Abstract
Falls frequently cause injury-related hospitalization or death among older adults. This article reviews a new conceptual framework on dynamic stability and weight support in reducing the risk for falls resulting from a forward slip, based on the principles of motor control and learning, in the context of adaptation and longer-term retention induced by repeated-slip training. Although an unexpected slip is severely destabilizing, a recovery step often is adequate for regaining stability, regardless of age. Consequently, poor weight support (quantified by reduction in hip height), rather than instability, is the major determinant of slip-related fall risk. Promisingly, a single session of repeated-slip training can enhance neuromechanical control of dynamic stability and weight support to prevent falls, which can be retained for several months or longer. These principles provide the theoretical basis for establishing task-specific adaptive training that facilitates the development of protective strategies to reduce falls among older adults.
The increased susceptibility to falling with increasing age,1 associated with the reduced capacity in sensorimotor function,2 poses a significant health threat to the older adult population. Injuries as the result of falls affect a broad spectrum of older adults—not only those individuals who are frail or have impairments but also those who are healthy and living independently.3,4 Falls are the leading cause of injury-related hospitalization and death in this older population.5 Such fall-related injuries can lead to decreased mobility or to a reduced activity level because of an instilled fear of falling.6 In either case, the result is a decreased quality of life. There is no question that prevention of falls is a pressing problem that biomedical research must solve.
One intervention strategy that is currently underutilized and that has far-reaching potential is to promote an older adult's own neuromuscular protective mechanisms appropriate for reducing the incidence of falls. This strategy emphasizes motor training under conditions resembling real-life situations. This review focuses on literature relevant to the prevention of slip-related falls resulting from the repeated, unannounced exposure to slips during the performance of activities of daily living, such as in moving from a sitting position to a standing position and in walking. Such a repeated-slip paradigm represents one class of external perturbation induced through reducing surface friction (versus unperturbed, volitional movement or a different class of external perturbation, such as a trip) under therapeutically manipulated safe conditions. Furthermore, it mimics the accidental (unpredictable) nature of slips experienced in real life by mixing the slips with nonslip conditions, unbeknownst to the participating individuals.
Although slip-like perturbations can be applied during standing or walking by moving the support surface forward with an actively controlled, motorized mechanism,7–9 such forced sliding is theoretically different from slips due to reduced friction.10 Moreover, the fixed motion profile used during forced sliding contradicts the commonly observed adaptive strategies for stability control, which is essentially achieved by actively controlling the peak slip displacement and velocity, as these motor skills can be acquired spontaneously under low-friction conditions.11 It is reasonable to postulate that the motor skills required for overcoming real-life challenges (ie, slip accidents) are best acquired under conditions that resemble real-life situations. Our review consists of 3 parts that further explore the mechanisms and feasibility for such an approach:
We first define commonly used terms such as “stability,” “balance loss,” “weight support,” and “limb collapse.” We review the conceptual framework upon which their relationship with falls can be elucidated.
We synthesize both empirical and analytical evidence indicating that the central nervous system (CNS) might be trained to simultaneously prevent backward balance loss and reduce downward descent of the body resulting in falls. It is suggested that, with repeated perturbations affecting posture and gait, the CNS likely builds new, or updates existing, internal representations to improve its feedforward control, while decreasing a person's reliance on feedback corrective mechanisms for successful recovery. The latter mechanism also can be enhanced and is effective in reduction of falls.
We demonstrate meaningful preservation, over months, of the proactive and reactive locomotion strategies that emerge from a single training session via adaptive enhancements in feedforward and feedback control.
Dynamic Stability and Weight Support
We will first clarify the meaning of several terms essential to this review. In Newtonian mechanics, balance occurs only when equilibrium conditions are met (ie, when the net external force and moment acting on a person diminish). Balance is only transient; however, humans cannot uphold these equilibrium conditions constantly nor indefinitely, even during quiet standing. We, therefore, refer to stability as a person's ability to restore balance without resorting to the alteration of his or her base of support (BOS) following an externally imposed perturbation or even during volitional movement.10,12–15 Logically, there must be limits on a person's stability. Outside of these limits, loss of balance or instability occurs. The relationship between a standing person's center of mass (COM) and BOS defines the person's stability limits, which can be termed as the stability threshold or boundary that outlines a “stability region.” The BOS consists of the outline area of each foot in contact with the ground and the area between the feet in bipedal stance. When the horizontal component of the velocity of the COM is negligible, this person is stable when the COM projection stays inside the BOS, which defines the stability limits in a static sense. Forward loss of balance must occur when the COM traverses anteriorly to the BOS and vice versa for backward loss of balance.10
A fall is initiated when loss of balance occurs. Fall initiation, in this context, is the same as the term “falling,” which describes a process. Because of the availability and the application of a recovery step or other protective responses, such falling is different from an actual fall. The latter is a consequence rather than a process, and it is defined by a sudden, unintended change in position causing an individual to land inadvertently at a lower level, on an object, the floor, or the ground. When loss of balance (falling) occurs following an external perturbation, a person usually responds with changing BOS to avert an actual fall by taking a recovery step or by grasping onto nearby structures if they exist.
Postural sway reflects our inability to keep ourselves in constant equilibrium conditions; thus, it is often used as a convenient measure of stability. It can be assessed by the displacement of the center of pressure pertaining to the BOS in quiet standing.16–18 The implication is that the greater the body sway, the closer a person is to approaching his or her stability limits, thus the less stable this person is becoming.19–23 Sway-referenced measurements have been shown to be sensitive to problems of falling.24,25 An age-related increase in body sway often is cited as an indication of a decline in stability,26–29 and it has, in several instances, been associated with falling among older adults.30,31 No conclusive evidence, however, indicates that people who sway with greater magnitude are less likely to recover balance after perturbation. Because most falls occur during locomotion,3,29,32–34 body sway evaluated during quiet standing may not be the most appropriate indicator of stability during activities of daily living.20
What are a person's dynamic stability limits during movement when the horizontal component of the COM velocity is not negligible? We have recently extended the above-stated static definition of stability initially proposed by Borelli,35 the founding father of biomechanics, to dynamic conditions. This was done by simultaneously considering the instantaneous COM position and velocity that define a person's motion state (ie, in the COM state-space). When the forward velocity of the COM exceeds a set of stability limits and when the forward momentum (ie, the product of the velocity and body mass) cannot be diminished before the COM reaches the anterior border of the BOS, forward balance loss must occur. Conversely, when the COM is located posterior to the BOS and when its forward velocity (and its forward momentum) is lower than the threshold required to bring the COM into the BOS, backward balance loss must occur (Fig. 1).
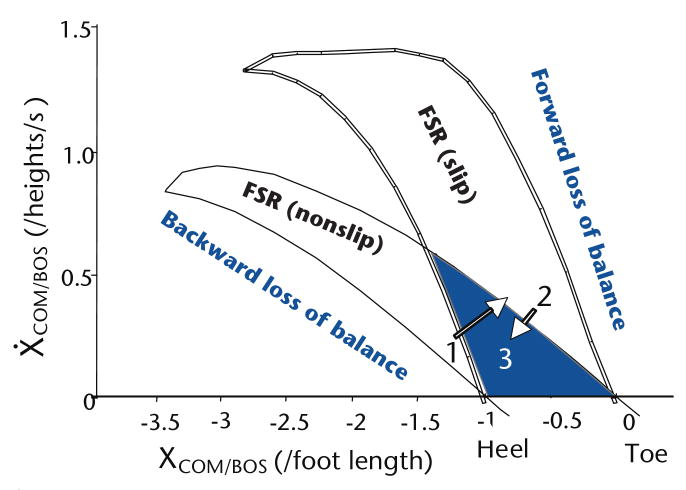
Figure 1.
The predicted feasible stability region (FSR) for both slip and nonslip conditions in the center of mass (COM) state-space (ie, its position and velocity). The position of the COM in the anteroposterior direction was expressed relative to the rear of the base of support (BOS) (XCOM/BOS) of the most recent foot to touch-down (ie, the heel of the sliding foot for slip onset) and normalized to foot length. The COM velocity in the anteroposterior direction was expressed relative to the velocity of the BOS (Ẋ COM/BOS) and normalized to body height. During regular sit-to-stands prior to their exposure to slipping, 41 older adults' COM state at seat-off was around area 1, which was very near the boundary for backward balance loss for a slip. Subsequently, they all experienced backward balance loss during the first, novel slip. Following repeated exposures to slipping, their COM state at seat-off was shifted from area 1 to area 2, which was inside the stability region for slipping but near the forward balance loss region for the nonslip condition. Nearly all of them experienced forward balance loss when the slip stopped. After the nonslip block, they readjusted their COM state from area 2 to area 3, which is in the middle of the shaded area where stability can be maintained regardless whether a slip occurs or does not occur.56
Across a range of COM positions, these 2 sets of the threshold divide the COM state-space into respective regions of forward and backward loss of balance. Between the 2 thresholds lies the feasible stability region (FSR) or the dynamic stability region, within which balance can possibly be restored (Fig. 1). The environmental constraints play a major role in shaping the FSR. For example, the FSR differs between nonslip conditions and low-friction induced slip conditions (Fig. 1), which also is different from that under forced-slide perturbations created by a motorized moving platform.10 It is noteworthy that there is a region of overlap between the FSR for nonslip conditions and that for slip conditions, where a person can be stable regardless of which condition is applied (shaded region in Fig. 1). We will discuss the implications of this region later.
