Abstract
Background
The onset of sexual maturation at puberty is a unique developmental period from a neuroendocrine perspective in that it is characterized by enhanced FSH secretion and FSH responsiveness to exogenous GnRH (vs. LH) from the gonadotrope, yet the mechanism of these dynamics remains unclear. This study aimed to elucidate this phenomenon using a human disease model of GnRH deficiency (idiopathic hypogonadotropic hypogonadism, IHH) in which GnRH input can be experimentally controlled.
Methods
25 GnRH-deficient men were selected for study based upon their baseline testicular volumes (TV) and serum inhibin B (IB) levels to represent a spectrum of pubertal/testicular development. Subjects underwent: (i) a 12-hour overnight neuroendocrine evaluation for hormonal profiling and determination of endogenous LH secretion pattern, and (ii) a 7-day exposure to a physiologic regimen of exogenous pulsatile GnRH (25 ng/kg every 2 h). Daily measurements of serum testosterone (T) and IB levels were made and a 2-hour window of frequent blood sampling was monitored to measure LH and FSH following a single i.v. GnRH bolus (25 ng/kg). All subjects were screened for known loci underlying GnRH deficiency and the response to GnRH was tracked according to genotype.
Results
Among the entire cohort, no changes were noted in serum T or IB during the 7 days, thus keeping gonadal feedback relatively constant. However, serum LH and FSH levels increased significantly (p < 0.0001) in the entire cohort. When analyzed by degree of pubertal/testicular development, men with no evidence of prior spontaneous pubertal development (TV ≤3 ml, Group I) showed sharp increases in serum FSH compared to men with some prior evidence of partial puberty (TV >3 ml, Group II, p < 0.0001). Group I exhibited a decreased LH response to GnRH on day 2 compared to day 1 (p < 0.01), which did not recover until day 5 (1–4 vs. 5–7 days, p < 0.0001). Group II displayed robust and equivalent LH responses to GnRH throughout the 7-day study. Genetic studies identified 8 mutations in 4 different loci (DAX1, KAL1, GNRHR, and FGFR1) in this cohort.
Conclusions
GnRH-deficient men undergoing GnRH-induced sexual maturation display an inverse relationship between FSH responsiveness to GnRH and baseline testicular size and IB levels. This observation implies that increasing seminiferous tubule maturity represents the major constraint on FSH responsiveness to GnRH in early puberty. In contrast, LH responsiveness to GnRH correlates directly with duration of GnRH exposure. Attenuated pituitary gonadotropin responses were noted in subjects harboring DAX1 mutations, consistent with known pituitary defects.
Key Words: GnRH, LH, FSH, Puberty, Gonadal development
Introduction
N.P. and A.T. contributed equally to this work.
Sexual maturation is initiated by the activation of the hypothalamic-pituitary-gonadal axis induced by endogenous GnRH secretion, typically in a sleep-entrained fashion, in early puberty [1]. In boys, this earliest period of sexual development is correlated with relatively isolated growth of the components of the seminiferous tubules of the testes [2]. As such, spermarche is often achieved prior to significant systemic androgenization from Leydig cell secretion of testosterone (T) as evidenced by mature sperm in the morning urine specimen of boys prior to other manifestations of sexual maturation [3]. This early pubertal program of GnRH secretion is characterized by a relatively unique period of FSH predominant release from the pituitary [4]. In contrast, during later puberty and adulthood, LH responses to endogenous GnRH stimulation predominate and FSH responsiveness is relatively suppressed [1,5,6]. Previous investigations into the hormonal dynamics of this early pubertal period have suggested a relatively enhanced secretion of FSH versus LH in response to GnRH that may be influenced by the status of gonadal sex steroid feedback [7,8,9,10,11]. However, the impact from non-steroidal gonadal proteins, such as inhibin B (IB), has been less well studied. In a cross-sectional study of pubertal boys, Manasco et al. [10] observed that the greatest increase in FSH levels occurred in early puberty when negative feedback was the lowest, suggesting a critical role of IB in the feedback control of FSH during later puberty.
Idiopathic hypogonadotropic hypogonadism (IHH) is a human disorder characterized by an isolated defect in GnRH secretion or action [12]. Administration of exogenous GnRH to these GnRH-deficient men at dosages and frequencies modeled after the GnRH-induced LH secretory profiles of normal adult males [13,14,15,16] activates their pituitary-gonadal axis with resulting long-term normalization of steroidogenesis, testicular growth, and spermatogenesis which faithfully recapitulates normal puberty [13,15,17]. Because the regimen of exogenous GnRH stimulation can be fixed and hence quantified in GnRH-deficient men, their initial gonadotrope responsiveness to such a ‘clamped’ GnRH input provides a unique opportunity to dissect the negative feedback influences of gonadal sex steroids versus seminiferous tubule maturation in controlling the relative responses of LH and FSH from the gonadotrope during early puberty. As such, this novel investigative model resolves the human investigative considerations that make studying normal children across periods of sexual development problematic.
