Abstract
Background and Aims
In perennial plants (especially post-fire resprouters), extant populations may reflect recruitment events in the distant past. This is true of hybrid zones formed by two Banksia species of swamps and woodlands in south-eastern Australia, Banksia robur and B. oblongifolia. Both resprout after fire but recruitment is dependent on periodic fires. Although plants of intermediate morphology have also been identified as hybrids using allozyme markers, the extent of ongoing hybridization is unknown. This study investigates whether both microsatellite markers and morphological measurements can be used to distinguish between the two species and their hybrids. A recent recruitment event and microsatellite markers allow the frequency of ongoing hybridization to be estimated, and also the effects of environmental variation on the morphology of plants and seedlings to be tested.
Methods
Variation at seven microsatellite loci was scored and seven leaf characteristics within putatively pure stands and mixed stands of both species were measured, revealing that the two species were genetically and morphologically distinct and that mixed stands also contained genetically and sometimes morphologically distinct hybrids. An opportunity created by wildfires was used to analyse the genetics and morphometrics of adults and seedlings from two hybrid zones.
Key Results
Approximately 9 % of adults and 21 % of seedlings were identified as genetic hybrids in both hybrid zones. Within these sites, the genotype of mature plants correlated well with morphology, except for some hybrid plants that had parental morphology. However, seedling morphology was highly variable and insufficient to describe the composition of the hybrid zone in this cohort. Greater phenotypic plasticity was evident among seedlings growing within the hybrid zones than seedlings growing in pots.
Conclusions
The hybrid zones are complex and the range of genotypes detected in seedlings reveals both continuing hybridization and introgression.
Keywords: Banksia oblongifolia, Banksia robur, genotype, hybrid, morphology, phenotypic plasticity, plant, recruitment, seedling
INTRODUCTION
Hybrid zones are often viewed as dynamic systems in which speciation and introgression (the incorporation of genes from one species into another) can proceed rapidly (Ungerer et al., 1998; Arnold, 2004; Gross and Rieseberg, 2005; Mallet, 2007; Bunje et al., 2007). An understanding of the processes leading to evolutionary change during hybridization requires knowledge of the frequency of interbreeding between the hybridizing species, and the intensity of selection operating on the hybrids and their parental species. In many published studies on both plants and animals, hybrids are relatively easy to recognize morphologically, and hybrid fitness can therefore be evaluated within annual recruitment events (Grant and Grant, 1994; Jiggins et al., 1996; Burke et al., 1998; Carney et al., 2000; Bridle et al., 2001; Campbell, 2003; Reed et al., 2003; Yanchukov et al., 2006). However, there are other systems in which hybrid and parental phenotypes are not easily distinguishable (Hopper, 1977; McKinnon et al., 2001) and in which recruitment of hybrids can be rare or episodic (Myerscough et al., 2000; Knox and Morrison, 2005). Moreover, the phenotypes of plants can vary in response to environmental variation (Bradshaw, 1965; Sultan and Bazzaz, 1993a, b, c; Ackerly et al., 2000; Donohue et al., 2001), and in response to environmental stress such as drought (Ackerly et al., 2000; Dudley, 2003; Close et al., 2003). An understanding of the dynamics of hybridization within mixed stands of Banksia oblongifolia and Banksia robur therefore requires evaluation of the capacity of genetic and morphological data to identify the two species and their hybrids in the field, and upon having the opportunity to study an episode of recruitment.
Morphological intermediates between these monoecious shrubs, B. robur and B. oblongifolia (Fig. 1), occur across a series of widely distributed swamps along the east coast of Australia (Taylor and Hopper, 1988). Hybridization may have been occurring for a long time, because a specimen collected in 1793 has intermediate morphology (George, 1987). However, although Schibeci (1994) used allozyme data to provide evidence that hybrid plants are present, the composition and dynamics of these zones remained obscure. Only adult plants were present within her study sites; and the relatively low variation revealed by allozyme markers (Schibeci, 1994) has meant that there has been little capacity to quantify the composition of hybrids within the hybrid zones. Moreover, individual plants may be very long-lived, having the capacity to resprout from lignotubers after fire destroys the canopy. Seed stored in the canopy is usually released only after fire, which can be infrequent and has occurred only once in the last 20 years in the study area. Very long-lived plants may be slow to respond to selection (Ackerly et al., 2000) particularly if selection occurs mainly during recruitment events.
Fig. 1.
Banksia seedlings showing different phenotypes with B. robur on the left, B. oblongifolia on the right and intermediate forms in the middle.
Two developments have allowed a unique opportunity to study the dynamics of the Banksia hybrid zones in more detail. First, an extensive wildfire, which burned through two sites containing B. robur and B. oblongifolia and their putative hybrids, has provided the opportunity to seek evidence of recruitment of hybrid individuals from the seed bank. Second, the development of primers for microsatellite markers (providing greater variation than allozyme markers) allowed the genetic composition of these hybrid zones to be described more completely. Importantly, this recruitment event also allowed a test of the match between seedling morphology and genotype within variable and invariable environments, which will facilitate future studies of this hybrid zone.
This study had several interrelated aims: (1) to determine the effectiveness of microsatellite markers and morphological characters in distinguishing pure populations of two species of Banksia, B. robur and B. oblongifolia; (2) to determine the genotypic composition of sets of mature plants and recruits within two Banksia hybrid zones; (3) to determine whether or not mature hybrid plants and seedlings exhibit intermediate morphology and hence whether morphology can be used to identify hybrids; and (4) to determine the effect of environment on seedling morphology by comparing the morphology of seedlings grown in pots with those found growing naturally within the hybrid zone.
The microsatellite genotypes and morphology of mature plants in apparently pure and mixed stands were first categorized. Second, the genotypes and morphology of seedlings growing in mixed stands, and of seedlings grown in pots were categorized. Seeds for these latter seedlings were taken from plants of known maternal genotype growing across the mixed stands.