How is the dynamic stability region quantified? The boundaries of the FSR are shaped by a person's anatomical and physiological limitations, as well as by environmental constraints.13 These boundaries can be determined numerically with the aid of mathematical simulation and optimization.10,12,15 The latter is an iterative computational process that systematically searches the entire COM state-space within those physical limitations and constraints to “rule out” all possible COM position-velocity combinations that will ultimately lead to balance loss and violate the task objectives that define successful performance.
How does the FSR apply to standing conditions? When a perturbation from a moving platform, a tug, or a push is insufficient to displace a person's COM state outside the FSR, “in-place” responses such as “ankle or hip strategy”8,36 are sufficient to restore balance without resorting to taking a step. Balance loss occurs when a large-scale perturbation displaces the body's COM state outside the FSR. When that happens, only establishing a new BOS, often by taking a recovery step, can then restore balance and avert an actual fall.37,38
How does the FSR apply to locomotion? Humans volitionally displace their COM state outside of stability limits to achieve mobility in locomotion. In unperturbed walking, forward progression is achieved by “controlled” forward falling, which is accompanied by a forward step. Each step prevents an actual fall and improves dynamic stability by bringing the person's COM state either inside the FSR or at least closer toward rather than farther from the threshold for forward balance loss. In contrast, backward falling is not an intended task objective in regular gait, which is commonly stable against backward loss of balance under unperturbed conditions.15 As mentioned for standing, however, backward balance loss occurs during perturbed gait when a person's COM state is located posterior to the BOS, below the corresponding FSR threshold. This happens when that person is subject to a large-scale, unexpected, novel forward slip, as discussed below extensively.
The development of this conceptual framework and the quantification of dynamic stability have enabled us to differentiate, for the first time, the individual roles of stability and weight support in averting an actual fall. Hip height (measured as the vertical distance from the ground to the midpoint between 2 hips) can readily quantify weight support. Perturbation-induced “buckling” of the knee or limb collapse occurs when a person's hip height descends below his or her usual amount of variability following a perturbation (ie, unintended hip descent). In walking, following an unexpected forward slip after touch-down, the unintended hip descent is defined as when the hip is located 3 standard deviations below this person's average hip height in unperturbed gait and when the hip velocity prematurely reverses in the downward direction (Fig. 2). In the sit-to-stand task, because the task requires continuous ascent, unintended limb collapse can easily be identified when descent (instead of continuous ascent) begins at the hip a few hundred milliseconds after a slip, which is induced immediately after a person loses contact with the chair (seat-off).39,40 Most of the examples in the rest of this section relate to this slip paradigm in the sit-to-stand task.
Figure 2.
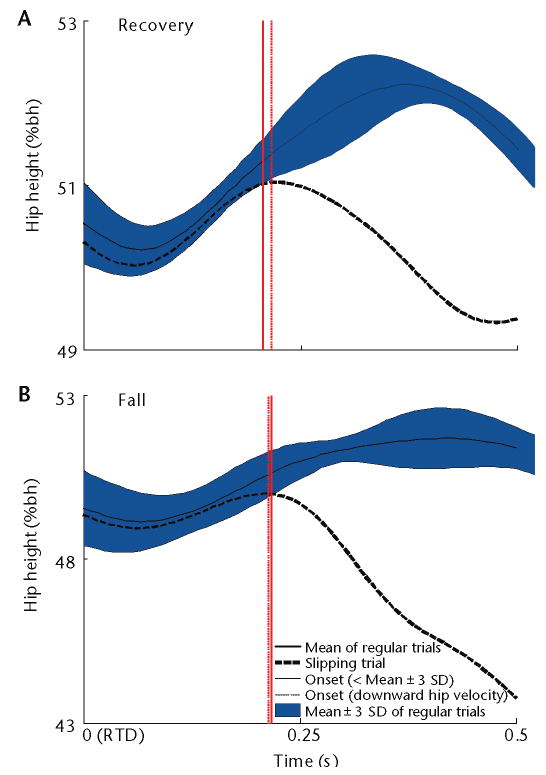
Onset of the unintended hip descent (thin solid vertical line) after the slipping (right) limb touch-down (RTD) from 2 typical young subjects: (A) one who recovered and (B) one who fell. This instant is identified as the point when the hip height (ie, the vertical distance from the ground to the midpoint between 2 hip centers) trajectory (thick broken line) traversed below 3 standard deviations (shaded area) from its mean trajectory (thick solid line) taken from the subjects' regular (unperturbed) gait. This onset timing is nearly identical to the onset of negative vertical (downward) hip velocity (thin broken vertical line). Hip height is normalized as percentage of body height (%bh).
The concept of limb collapse is similar to that of balance loss (falling), describing a process that does not necessarily lead to an actual fall. In a subgroup of 23 young adults who took only one recovery step upon a slip, hip descent began at about 300 milliseconds after slip onset (ie, about 100 milliseconds before step lift-off41), from about the same height of 44% body height, regardless of who recovered or fell.39 At recovery step touch-down, both groups experienced unintended hip descent (ie, knee buckling or limb collapse). However, the difference in hip height (X̄±SD) between recoveries and falls was significant, at 42.8%±1.7% and 37.6%±3.0% of body height, with a downward speed of 11.8%±16.0% and 62.0%±22.1% body height/s, respectively.39 Thus, although unintended hip descent occurs in all individuals following slip onset, the degree of decent in fallers is significantly greater than in recovery. For both young and older adults who fell, their hip height already descended more than 5% body height below their corresponding seated height at the time of harness arrest.42,43
Interestingly, although instability is often a precursor to an actual fall, it is neither a sufficient nor a necessary condition at the time of an actual fall, because the COM state stability can be restored rapidly (in a few hundred milliseconds) by taking a recovery step.14,44–46 It is when instability is combined with inadequate weight support that recovery from a large-scale perturbation such as a slip becomes virtually impossible. A lowered hip height at recovery step lift-off can hinder foot clearance, affecting the step length and foot placement at touch-down. Inability to provide timely and sufficient weight support is indeed associated with increased risk for falling from trips47 and slips.41 Decreases in hip height at post–slip recovery step touch-down had over a 20 times greater effect on the odds of falls than equivalent decreases in stability among young and older adults during an unexpected slip induced in the sit-to-stand task.40
Unlike stability-related issues, the reasons why fallers with apparent good strength (force-generating capacity) fail to provide adequate weight support after experiencing a sudden, unexpected, usually large-scale perturbation have been studied less and are little understood. Only recently have results indicated that unintended hip descent is caused by inadequate concentric work generated by the knee extensors and, to a lesser extent, the hip extensors from slip onset to step lift-off (Fig. 3).41 A dilemma arising in this sit-to-stand paradigm is that initiating the recovery step with one limb to regain stability following a slip takes away its ability to assist the other limb to provide weight support during the slip.41 All of the 65 subjects included in this analysis took a step for recovery, but from step lift-off to touch-down, fallers could not resist knee buckling under the gravity, which forced their knee extensors to do more negative (eccentric) work at the stance limb than those who recovered. This finding suggests that the need to simultaneously provide weight support and stability may represent competing task objectives under such circumstances, regardless of age (Fig. 3). The CNS may be unable to resolve this dilemma due to lack of experience for proper prioritization.40 It also may be due to a conflict with an ongoing motor program, demonstrated, for example, by the inability to modify the central pattern generator to prevent lift-off of the trailing limb (ie, by aborting a forward step following a slip in walking).46 Such an “aborted” step would increase stability by maintaining the extended posterior BOS.
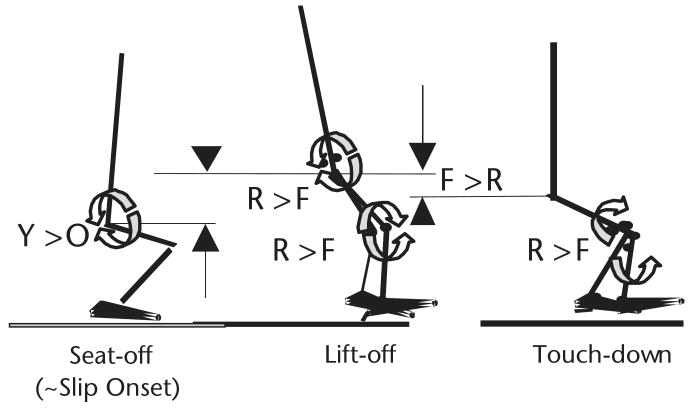
Figure 3.