Materials and Methods
IHH Subjects
25 men (age 26.5 ± 1.8 years) with congenital GnRH deficiency were included in the study. The diagnosis of IHH was based on the following criteria: (i) absent or incomplete endogenous pubertal development; (ii) serum T ≤100 ng/dl (3.5 nmol/l) in association with inappropriately low gonadotropin levels; (iii) absence of normal endogenous gonadotropin pulsations during a 12- to 24-hour period of frequent blood sampling; (iv) otherwise normal reserve testing of anterior pituitary function, and (v) a normal hypothalamic-pituitary region by MRI. None of the men participating in the study received prior GnRH therapy or gonadotropin treatment.
Healthy Genetic Control Subjects
A healthy adult male control cohort (≥200 subjects) was screened to determine whether observed base pair changes in the screened genes were normal variants. The study was approved by the Human Research Committee at the Massachusetts General Hospital, and all subjects provided written informed consent prior to the initiation of any study-related procedures.
Study Design
All the GnRH-deficient men discontinued any prior T therapy prior to receiving exogenous GnRH (washout period of ≥4 weeks for transdermal T, ≥6 weeks for T injections). Subjects underwent an initial clinical and biochemical evaluation prior to receiving a 7-day regimen of pulsatile GnRH. Subjects also provided a peripheral blood sample for genetic screening.
Baseline Clinical Assessment of IHH
A detailed history was obtained from each subject including history of cryptorchidism, microphallus, prior sexual development, and prior therapy. A complete physical examination was performed including arm-span measurements, Tanner staging of pubic and axillary hair, and measurement of testicular volume (TV) using a Prader orchidometer. Subjects underwent quantitative smell testing [18] and individuals scoring <5th percentile for age were classified as having Kallmann syndrome (KS).
Baseline Biochemical Assessment
Subjects were admitted to the Massachusetts General Hospital Clinical Research Center for an overnight frequent blood sampling study (every 10 min ×12 h) to assess their baseline pattern of endogenous GnRH-induced LH secretion. Serum LH, FSH, T, and IB were measured in pooled samples constituted from equal aliquots of each of the 10-min samples.
GnRH Administration
Patients received GnRH (25 ng/kg/bolus) subcutaneously every 2 h via microinfusion pump (Ferring AG, Baar, Switzerland) for 7 days. Each day, subjects were admitted to the Clinical Research Center for an i.v. bolus of GnRH (25 ng/kg/bolus) followed by blood sampling at 0, 15, 30, 45, 60, 90, and 120 min following the GnRH bolus for LH and FSH measurement. Serum T and IB were measured at time 0 on each of the 7 days.
Genetic Studies
Subjects were screened for 8 genes known to date to cause IHH when mutated: GNRHR (NM_000406) [19], KAL1 (NM_000216) [20], GPR54 (NM_032551) [21], DAX1 (NM_000475) [22], FGFR1 (NM_000604) [23], FGF8 (NM_033163) [24], PROK2 (NM_021935) [25], and PROKR2 (NM_144773) [26]. Genetic studies were performed on DNA extracted from whole blood. The details of the PCR amplifications and sequencing were performed according to previously reported techniques [27]. Nonsense changes resulting in a truncated protein, frameshift, insertion, or deletion were categorized as definitive mutations. Nucleotide changes, which were (a) absent from the Single Nucleotide Polymorphism database (http://www.ncbi.nlm.nih.gov/projects/SNP/) and expressed sequence tags and (b) absent in at least 170 ethnically matched healthy controls were identified as mutations. All genes and proteins are described using standard nomenclature [28].
