Abstract
Purpose: The goal of this study was to estimate organ doses for chest CT examinations using volume computed tomography dose index (CTDIvol) data as well as accounting for patient weight.
Methods: A CT dosimetry spreadsheet (ImPACT CT patient dosimetry calculator) was used to compute organ doses for a 70 kg patient undergoing chest CT examinations, as well as volume computed tomography dose index (CTDIvol) in a body CT dosimetry phantom at the same CT technique factors. Ratios of organ dose to CTDIvol (forgan) were generated as a function of anatomical location in the chest for the breasts, lungs, stomach, red bone marrow, liver, thyroid, liver, and thymus. Values of forgan were obtained for x-ray tube voltages ranging from 80 to 140 kV for 1, 4, 16, and 64 slice CT scanners from two vendors. For constant CT techniques, we computed ratios of dose in water phantoms of differing diameter. By modeling patients of different weights as equivalent water cylinders of different diameters, we generated factors that permit the estimation of the organ doses in patients weighing between 50 and 100 kg who undergo chest CT examinations relative to the corresponding organ doses received by a 70 kg adult.
Results: For a 32 cm long CT scan encompassing the complete lungs, values of forgan ranged from 1.7 (thymus) to 0.3 (stomach). Organs that are directly in the x-ray beam, and are completely irradiated, generally had forgan values well above 1 (i.e., breast, lung, heart, and thymus). Organs that are not completely irradiated in a total chest CT scan generally had forgan values that are less than 1 (e.g., red bone marrow, liver, and stomach). Increasing the x-ray tube voltage from 80 to 140 kV resulted in modest increases in forgan for the heart (9%) and thymus (8%), but resulted in larger increases for the breast (19%) and red bone marrow (21%). Adult patient chests have been modeled by water cylinders with diameters between ∼20 cm for a 50 kg patient and ∼28 cm for a 100 kg patient. At constant x-ray techniques, a 50 kg patient is expected to have doses that are ∼18% higher than those in a 70 kg adult, whereas a 100 kg patient will have doses that are ∼18% lower.
Conclusions: We describe a practical method to use CTDI data provided by commercial CT scanners to obtain patient and examination specific estimates of organ dose for chest CT examinations.
Keywords: chest CT, organ dose, CTDI, patient weight
INTRODUCTION
The computed tomography dose index (CTDI) is a metric that is widely used in CT. All commercial scanners provide CTDIvol, expressed in terms of air kerma (mGy), which is measured in a single rotation of the x-ray tube and generally depends on the choice of x-ray techniques (kV and mAs) selected to perform any examination.1 CTDIvol quantifies the intensity of the radiation beam being produced by the CT scanner, whose value is independent of the scan length. Operators can adjust techniques to modify CTDIvol values so that the radiation intensity used for any examination is appropriate for the diagnostic task at hand and has been appropriately adjusted to take into account the size of the patient being scanned.2
CTDIvol doses for chest imaging are measured in 32 cm diameter acrylic phantoms. Acrylic has a high density (1.19 g∕cm3), and these phantoms are larger than average-sized adult patients. Therefore, the CTDIvol reported on CT scanners will generally be lower than the absorbed radiation doses delivered to any organ in an average-sized patient undergoing body CT scanning.3 Calculation of a more realistic value of the radiation dose requires accounting for differences between the patient diameter and that of the acrylic phantom. Furthermore, organ doses will also be influenced by technique factors (e.g., x-ray tube voltage), as well as on the scanned region and the total scan length.
Currently, there are no convenient methods available for obtaining organ doses directly from CTDIvol dose metric provided on most commercial CT scanners. In this paper, we propose a method that enables CTDIvol to be converted into estimates of patient organ dose. Our approach takes into account the x-ray tube voltage, the scanned area, and patient size. Organ doses obtained in this manner may be used to estimate patient specific risks of carcinogenesis.