During preparation for chair-rise from movement initiation to losing contact with chair (seat-off) when a slip was unexpectedly induced (∼slip onset), 22 older adults (O) did less net concentric (positive) work, on average, than that generated by the hip extensors (2 half circles: counterclockwise-clockwise) of 43 young adults (Y). This age-related difference (Y>O) caused less increase in upward momentum and in potential energy required for ascending (upward arrow and triangle) and might have predisposed a greater proportion of older adults to falls. All of them took a step for recovery, but from seat-off to step lift-off, fallers (F) did less concentric work generated by the hip extensors (counterclockwise-clockwise circles) and knee extensors (clockwise-counterclockwise circles) than those who recovered (R) (R>F). From step lift-off to touch-down, fallers' knee extensors (clockwise-counterclockwise circles) failed to resist gravitational effect, resulting in more negative (eccentric) work than in those who recovered (R>F), causing knee buckling. Although those who fell and those who recovered both experienced unintended hip descent (limb collapse), hip height for those who fell was significantly lower than that for those who recovered at the step touch-down (downward arrow and triangle for descent, F>R). This mechanism of fall applied to all fallers regardless of their age.41
Unfortunately, little has been postulated to account for such failure in neural control mechanisms. A spectrum of reflex and triggered responses, that can be elicited following the perturbation, exists within and across limbs. These responses are likely influenced by the task-related modulation from supraspinal or cortical control.8,48–50 Thus, the deficient weight support of fallers may reflect a failure to exhibit an appropriate neuromuscular response during the initial hundred milliseconds following perturbation onset (unpublished research). In fact, the most robust electromyographic (EMG) magnitudes are often registered a few hundred milliseconds (100–200 milliseconds) after the onset of a novel perturbation and range from 4 to 9 times the EMG magnitudes achieved during normal walking, with a duration lasting for a relatively long time (70–200 milliseconds) (unpublished research).51–55 Yet, such responses may be inefficient or come too late to reverse hip descent. Therefore, fall incidence was about 30% among young adults and more than twice that rate among older adults upon the first unexpected slip during the sit-to-stand task.40
Promisingly, this conceptual framework also offered us a theoretical basis for motor training, suggesting that a person can adaptively enhance his or her stability. Three lines of evidence highlight the feasibility of adaptive training to improve stability through exposure to repeated slips induced after seat-off during the sit-to-stand task. Initially, upon the first unexpected slip in the sit-to-stand task in a study by Pai et al,56 the averaged COM state of 41 older adults at seat-off was located in area 1 shown in Figure 1. It was very stable under nonslip conditions, but very near the boundary for backward balance loss under slip conditions. Consequently, the perturbation forced the COM state outside the FSR, and all of the participants experienced backward balance loss (100%), as predicted. Following a block of 5 repeated slips, they rapidly adapted to increase their stability at seat-off from area 1 to area 2 shown in Figure 1, resulting in only 12% backward balance loss.
Although area 2 shown in Figure 1 is very stable under slip conditions, it is very near the boundary for forward balance loss under nonslip conditions, representing potential “over-compensation.” That was exactly what happened upon the first nonslip trial after the slip block, in which most individuals experienced a forward balance loss. The model prediction again was correct. Finally, older adults rapidly made readjustments to their COM state upon the second nonslip trial to increase their stability at seat-off against forward balance loss by scaling back the amount of their forward COM shift and velocity increase.56,57 Notably, they did not return to their original movement pattern prior to the first slip, but adapted instead to an intermediate pattern that, on average, was stable under both slip and nonslip conditions (with their stability located in area 3 of Fig. 1). These readjustments had a significant influence on the recovery outcome, such that the falls incidence among older adults decreased from 73% upon the first slip of the initial slip block to only 20% upon the first re-slip trial (that is, the first slip of the second slip block, which followed a block of nonslip trials).57 Stability can be maintained with such desirable adaptive strategies inside this shaded area of Figure 1, regardless of whether a slip occurs or not.
Improvements in stability reduced the reliance on reactive responses to provide weight support. Nonetheless, both older and young adults were able to improve weight support in reaction to a slip and prevent unintended hip descent through adaptive training. In subgroups of 22 young and 14 older adults who undertook repeated slip, then nonslip and re-slip trials, in the sit-to-stand task, all participants were able to recover upon the re-slip by significantly increasing their hip height, regardless of their age and whether they fell or did not fall during the first slip.43 As demonstrated, a person's own neuromuscular protective mechanisms against falls can be developed or enhanced with appropriate adaptive training. The neuromechanical principles of adaptive training will be discussed further in the next section.
Adaptive Control and Training
It is well established that humans are adaptable to sudden or unexpected changes in environmental constraints during different activities, such as standing or locomotion.11,36,48,54,58 Encouragingly, this ability can remain intact even at an older age (Fig. 4).42 Such motor adaptation is a process that occurs in the initial acquisition phase of motor learning and often requires novel associations between the externally imposed perturbations, such as a slip, and motor actions. An increase in stability resulting from an alteration in regular gait pattern, such as the emergence of a walkover or skate-over strategy after repeated exposure to a slippery surface, is an example of such an adaptation used for preventing the incidence of backward balance loss and falls.11 In the walkover pattern, the subject's response to a slip from reduced surface friction resembled a natural walking pattern, with minimal forward BOS displacement (≤0.05 m). In the skate-over pattern, the forward BOS displacement (>0.05 m) was less than that during a trial in which loss of balance occurred but greater than that in the walkover pattern. Similar to the unperturbed gait, the trailing limb in both walkover and skate-over patterns lands anterior to the slipping limb. Such an alteration in gait pattern is a prerequisite to a permanent change in motor behavior and represents an early “form of learning that evolves over a series of movements to restore the original performance of a task in the presence of an external perturbation.”59(p972)
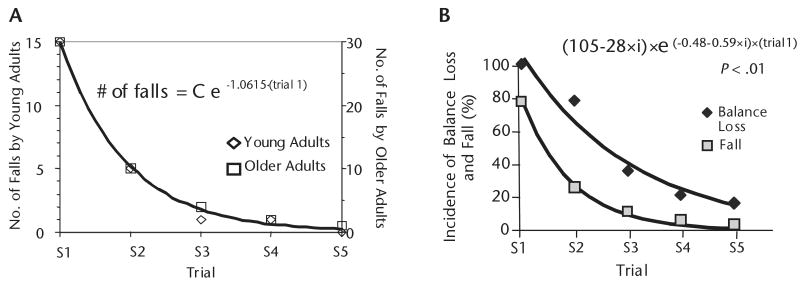
Figure 4.
(A) Fall incidence decreased exponentially with repeated exposure to a slip during a sit-to-stand task and at a similar rate in young and older adults. Shown are the number of falls by each age group in each of the 5 trials of the first slip block and the corresponding best-fit exponential relationship identified through nonlinear regression. The parameter for initial fall incidence (C) equaled 14.9 and 30.0 in young and old adults, respectively. The exponential relationship explained 99.7% of the variance in the number of falls, with P<.05 for all parameters. Fall incidence among both groups decreased by the same factor of 3 following each slip exposure. (From: Pavol MJ, Runtz EF, Edwards BJ, Pai YC. Age influences the outcome of a slipping perturbation during initial but not repeated exposures. J Gerontol A Biol Sci Med Sci. 2002;57:M496–M503. Copyright © The Gerontological Society of America. Reproduced by permission of the publisher.) (B) Although instability is neither a sufficient nor a necessary condition to cause falls that result from vertical hip descent and limb collapse, improved stability directly resulted in the reduction in fall incidence among older adults (i=0 for balance loss and 1 for fall in the exponential equation). The rate of incidence decline was almost twice as fast for falls as for balance loss (exponential rate constants of −1.07/trial and −0.48/trial, respectively).