MATERIALS AND METHODS
Study area
The study area is located within the O'Hares Creek Catchment, on the Woronora Plateau near Darkes Forest (34°14′S 150°54′E), 45 km south-west of Sydney. The site has been described as a mosaic of moorlands and eucalypt woodlands and forests (Davis, 1941; Keith and Myerscough, 1993). Banksia robur, bearing large shiny leaves, grows in the upland swamps and B. oblongifolia, bearing smaller, more numerous leaves, grows along swamp margins and in surrounding eucalypt woodland. The study area contained pure and mixed stands of B. oblongifolia and B. robur.
Banksia paludosa plants, often found in close proximity to B. oblongifolia and B. robur, were found on the periphery of one of the hybrid zone sites in B. oblongifolia habitat. The genetic composition and morphological appearance (Fig. 2) of four mature plants revealed that B. paludosa may also hybridize with the other two species. Therefore, these plants and their seedlings were excluded from this study.
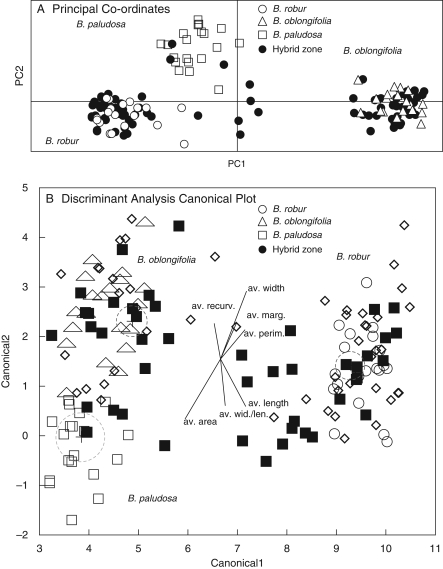
Fig. 2.
(A) Genetic and (B) morphological distinction between mature plants in B. robur, B. oblongifolia and B. paludosa pure populations, and mature plants within the two hybrid zones, as indicated. Circles in (B) show 95 % confidence limit for the mean.
Population samples
Mature plants
To determine whether the mature plants of B. oblongifolia and B. robur were genetically distinct, and to measure the morphological variation within the pure stands, morphology was used to select two large, apparently pure stands of each species. In 2005, after 3 years of re-growth from lignotubers, leaves were collected from ten plants from each stand (for a total of 20 plants from each species), all towards the centre of each stand (at least 300 m from plants of the other species).
To determine the genetic and morphological composition of mature plants within Banksia hybrid zones, and to determine whether hybrid plants exhibit intermediate morphology, two mixed stands were sampled in 2005, including 45 plants at one site and 48 plants at the other site. Plant tissue and leaves was collected for genetic and morphometric analyses.
Seedlings
It was possible to visit the two hybrid zones so soon after fire in 2002 that seeds had still not been broadcast from the burned fruits, although they were readily removed from follicles. Seeds were collected from a random selection of plants (which were subsequently genotyped) across the hybrid zones and raised in pots. After the fire, the above-ground biomass of mature plants consisted only of burnt stems and canopy-stored fruits. Consequently, during seed collection, it was not possible to use leaf morphology to indicate hybrid plants. Some 840 of the seeds collected were placed in seedling trays to germinate. After 2 months, the seedlings that germinated (70 %) were potted into 6-cm pots and kept in a glasshouse for 4 months. They were then re-potted into 12·5-cm pots and placed in the open for the next 8 months. At this time they became too big for the pots so they were removed and measured. As the maternal genotypes were already determined, 31 seedlings were selected, 15 at random from B. oblongifolia and 15 from B. robur maternal plants along with one seedling selected from a hybrid plant.
The genotype and morphology of each seedling that developed in the glasshouse trial and of a subset of 53 seedlings growing within the hybrid zones 3 years after the fire, were determined. These seedlings, growing within the hybrid zone, were selected randomly (maternal genotypes were unknown) from across the width of the hybrid zones along 1-m transects. Although the seedlings raised in pots were measured almost a year and a half before the wild seedlings, they were at a more advanced stage of development in terms of overall size and branching.
Analysis of microsatellite data
For genetic analysis, tissue was collected from new leaves and stored at –80 °C. Seven microsatellite loci, Bo3, Bo7, Bo17, Bo22, Br3, Br13 and Br23, were used to genotype the plants collected for this study. The molecular markers and genotyping methods are described in Usher et al. (2005). The average number of alleles per locus was 3·6 for both B. oblongifolia and B. robur. Each species had a unique set of alleles at five of the seven loci, and both shared and unique alleles were detected at the remaining two loci.
As a measure of genetic distance between the two species, Nei's genetic distance and FST values were calculated using the population genetics software GenAlEx 6 (Peakall and Smouse, 2006). To visualize the genetic grouping of plants in pure stands, in hybrid zones and in potted and field seedlings, principal components analysis (PCA) plots were generated using the same software. Pure-stand plants were included in the analysis of hybrid-zone mature plants to compare the genetic composition of pure and mixed stands.