METHOD
CTDI
Commercial scanners provide body dosimetry data that are obtained in 32 cm diameter acrylic cylinders.4 Dose measurements for selected techniques (kV∕mAs) are obtained for a single rotation of the x-ray tube using a 100 mm long pencil shaped ionization chamber calibrated in terms of air kerma (mGy). The measured values are known as the CTDI, which can be obtained at the periphery (CTDIp) and center of the phantom (CTDIc). The weighted CTDIw is obtained using5
| (1) |
Table 1 shows values of normalized CTDIw (mGy∕100 mAs), measured at the maximum available beam width, for the body CT dosimetry phantom for CT scanners from two vendors obtained using the ImPACT dosimetry calculator. These CT scanners range from single slice systems to 64 slice systems. The ImPACT dosimetry calculator allows males and females to be specified, but this only affects the organs used for gonad dose calculation which are negligible for chest CT examinations.
Table 1.
Values of normalized CTDIw at the widest available beam width, for eight commercial CT scanners. N∕A: Not available.
| Vendor | Scanner model | CTDIw (mGy∕100 mAs) | |||
|---|---|---|---|---|---|
| 80 kV | 100 kV | 120 kV | 140 kV | ||
| GE | HiSpeed CT∕i | 1.6 | 3.6 | 5.2 | 7.6 |
| LightSpeed Plus | 2.9 | 5.6 | 9.0 | 12.9 | |
| LightSpeed 16 | 2.7 | 5.8 | 9.1 | 13.7 | |
| VCT | 3.4 | 6.2 | 9.5 | 13.3 | |
| Siemens | Somaton Plus 4 | 2.3 | N∕A | 7.9 | 11.4 |
| Sensation 4 | 2.5 | N∕A | 7.7 | 10.9 | |
| Sensation 16 | 2.4 | 4.8 | 7.6 | 10.9 | |
| Sensation 64 | 1.8 | 4.1 | 7.0 | 11.4 | |
| Average ±σ | 2.5±0.6 | 5.0±1.0 | 7.9±1.4 | 11.5±1.9 | |
All current commercial CT scanners provide CTDIvol. The CTDIvol is the weighted CTDIw divided by the pitch, where pitch is the table increment distance per x-ray tube rotation divided by the nominal beam width at the scanner isocenter.1 CTDIvol provides an estimate of the average dose within the 32 cm diameter acrylic phantom in helical scanning.
Calculation of organ doses
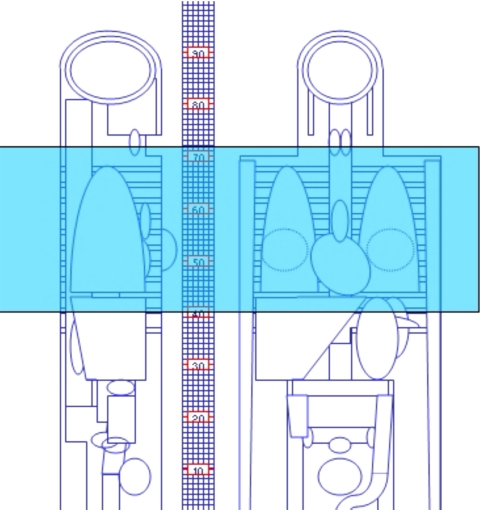
To compute values of the organ dose to adult patients undergoing CT examinations, we used version 0.99x (20∕01∕06) of the ImPACT CT patient dosimetry calculator.6 This spreadsheet makes use of the NRPB Monte Carlo dose data7, 8 for normalized organ dose data for a mathematical phantom modeling a normal sized (70 kg) adult patient. Figure 1 shows the mathematical phantom used in this dosimetry software and Table 2 lists important anatomical markers relevant to chest CT scanning. The anatomical region irradiated for a representative chest CT examination is shown as the shaded region in Fig. 1 that extends for 32 cm (i.e., from z=40 to z=72).
Figure 1.