Necessary for adaptive control that underlies movement alteration are sensory information processing and sensorimotor transformation.60 With repeated exposure to perturbation, a newly acquired, predominantly predictive form of adaptive control emerges. Such control exhibits feedforward behavior by responding to this perturbation in a predefined way that improves performance by modifying present and future motor commands, relying on stored information from previous experience.59 To perform the transformation, the CNS builds, refines, or updates an internal representation of the potential threats that may occur in the external environment (eg, stepping onto a patch of wet surface or traversing an icy parking lot). The motor commands and sensory prediction must accurately reflect the changes occurring in the environment. When the sensory prediction is consistent with the actual sensory information generated by the ongoing movement, the feedback controller will elicit little online corrective commands.61 Otherwise, these sensory inputs will not only elicit online reactive responses to compensate for motion errors, but also continue to be used, adaptively, in an offline mode, to reshape the sensory representation of the environment and the motor commands in a feedforward manner.2 Offline modification is needed for correct future performance under similar contexts. Although the reliance on feedback control decreases with the maturation of the adaptation process, sensory inflow continues to play a role in fine-tuning the movement during this acquisition phase.
The feedforward control underlying successful proactive adjustments, occurring before or in anticipation of perturbation onset and relying usually on previously acquired knowledge, can affect the movement outcomes in 2 different ways during this adaptive process. First, it can alter posture and limb control prior to an encounter with an environmental hazard.54,56,62 Our experimental results indicated that improved dynamic stability prior to the onset of perturbation can reduce or eliminate the need for a reactive response following the onset of perturbation (Fig. 4).56 Both young and older adults adapted to preventing falls without resorting to recovery stepping.
The second aspect of the feedforward control is that it can influence and alter the feedback-control-related reactive response following the onset of perturbation to increase the likelihood of a successful reactive response.11,43,54 As an example in responding to a slip during walking,11 an increase in both pre– and post–slip-onset gait stability is significantly correlated to a reduction in the incidence of balance loss. In this case, pre- and post-slip gait stability for all slip trials was measured, respectively, at touch-down of the slipping limb and at lift-off of the contralateral trailing limb based on the COM state relative to the predicted thresholds for backward loss of balance. Although increases in pre-slip gait stability were affected by a feedforward anterior shift in COM position, the post–slip-onset improvements derived from adaptive training were even greater and benefited from reductions in the perturbation (slip) intensity (ie, a reduced displacement and peak velocity of the BOS). Such reactive changes, characterized by an active control of slip intensity, came from a reduction in the demand on the post–slip-onset braking impulse.
These post-slip changes were nonetheless preceded by and correlated with proactive (feedforward) control of posture and gait pattern prior to slip onset (eg, more anteriorly positioned COM resulting from smaller step length, flat-footed landing, and increased knee flexion). These changes also revealed the maturing process of the adaptive control, characterized by a shift from a reliance on feedback control for postural correction to a distinctive feedforward control that improves pre-slip gait stability and alters slip intensity following its onset, leading to skate-over and walkover adaptive strategies.63 In the walkover strategy, a person could almost eliminate forward BOS displacement even in the presence of the same low surface friction by exhibiting significantly greater knee flexion, a more flat-footed landing, and a lower net momentum during double stance than in a “normal” gait pattern. In contrast, subjects in the study by Bhatt et al11 were able to glide forward on low surface friction against backward balance loss using the skate-over strategy, effectively placing their trailing limb ahead of the slipping limb and exhibiting a flat-footed landing, but a significantly greater momentum than was achieved using the walkover strategy, during double stance.11 It must be noted that there could be differences in many other movement parameters between these 2 strategies.
It has been proposed that feedforward control adaptively influences feedback control by the formation of a “set,” which refers to the tendency of the CNS to behave or respond in a particular way as a result of previous experience or context.48 This type of set formation by the CNS, perhaps similar to its building of the internal representation and accurate prediction of context, plays a crucial role in the success of the feedforward control.59,64
The importance of the post-perturbation reactive response, however, must be acknowledged. Rapid adaptive changes in reactive responses upon repeated perturbation exposure, consistent with an increased ability to recover from the perturbation, have been observed in young9,54,63 and older43,58,65 adults who are healthy. The role of feedback control in such adaptation of reactive postural responses is evident from the attenuation of both muscle EMG magnitudes and degree of postural sway with repeated BOS translations in earlier research on support-surface perturbations.36,48 Attenuation of such responses often has been attributed to habituation, reducing the gain of the neural feedback circuitry.8 Some researchers also have suggested a role of “feedback-error–based” learning models in motor adaptation.60
Such models could explain the findings by Nashner and colleagues8,36 that a session of repeated “toes-up” platform rotations resulted in the attenuation of muscle responses inappropriate to the perturbation, whereas the responses appropriate to the perturbation still persisted. Decreased length of the recovery step,9,58 decreases in reaction time,58 reduction in the incidence of multiple stepping,9 and increasing success of a single recovery step43 with repeated perturbations, during both quiet stance and dynamic activities, could similarly be explained. On similar lines, our recent results revealed that adaptive changes in reaction following a slip induced during chair-rise not only reduce the likelihood of a balance loss but also enhance weight support to prevent vertical descent of the body, which otherwise would result in a fall.43 Our preliminary work indicates that such adaptive enhancement in weight support was achieved primarily by increasing the positive (concentric) mechanical work generated by the knee extensors.
The possibility of alternative mechanisms for these adaptive changes, however, should be considered. For instance, could all of the observed adaptive changes have resulted merely from an increased knowledge of the perturbation and its effects? The adaptive changes observed then might be dependent on subjects' familiarity with the specific laboratory environment, experimental protocol, and characteristics of the potential perturbation. If this is the case, could watching educational material on how to successfully adapt to a perturbation achieve similar effects to direct experience? These prospects are attractive because increasing older adults' knowledge and awareness of the environment is a safer and easier alternative to exposure to repeated slips.
Awareness of potential slippery conditions can induce a cautious gait pattern.54,62 Nevertheless, it may not adequately prepare a person to respond to backward balance loss and falls when a slip occurs (unpublished research). We have demonstrated that, even when subjects were informed that a slip might occur, most experienced a backward balance loss upon their first, novel slip during walking.11,63 Similarly, explicit awareness and the actual experience of repeated-slip exposure clearly yield different preventive and reactive responses to a slip, with the latter being more effective.11,66 Awareness of an upcoming slip may result only in kinematic changes at pre-slip touch-down of the slipping limb, whereas experiencing an actual slip results in changes in pre-slip muscle activation as well as changes in foot-floor interaction forces upon touch-down.66 Without actual experience and adaptive modification of movement, explicit knowledge of upcoming perturbation may not be sufficient for humans to exhibit appropriate behavior.67 With repeated perturbations affecting posture or gait, however, the CNS builds new, or updates existing, internal representations to increase its feedforward control while decreasing a person's reliance on feedback corrective mechanisms for successful recovery. It is highly likely that such motor behavior modification can be meaningfully retained over months, if not years, as discussed below.
Retention of Adaptive Control
Adaptive training that improves stability and weight support would be of very limited practical benefit if the effects were not retainable. Retention is operationally defined as persistence of the observed behavioral or structural changes on either a shorter-term (minutes to hours) or longer-term (days, months, years) basis. In the context of adaptive training, longer-term retention will be the hallmark of a learned motor behavior. Retention within the CNS usually is considered a function of long-term changes that occur within the neural circuitry, a consequence of the process of consolidation, historically referred to as a process reflecting increased resistance of memory with passage of time to interference from competing or disrupting factors, resulting in stabilization of long-term memory.68,69 This process accompanies the formation of new synapses, synthesis of new protein, and an increase in the strength of existing synapses (ie, long-term potentiation).70
These changes usually occur in cortical and subcortical structures (basal ganglia, cerebellum) for tasks involving voluntary movements.71 The process of building or updating the internal representation of one's stability limits11,56 is probably associated with a shift from reliance on long-loop reflex pathways within the spinal cord and the brain stem50,72 to increased subcortical and cortical influence.73 Such a shift also would result in the development of an increase in memory from the short-term labile state to a longer-lasting stable state.74 There is considerable literature establishing that adaptation occurs within the balance-locomotor control system,7,8,11,36,48,54,58 but little is known about the extent to which adaptive improvements in stability and fall-resisting behavior to external disturbances, once acquired, can be retained.
The presence of an “aftereffect” of repeated perturbations has been well established.42,43,57,59,67,75–78 The “aftereffect” phenomenon is said to occur when the acquired behavior after repeated perturbation is reproduced even when the perturbation is no longer present on a subsequent trial. Similarly, the persistence of an acquired obstacle avoidance skill from one block to another, separated by only minutes of rest, also has been demonstrated.79 However, it is by no means clear that the above-mentioned examples can be considered a form of “retention.” Recently, Tjernström et al80 provided evidence for motor learning within the postural control system by showing long-term (30 days) retention of changes in the magnitude of body sway in response to calf vibrations during stance. In contrast, another study81 showed no retention from one day to the next, over 5 days, of an acquired adaptation in “postural response size” (amplitude of gastrocnemius muscle EMG activity) as the result of repeated “toes-up” platform rotations. Such differences could have arisen from many factors, including the nature of the stimulus, the intensity (repetitions per session) and duration (number of sessions) of the adaptive training, and the neural mechanisms involved in the adaptation response. The differences also could have resulted from differences in the outcome variables used to quantify the retention.