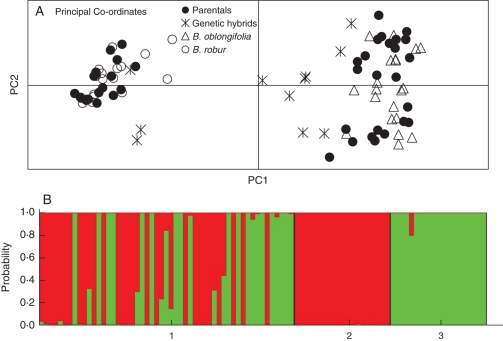
The Bayesian methods implemented in the software structure version 2·0 (Pritchard et al., 2000) were used to perform population assignment of individual plants and to identify putative hybrid individuals. This model-based clustering method uses multilocus data to assign individuals to clusters. Probabilities were used to assign individuals to one of two populations (K = 2). The program was run with 10 000 iterations as the burn-in period, and 100 000 iterations for Markov chain convergence. Plants were assigned as hybrids if they had an assignment value (qi) of less than 0·90. Once hybrids were identified, the individual genotypes of hybrids were scrutinized to determine the origin of alleles at each locus. Pure populations of B. oblongifolia and B. robur proved to have fixed differences at five of the seven microsatellite loci and it was possible to infer whether hybrids were most probably F1 hybrids (displaying an allele from each species at each locus), backcrossed (more similar to one of the parental species) or later generation hybrids (with some loci only having alleles unique to one species and some loci only having alleles unique to the other species).
Morphology
The same plants selected for genetic analysis were used for morphological analysis. For each mature plant, four mature leaves were collected from different branches and from each direction of the compass (following the method of Schibeci, 1994). For each seedling the four largest leaves were measured. The leaves were scanned while still fresh using a flatbed scanner, and the length, width (at the widest section), perimeter and area were measured using NIH Image software (http://rsb.info.nih.gov/nih-image/Default.html) for Macintosh computers. The number of veins that curved away from the margin of the leaf and the number of veins that ended on the margin of the leaf were also counted along one side of each leaf. The width (at the widest point) and length ratio was used to represent leaf shape. Measurements of four leaves from each plant were averaged for analysis.
A one-way ANOVA was performed on each leaf measurement for both pairs of pure stands from each species to test the significance of inter-stand variation in individual leaf measurements (SAS – JMP 5·1; SAS Institute Inc., 2003). A canonical discriminant analysis (CDA) (SAS – JMP 5·1) was performed to determine the power of all leaf measurements to distinguish between the two species and hence to determine whether it is possible to distinguish hybrids of intermediate morphology from pure species. Plants with a probability of assignment to the parental species of less than 0·90 were assigned as hybrids. Before analysis, plants were classified into species (the classification variable) according to their genotype. As with the genetic analysis, pure-stand plants were included in the analysis of the hybrid-zone mature plants to improve the accuracy of the assignment inference and to determine any phenotypic differences between pure and mixed stands.
Genetic and morphometric correlations
To determine the relationship between morphology and genotype of mature plants in pure stands and in mixed stands and to determine the relationship between morphology and genotype of seedlings in the field and in pots, Mantel tests were performed (permutations, n = 999) using the software ‘GenAlEx 6’ (Peakall and Smouse, 2006). The first two canonical points from the morphological discriminant analysis (SAS – JMP 5·1) were used in place of geographical distances in the GenAlEx Mantel test. Tests were also performed without genetic hybrids to determine whether any change in the correlation between morphology and genotype between treatments was due to hybrids or to phenotypic plasticity.
RESULTS
A summary of the number of individuals classified as pure and hybrid based on either genetic or morphological analyses for pure stands, mixed stands and seedlings is shown in Table 1.
Table 1.
Number of pure species and hybrid plants found within each population as determined by genotypes and leaf morphology.
| Genetic analyses (qi < 0·90) | Morphological analyses (P < 0·90) | |
|---|---|---|
| Pure populations | ||
| B. oblongifolia | 20 | 20 |
| B. robur | 19 | 20 |
| Hybrids | 1 | 0 |
| Mixed populations | ||
| B. oblongifolia | 36 | 36 |
| B. robur | 42 | 49 |
| Hybrids | 11 | 4 |
| Potted seedlings | ||
| B. oblongifolia | 16 | 16 |
| B. robur | 14 | 19 |
| Hybrids | 5 | 0 |
| Natural seedlings | ||
| B. oblongifolia | 24 | 31 |
| B. robur | 17 | 19 |
| Hybrids | 12 | 3 |
Pure stands
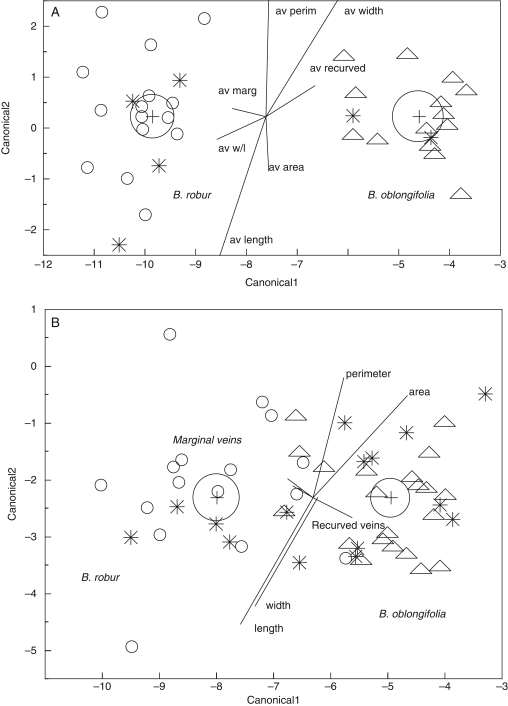
Genetic (Fig. 3A, C) and morphometric (Fig. 4A) analyses revealed that, with one exception, the 40 plants within the putatively pure stands formed two distinct groups. CDA clusters thus allowed us to characterize mature plants from the two parental species.
Fig. 3.