Mathematical phantom used to compute effective doses. The shaded region depicts a 32 cm long chest CT examination that ranges from z=40 up to z=72 that was used to compute forgan values.
Table 2.
Anatomical markers in the mathematical anthropomorphic phantom (Fig. 1).
| z location in Fig. 1 (cm) | Anatomical descriptor |
|---|---|
| 68 | Apex of the lungs |
| 64 | Knob of aortic archa |
| 60 | Trachea bifurcationa |
| 43 | Apex of the heart |
Estimated from image of schematic phantom.
The ImPACT calculator provides values of organ dose as well as CTDIvol for a given scan location and defined scan length. For a scan starting at z1, and ending at z2, we define the fractional organ dose, forgan, by
| (2) |
Values of forgan were obtained with each starting atz1=40 cm in the anthropomorphic phantom and with a scan length that increased in 4 cm increments in the cranial direction. For all scans a pitch ratio of 1 was chosen. Values of forgan were obtained for organs of interest in medical radiation dosimetry for chest CT scanning because they are recognized as being radiosensitive9 [i.e., heart, lung, breast, thymus, stomach, red bone marrow (rbm), liver, and the thyroid gland]. For each organ of interest, values of forgan were obtained at x-ray tube voltages ranging from 80 to 140 kV for each of the eight CT scanners listed in Table 1, and used to obtain the mean value, as well as the corresponding standard deviation.
Patient weight
Consider a water cylinder with radius r mm at a CT scanner isocenter irradiated during one 360° rotation of the x-ray tube. The mean section dose Dm may be defined as the total energy deposited in the water cylinder divided by the directly irradiated mass. Dm approximates the average dose in the directly irradiated region of the water cylinder for contiguous scanning in axial scanning mode, or using a pitch ratio of 1 in helical scanning. Published values of Dm as a function of water cylinder size for a commercial CT scanner were used to obtain the ratios R generally defined as10
| (3) |
where Dm (diameter x) relates to the mean section dose in a water cylinder with a diameter x cm. Values of R quantify relative changes in dose to the water cylinder as the size of the water cylinder is varied.
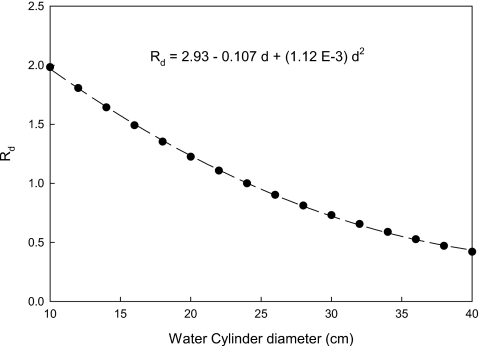
The chest of a 70 kg adult may be modeled as a 24 cm diameter uniform cylinder of water,11 and at diagnostic photon energies, energy deposition in the water cylinder and a patient is expected to be similar at the same CT techniques. We computed values of Rd using Eq. 3 for cylinder diameters ranging from 10 to 40 cm, with the denominator kept a constant 24 cm. Rd will increase for cylinders that are smaller than 24 cm, and vice versa.
The water cylinder diameter d cm that models the chest of an adult patient weighing W kg is given by the formula11
| (4) |
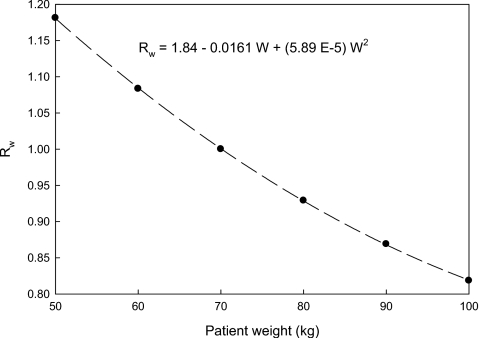
which may be applied to adults whose weights range between 50 and 100 kg. We computed values of Rw, using Eq. 3 where the cylinder diameter in the numerator was obtained using Eq. 4, and the cylinder diameter in the denominator was 24 cm. Rw will increase for patients who weigh less than 70 kg, and vice versa.