Hormone Assays
Both serum LH and FSH concentrations were determined by microparticle enzyme immunoassay (MEIA) using an automated Abbott AxSYM system (Abbott Laboratories, Chicago, Ill., USA). The Second International Reference Preparation was used as the reference standard. The assay sensitivities for LH and FSH were 1.6 IU/l with intraassay CVs of <7% and interassay CVs of <7.4%. A level of detection of 1.6 IU/l based on a HMG standard is equivalent to 0.34 mIU/ml LH or 0.66 mIU/ml FSH based on pituitary standards (80/552 and 92/510, respectively). We elected to use the HMG standard (1.6 IU/l level of detection) as we have published using this reference over the past decades and chose to keep it for the sake of consistency. The limits of detection (lowest dose distinguishable from zero) for the methods we use were 0.07 and 0.05 mIU/ml for LH and FSH, respectively. Serum T concentrations were measured using the DPC Coat-A-Count RIA kit (Diagnostics Products Corp., Los Angeles, Calif., USA). The T assay sensitivity was 4 ng/dl with an intra- and interassay CV of <10%. Estradiol (E2) was measured by the Abbott AxSYM system, which had an assay sensitivity of 20 pg/ml. The intraassay CV was 6.4% and the interassay CV was 10.6%. IB was measured using a previously described commercially available double-antibody enzyme-linked immunosorbent assay (Serotec, Oxford, UK) [29]. In our experience with this assay, the clinical detection limit is 15.6 pg/ml, with a CV of 4–6% within plate and 15–18% between plates.
Statistical Methods
For analysis, pulsatile hormone secretion was analyzed using a modification of the Santen and Bardin method [30,31]. The cohort was divided into two groups according to degree of prior spontaneous pubertal/testicular development. As T treatment induces virilization, determination of spontaneous pubertal development was based on TV. Group I included men with a complete absence of spontaneous puberty, i.e., prepubertal TV ≤3 ml, while Group II included men with some evidence of partial spontaneous pubertal development (TV >3 ml) as we have described previously [17]. Serum LH and FSH responses to GnRH administration were expressed as the geometric mean of the 7 time points (0, 15, 30, 45, 60, 90, and 120 min) comprising a single monitored bolus of i.v. GnRH delivered at the same time each day. When assay results were below the level of detection, the limit of the assay was used. Serum T and IB on day 1 were compared with the levels on each of the subsequent study days by paired t tests. The rate of rise in LH and FSH across the study was expressed as the slope of the lines of regression through the mean of the daily hormone levels estimated by the longitudinal mixed effects model with random intercept and fixed time effect. We further made a comparison of the response days 1–4 and 5–7 by using the linear contrast. The correlation of the rate of rise of LH and FSH with TV, IB, and rate of rise of daily serum T were respectively determined. All data are expressed as the geometric mean ± SE unless otherwise stated and a p value <0.05 was considered statistically significant.
Results
Baseline Clinical and Biochemical Features