Evidence from the motor learning literature, mainly from skilled voluntary tasks, indicates that practice schedule can considerably affect motor learning. In particular, incorporating random practice (contextual interference)82 and “over-learning” (ie, continued practice of a task after having reached some success criterion)83 can lead to improved retention effects. Indeed, we found that a single acquisition session, utilizing principles such as combined blocked and randomized alternating practice and incorporating “over-learning” (with an alternating slip, no-slip, and re-slip paradigm), can yield significant retention of improvements in gait stability and lead to a reduction in the incidence of balance loss.84
In that study,84 22 safety-harnessed young adults were exposed to slips during walking. Slips were induced using a low-friction platform. After a block of 10 regular walking trials, subjects were exposed to a block of 8 repeated-slip trials (S1–8), a block of 3 nonslip trials (NS1–3), another block of 8 repeated slip trials (S9–16), and then 3 more nonslip trials (NS4–6). This was followed by a set of 15 randomly “mixed” slip and nonslip trials, of which 8 were slip trials (S17–24). In total, from the initial slip (S1) through the final slip (S24), subjects experienced 24 slip trials, which were mixed with 13 nonslip trials in keeping with motor learning principles. The initial slip was unexpected and induced during self-selected, “natural” walking. This initial training session was followed by 4 retest sessions, conducted at 1 week, 2 weeks, 1 month, and 4 months after the initial session. Each retest consisted of a single unannounced slip that followed a random number of 8 to 13 regular walking trials, designed to reduce the probability of predicting the occurrence of the slip trial. Subjects were aware only that a slip might or might not occur during any trial.
The results of that study84 indicated that, by the end of the training session, subjects substantially improved their pre– and post–slip-onset stability and weight support, with both proactive and reactive control, leading to nearly no balance loss toward the end of the session. Effects derived from the initial session of motor training were retained for 4 months among these young adults. Only 40% of these 22 young adults exhibited a backward balance loss upon their slip at 1 week, compared with 100% loss of balance upon the first slip (S1) (Fig. 5). Although more than 15% of the young adults fell at the initial session, none of them ever fell again at any of the retest sessions. Post-slip stability and weight support were greater at the first retest (at 1 week) than they were for the first slip of the initial session (S1), but lower than they were for S24 (explaining the higher incidence of balance loss at 1 week than at S24). That study revealed that the less-than-perfect retention resulted, to some extent, from individual variability rather than across-the-board generalized behavior. It is noteworthy that the subjects' performance did not improve during the retests. It appears that the motor training provided a sufficiently strong initial effect, such that subjects had no further benefit from the “add-on effect” of the single slip in each retest.
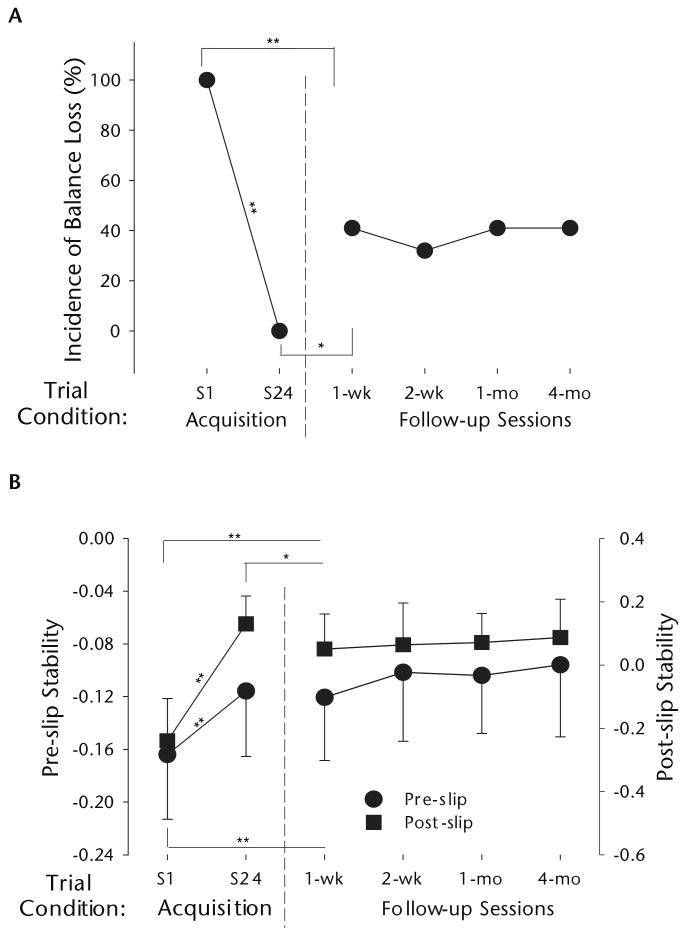
Figure 5.
(A) Changes in incidence of balance loss (percentage) and (B) in pre- and post-slip stability (means ± SD), on the first and last slip trials of the acquisition session (S1 and S24, respectively) and the slip trial in each of the follow-up sessions conducted at 1 week, 2 weeks, 1 month, and 4 months after the acquisition session. More positive values indicate greater stability. N=22 for all trials except for the follow-up session at 4 months (n=17). Significant differences with respect to the preceding trial included in the statistical analysis are indicated (*=P<.05, **=P<.001). Data illustrated in Figure 5 are from Bhatt TS, Wang E, Pai YC. Retention of adaptive control over varying intervals: prevention of slip-induced backward balance loss during gait. J Neurophysiol. 2006;95: 2913–2922. Figure 5B, modified and reprinted from the article by Bhatt et al,84 is used with permission of the American Physiological Society.
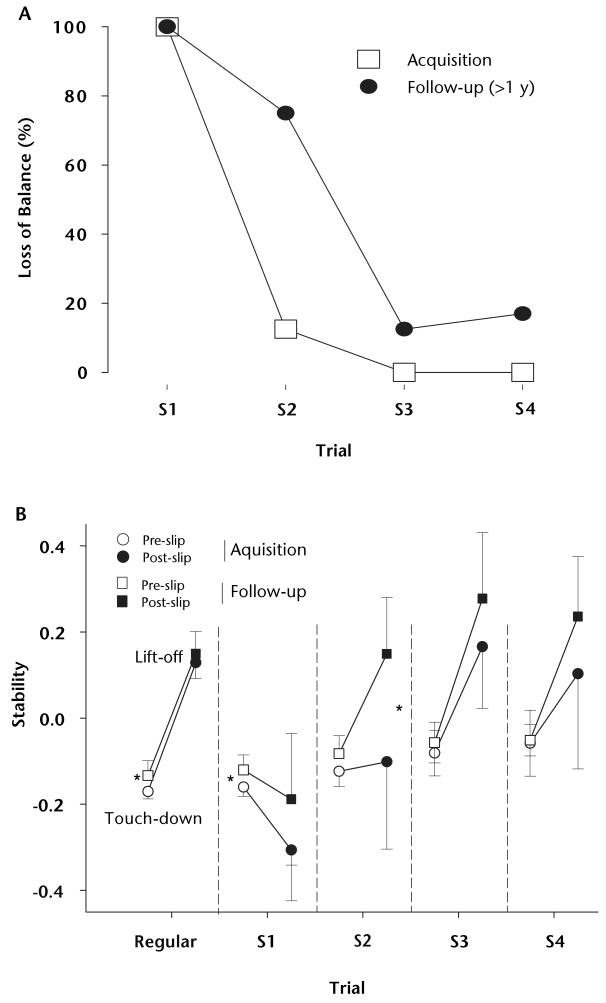
The retention of adaptive behavior may be conditioned by the penalties imposed upon an inappropriate response by the CNS and the increased potential of injury.85,86 Based on this, the authors postulated that a single acquisition session with a highly threatening environment would be sufficient to induce long-term retention of the acquired motor behavior. Alternatively, they postulated that with reduced-intensity training consisting of only 5 repeated slips and without multiple retests in between, which could potentially generate an “add-on” training effect, the adaptive motor skills could decay over a 12-month period. To test this, 8 young adults were exposed to a reduced training paradigm of only 5 repeated slips during walking.63 The same subjects returned for a retest using the same protocol only once 12 months later. The setup and the instructions were similar to those in the previously described study,84 with the major exceptions being the number of slip exposures and the frequency and interval of follow-ups.