RESULTS
Organ doses
Table 3 shows average values of forgan as a function of scan length ranging from 4 to 32 cm at 120 kV. All data were obtained for scans starting at the bottom of the lung where z=40 cm. For a 32 cm long CT scan encompassing the complete lungs, values of forgan ranged from 1.7 (thymus) to 0.3 (stomach). Organs that are directly in the x-ray beam, and are completely irradiated, generally had forgan values for a complete chest CT scan that were well above 1 (i.e., breast, lung, heart, and thymus). Organs that are not completely irradiated in a total chest CT scan generally had forgan values that were less than 1 (e.g., red bone marrow, liver, and stomach).
Table 3.
Average value of forgan for scan lengths ranging from 4 to 32 cm performed at 120 kV.
| Scan from z=40 cm to z≕ | fbreast | flung | fthyroid | fthymus | fheart | frbm | fliver | fstomach |
|---|---|---|---|---|---|---|---|---|
| 44 | 0.03 | 0.12 | 0.0 | 0.02 | 0.14 | 0.05 | 0.31 | 0.18 |
| 48 | 0.07 | 0.42 | 0.01 | 0.07 | 0.63 | 0.11 | 0.39 | 0.24 |
| 52 | 0.62 | 0.72 | 0.01 | 0.16 | 1.08 | 0.16 | 0.44 | 0.27 |
| 56 | 1.16 | 0.98 | 0.02 | 0.61 | 1.29 | 0.23 | 0.46 | 0.28 |
| 60 | 1.21 | 1.23 | 0.04 | 1.39 | 1.37 | 0.28 | 0.47 | 0.29 |
| 64 | 1.23 | 1.39 | 0.09 | 1.58 | 1.41 | 0.35 | 0.47 | 0.29 |
| 68 | 1.25 | 1.47 | 0.20 | 1.64 | 1.43 | 0.41 | 0.48 | 0.30 |
| 72 | 1.25 | 1.50 | 1.07 | 1.67 | 1.43 | 0.44 | 0.48 | 0.30 |
Table 4 shows how values of forgan for complete chest CT scans vary with x-ray tube voltage. Increasing the x-ray tube voltage from 80 to 140 kV resulted in modest increases in forgan for the heart (6%) and thymus (4%), but resulted in larger increases for the breast (23%) and red bone marrow (15%).
Table 4.
Average value of forgan with 32 cm scan length as a function of x-ray tube voltage.
| Tube voltage (kV) | fbreast | flung | fthyroid | fthymus | fheart | frbm | fliver | fstomach |
|---|---|---|---|---|---|---|---|---|
| 80 | 1.11 | 1.39 | 1.14 | 1.66 | 1.42 | 0.41 | 0.45 | 0.27 |
| 120 | 1.25 | 1.50 | 1.07 | 1.67 | 1.43 | 0.44 | 0.48 | 0.30 |
| 140 | 1.36 | 1.59 | 1.10 | 1.73 | 1.50 | 0.47 | 0.51 | 0.32 |
Average coefficients of variation for forgan values obtained for the eight CT commercial scanners are summarized in Table 5. For a whole lung scan performed at 120 kV, the typical uncertainty in the organ dose derived from CTDIvol may be taken to be ∼5%, which is the average coefficient of variation for the eight organ values listed in Table 5. The average uncertainty is highest at 80 kV (∼11%). It is also notable that there are larger uncertainties for the breast (average of ∼13%) and thyroid gland (average of ∼10%) than all other organs listed in Table 5.
Table 5.