The baseline characteristics of the GnRH-deficient men are profiled in table 1. 13 subjects were anosmic and given the diagnosis of KS whereas 12 had normosmic IHH. 16 had received prior T therapy whereas 9 had none. When the cohort was classified according to prior spontaneous testicular development (prepubertal testes TV ≤3 ml), the groups differed at baseline in terms LH (p < 0.005) and IB (p < 0.01) (table 1). 13 of the 15 (87%) men with absent puberty had undetectable LH secretion, while 7 of 10 (70%) men with prior evidence of partial puberty had detectable baseline LH levels, 3 of whom exhibited enfeebled but present pulsatile LH secretion. These findings are consistent with our prior report of 80% of IHH men lacking pubertal development demonstrating undetectable LH levels, while only 60% of IHH men with partial puberty have undetectable LH [26].
Table 1.
Clinical, biochemical and genetic characteristics of 25 IHH men at baseline prior to GnRH therapy
| Patient | Diagnosis | Crypt- orchidism | Prior treatment | TV ml | LH IU/l | LH secretion pattern | T ng/dl | FSH IU/l | IB pg/ml | Genetic analysis |
|---|---|---|---|---|---|---|---|---|---|---|
| No prior puberty | ||||||||||
| 1 | KS | 1 | <1.6 | 42 | 2 | |||||
| 2 | KS | 1 | <1.6 | 30 | 2.3 | |||||
| 3 | KS | bilateral | 1 | <1.6 | 16 | <1.6 | ||||
| 4 | KS | T | 2 | <1.6 | 56 | <1.6 | ||||
| 5 | KS | bilateral | T | 2 | <1.6 | 40 | 2.2 | |||
| 6 | KS | bilateral | 2 | <1.6 | 73 | <1.6 | 21.2 | |||
| 7 | nIHH | unilateral | T | 2 | <1.6 | 89 | 1.7 | 39.7 | ||
| 8 | nIHH | 2 | <1.6 | 14 | <1.6 | 26.7 | DAX1 [34] | |||
| 9 | KS | 3 | 2.9 | pulsatile | 40 | 6.7 | 18.5 | |||
| 10 | KS | T | 3 | <1.6 | 34 | <1.6 | 32 | |||
| 11 | KS | T | 3 | <1.6 | 42 | 2.3 | 43.3 | |||
| 12 | KS | bilateral | T | 3 | <1.6 | 14 | <1.6 | 26.7 | ||
| 13 | KS | bilateral | T | 3 | <1.6 | 60 | <1.6 | <15.6 | ||
| 14 | KS | bilateral | T | 3 | 2.3 | apulsatile | 32 | 1.6 | 29.6 | KAL1 |
| 15 | nIHH | 3 | <1.6 | 16 | 2.5 | 90.1 | ||||
| Mean | 2.3 | 1.7 | 40 | 2.2 | 30.4 | |||||
| SEM | 0.2 | 0.1 | 5.7 | 0.3 | 5.2 | |||||
| Partial puberty | ||||||||||
| 16 | nIHH | 4 | 1.8 | apulsatile | 13 | <1.6 | <15.6 | |||
| 17 | nIHH | T | 5 | <1.6 | 10 | <1.6 | 75.7 | |||
| 18 | nIHH | T | 7 | 7.6 | pulsatile | 129 | 5.6 | − | DAX1 [34] | |
| 19 | nIHH | T | 8 | 3.3 | apulsatile | 83 | <1.6 | <15.6 | ||
| 20 | KS | T | 10 | 4.1 | apulsatile | 71 | 3.9 | 66.3 | ||
| 21 | nIHH | T | 10 | <1.6 | 21 | <1.6 | <15.6 | FGFR1 | ||
| 22 | nIHH | T | 11 | 6.8 | pulsatile | 85 | 2.8 | 203 | FGFR1 [27] | |
| 23 | nIHH | 13 | 2.3 | apulsatile | 101 | 4 | 88.5 | |||
| 24 | nIHH | T | 13 | <1.6 | 57 | <1.6 | 388.5 | |||
| 25 | nIHH | T | 18 | 3.5 | pulsatile | 52 | 2.4 | 63.3 | GNRHR [35] | |
| Mean | 9.9 | 3.4 | 62 | 2.7 | 104 | |||||
| SEM | 1.3 | 0.7 | 12.5 | 0.4 | 40.5 | |||||
KS = Kallmann's syndrome; nIHH = normosmic idiopathic hypogonadotrpic hypogonadism; T = testosterone; TV = mean testicular volume.
Responsiveness to GnRH
During the 7 days of treatment, both groups displayed significant increases in serum LH (p < 0.0001) and FSH (p < 0.0001) (fig. 1). When examined as a whole, no significant changes were noted in either serum T or IB during the 7 days of treatment. Interestingly, when the groups were examined according to level of pubertal development, the gonadotropin response to GnRH differed markedly (fig. 1).
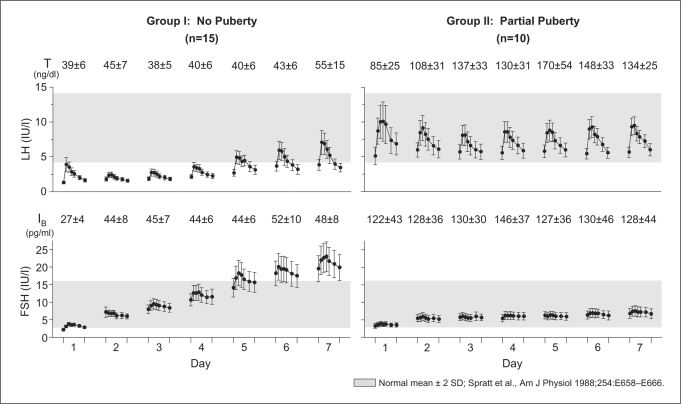
Fig. 1.
Mean (± SE) response to the first 7 days of pulsatile GnRH therapy in 25 GnRH-deficient men. The left panels display the responses to GnRH treatment in 15 IHH men with no pubertal development (Group I) and the right panels display the responses of 10 IHH men with partial puberty (Group II). Graphs depict LH and FSH response to a single i.v. dose of GnRH (25 ng/kg) during a 2-hour window on each of the 7 days. Inhibin B (IB) and testosterone (T) levels were measured each day prior to the i.v. GnRH bolus. The shaded region is the reference range for healthy adult males [16].