The results indicated that following such reduced-intensity training, in the acquisition session, all subjects exhibited a backward balance loss on their first slip 12 months later63 (Fig. 6). They nonetheless were able to retain the acquired pre-slip stability with feedforward control during the follow-up session, but not the post-slip stability related to reactive control. A training effect also was evident in that the subjects demonstrated a faster reacquisition, with only one balance loss on the second slip of the follow-up session, as compared with 7 balance losses upon that slip 12 months earlier. Such rapid improvements were achieved by significantly greater improvements in post-slip stability than in pre-slip stability, which were achieved for the most part by reducing the BOS velocity. A single low-intensity training session could still prime87 the CNS to more rapidly adapt and update its internal representation of gait stability in response to the same perturbation during the same task as compared with the initial training session.63
Figure 6.
(A) Decrease in number of balance losses from first to fourth slip trials for the acquisition (open squares) and the 1-year follow-up (closed circles) sessions. (B) Gait stability (means ± 1 SD) at pre-slip touch-down of slipping limb (open symbols) and post-slip lift-off of contralateral limb (closed symbols) from the acquisition (circles) and the follow-up (squares) sessions for regular walking and first through fourth slip trials (S1 to S4). Significant differences in gait stability (for both pre- and post-slip events) between the 2 sessions for each trial are indicated (*=P<.05). Note that there was no significant difference in post-slip stability and slip outcome, with all subjects experiencing a backward balance loss on the first slip induced 1 year after the acquisition session. Also note the differential increase from pre- to post-slip stability for the follow-up session compared with the acquisition session on the second slip trial (S2), indicating the presence of a priming effect induced by the first follow-up slip. Data illustrated in Figure 6 are from: Bhatt TS, Pai YC. Long-term retention of gait stability improvements. J Neurophysiol. 2005;94:1971–1979. Figure 6B, modified and reprinted from the article by Bhatt and Pai,63 is used with permission of the American Physiological Society.
Bridging a Gap
This review has outlined the emergent evidence of applying perturbations mimicking real-life situations as a form of motor training, with long-term effects on postural stability and weight support for prevention of loss of balance and falls. With repeated-slip training, subjects adapt and update their internal representation of stability limits to prevent the incidence of backward balance loss under unpredictable context conditions with the CNS learning to anticipate and adopt movement options that satisfy constraints under both slip and nonslip contexts experienced. Furthermore, new findings charted a temporal course. With sufficient training intensity, adaptive changes in COM state stability can be acquired rapidly with a single intervention session, and the significant positive retention of the acquired adaptation effects in stability leads to a significant (60%) reduction of balance loss incidence for at least 4 months. This emerging paradigm, therefore, should be considered highly effective (reducing the frequency, and thus the cost, of intervention needed) and clearly beyond the existing standard of physical therapist practice and intervention for balance training.88
Most encouragingly, our results revealed that older adults can rapidly develop adaptive skills for fall prevention in a similar manner as young adults after their exposure to the repeated-slip paradigm. Although direct evidence on retention in older adults has not been presented, our preliminary findings suggest that a similar trend existed regardless of age. With a reduced intensity (eg, slip distance and number of repetitions), the novel training paradigm of repeated perturbation–no perturbation– perturbation also may provide a common intervention for training a broad spectrum of individuals ranging from active individuals at high risk for falling to frail elderly people with limited or impaired mobility. It may even have future application in the area of individuals with sensory, motor, or cognitive deficits or disabilities (eg, from stroke, traumatic brain injury, and so on). Development of such protocols, resulting in long-term changes within the locomotor-posture control system, would have a significant practical impact on health care professionals' ability to intervene against falls, allowing a significant reduction in the financial health care costs to society.
Supplementary Material
Acknowledgments
Dr Pai provided concept/idea/project design and fund procurement. Both authors provided data collection and analysis and project management. The authors thank Michael J Pavol, PhD, for providing data collection and for editorial assistance and Dr Feng Yang for generating a figure.
This work was supported by the Whitaker Foundation and by National Institutes of Health grants 1R01 and 2R01 AG16727 to Dr Pai.
Contributor Information
YC Pai, Department of Physical Therapy, University of Illinois at Chicago, 1919 W Taylor St, Room 426 (M/C 898), Chicago, IL 60612 (USA).
TS Bhatt, Department of Physical Therapy, University of Illinois at Chicago.
References
- 1.Rubenstein LZ, Josephson KR. The epidemiology of falls and syncope. Clin Geriatr Med. 2002;18:141–158. doi: 10.1016/s0749-0690(02)00002-2. [DOI] [PubMed] [Google Scholar]
- 2.Stelmach GE, Worringham CJ. Sensorimotor deficits related to postural stability: implications for falling in the elderly. Clin Geriatr Med. 1985;1:679–694. [PubMed] [Google Scholar]
- 3.Rubenstein LZ, Josephson KR, Robbins AS. Falls in the nursing home. Ann Intern Med. 1994;121:442–451. doi: 10.7326/0003-4819-121-6-199409150-00009. [DOI] [PubMed] [Google Scholar]
- 4.Morley JE. A fall is a major event in the life of an older person. J Gerontol A Biol Sci Med Sci. 2002;57:M492–M495. doi: 10.1093/gerona/57.8.m492. [DOI] [PubMed] [Google Scholar]
- 5.Baker SP, Harvey AH. Fall injuries in the elderly. Clin Geriatr Med. 1985;1:501–512. [PubMed] [Google Scholar]
- 6.Tinetti ME, Speechley M, Ginter SF. Risk factors for falls among elderly persons living in the community. N Engl J Med. 1988;319:1701–1707. doi: 10.1056/NEJM198812293192604. [DOI] [PubMed] [Google Scholar]
- 7.Nashner LM. Balance adjustments of humans perturbed while walking. J Neurophysiol. 1980;44:650–664. doi: 10.1152/jn.1980.44.4.650. [DOI] [PubMed] [Google Scholar]
- 8.Horak FB, Nashner LM. Central programming of postural movements: adaptation to altered support-surface configurations. J Neurophysiol. 1986;55:1369–1381. doi: 10.1152/jn.1986.55.6.1369. [DOI] [PubMed] [Google Scholar]
- 9.McIlroy WE, Maki BE. Adaptive changes to compensatory stepping responses. Gait Posture. 1995;3:43–50. [Google Scholar]
- 10.Pai YC, Iqbal K. Simulated movement termination for balance recovery: Can movement strategies be sought to maintain stability even in the presence of slipping or forced sliding? J Biomech. 1999;32:779–786. doi: 10.1016/s0021-9290(99)00074-3. [DOI] [PubMed] [Google Scholar]
- 11.Bhatt T, Wening JD, Pai YC. Adaptive control of gait stability in reducing slip-related backward loss of balance. Exp Brain Res. 2006;170:61–73. doi: 10.1007/s00221-005-0189-5. [DOI] [PubMed] [Google Scholar]
- 12.Pai YC, Patton JL. Center of mass velocity-position predictions for balance control. J Biomech. 1997;30:347–354. doi: 10.1016/s0021-9290(96)00165-0. [DOI] [PubMed] [Google Scholar]
- 13.Pai YC. Movement termination and stability in standing. Exerc Sport Sci Rev. 2003;31:19–25. doi: 10.1097/00003677-200301000-00005. [DOI] [PubMed] [Google Scholar]