Values of the coefficient of variation (%) in forgan values obtained for eight CT scanners (Table 1) performing a full 32 cm long CT scan of the chest, at three x-ray tube voltages.
| Tube voltage (kV) | Heart | Lung | Breast | Thymus | Stomach | RBM | Liver | Thyroid |
|---|---|---|---|---|---|---|---|---|
| 80 | 10 | 8.5 | 17 | 10 | 7.2 | 5.9 | 7.6 | 12 |
| 120 | 5.4 | 3.9 | 10 | 5.6 | 4.0 | 4.0 | 3.8 | 6.4 |
| 140 | 5.6 | 6.1 | 12 | 7.0 | 5.2 | 6.0 | 5.6 | 9.7 |
R values
Figure 2 shows values of Rd for cylinders ranging from 10 to 40 cm at generated an x-ray tube voltages of 120 kV. The dashed line is a least-squares fit to the experimental data and the equation provided in this figure for Rd permits the dose in a water phantom to be quantitatively determined relative to the corresponding dose in a 24 cm diameter phantom. The chests of most adult patients are likely be modeled by water cylinders with diameters between ∼20 and ∼28 cm.12 Over this range of water phantom diameters, changing the x-ray tube voltage from 80 to 140 kV changes Rd by less than 5%.
Figure 2.
Plot of Rd as a function of water cylinder diameter, which shows how the water phantom dose varies with water cylinder diameter relative to a 24 cm diameter (where R=1). The dashed line is a least-squares fit to the data (r2>0.99) whose coefficients are given in the equation relating Rd to d.
Figure 3 shows how the ratio Rw varies with patient weight between 50 and 100 kg. The dashed line shown in Fig. 3 is a least-squares fit to the experimental data, and the equation given in Fig. 3 permits chest organ doses in a patient of any weight to be estimated relative to the corresponding dose in a 70 kg patient. Chest CT examination performed at a constant x-ray technique would increase organ doses in 50 kg patients by ∼18% and reduce organ doses by ∼18% in 100 kg patients, relative to those of a 70 kg adult.
Figure 3.
Plot of Rw as a function of adult patient weight (w), which shows how the organ doses vary with patient weight relative to a 70 kg patient (where R=1). Rw was generated by modeling patients as equivalent cylinders of water using Eq. 4. The dashed line is a least-squares fit to the data (r2>0.99) whose coefficients are given in the equation relating Rw to W.
DISCUSSION
The ImPACT spreadsheet utilizes the NRPB SR250 Monte Carlo dosimetry data for 23 scanners from the early 1990s. To accommodate modern scanners, ImPACT “matches” dosimetric characteristics of each new scanner to one of the available sets of MC generated data. This matching process uses a combination of ratios of phantom CTDI to the corresponding “in air” CTDI, and which are known as ImPACT Factors.6 For each new CT scanner that is introduced into the marketplace, it is possible to obtain ImPACT factors from measured CTDI values, and thereby identify a MC data set that best matches this new CT scanner. Consequently, forgan listed in Tables 3, 4 are only approximations. It is notable that none of the 23 original data sets were obtained at an x-ray tube voltage of 80 kV, which is the most likely explanation for the higher values of the coefficient of variation obtained at 80 kV. At 120 kV, the average differences in forgan (Table 5) were ∼5%, which is likely to result in satisfactory organ dose estimates for most CT applications.
