± 0·07 | 0·10 ± 0·02 | ||||
| Control | Vmax (μmol m−2 s−1) | 30·9 ± 3·7 | 22·4 ± 1·0 | 32·5 ± 2·6 | F2,30 = 4·1** | F2,30 = 68·6*** | F1,30 = 0·9 n.s. |
| Drought | 12·8 ± 1·6 | 11·0 ± 2·9 | 14·8 ± 2·5 | ||||
| C3 species | A. semialata | P. aequinerve | P. ecklonii | ||||
| Control | Vcmax (μmol m−2 s−1) | 107·8 ± 7·3 | 57·2 ± 5·7 | 148·8 ± 10·2 | F2,30 = 3·0 n.s. | F1,30 = 20·3*** | F2,30 = 0·9 n.s. |
| Drought | 48 ± 6·5 | 36·9 ± 11·4 | 77·1 ± 10 | ||||
| Control | Jmax (μmol m−2 s−1) | 44·9 ± 1·5 | 38·3 ± 15·4 | 64·0 ± 4·8 | F2,30 = 31·2*** | F1,30 = 56·0*** | F2,30 = 13·3*** |
| Drought | 23·9 ± 3·5 | 14·2 ± 5·3 | 31·5 ± 4·6 | ||||
For each photosynthetic type, these analyses tested for the interacting effects of species and drought. Abbreviations for A/Ci parameters are: initial slope of the C4 photosynthetic response (k), C4 maximum Rubisco capacity (Vmax), C3 maximum rate of Rubisco carboxylation (Vcmax) and the apparent C3 maximum rate of photosynthetic electron transport (Jmax). Levels of significance are indicated as: n.s. (not significant) P > 0·05;*P< 0·05;**P < 0·01;***P < 0·001.
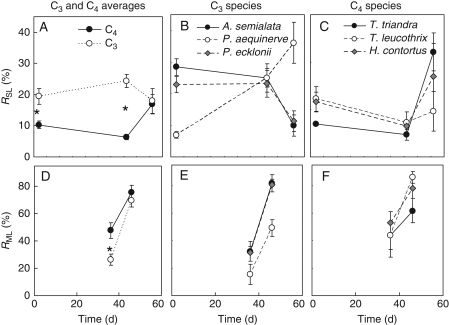
Thirty-six days of drought reduced average C4 photosynthetic rate by 54 %, of which 6 % could be attributed to stomatal limitations (RSL) and 48 % to metabolic limitations (RML; Fig. 6A). At the same stage of drought the C3 average photosynthetic rates were similarly decreased by 50 %, but 24 % was due RSL and 26 % due to RML (Fig. 6A).
Fig. 6.
Relative stomatal (RSL) and metabolic (RML) limitations of photosynthesis for Panicoid grasses subjected to controlled drought. Data are averaged by photosynthetic type (A, D) or are presented for individual C3 (B, E) or C4 (C, F) species. Values are means ± s.e. and n ≥ 4 for each species. *Significant differences between means on the different days at P < 0·05 (Fisher LSD test).
Prolonging the drought to 48 d resulted in a 92 % decline in C4 photosynthetic rates with increases in RSL to 17 % and RML to 75 %. Under the same conditions C3 photosynthesis decreased by 88 %, of which 18 % was due to RSL and 70 % to RML (Fig. 6A). These differences in the change in the magnitude of RSL and RML with increasing drought resulted in significant type-by-date interactions, although the magnitude of these changes was not always consistent between species within a photosynthetic type (Table 3), the notable exception being P. aequinerve which developed a large RSL even at the severest level of drought (Fig. 6B, C).
Table 3.