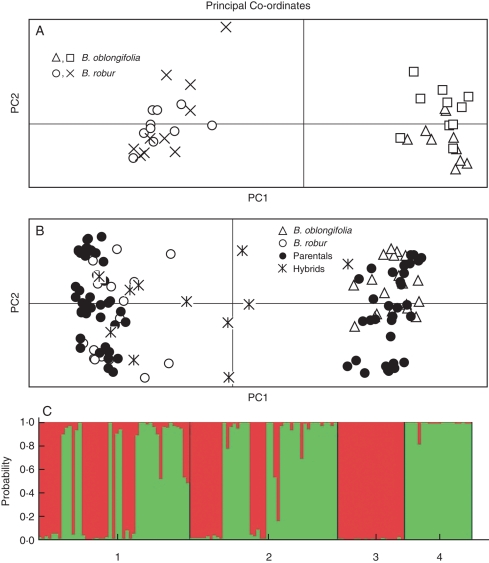
(A) Genetic distinction between the four pure stands, two B. oblongifolia and two B. robur stands, as indicated. (B) Genetic distinction between plants from the pure stands [B. oblongifolia and B. robur, and each of the hybrid zones (parentals and genetic hybrids, as determined by structure), as indicated. (C) Bar plot of the probabilities of assignment for the 93 plants in the two hybrid zones (1 and 2), and in the 20 plants each in pure stands of B. oblongifolia (3) and B. robur (4).
Fig. 4.
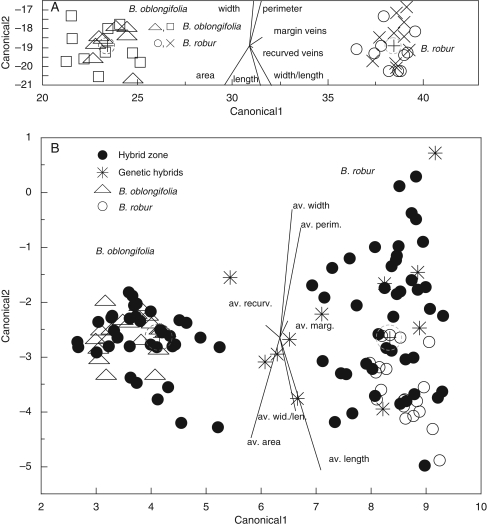
(A) Canonical plot of morphological discrimination between the four pure stands, two B. oblongifolia and two B. robur stands, as indicated. (B) Canonical plot of morphological discrimination within the two hybrid zones, with genetic hybrids (as determined by structure) from both zones; the pure stands of B. oblongifolia and B. robur are included, as indicated. Circles show 95 % confidence limits of the mean.
The program structure confirmed the genetic pattern that the two putative B. oblongifolia stands were genetically distinct from the two putative B. robur stands (Fig. 3C) and this was reflected in Nei's genetic distance values (D = 2·28–2·42) and the mean FST of 0·414. Within species, the two B. robur stands and the two B. oblongifolia stands showed little differentiation (D = 0·09 and 0·02, and FIS = 0·048). Five loci, Bo17, Br23, Bo22, Br13 and Bo7, each showed fixed differences between the two species. The other two loci revealed shared and unique alleles. There was some evidence for introgression within a pure stand of B. robur plants with the presence of a B. oblongifolia allele in one plant (Fig. 3A, C).
Moreover, our discriminant analysis of the morphological data from the pairs of pure stands confirmed that the two species formed distinct clusters (Wilks' lambda = 0·016, F7, 32 = 272·69, P < 0·0001) (Fig. 4A). This analysis also revealed some morphological differentiation of the two B. robur stands (P = 0·039) and this was supported by ANOVA for four of the seven leaf characters, length (P = 0·005), area (P = 0·048), perimeter (P = 0·003) and width/length ratio (P = 0·026). In contrast, both CDA (P = 0·75) and ANOVAs (each measurement P < 0·0001) for individual characters revealed no significant morphological differences between the two B. oblongifolia stands.
Hybrid zones
In contrast to the clear separation of parental species in the pure stands, genetic (Fig. 3B, C) and morphometric (Fig. 4B) analyses of mature plants occupying the two mixed sites revealed both parental types and a range of intermediates.
Analysis of the genetic data revealed that the 89 mature plants within the mixed stands displayed both pure species plants (42 B. robur and 36 B. oblongifolia plants) and a range of hybrid genotypes (11 plants) (Fig. 3B, C). Four plants possessed one B. robur and one B. oblongifolia allele at each locus (as expected for first-generation hybrids) and two plants displayed the genotype of one species with the exception of a single allele from the other species (as expected for introgressed plants). One plant displayed alleles indicative of B. oblongifolia at all but one locus, which displayed two alleles expected only in B. robur plants (as expected for a later generation hybrid). One plant displayed four loci with alleles indicative of B. robur and three loci with one allele from each species (a pattern predicted for a backcrossed hybrid). The remaining three plants were classified as hybrids by structure (qi < 0·90). However, when individual loci were scrutinized, it was unclear why they were classified as hybrids and appeared to be like B. robur (Fig. 3B).
Similarly, the contact zone stands contained some plants with either typical B. robur or typical B. oblongifolia morphologies and plants with a range of intermediate morphologies (Wilks' lambda = 0·18, F7,121 = 77·16, P < 0·0001) (Fig. 4B). The discriminant analysis revealed that only four of the 11 plants with hybrid genotypes were morphologically intermediate along with one plant that was genetically classified as B. oblongifolia (qi = 0 0·91).
Potted seedlings
As expected, the genetic analysis of 31 seedlings from known mature plants growing in the hybrid zones revealed a majority (29) of parental genotypes that were indistinguishable from the clusters formed by pure species adults (Fig. 5A, B). Within this sample, two hybrid seedlings were originally detected, one from the single hybrid adult and one from a B. robur maternal plant. As there were so few hybrids, four more seedlings from the same maternal plants were also genotyped, two more from each plant. As the genotype of the maternal parent was known, it was possible to infer the genotype of the paternal parents by assessment of the maternal and their seedling genotypes. All hybrid seedlings were sired either by B. oblongifolia or by hybrid plants. The range of hybrid genotypes reflected the diversity of known maternal genotypes along with further interspecific crosses. For example, of three hybrids produced by a B. robur maternal plant, two were first-generation hybrids sired by a B. oblongifolia plant (and genetically intermediate between the two parental species) and one was apparently a backcrossed hybrid (with five complete loci from B. robur and two loci bearing one B. oblongifolia allele) sired by a hybrid plant. One of the seedlings from the hybrid parent plant was classified as B. oblongifolia by structure (P < 0·90) and displayed one B. robur allele.