- 14.Wu M, Ji L, Jin D, Pai YC. Minimal step length necessary for recovery of forward balance loss with a single step. J Biomech. 2007;40:1559–1566. doi: 10.1016/j.jbiomech.2006.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang F, Anderson FC, Pai YC. Predicted threshold against backward balance loss in gait. J Biomech. 2007;40:804–811. doi: 10.1016/j.jbiomech.2006.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shimba T. An estimation of center of gravity from force platform data. J Biomech. 1984;17:53–60. doi: 10.1016/0021-9290(84)90080-0. [DOI] [PubMed] [Google Scholar]
- 17.Era P, Hiekkinen E. Postural sway during standing and unexpected disturbance of balance in random samples of men of different ages. J Gerontol. 1985;40:287–295. doi: 10.1093/geronj/40.3.287. [DOI] [PubMed] [Google Scholar]
- 18.Raymakers JA, Samson MM, Verhaar HJ. The assessment of body sway and the choice of the stability parameter(s) Gait Posture. 2005;21:48–58. doi: 10.1016/j.gaitpost.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 19.Lord SR, Clark RD, Webster IW. Postural stability and associated physiological factors in a population of aged persons. J Gerontol A Biol Sci Med Sci. 1991;46:M69–M76. doi: 10.1093/geronj/46.3.m69. [DOI] [PubMed] [Google Scholar]
- 20.Maki BE, Holliday PJ, Topper AK. A prospective study of postural balance and risk of falling in an ambulatory and independent elderly population. J Gerontol A Biol Sci Med Sci. 1994;49:M72–M84. doi: 10.1093/geronj/49.2.m72. [DOI] [PubMed] [Google Scholar]
- 21.Robin DW, Hasan SS, Edeki T, et al. Increased baseline sway contributes to increased losses of balance in older people following triazolam. J Am Geriatr Soc. 1996;44:300–304. doi: 10.1111/j.1532-5415.1996.tb00919.x. [DOI] [PubMed] [Google Scholar]
- 22.Vittas D, Larsen TK, Jansen EC. Body sway in below-knee amputees. Prosthet Orthot Int. 1986;10:139–141. doi: 10.3109/03093648609164518. [DOI] [PubMed] [Google Scholar]
- 23.Williams HG, McClenaghan BA, Dickerson J. Spectral characteristics of postural control in elderly individuals. Arch Phys Med Rehabil. 1997;78:737–744. doi: 10.1016/s0003-9993(97)90082-4. [DOI] [PubMed] [Google Scholar]
- 24.Cohen H, Blatchly CA, Gombash LL. A study of the Clinical Test of Sensory Interaction and Balance. Phys Ther. 1993;73:346–351. 351–354. doi: 10.1093/ptj/73.6.346. discussion. [DOI] [PubMed] [Google Scholar]
- 25.Yoneda S, Tokumasu K. Frequency analysis of body sway in the upright posture: statistical study in cases of peripheral vestibular disease. Acta Otolaryngol. 1986;102:87–92. doi: 10.3109/00016488609108650. [DOI] [PubMed] [Google Scholar]
- 26.Crosbie WJ, Nimmo MA, Banks MA. Standing balance responses in two populations of elderly women: a pilot study. Arch Phys Med Rehabil. 1989;70:751–754. [PubMed] [Google Scholar]
- 27.Heitmann DK, Gossman MR, Shaddeau SA, Jackson JR. Balance performance and step width in noninstitutionalized, elderly, female fallers and nonfallers. Phys Ther. 1989;69:923–931. doi: 10.1093/ptj/69.11.923. [DOI] [PubMed] [Google Scholar]
- 28.Lichtenstein MJ, Burger MC, Shields SL, Siavi RG. Comparison of biomechanics platform measures of balance and videotaped measures of gait with a clinical mobility scale in elderly women. J Gerontol A Biol Sci Med Sci. 1990;45:M49–M54. doi: 10.1093/geronj/45.2.m49. [DOI] [PubMed] [Google Scholar]
- 29.Robbins AS, Rubenstein LZ, Josephson KR, et al. Predictors of falls among elderly people: results of two population-based studies. Arch Intern Med. 1989;149:1628–1633. [PubMed] [Google Scholar]
- 30.Sheldon JH. The effect of age on the control of sway. Gerontologia Clinica. 1963;5:129–138. doi: 10.1159/000244784. [DOI] [PubMed] [Google Scholar]
- 31.Overstall PW, Exton-Smith AN, Imms FJ, Johnson AL. Falls in the elderly related to postural imbalance. Br Med J. 1977;1:261–264. doi: 10.1136/bmj.1.6056.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Berg WP, Alessio HM, Mills EM, Tong C. Circumstances and consequences of falls in independent community-dwelling older adults. Age Ageing. 1997;26:261–268. doi: 10.1093/ageing/26.4.261. [DOI] [PubMed] [Google Scholar]
- 33.Topper AK, Maki BE, Holliday PJ. Are activity-based assessments of balance and gait in the elderly predictive of risk of falling and/or type of fall? J Am Geriatr Soc. 1993;41:479–487. doi: 10.1111/j.1532-5415.1993.tb01881.x. [DOI] [PubMed] [Google Scholar]
- 34.Woolley SM, Czaja SJ, Drury CG. An assessment of falls in elderly men and women. J Gerontol A Biol Sci Med Sci. 1997;52:M80–M87. doi: 10.1093/gerona/52a.2.m80. [DOI] [PubMed] [Google Scholar]
- 35.Borelli GA. De Motu Animalium. Vol. 1680. Berlin, Germany: Springer Verlag; p. 130. [Google Scholar]
- 36.Nashner LM. Adapting reflexes controlling the human posture. Exp Brain Res. 1976;26:59–72. doi: 10.1007/BF00235249. [DOI] [PubMed] [Google Scholar]
- 37.Maki BE, McIlroy WE. The role of limb movements in maintaining upright stance: the “change-in-support” strategy. Phys Ther. 1997;77:488–507. doi: 10.1093/ptj/77.5.488. [DOI] [PubMed] [Google Scholar]
- 38.Pai YC, Maki BE, Igbal K, et al. Thresholds for step initiation induced by support-surface translation: a dynamic center-of-mass model provides much better prediction than a static model. J Biomech. 2000;33:387–392. doi: 10.1016/s0021-9290(99)00199-2. [DOI] [PubMed] [Google Scholar]
- 39.Pavol MJ, Runtz EF, Pai YC. Diminished stepping responses lead to a fall following a novel slip induced during a sit-to-stand. Gait Posture. 2004;20:154–162. doi: 10.1016/j.gaitpost.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 40.Pavol MJ, Pai YC. Deficient limb support is a major contributor to age-differences in falling. J Biomech. 2007;40:1318–1325. doi: 10.1016/j.jbiomech.2006.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pai YC, Yang F, Wening JD, Paveol MJ. Mechanisms of limb collapse following a slip among young and older adults. J Biomech. 2006;39:2194–2204. doi: 10.1016/j.jbiomech.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 42.Pavol MJ, Runtz EF, Edwards BJ, Pai YC. Age influences the outcome of a slipping perturbation during initial but not repeated exposures. J Gerontol A Biol Sci Med Sci. 2002;57:M496–M503. doi: 10.1093/gerona/57.8.m496. [DOI] [PubMed] [Google Scholar]
- 43.Pavol MJ, Runtz EF, Pai YC. Young and older adults exhibit proactive and reactive adaptations to repeated slip exposure. J Gerontol A Biol Sci Med Sci. 2004;59:494–502. doi: 10.1093/gerona/59.5.m494. [DOI] [PubMed] [Google Scholar]
- 44.Rogers MW, Hedman LD, Johnson ME, et al. Triggering of protective stepping for the control of human balance: age and contextual dependence. Brain Res Cogn Brain Res. 2003;16:192–198. doi: 10.1016/s0926-6410(02)00273-2. [DOI] [PubMed] [Google Scholar]
- 45.McIlroy WE, Maki BE. Age-related changes in compensatory stepping in response to unpredictable perturbations. J Gerontol. 1996;51:M289–M296. doi: 10.1093/gerona/51a.6.m289. [DOI] [PubMed] [Google Scholar]
- 46.Bhatt TS, Wening JD, Pai YC. Influence of gait speed on stability: recovery from anterior slips and compensatory stepping. Gait Posture. 2005;21:146–156. doi: 10.1016/j.gaitpost.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 47.Pavol MJ, Owings TM, Foley KT, Grabiner MD. Mechanisms leading to a fall from an induced trip in healthy older adults. J Gerontol A Biol Sci Med Sci. 2001;56:M428–M437. doi: 10.1093/gerona/56.7.m428. [DOI] [PubMed] [Google Scholar]
- 48.Horak FB, Diener HC, Nashner LM. Influence of central set on human postural responses. J Neurophysiol. 1989;62:841–853. doi: 10.1152/jn.1989.62.4.841. [DOI] [PubMed] [Google Scholar]
- 49.Bretzner F, Drew T. Motor cortical modulation of cutaneous reflex responses in the hindlimb of the intact cat. J Neurophysiol. 2005;94:673–687. doi: 10.1152/jn.01247.2004. [DOI] [PubMed] [Google Scholar]
- 50.Hiebert GW, Gorassini MA, Jiang W, et al. Corrective responses to loss of ground support during walking, II: comparison of intact and chronic spinal cats. J Neurophysiol. 1994;71:611–622. doi: 10.1152/jn.1994.71.2.611. [DOI] [PubMed] [Google Scholar]