GLM results of a comparison of relative stomatal (RSL) and metabolic (RML) limitations between C3 and C4 Panicoid species (represented as species nested in photosynthetic type) in response to conditions of adequate water supply and decreasing SWC measured on days 0, 36 and 48
| Parameter | Photosynthetic type | Species | Date | Type × date | Species × date |
|---|---|---|---|---|---|
| RSL | F1,66 = 6·7* | F4,66 = 4·7** | F2,66 = 53*** | F2,66 = 39*** | F8,66 = 1·6 n.s. |
| RML | F1,37 = 43·7*** | F4,37 = 0·7 n.s. | F1,196 = 1·3 n.s. | F1,196 = 7·2** | F4,37 = 10·0*** |
The analysis tested for the interacting effects of species, photosynthetic type and date. Levels of significance are indicated as: n.s. (not significant) P > 0·05;*P< 0·05;**P < 0·01;***P < 0·001.
DISCUSSION
Our results demonstrate that for co-occurring grasses within this single subfamily (Panicoideae), the sensitivity of photosynthesis to drought and the limitations responsible for this are different between C3 and C4 (NADP-ME) species. These differences between photosynthetic types are distinct despite interspecific variation and because our methods meant that slow-dehydrating species were subject to continuous drought, whereas others were occasionally given small volumes of water to ensure a uniform rate of drying. Under well-watered conditions, and consistent with an extensive literature (e.g. Long, 1999), the C4 species had higher photosynthetic rates and A/gST than the C3 species. This advantage was maintained during the initial period of the drought, but was lost as the drought became more severe, and took in excess of 20 d to be regained after re-watering. The loss of this advantage could be attributed to differences in the response of C3 and C4 stomatal conductance and photosynthetic potential to drought. The more sensitive and larger metabolic limitation in the C4 plants was correlated with slower recovery from drought, while the predominance of stomatal limitation in the C3 species allowed more rapid recovery. These findings provide comparative results between C3 and C4 grass species, data lacking in the current debate on the relative roles of these mechanisms in plant drought responses (Chaves et al., 2009; Lawlor and Tezara, 2009). The results also suggest that the significance of the C4 photosynthetic and water-use efficiency will depend both on the severity of the drought and frequency of rainfall events, determining both the degree of inhibition of gas exchange and its rate of recovery. This is important as C4 water-use efficiency is considered a mechanism for maintaining soil water status and prolonging productivity after rainfall (Ehleringer and Monson, 1993; Kalapos et al., 1996).
As has been characterized in many C3 and C4 species, drought decreased A through a combination of stomatal and metabolic limitations (Lawlor, 2002; Ghannoum et al., 2003; Flexas et al., 2006), but the magnitude of these responses differed in these C3 and C4 grasses, and changed as drought progressed. Under well-watered conditions, the C3 species had RSL values twice as high as those of the C4 species and the importance of RML increased with drought. In the C4 species, RML predominated under much less severe conditions, demonstrating the sensitivity of C4 photosynthesis to drought. Similar differences were noted when comparing the drought response of C3 and C4 subspecies of Alloteropsis semialata (Ripley et al., 2007) and, as with the present study, increased metabolic limitations were attributed to changes in the initial slope, curvature and CO2-saturated values of the A/Ci curves. These changes indicate reductions in the rate of the C3 and C4 cycles, decreased bundle sheath or mesophyll conductance to CO2, decreased Rubisco activity, and decreased rates of RuBP regeneration (Krieg and Hutmacher, 1986; von Caemmerer, 2000). The underlying mechanism responsible for these changes in C3 species has been attributed to decreased ATP production (Lawlor and Tezara, 2009), although other mechanisms may be involved and have recently been reviewed by various authors (e.g. Flexas and Medrano, 2002; Lawlor, 2002; Flexas et al., 2004; Lawlor and Tezara, 2009). Similar mechanisms have been proposed for C4 species (Ghannoum, 2009; Lawlor and Tezara, 2009), and are probably complicated by the additional metabolism associated with the CO2-concentrating mechanism, but as yet are unresolved. Furthermore, the interpretation of these A/Ci data are reliant on assumptions that do not necessarily hold under conditions of severe drought and may result in the over-estimation of Ci (Terashima et al., 1988). The interpretation is further complicated by the affects of drought on mesophyll conductance (gM), the quantification of which remains controversial in C3 species (Warren and Adams, 2006; Lawlor and Tezara, 2009) and is not yet possible for C4 plants. However despite these concerns, A/Ci curves retain their value in demonstrating a drought-induced reduction in photosynthetic potential (Lawlor and Tezara, 2009).