Fig. 5.
(A) Genetic discrimination of seedlings raised in pots (parental and genetic hybrids as determined by structure) and mature plants from pure stands B. oblongifolia and B. robur, as indicated. (B) Plot of the probabilities of assignment for the 35 potted seedlings (1) and for the 40 mature plants from pure stands of B. oblongifolia (2) and B. robur (3).
There was clear morphological distinction between B. oblongifolia and B. robur potted seedlings (Wilks' lambda = 0·12, F7,27 = 27·56, P < 0·0001) (Fig. 6A). However, leaf morphology proved ineffective in detecting hybrid seedlings. None of the hybrids detected by genotyping had intermediate morphology (P > 0·90) (Fig. 6A). Even apparently first-generation hybrids were phenotypically similar to parental species.
Fig. 6.
Canonical plots of morphological discrimination between seedlings (A) grown in pots and (B) growing naturally in the field. Plants were classified genotypically by structure as B. robur, B. oblongifolia or hybrids, as indicated. Circles show 95 % confidence limits of the mean.
Seedlings in the field
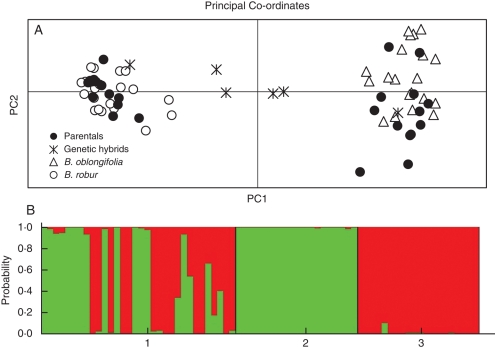
Twelve hybrid seedlings (qi < 0·90) and 41 parental seedlings were detected among the seedlings that germinated within the hybrid zones (Fig. 7A, B). However, none of the hybrids in this sample appeared to be first-generation. Hybrid seedlings comprised what appeared to be seven B. oblongifolia backcrosses, one B. robur backcross and four complex later generation hybrids
Fig. 7.
(A) Genetic discrimination of the seedlings growing naturally in the two hybrid zones (parental and genetic hybrids as determined by structure). Pure stand plants of B. oblongifolia and B. robur were included, as indicated. (B) Plot of the probabilities of assignment for the 53 seedlings growing naturally in the hybrid zones (1), and for the 40 mature plants from pure stands of B. oblongifolia (2) and B. robur (3).
Leaf morphology proved very ineffective in detecting hybrid plants. Although most individuals with parental B. robur or B. oblongifolia genotypes formed pure species clusters (Wilks' lambda = 0·30, F7,45 = 15·09, P < 0·0001) (Fig. 6B) these were relatively indistinct. The discriminant analysis misclassified seven of the parental seedlings as hybrids (P < 0·90) and only three of the 12 genetic hybrids were classified as morphological hybrids.
Genetic and morphometric correlations
The environment of the hybrid zone does not appear to affect the range of morphologies displayed by mature pure species plants as this was similar to that seen within pure populations. There was a strong correlation between genetic distance and morphological distance when B. oblongifolia and B. robur pure-stand plants were analysed together (r = 0·92; P < 0·001). The morphological and genetic markers also correlated well for hybrid-zone plants (r = 0·79; P < 0·001) and this correlation improved when hybrid plants were removed from the analysis of the hybrid zone (r = 0·90; P < 0·001). This result was not surprising as most of the plants had parental genotypes. When hybrid plants were analysed separately there was no correlation between genotype and morphology (r = –0·10; P = 0·62) as predicted by a lack of intermediacy in some hybrid plants, although the sample size was small (11 plants). There was also strong correlation between genetic distance and morphological distance for potted seedlings (r = 0·69; P < 0·001) and this again improved when hybrids were removed from the analysis (r = 0·87; P < 0·001). When hybrid seedlings were analysed separately there was no correlation between genotype and morphology (r = –0·03; P = 0·61), although the sample size was very small (six seedlings from two maternal plants). The correlation between genetic distance and morphological distance for seedlings growing in the field was less than for potted seedlings (r = 0·57; P < 0·001) and there was little change when hybrid genotypes were removed (r = 0·59; P < 0·001) indicating that hybrid plants were not contributing to the observed morphological variation. Indeed there was a slight correlation between genotypic and morphometric distances (r = 0·33; P = 0·06) amongst the 12 hybrid seedlings growing in the field, but again the sample size was small.
DISCUSSION
The present study confirmed that areas of mixed stands between B. robur and B. oblongifolia are indeed zones of active hybridization. Of the seedlings that appeared in response to fire, at least 21 % were hybrids, and first-generation hybrid genotypes were found amongst the seeds collected from plants in the hybrid zones. Although pulses of recruitment may be rare in populations of these re-sprouting plants, the presence of hybrid plants (approx. 9 % amongst mature plants within contact zones) does not simply reflect historical hybridization events. Moreover, the genotypes of mature plants proved a powerful indicator of morphology, and both data sets can be used to distinguish pure species. The findings here support those of a previous study (Schibeci, 1994), which found a good correlation between genotype and morphology using morphological and allozyme markers to characterize the hybrid zones. However, although hybrid plant sample sizes were small and generalizations may be premature, the morphology of hybrids was found to be less predictable. Not all genetic hybrids were morphologically intermediate between the two species and even one apparently first-generation hybrid was morphologically indistinguishable from other B. robur adults. For seedlings growing in pots, there was good morphological distinction between the two species. However, seedlings of the two species growing in the field, within mixed populations, were morphological less distinct than the potted seedlings. In both potted and field seedlings, most genetically intermediate hybrid seedlings were morphologically indistinguishable from pure species seedlings.