The ImPACT dosimetry calculator scales all doses inversely proportional to the pitch, and reducing the pitch from 1.0 to 0.5 doubles organ dose. However, CTDIvol is scaled in exactly the same manner as organ dose, so values of forgan obtained from the ImPACT dosimetry calculator are independent of CT pitch. Values of forgan are also independent of selected nominal beam collimation in the ImPACT spreadsheet, since changes in collimation also result in the same modifications to both CTDIvol and organ dose. Data in Table 1 show that CTDIw (mGy∕mAs) can differ by a factor of 2 between different types of scanner. At the same x-ray beam quality, doses to patients and CT dosimetry phantoms will both be directly proportional to the x-ray beam intensity. As a result, the ratio of organ dose to CTDIvol should be relatively independent of factors such as the x-ray tube characteristics, x-ray beam filtration, and specific characteristics of any beam shaping filter. These expectations are supported by modest intervendor and interscanner differences found in this study, and in similar studies in the scientific literature.13
Data shown in Table 3 clearly indicate that doses to patients can be markedly higher than the CTDIvol data that are measured in 32 cm cylindrical acrylic phantoms (i.e., forgan>1.0). Organs that are directly (and wholly) irradiated in a chest CT scan have doses that on average are ∼50% greater than CTDIvol. These findings are not unexpected given that the chest of a 70 kg adult is modeled as a water cylinder with a diameter of 24 cm whereas the body phantom has an equivalent water cylinder diameter of 35 cm because of the increased density of acrylic (1.19 g∕cm3). Accordingly, CTDIvol should never be used as a surrogate for any patient dose. CTDIvol is useful as quantifying the intensity of the CT x-ray beam that is being used to perform any given CT examination.2 When organ doses are required, they should be obtained directly using a CT dosimetry software package6, 14, 15 or by the use of forgan factors of the type provided in Table 3.
The data in Table 3 can be used to estimate the organ dose for any scan length, and in any selected anatomical region of the chest. This can be illustrated by estimating the heart dose when scanning from z=44 to z=52. At 120 kV, a scan from z=40 to z=44 would result in a heart dose of 0.14×CTDIvol, and a scan from z=40 to z=52 would result in a dose of 1.08×CTDIvol. The difference of these two doses (i.e., 0.94 CTDIvol) is the contribution to the heart dose from the scan performed between z=44 and z=52. Data presented in Table 3 can also be used to estimate organ dose reductions achievable by reducing the scan length.
When longitudinal mA modulation is employed,16 accurate organ doses would require dividing the chest CT scan into a series of shorter scan where each part has its individual average mAs value, together with a corresponding value of CTDIvol. Our computations also assume a constant x-ray tube output as the x-ray tube rotates around the patient and neglect the effects of automatic exposure control (AEC) systems that are available on most current commercial CT scanners. Use of rotational AEC systems in chest imaging is likely to reduce the values of forgan, but the magnitude of any such changes will likely be less than ∼11%.13
The choice of x-ray tube voltage has a major effect on the amount of radiation used to perform any CT examinations. Data in Table 1 show that at constant x-ray technique (mAs), increasing the x-ray tube voltage from 80 to 140 kV will increase the dose (CTDIw) by a factor of 4.6. For chest CT studies performed with the administration of iodinated contrast material, reducing the x-ray tube voltage would also be expected to markedly increase image contrast, as well as the contrast to noise ratio, of vessels containing iodine.17, 18 For larger patients typically encountered in chest CT, however, the x-ray tube voltage may need to be increased to ensure that there is adequate patient penetration by the x-ray beam.19 Accordingly, it is important that methods for dosimetry in CT imaging are equipped to deal with the complete range of x-ray tube voltages (i.e., 80 to 140 kV) that current scanners offer.