Evidence for more complex mechanisms contributing to the metabolic limitations in C4 plants comes from examination of the A–gST responses of these drought-stressed grasses. In the C3 species this response was described by a single relationship, demonstrating that irrespective of drought conditions, CO2 supply and use are closely regulated, a phenomenon that is well described in the literature on C3 plants (Schulze and Hall, 1982; Brodribb, 1996). In contrast, the C4 grass species did not show this simple relationship and photosynthesis deviated from the initial A–gST relationship as drought progressed, showing that this regulation was uncoupled under conditions of more severe drought.
The loss of superior C4 photosynthetic rate only occurred under the most severe conditions of drought, while their A/gST advantage was lost under less severe conditions. The loss of the C4 A/gST advantage could not be ascribed to differences in the contributions of RSL and RML given that A declined by a similar amount in both C3 and C4 species over the initial 36 d. Instead the loss occurred because of differences in the stomatal responses and because C4 species maintained higher gST than C3 species. This might reflect differences in plant hydraulic architecture (Kocacinar and Sage, 2003) and strategies of water use (Long, 1999), and the higher gST in C4 species combined with less negative values of Ψleaf implies a higher hydraulic conductance than was evident for the C3 species. The initial response of A/gST contrasted with that noted under more severe conditions, when the reduction in A/gST was due to dramatically reduced C4 photosynthetic rates combined with higher gST. Decreased instantaneous water-use efficiency in response to drought has been reported for various C4 species (Marques da Silva and Arrabaça, 2004; Xu et al., 2006), but data comparing closely related C3 and C4 species are limited to our work on A. semialata, where a similar loss of the A/gST advantage was demonstrated both in a controlled pot drought (Ripley et al., 2007), and in a common garden experiment under natural rainfall conditions (Ibrahim et al., 2008).
C4 water-use efficiency contributes to a competitive advantage in certain environments (Ehleringer and Monson, 1993; Kalapos et al., 1996) and its loss and the length of time required for its recovery would alter competition, and may constrain C4 species distributions. The present study demonstrated the loss of this C4 A/gST superiority, which took 20 d of well-watered conditions to be regained. The treatment applied was representative of field conditions and at day 36 produced a similar range of Ψleaf to those measured in the field (−1·8 to −3 MPa), when SWC was approx. 5 %. Even the more severe conditions on day 48 are likely to occur in the field, and over the last 6 years the Faraway Farm site has experienced nine occasions when less than 10 mm month−1 of rain fell during the growing season (South Africa Weather Services). During the present study, when 17 mm of rain fell over the 29-d period (16 December to 24 January) SWC was reduced to 5 % (Fig. 1A), and hence these nine periods of more severe drought would probably have resulted in SWC of less than 5 %. Data on the recovery of other C4 species are mainly available for maize (Lal and Edwards, 1996; Saccardy et al., 1996; Foyer et al., 1998) and sorghum (Loreto et al., 1995), where the treatments imposed were mild and recovery was both rapid and complete. Hence, further research is needed on the responses of non-crop C4 species to drought treatments of severities that are ecologically relevant.
Conclusions
The present results demonstrate that the loss of the photosynthetic advantage of C4 NADP-ME Panicoid grasses relative to C3 Panicoid grasses occurs only under conditions of severe drought, while the A/gST advantage was lost under less severe conditions. The underlying mechanisms for these responses were differences in the dominance of C3 and C4 stomatal and metabolic limitations and how these changed under conditions of drought. Differences in the A–gST response to drought suggested the operation of alternative or additional mechanisms of photosynthetic inhibition in the C4 species, which might explain why the recovery from drought differed between the C3 and C4 species.