- 51.Tang PF, Woollacott MH. Inefficient postural responses to unexpected slips during walking in older adults. J Gerontol A Biol Sci Med Sci. 1998;53:M471–M80. doi: 10.1093/gerona/53a.6.m471. [DOI] [PubMed] [Google Scholar]
- 52.Cham R, Redfern MS. Lower extremity corrective reactions to slip events. J Biomech. 2001;34:1439–1445. doi: 10.1016/s0021-9290(01)00116-6. [DOI] [PubMed] [Google Scholar]
- 53.Eng JJ, Winter DA, Patla AE. Strategies for recovery from a trip in early and late swing during human walking. Exp Brain Res. 1994;102:339–349. doi: 10.1007/BF00227520. [DOI] [PubMed] [Google Scholar]
- 54.Marigold DS, Patla AE. Strategies for dynamic stability during locomotion on a slippery surface: effects of prior experience and knowledge. J Neurophysiol. 2002;88:339–353. doi: 10.1152/jn.00691.2001. [DOI] [PubMed] [Google Scholar]
- 55.Dietz V, Trippel M, Discher M, Horstmann GA. Compensation of human stance perturbations: selection of the appropriate electromyographic pattern. Neurosci Lett. 1991;126:71–74. doi: 10.1016/0304-3940(91)90374-3. [DOI] [PubMed] [Google Scholar]
- 56.Pai YC, Wening JD, Runtz EF, et al. Role of feedforward control of movement stability in reducing slip-related balance loss and falls among older adults. J Neurophysiol. 2003;90:755–762. doi: 10.1152/jn.01118.2002. [DOI] [PubMed] [Google Scholar]
- 57.Pavol MJ, Pai YC. Feedforward adaptations are used to compensate for a potential loss of balance. Exp Brain Res. 2002;145:528–538. doi: 10.1007/s00221-002-1143-4. [DOI] [PubMed] [Google Scholar]
- 58.Owing TM, Pavol MJ, Grabiner MD. Mechanisms of failed recovery following postural perturbations on a motorized treadmill mimic those associated with an actual forward trip. Clin Biomech. 2001;16:813–819. doi: 10.1016/s0268-0033(01)00077-8. [DOI] [PubMed] [Google Scholar]
- 59.Scheidt RA, Dingwell JB, Mussa-Ivaldi FA. Learning to move amid uncertainty. J Neurophysiol. 2001;86:971–985. doi: 10.1152/jn.2001.86.2.971. [DOI] [PubMed] [Google Scholar]
- 60.Atkeson CG. Learning arm kinematics and dynamics. Annu Rev Neurosci. 1989;12:157–183. doi: 10.1146/annurev.ne.12.030189.001105. [DOI] [PubMed] [Google Scholar]
- 61.Wolpert DM, Ghahramani Z. Computational principles of movement neuroscience. Nat Neurosci. 2000;3(suppl):1212–1217. doi: 10.1038/81497. [DOI] [PubMed] [Google Scholar]
- 62.Cham R, Redfern MS. Changes in gait when anticipating slippery floors. Gait Posture. 2002;15:159–171. doi: 10.1016/s0966-6362(01)00150-3. [DOI] [PubMed] [Google Scholar]
- 63.Bhatt TS, Pai YC. Long-term retention of gait stability improvements. J Neurophysiol. 2005;94:1971–1979. doi: 10.1152/jn.00266.2005. [DOI] [PubMed] [Google Scholar]
- 64.Vetter P, Wolpert DM. Context estimation for sensorimotor control. J Neurophysiol. 2000;84:1026–1034. doi: 10.1152/jn.2000.84.2.1026. [DOI] [PubMed] [Google Scholar]
- 65.Woollacott MJ, Shumway-Cook A, Nashner LM. Aging and posture control: changes in sensory organization and muscular coordination. Int J Aging Hum Dev. 1986;23:97–114. doi: 10.2190/VXN3-N3RT-54JB-X16X. [DOI] [PubMed] [Google Scholar]
- 66.Heiden TL, Sanderson DJ, Inglis JT, Siegmund GP. Adaptations to normal human gait on potentially slippery surfaces: the effects of awareness and prior slip experience. Gait Posture. 2006;24:237–246. doi: 10.1016/j.gaitpost.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 67.Reynolds RF, Bronstein AM. The broken escalator phenomenon: aftereffect of walking onto a moving platform. Exp Brain Res. 2003;151:301–308. doi: 10.1007/s00221-003-1444-2. [DOI] [PubMed] [Google Scholar]
- 68.Walker MP, Brakefield T, Hobson JA, Stickgold R. Dissociable stages of human memory consolidation and reconsolidation. Nature. 2003;425:616–620. 571–572. doi: 10.1038/nature01930. comment. [DOI] [PubMed] [Google Scholar]
- 69.Krakauer JW, Shadmehr R. Consolidation of motor memory. Trends Neurosci. 2006;29:58–64. doi: 10.1016/j.tins.2005.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kandel ER. The molecular biology of memory storage: a dialogue between genes and synapses. Science. 2001;294(5544):1030–1038. doi: 10.1126/science.1067020. [DOI] [PubMed] [Google Scholar]
- 71.Kawato M, Furukawa K, Suzuki R. A hierarchical neural-network model for control and learning of voluntary movement. Biol Cybern. 1987;57:169–185. doi: 10.1007/BF00364149. [DOI] [PubMed] [Google Scholar]
- 72.Forssberg H, Grillner S, Rossingnol S. Phase dependent reflex reversal during walking in chronic spinal cats. Brain Res. 1975;85:103–107. doi: 10.1016/0006-8993(75)91013-6. [DOI] [PubMed] [Google Scholar]
- 73.Drew T, Jiang W, Widajewicz W. Contributions of the motor cortex to the control of the hindlimbs during locomotion in the cat. Brain Res Brain Res Rev. 2002;40(1–3):178–191. doi: 10.1016/s0165-0173(02)00200-x. [DOI] [PubMed] [Google Scholar]
- 74.Shadmehr R, Holcomb HH. Neural correlates of motor memory consolidation. Science. 1997;277(5327):821–825. doi: 10.1126/science.277.5327.821. [DOI] [PubMed] [Google Scholar]
- 75.Anstis S. Aftereffects from jogging. Exp Brain Res. 1995;103:476–478. doi: 10.1007/BF00241507. [DOI] [PubMed] [Google Scholar]
- 76.Earhart GM, Jones GM, Horak FB, et al. Podokinetic after-rotation following unilateral and bilateral podokinetic stimulation. J Neurophysiol. 2002;87:1138–1141. doi: 10.1152/jn.00464.2001. [DOI] [PubMed] [Google Scholar]
- 77.Shadmehr R, Mussa-Ivaldi FA. Adaptive representation of dynamics during learning of a motor task. J Neurosci. 1994;14:3208–3224. doi: 10.1523/JNEUROSCI.14-05-03208.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cunningham HA, Welch RB. Multiple concurrent visual-motor mappings: implications for models of adaptation. J Exp Psychol Hum Percept Perf. 1994;20:987–999. doi: 10.1037//0096-1523.20.5.987. [DOI] [PubMed] [Google Scholar]
- 79.van Hedel HJ, Biedermann M, Erni T, Dietz V. Obstacle avoidance during human walking: transfer of motor skill from one leg to the other. J Physiol. 2002;543(Pt 2):709–717. doi: 10.1113/jphysiol.2002.018473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tjernström F, Fransson PA, Hafström A, Magnusson M. Adaptation of postural control to perturbations: a process that initiates long-term motor memory. Gait Posture. 2002;15:75–82. doi: 10.1016/s0966-6362(01)00175-8. [DOI] [PubMed] [Google Scholar]
- 81.Schwabe A, Drepper J, Maschke M, et al. The role of the human cerebellum in short- and long-term habituation of postural responses. Gait Posture. 2004;19:16–23. doi: 10.1016/s0966-6362(03)00006-7. [DOI] [PubMed] [Google Scholar]
- 82.Del Rey P. Training and contextual interference effects on memory and transfer. Res Q Exerc Sport. 1989;60:342–347. doi: 10.1080/02701367.1989.10607461. [DOI] [PubMed] [Google Scholar]
- 83.Markowitsch HJ, Kessler J, Streicher M. Consequences of serial cortical, hippocampal, and thalamic lesions and of different lengths of overtraining on the acquisition and retention of learning tasks. Behav Neurosci. 1985;99:233–256. [PubMed] [Google Scholar]
- 84.Bhatt TS, Wang E, Pai YC. Retention of adaptive control over varying intervals: prevention of slip-induced backward balance loss during gait. J Neurophysiol. 2006;95:2913–2922. doi: 10.1152/jn.01211.2005. [DOI] [PubMed] [Google Scholar]
- 85.Adkin AL, Frank JS, Carpenter MG, Peysar GW. Postural control is scaled to level of postural threat. Gait Posture. 2000;12:87–93. doi: 10.1016/s0966-6362(00)00057-6. [DOI] [PubMed] [Google Scholar]
- 86.Carpenter MG, Frank JS, Silcher CP, Peysar GW. The influence of postural threat on the control of upright stance. Exp Brain Res. 2001;138:210–218. doi: 10.1007/s002210100681. [DOI] [PubMed] [Google Scholar]
- 87.Schacter DL, Dobbins IG, Schnyer DM. Specificity of priming: a cognitive neuroscience perspective. Nat Rev Neurosci. 2004;5:853–862. doi: 10.1038/nrn1534. [DOI] [PubMed] [Google Scholar]
- 88.Phys Ther. 2nd. Vol. 81. 2001. Guide to Physical Therapist Practice; pp. 9–746. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.