The data shown in Fig. 2 permit relative changes in dose to be estimated for any two water equivalent phantom sizes. These data could be used by researchers who wish to develop alternative methods for modeling any sized patient or body region as an “equivalent water cylinder.” The curve in Fig. 2 shows how changing the water phantom diameter modifies the corresponding average water phantom dose and is independent of the normalizing value of water cylinder diameter that we used to compute Rd (i.e., 24 cm). Modeling patients as the mass equivalent cylinders of water is reasonable for CT dosimetry purposes, where the use of high x-ray tube voltages and heavy filtrations results in x-ray beams that ensure that Compton interactions dominate. Although the accuracy of the approaches offered by the data shown in Figs. 23 have not been investigated, it is clear that use of data in Figs. 23 should result in improved organ dose estimates over current practice that fails to explicitly account for patient size (weight). The recent development of voxelized patient models, coupled with Monte Carlo dosimetry calculations for commercial CT scanners, is a means whereby the patient size modifications proposed in this paper could be empirically tested.20
Hitherto, it has been difficult to obtain dose data for individual patients in chest CT imaging because such doses depend on patient characteristic (e.g., weight) and the CT scan factors (e.g., kV, scan length, and scan region). In this paper, we propose a robust method that permits CTDIvol and forgan to be used to determine doses to the most radiosensitive organs and tissues in chest CT. Our methodology explicitly takes into account critical factors that impact patient doses including x-ray tube voltage, scan location, and scan length, as well as the size of the patient undergoing the chest CT examination. Organ dose obtained in this manner can be combined with age and sex dependent risk factors that have been recently published in the BEIR VII report21 to estimate the cancer risks associated with adult chest CT examinations. Table 6 shows lung cancer risk for males and females as a function of patient size and age, that were obtained for a chest CT examination performed at a constant CTDIvol of 15 mGy, a DLP of 480 mGy cm, and where the lung dose to a 70 kg adult is estimated to be 24 mGy. Generating radiation risks as depicted in Table 6 is of interest too because this helps determine whether a given radiological examination is indicated by generating a net patient benefit. Furthermore, understanding radiation risks also helps focus attention on the design of imaging protocols that keep doses as low as reasonably achievable.22, 23
Table 6.
Estimated risk of lung cancer incidence for males (M) and females (F) for a routine chest CT scan (CTDIvol 15 mGy; DLP 480 mGy) obtained using the lung cancer risk estimates provided in BIER VII and expressed per 10 000 patient examinations.
| Patient age (years) | Patient weight (kg) | |||||
|---|---|---|---|---|---|---|
| 50 | 70 | 100 | ||||
| M | F | M | F | M | F | |
| 20 | 4.2 | 9.8 | 3.6 | 8.3 | 3.0 | 6.8 |
| 40 | 3.0 | 6.8 | 2.5 | 5.8 | 2.1 | 4.8 |
| 60 | 2.5 | 5.7 | 2.1 | 4.8 | 1.7 | 3.9 |
ACKNOWLEDGMENTS
The research was supported, in part, by the NIH (Grant No. R01 EB000460). We would like to thank Paul Shrimpton and Sue Edyvean for permission to reproduce Fig. 1.
References
- International Electro-chemical Commission (IEC), International standard of IEC 60601–2-44 Ed2 Amendment 1: Medical electrical equipment—Part 2–44: Particular requirements for the safety of x-ray equipment for computed tomography, 2003.
- Brenner D. J. and McCollough C. H., “It is time to retire the computed tomography dose index (CTDI) for CT quality assurance and dose optimization,” Med. Phys. 33, 1189–1190 (2006). 10.1118/1.2173933 [DOI] [PubMed] [Google Scholar]
- Nickoloff E. L., Dutta A. K., and Lu Z. F., “Influence of phantom diameter, kVp and scan mode upon computed tomography dose index,” Med. Phys. 30, 395–402 (2003). 10.1118/1.1543149 [DOI] [PubMed] [Google Scholar]
- McNitt-Gray M. F., “AAPM/RSNA Physics Tutorial for Residents: Topics in CT. Radiation dose in CT,” Radiographics 22, 1541–1553 (2002). 10.1148/rg.226025128 [DOI] [PubMed] [Google Scholar]
- Leitz W., Axelsson B., and Szendro G., “Computed tomography dose assessment - a practical approach,” Radiat. Prot. Dosim. 57, 377–380 (1995). [Google Scholar]
- ImPACT, http://www.impactscan.org/ctdosimetry.htm.
- Shrimpton P. C., Jones D. G., Hillier M. C., Wall B. F., Le Heron J. C., and Faulkner K., “Survey of CT practice in the UK Part 2: Dosimetric aspects,” NRPB Report No. 249, 1991.