SUPPLEMENTARY DATA
Supplementary Material
ACKNOWLEDGEMENTS
We gratefully acknowledge funding from the South African National Research Foundation (NRF) and the Rhodes University Joint Research Council (JRC).
LITERATURE CITED
- Barbour MJ, Burk JH, Pitts WD. Terrestrial Plant Ecology. 2nd edn. Menlo Park, CA: Benjamin Cummings, Inc; 1987. [Google Scholar]
- Bernacchi CJ, Singsaas EL, Pimental C, Portis AR, Long SP. Improved temperature response functions for models of Rubisco-limited photosynthesis. Plant, Cell and Environment. 2001;24:253–259. [Google Scholar]
- Bernacchi CJ, Pimentel C, Long SP. In vivo temperature response functions of parameters required to model RuBP-limited photosynthesis. Plant, Cell and Environment. 2003;26:1419–1430. [Google Scholar]
- Brodribb T. Dynamics of changing intercellular CO2 concentration (ci) during drought and determination of minimum functional ci. Plant Physiology. 1996;111:179–185. doi: 10.1104/pp.111.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabido M, Pons E, Cantero JJ, Lewis JP, Anton A. Photosynthetic pathway variation among C4 grasses along a precipitation gradient in Argentina. Journal of Biogeography. 2008;35:131–140. [Google Scholar]
- von Caemmerer S. Biochemical models of leaf photosynthesis. Canberra: CSIRO Publishing; 2000. [Google Scholar]
- von Caemmerer S, Farquhar GD. Some relationships between the biochemistry of photosynthesis and the gas exchange of leaves. Planta. 1981;153:376–387. doi: 10.1007/BF00384257. [DOI] [PubMed] [Google Scholar]
- Chaves MM, Flexas J, Pinheiro C. Photosynthesis under drought and salt stress: regulation mechanisms from whole plant to cell. Annals of Botany. 2009;103:551–560. doi: 10.1093/aob/mcn125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton WD, Harman KT, Williamson H. GrassBase – The Online World Grass Flora. 2006 http://www.kew.org/data/grasses-db.html . [accessed 17 November 2006] [Google Scholar]
- Collatz GJ, Ribas-Carbo M, Berry JA. Coupled photosynthesis–stomatal conductance model for leaves of C4 plants. Australian Journal of Plant Physiology. 1992;19:519–538. [Google Scholar]
- Ehleringer JR, Monson RK. Evolutionary and ecological aspects of photosynthetic pathway variation. Annual Review of Ecology and Systematics. 1993;24:411–439. [Google Scholar]
- Ellis RP, Vogel JC, Fuls A. Photosynthetic pathways and the geographical distribution of grasses in South West Africa/Namibia. South African Journal of Science. 1980;76:307–312. [Google Scholar]
- Flexas J, Medrano H. Drought-inhibition of photosynthesis in C3 plants: stomatal and non-stomatal limitation revisited. Annals of Botany. 2002;89:183–189. doi: 10.1093/aob/mcf027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flexas J, Bota J, Cifre J, et al. Understanding down-regulation of photosynthesis under water stress: future prospects and searching for physiological tools for irrigation management. Annals of Applied Biology. 2004;144:273–283. [Google Scholar]
- Flexas J, Bota J, Galmés J, Medrano H, Ribas-Carbό M. Keeping a positive carbon balance under adverse conditions: responses of photosynthesis and respiration to water stress. Physiological Plantarum. 2006;127:343–352. [Google Scholar]
- Foyer CH, Valadier M-H, Migge A, Becker TW. Drought-induced effects on nitrate reductase activity and mRNA and on the coordination of nitrogen and carbon metabolism in maize leaves. Plant Physiology. 1998;117:283–292. doi: 10.1104/pp.117.1.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galle A, Haldimann P, Feller U. Photosynthetic performance and water relations in young pubescent oak (Quercus pubescens) trees during drought stress and recovery. New Phytologist. 2007;174:799–810. doi: 10.1111/j.1469-8137.2007.02047.x. [DOI] [PubMed] [Google Scholar]