Patterns of hybridization and introgression: as revealed by genetic data
Although, the genetic categorization of ‘pure’ and ‘hybrid’ individuals is predictably difficult in species that hybridize freely and form sympatric populations (Rieseberg et al., 1998), the genetic data presented here revealed that Banksia populations in their native heathland can form a patchy mosaic of almost pure species stands and complex hybrid swarms. This implies that, although hybridization may be common under favourable conditions (that appear to be repeated within many drainage basins), isolating mechanisms are sufficient to prevent widespread introgression and imply that hybrid genotypes must be less fit outside environmentally intermediate hybrid zones. Indeed, only one individual of the 40 B. robur and B. oblongifolia sampled in the ‘pure’ stands (initially based on leaf morphology) appeared to be of mixed ancestry.
Within areas of hybridization, mixed stands of B. robur and B. oblongifolia appear to consist of pure species plants, as well as first- and later generation hybrid plants. Boecklen and Howard (1997) used mathematical models to determine the number of diagnostic markers needed to correctly classify a series of backcrossed (BC) individuals as hybrids and not pure species and found that, for an error rate less than 10 %, eight markers would be needed to detect a second-generation BC individual and up to 70 markers for a fifth-generation BC individual. Self-fertilization further complicates the distinction between later generation hybrids and BC individuals. Nevertheless, simple inspection of the present data revealed plants that were genetically intermediate between pairs of pure species, displaying one allele characteristic of each species at each locus. These plants perfectly match our expectations for F1 hybrids whereas other plants displayed genotypes predicted for backcrosses to either of the species and also more complex genotypes.
Schibeci (1994) found that overlapping flowering times of B. oblongifolia and B. robur plants along with common pollinators provide opportunity for interspecific pollination. The flowering times of hybrid plants overlap much of the flowering times of the two parental species, and several examples in this study demonstrate a role for backcrossing in the facilitation of gene flow from one species to another. Although direct observation of a small number of hybrid seedlings from two known maternal plants revealed only backcrossing toward B. oblongifolia, the genotypes of field seedlings suggested that introgression occurs in both directions at the contact zones.
Morphology as an indicator of hybridization
Although hybrid plants are often recognized on the basis of morphologically intermediate mature plants, Rieseberg and Ellstrand (1993) noted that the unpredictability of hybrid character expression makes the use of morphology for hybrid identification unreliable. The data presented herein show that classification based on morphological intermediacy alone would result in an underestimation of the frequency of hybridization between B. robur and B. oblongifolia within groups of mature plants, seedlings raised in pots and seedlings growing naturally within the hybrid zone. This agrees with the findings of Craft et al. (2002) that for Quercus lobata and Quercus douglassi not all intermediate plants were hybrids and not all hybrids were intermediate. This suggests that hybridization in general could be more common than is apparent by morphological observation and emphasizes the need for genetic analyses in the detection of hybridization.
Rieseberg and Ellstrand (1993) found that a large proportion of first- and later generation hybrids exhibited extreme or novel characters and that hybrids of all generations were generally no more likely to display intermediate character states than parental character states [see Rieseberg and Ellstrand (1993) for a summary of some causes for transgressive segregation and/or novel characters]. Three of the four mature first-generation genetic hybrids between B. robur and B. oblongifolia plants had leaf morphology intermediate between the two species; the fourth had B. robur morphology. Later generation hybrids showed a range of intermediate to parental characteristics. These results are consistent with multigenic control (Grant, 1975) of the leaf characteristics measured and mostly quantitative traits (Rieseberg and Ellstrand, 1993). However, the morphology of the potted seedlings suggests that leaf morphology is determined by more than simple additive genetic variation. All hybrid seedlings, including putatively first-generation hybrids, had parental morphology and all but one had B. robur morphology like the maternal parent. This could reflect either nuclear or cytoplasmic effects or interactions between maternally inherited cytoplasmic genes and nuclear genes (Campbell et al., 2008).
Phenotypic plasticity amongst seedlings
Identification of hybrids can be obscured by phenotypic plasticity within a variable environment (Bradshaw, 1965; Dudley, 1996) and phenotypic plasticity is hypothesized to influence ecological niche breadth (Bradshaw, 1965; Sultan and Bazzaz, 1993a, b, c; Oyama, 1994; Sultan et al., 1998). For Banksia seedlings, morphology proved ineffective in predicting hybrid seedling genotypes, particularly for seedlings growing naturally in the hybrid zones. Phenotypic plasticity appears to play a role in seedling survival in the variable environment of the hybrid zones, as seedlings of the two species were closer in size and more variable in field conditions than those grown in pots. The ability of Banksia seedlings to adapt to various stresses may also extend the niche range of seedlings of each species.
During this study, there was a severe drought. Prolonged drought conditions can have severe deleterious affects on plant metabolism (Bradford et al., 1982; Sultan and Bazzaz, 1993b). Furthermore, growth can be restricted by biochemical disruption and reduced cell enlargement, leading to smaller leaves and delayed ontogenetic development (Gedroc et al., 1996). Many traits can change with size and age (Gedroc et al., 1996; Dudley, 2003). Compared with the development of the potted seedlings, the development of the field seedlings was significantly retarded. It could be argued that different developmental stages and water stress experienced by the field seedlings contributed to the difference in comparative sizes of B. oblongifolia and B. robur seedlings growing in the field and in pots.