- Jones D. G. and Shrimpton P. C., “Normalized organ doses for x-ray computed tomography calculated using Monte Carlo techniques,” NRPB Report No. SR 250, 1993.
- International Commission on Radiological Protection Publication 103, The 2007 Recommendations of the ICRP,” Annals of the ICRP, 2007, Vol. 37. [DOI] [PubMed]
- Huda W., Atherton J. V., Ware D. E., and Cumming W. A., “An approach for the estimation of effective radiation dose at CT in pediatric patients,” Radiology 203, 417–422 (1997). [DOI] [PubMed] [Google Scholar]
- Huda W., Scalzetti E. M., and Roskopf M., “Effective doses to patients undergoing thoracic computed tomography examinations,” Med. Phys. 27, 838–844 (2000). 10.1118/1.598949 [DOI] [PubMed] [Google Scholar]
- International Commission on Radiological Protection Publication 89: Basic anatomical and physiological data for use in radiological protection: Reference values, Annals of the ICRP, 2002, Vol. 32. [PubMed]
- Huda W., Ogden K. M., and Khorasani M., “Converting dose length product to effective dose at CT,” Radiology 248(3), 995–1003 (2008). 10.1148/radiol.2483071964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalender W. A., Schmidt B., Zankl M., and Schmidt M. A., “PC program for estimating organ dose and effective dose values in computed tomography,” Eur. Radiol. 9, 555–562 (1999). 10.1007/s003300050709 [DOI] [PubMed] [Google Scholar]
- Stamm G. and Nagel H. D., “CT-expo–a novel program for dose evaluation in CT,” Fortschr Geb Rontgenstrahlen Nuklearmed Erganzungsbd 174, 1570–1576 (2002). 10.1055/s-2002-35937 [DOI] [PubMed] [Google Scholar]
- Althen J. N., “Automatic tube-current modulation in CT–a comparison between different solutions,” Radiat. Prot. Dosim. 114, 308–312 (2005). 10.1093/rpd/nch501 [DOI] [PubMed] [Google Scholar]
- Wintermark M. et al. , “Using 80 kVp versus 120 kVp in perfusion CT measurement of regional cerebral blood flow,” AJNR Am. J. Neuroradiol. 21, 1881–1884 (2000). [PMC free article] [PubMed] [Google Scholar]
- Huda W., Lieberman K. A., Chang J., and Roskopf M. L., “Patient size and x-ray technique factors in head computed tomography examinations. II. Image quality,” Med. Phys. 31, 595–601 (2004). 10.1118/1.1646233 [DOI] [PubMed] [Google Scholar]
- Ogden K., Huda W., Scalzetti E. M., and Roskopf M. L., “Patient size and x-ray transmission in body CT,” Health Phys. 86, 397–405 (2004). 10.1097/00004032-200404000-00009 [DOI] [PubMed] [Google Scholar]
- Angel E., Yaghmai N., Matilda Jude C., DeMarco J. J., Cagnon C. H., Goldin J. G., Primak A., Stevens D. M., Cody D. D., McCollough C. H., and McNitt-Gray M. F., “Monte Carlo simulations to assess the effects of tube current modulation on breast dose for multidetector CT,” Phys. Med. Biol. 54, 497–512 (2009). 10.1088/0031-9155/54/3/003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- “Health effects of exposure to low levels of ionizing radiations: Time for reassessment?,” National Academy of Sciences Committee on the Biological Effects of Ionizing Radiation (BEIR), Report No. VII, 2005.
- International Commission on Radiological Protection (ICRP) Publication 87, “Managing patient dose in computed tomography,” Annals of the ICRP, 2000, Vol 30. [DOI] [PubMed]
- Linton O. W. and F. A.Mettler, Jr., “National conference on dose reduction in CT, with an emphasis on pediatric patients,” AJR, Am. J. Roentgenol. 181, 321–329 (2003). [DOI] [PubMed] [Google Scholar]