- Ghannoum O. C4 photosynthesis and water stress. Annals of Botany. 2009;103:635–644. doi: 10.1093/aob/mcn093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghannoum O, Conroy JP, Driscoll SP, Paul MJ, Foyer CH, Lawlor DW. Nonstomatal limitations are responsible for drought-induced photosynthetic inhibition in four C4 grasses. New Phytologist. 2003;159:599–608. doi: 10.1046/j.1469-8137.2003.00835.x. [DOI] [PubMed] [Google Scholar]
- Gibbs Russell GE, Watson L, Koekemoer M, et al. Grasses of southern Africa. South Africa: National Botanical Gardens/Botanical Research Institute; 1991. [Google Scholar]
- Hattersley PW. C4 photosynthetic pathway variation in grasses (Poaceae): its significance for arid and semi-arid lands. In: Chapman GP, editor. Desertified grasslands: their biology and management. London: Academic Press; 1992. pp. 181–212. [Google Scholar]
- Haxeltine A, Prentice IC. BIOME3: an equilibrium terrestrial biosphere model based on ecophysiological constraints, resource availability, and competition among plant functional types. Global Biogeochemical Cycles. 1996;10:693–709. [Google Scholar]
- Ibrahim DG, Gilbert ME, Ripley BS, Osborne C. Seasonal differences in photosynthesis between the C3 and C4 subspecies of Alloteropsis semialata are offset by frost and drought. Plant, Cell and Environment. 2008;31:1038–1050. doi: 10.1111/j.1365-3040.2008.01815.x. [DOI] [PubMed] [Google Scholar]
- Ignace DD, Huxman TE, Weltzin JF, Williams DG. Leaf gas exchange and water status responses of a native and non-native grass to precipitation across contrasting soil surfaces in the Sonoran Desert. Oecologia. 2007;152:401–413. doi: 10.1007/s00442-007-0670-x. [DOI] [PubMed] [Google Scholar]
- Kalapos T, van den Boogaard R, Lambers H. Effect of soil drying on growth, biomass allocation and leaf gas exchange of two annual grass species. Plant and Soil. 1996;185:137–149. [Google Scholar]
- Kocacinar F, Sage RF. Photosynthetic pathway alters xylem structure and hydraulic function in herbaceous plants. Plant, Cell and Environment. 2003;26:2015–2026. [Google Scholar]
- Krieg DR, Hutmacher RB. Photosynthetic rate control in sorghum: stomatal and nonstomatal factors. Crop Science. 1986;26:112–117. doi: 10.1104/pp.73.3.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lal A, Edwards GE. Analysis of inhibition of photosynthesis under water stress in the C4 species Amaranthus cruentus and Zea mays: electron transport, CO2 fixation and carboxylation capacity. Australian Journal of Plant Physiology. 1996;23:403–412. [Google Scholar]
- Lawlor DW. Limitation to photosynthesis in water-stressed leaves: stomata vs metabolism and the role of ATP. Annals of Botany. 2002;89:871–885. doi: 10.1093/aob/mcf110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawlor DW, Tezara W. Causes of decreased photosynthetic rate and metabolic capacity in water-deficient leaf cells: a critical evaluation of mechanisms and integration of processes. Annals of Botany. 2009;103:561–579. doi: 10.1093/aob/mcn244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long SP. Ecology of C4 photosynthesis. In: Sage RF, Monson RK, editors. C4 plant biology. San Diego: Academic Press; 1999. pp. 215–242. [Google Scholar]