Despite the abundance of hybridization and gene exchange within the Banksia hybrid zones, the genetic and morphological integrity of the different species appear to be maintained. Although there appears to be some evidence for selection against early generation hybrids amongst the field seedlings, physical and partial temporal separation along with pollinator preferences, and selection within different environments, could all contribute to the maintenance of pure species genotypes. The role of environmental variation in determining seedling survival, growth and susceptibility to herbivory has not been tested in the Banksia hybrid zones and will be investigated in a future study using transplant experiments, including independent measurements of the environment, and by comparing field and potted seedlings. A comparison between seedlings in pure stands with the seedlings in mixed stands may bring further clarity to the role of the environment in the morphological variation and selection amongst seedlings. Further studies should include the use of cytoplasmic markers and transplant experiments to ascertain any cytoplasmic influences on morphology of hybrids. The possibility of B. paludosa as a third species in a Banksia hybrid complex with B. robur and B. oblongifolia should also be further investigated.
ACKNOWLEDGEMENTS
This work was supported by a University of Wollongong ARC Discovery research grant to D.J.A. and R.J.W., the University of Wollongong's Institute for Conservation Biology and a University of Wollongong postgraduate scholarship to A.V.U. We thank the Sydney Catchment Authority and the NSW Department of Environment and Climate Change for access to and permission to use the study sites. Thanks to each member of the Ayre laboratory group for their expertise, comments and support.
LITERATURE CITED
- Ackerly DD, Dudley SA, Sultan SE, Schmitt J. The evolution of plant ecophysiological traits: recent advances and future directions. Bioscience. 2000;50:979–995. [Google Scholar]
- Arnold ML. Transfer and origin of adaptations through natural hybridization: were Anderson and Stebbins right? The Plant Cell. 2004;16:562–570. doi: 10.1105/tpc.HistPersp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridle JR, Baird SJE, Butlin RK. Spatial structure and habitat variation in a grasshopper hybrid zone. Evolution. 2001;55:1832–1843. doi: 10.1111/j.0014-3820.2001.tb00832.x. [DOI] [PubMed] [Google Scholar]
- Boecklen WJ, Howard DJ. Genetic analysis of hybrid zones: numbers of markers and power of resolution. Ecology. 1997;78:2611–2616. [Google Scholar]
- Bradford KJ, Hsiao TC, Yang SF. Inhibition of ethylene synthesis in tomato plants subjected to anaerobic root stress. Plant Physiology. 1982;70:1503–1507. doi: 10.1104/pp.70.5.1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw AD. Evolutionary significance of phenotypic plasticity in plants. Advances in Genetics. 1965;13:115–155. [Google Scholar]
- Bunje PME, Barluenga M, Meyer A. Sampling genetic diversity in the sympatrically and allopatrically speciating Midas cichlid species complex over a 16 year time series. BMC Evolutionary Biology. 2007;7:25–38. doi: 10.1186/1471-2148-7-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke JM, Carney SE, Arnold ML. Hybrid fitness in the Louisiana irises: analysis of parental and F1 performance. Evolution. 1998;52:37–43. doi: 10.1111/j.1558-5646.1998.tb05136.x. [DOI] [PubMed] [Google Scholar]
- Campbell DR. Natural selection in Ipomopsis hybrid zones: implications for ecological speciation. New Phytologist. 2003;161:83–90. [Google Scholar]
- Campbell DR, Waser NM, Aldridge G, Wu CA. Lifetime fitness in two generations of Ipomopsis hybrids. Evolution. 2008;62:2616–2627. doi: 10.1111/j.1558-5646.2008.00460.x. [DOI] [PubMed] [Google Scholar]
- Carney SE, Gardner KA, Rieseberg LH. Evolutionary changes over the fifty-year history of a hybrid population of sunflowers (Helianthus) Evolution. 2000;54:462–474. doi: 10.1111/j.0014-3820.2000.tb00049.x. [DOI] [PubMed] [Google Scholar]
- Close D, McArthur C, Paterson S, et al. Photoinhibition: a link between effects of the environment on eucalypt leaf chemistry and herbivory. Ecology. 2003;84:2952–2966. [Google Scholar]
- Craft KJ, Ashley MV, Koenig WD. Limited hybridization between Quercus lobata and Quercus douglasii (Fagaceae) in a mixed stand in central coastal California. American Journal of Botany. 2002;89:1792–1798. doi: 10.3732/ajb.89.11.1792. [DOI] [PubMed] [Google Scholar]
- Davis C. Plant ecology of the Bulli District Part II: Plant communities of the plateau and scarp. Proceedings of the Linnean Society of NSW. 1941;66:1–19. [Google Scholar]
- Donohue K, Pyle EH, Messiqua D, Heschel MS, Schmitt J. Adaptive divergence in plasticity in natural populations of impatiens capensis and its consequences for performance in novel habitats. Evolution. 2001;55:692–702. doi: 10.1554/0014-3820(2001)055[0692:adipin]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Dudley SA. Differing selection on plant physiological traits in response to environmental water availability: a test of adaptive hypotheses. Evolution. 1996;50:92–102. doi: 10.1111/j.1558-5646.1996.tb04475.x. [DOI] [PubMed] [Google Scholar]
- Dudley SA. The functional ecology of phenotypic plasticity in plants. In: DeWitt TJ, Scheiner SM, editors. Phenotypic plasticity: functional and conceptual approaches. New York: Oxford University Press; 2003. pp. 151–172. [Google Scholar]