- Loreto F, Tricoli D, Di Marco G. On the relationship between electron transport rate and photosynthesis in leaves of the C4 plant Sorghum bicolor exposed to water stress, temperature changes and carbon metabolism inhibition. Functional Plant Biology. 1995;22:885–892. [Google Scholar]
- Marques da Silva J, Arrabaça MC. Photosynthesis in the water-stressed C4 grass Setaria sphacelata is mainly limited by stomata with both rapidly and slowly imposed water deficits. Physiologia Plantarum. 2004;121:409–420. [Google Scholar]
- Medrano H, Escalona JM, Bota J, Gulias J, Flexas J. Regulation of photosynthesis of C3 plants in response to progressive drought: stomatal conductance as a reference parameter. Annals of Botany. 2002;89:895–905. doi: 10.1093/aob/mcf079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne CP. Atmosphere, ecology and evolution: what drove the Miocene expansion of C4 grasslands? Journal of Ecology. 2008;96:35–45. doi: 10.1111/j.1365-2745.2007.01323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearcy RW, Ehleringer J. Comparative ecophysiology of C3 and C4 plants. Plant, Cell and Environment. 1984;7:1–13. [Google Scholar]
- Ripley BS, Gilbert ME, Ibrahim DG, Osborne CP. Drought constraints on C4 photosynthesis: stomatal and metabolic limitations in C3 and C4 subspecies of Alloteropsis semialata. Journal of Experimental Botany. 2007;58:1351–1363. doi: 10.1093/jxb/erl302. [DOI] [PubMed] [Google Scholar]
- Saccardy K, Cornic G, Brulfert J, Reyss A. Effect of drought stress on net CO2 uptake by Zea leaves. Planta. 1996;199:589–595. [Google Scholar]
- Schulze E-D, Hall AE. Stomatal responses, water loss, and CO2 assimilation rates of plants in contrasting environments. In: Lange OL, Nobel PS, Osmond CB, Ziegler H, editors. Encyclopedia of plant physiology. Vol. 12B. Berlin: Springer-Verlag; 1982. pp. 181–230. new series. [Google Scholar]
- Taiz L, Zeiger E. Plant physiology. Redwood City, CA: The Benjamin/Cummings Publishing Company, Inc; 1991. [Google Scholar]
- Taub DR. Climate and the US distribution of C4 grass subfamilies and decarboxylation variants of C4 photosynthesis. American Journal of Botany. 2000;87:1211–1215. [PubMed] [Google Scholar]
- Terashima I, Wong S-C, Osmond CB, Farquhar GD. Characterisation of non-uniform photosynthesis induced by abscisic acid in leaves having different mesophyll anatomies. Plant and Cell Physiology. 1988;29:385–394. [Google Scholar]
- Tilman D, Downing JA. Biodiversity and stability in grasslands. Nature. 1994;367:363–365. [Google Scholar]
- Warren CR, Adams MA. Implications of internal conductance for carbon isotope discrimination and the economics of water and N use in photosynthesis. Plant, Cell and Environment. 2006;29:192–201. doi: 10.1111/j.1365-3040.2005.01412.x. [DOI] [PubMed] [Google Scholar]
- Warton DI, Wright IJ, Falster DS, Westoby M. Bivariate line-fitting methods for allometry. Biological Reviews. 2006;81:259–291. doi: 10.1017/S1464793106007007. [DOI] [PubMed] [Google Scholar]
- Weiner H, Burnell JN, Woodrow IE, Heldt HW, Hatch MD. Metabolite diffusion into bundle sheath cells from C4 plants: relation to C4 photosynthesis and plasmodesmatal function. Plant Physiology. 1988;88:815–822. doi: 10.1104/pp.88.3.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu B, Li F, Shan L, Ma Y, Ichizen N, Huang J. Gas exchange, biomass partitioning and water relationships of three grass seedlings under water stress. Weed Biology and Management. 2006;6:79–88. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.