- Gedroc JJ, McConnaughay KDM, Coleman JS. Plasticity in root/shoot partitioning: optimal, ontogenetic, or both? Functional Ecology. 1996;10:44–50. [Google Scholar]
- George AS. The Banksia Book. Sydney: Kangaroo Press; 1987. [Google Scholar]
- Grant PR, Grant BR. Phenotypic and Genetic effects of hybridization in Darwin's finches. Evolution. 1994;48:297–316. doi: 10.1111/j.1558-5646.1994.tb01313.x. [DOI] [PubMed] [Google Scholar]
- Grant V. Genetics of Flowering Plants. New York: Columbia University Press; 1975. [Google Scholar]
- Gross BL, Rieseberg LH. The ecological genetics of homoploid hybrid speciation. Journal of Heredity. 2005;96:241–252. doi: 10.1093/jhered/esi026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopper SD. Variation and natural hybridization in the Conostylis aculeata R.Br. species group near Dawesville, Western Australia. Australian Journal of Botany. 1977;25:395–411. [Google Scholar]
- Jiggins CD, McMillan WO, Neukirchen W, Mallet J. What can hybrid zones tell us about speciation? The case of Heliconius erato and H. himera (Lepidoptera: Nymphalidae) Biological Journal of the Linnean Society. 1996;59:221–242. [Google Scholar]
- Keith DA, Myerscough PJ. Floristics and soil relations of upland swamp vegetation near Sydney. Australian Journal of Ecology. 1993;18:325–344. [Google Scholar]
- Knox KJE, Morrison DA. Effects of inter-fire intervals on the reproductive output of resprouters and obligate seeders in the Proteaceae. Austral Ecology. 2005;30:407–413. [Google Scholar]
- Mallet J. Hybrid speciation. Nature. 2007;446:279–283. doi: 10.1038/nature05706. [DOI] [PubMed] [Google Scholar]
- McKinnon GE, Vaillancourt RE, Jackson HD, Potts BM. Chloroplast sharing in the Tasmanian Eucalypts. Evolution. 2001;55:703–711. doi: 10.1554/0014-3820(2001)055[0703:csitte]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Myerscough PJ, Whelan RJ, Bradstock RA. Ecology of Proteaceae with special reference to the Sydney region. Cunninghamia. 2000;6:952–1015. [Google Scholar]
- Oyama K. Local differentiation among populations of Arabis stelleri var. japonica in a sand dune habitat. Annals of Botany. 1994;74:103–109. [Google Scholar]
- Peakall R, Smouse P. GenAlEx V6: Genetic Analysis in Excel. Population Genetic Software for Teaching and Research. Molecular Ecology Notes. 2006;6:288–295. doi: 10.1093/bioinformatics/bts460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchard JK, Stephens M, Donnelly P. Inference of population structure from multilocus genotype data. Genetics. 2000;155:945–959. doi: 10.1093/genetics/155.2.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed DH, Lowe EH, Briscoe DA, Frankham R. Fitness and adaptation in a novel environment: effect of inbreeding, prior environment and lineage. Evolution. 2003;57:1822–1828. doi: 10.1111/j.0014-3820.2003.tb00589.x. [DOI] [PubMed] [Google Scholar]
- Rieseberg LH, Ellstrand NC. What can molecular and morphological markers tell us about plant hybridization? Critical Reviews in Plant Sciences. 1993;12:213–241. [Google Scholar]
- Rieseberg LH, Baird SJE, Desrouchers AM. Patterns of mating in wild sunflower hybrid zones. Evolution. 1998;52:713–726. doi: 10.1111/j.1558-5646.1998.tb03696.x. [DOI] [PubMed] [Google Scholar]
- Sultan SE, Bazzaz FA. Phenotypic plasticity in Polygonum persicaria. I. Diversity and uniformity in genotypic norms of reaction to light. Evolution. 1993a;47:1009–1031. doi: 10.1111/j.1558-5646.1993.tb02132.x. [DOI] [PubMed] [Google Scholar]
- Sultan SE, Bazzaz FA. Phenotypic plasticity in Polygonum persicaria. II. Norma of reaction to soil moisture and the maintenance of genetic diversity. Evolution. 1993b;47:1032–1049. doi: 10.1111/j.1558-5646.1993.tb02133.x. [DOI] [PubMed] [Google Scholar]
- Sultan SE, Brazzaz FA. The evolution of ecological breadth for nutrient environment (Phenotypic plasticity in Polygonum persicaria, part 3) Evolution. 1993c;47:1050–1072. doi: 10.1111/j.1558-5646.1993.tb02134.x. [DOI] [PubMed] [Google Scholar]
- Sultan SE, Wilczek AM, Bell DL, Hand G. Physiological response to complex environments in annual Polygonum species of contrasting ecological breadth. Oecologia. 1998;115:564–578. doi: 10.1007/s004420050554. [DOI] [PubMed] [Google Scholar]
- SAS Institute Inc. Version 5·1 of JMP. Cary, NC: SAS Institute; 2003. [Google Scholar]
- Schibeci S. Genetic and ecological analysis of the putative natural hybrid zone formed by Banksia robur Cav. and Banksia oblongifolia Cav. NSW: University of Wollongong; 1994. PhD thesis. [Google Scholar]
- Taylor A, Hopper S. The Banksia atlas. Canberra: Australian Government Publishing Service; 1988. [Google Scholar]
- Ungerer MC, Baird SJE, Pan J, Rieseberg LH. Rapid hybrid speciation in wild sunflowers. Proceedings of the National Academy of Science. 1998;95:11757–11762. doi: 10.1073/pnas.95.20.11757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usher AV, Ayre DJ, Whelan RJ. Microsatellites for eastern Australian Banksia species. Molecular Ecology Notes. 2005;5:821–823. [Google Scholar]
- Yanchukov A, Hofman S, Szymura JM, et al. Hybridization of Bombina bombina and B. variegate (Anura, Discoglossidae) at a sharp ectotone in western Ukraine: comparisons across transects over time. Evolution. 2006;60:583–600. [PubMed] [Google Scholar]