Group I: GnRH-Deficient Men without Evidence of Prior Testicular Maturation (TV ≤3 ml)
In response to exogenous GnRH (fig. 1), men in Group I exhibited a sharp and progressive rise in FSH from days 1 to 7 (3.3 ± 0.1 to 21.3 ± 1.4 IU/l, p < 0.0001). In contrast, the LH response was enfeebled in this group. Their mean LH response to GnRH decreased from day 1 to day 2 (2.8 ± 0.2 to 2.3 ± 0.1 IU/l, p < 0.01), remained low through day 4 (day 3 = 2.4 ± 0.1, day 4 = 3.0 ± 0.2 IU/l), and then recovered on day 5 (day 5 = 4.1 ± 0.3, day 6 = 4.6 ± 0.4, day 7 = 5.2 ± 0.5 IU/l; days 1–4 vs. days 5–7, p < 0.0001). Interestingly, those who received prior T treatment (n = 8) had a significantly greater increase in LH compared to the T-naive subjects (p < 0.0001) which was not observed with FSH. No significant change was noted in serum IB levels (26.9 ± 4.3 to 48.4 ± 8.3 pg/ml, p = 0.26) despite the striking increases in FSH during the 7 days. Serum T levels also remained unchanged (39 ± 6 to 55 ± 15 ng/dl, p = 0.24) in the setting of their significant increased LH levels into the normal adult male range (fig. 1). The absence of changes in IB and T secretion over this time period permitted a relatively clear insight into the interpretation of their FSH vs. LH secretory dynamics.
Group II: GnRH-Deficient Men with Evidence of Prior Testicular Development (TV >3 ml)
Group II exhibited a qualitative and quantitative difference in the pattern of gonadotropin response to GnRH (fig. 1). While they exhibited a greater FSH response to GnRH administration on day 1 (Group II = 3.7 ± 0.2 vs. Group I = 3.3 ± 0.1 IU/l, p < 0.05), they did not display the same robust increase in FSH responsiveness over the ensuing 7 days as seen in Group I (p < 0.0001). However, Group II showed a robust LH response on day 1 with a characteristic pulse architecture, which was unchanged during the 7 days (day 1 = 8.3 ± 0.8, day 7 = 7.8 ± 0.4 IU/l) in contrast to the dynamics observed in Group I (p < 0.0001). Gonadotropin responses within Group II were similar when stratified according to prior androgen exposure (past T treatment, n = 8; T naive, n = 2). No changes were observed in serum IB levels in Group II over the 7 days (day 1 = 122.3 ± 42.9, day 7 = 128.3 ± 43.6 pg/ml), mirroring the flat slope of the FSH response in this group (fig. 1). GnRH-induced LH-stimulated T production increased from 85 ± 25 ng/dl on day 1 to 134 ± 25 ng/dl on day 7 (p < 0.05).
Relationship of Genotype to Gonadotrope Responses
Genetic screening revealed mutations in 4 loci for IHH (table 1). Subject 4 has a heterozygous FGFR1 mutation in the immunoglobulin 2 domain of the receptor (p.E274G). Subjects 5, 21, and 22 harbor a heterozygous mutation in the immunoglobulin 1 domain of FGFR1 (p.V102I, p.Y99C, p.S91S, respectively) [27,32]. Subject 14 has a KAL1 missense mutation in the fibronectin repeat domain of the protein (p.V543I). Subjects 8 and 18 have a mutation and a deletion in DAX1 [33,34]. Subject 25 carried a homozygous missense mutation in GNRHR (p.Q106R) [35].
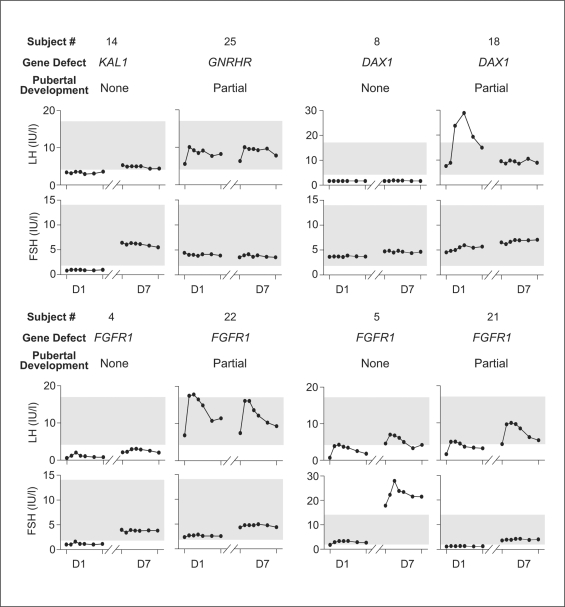
Eight subjects were found to harbor a mutation either in KAL1 (n = 1), FGFR1 (n = 4), DAX1 (n = 2), or GNRHR (n = 1). When stratified according to genotype, subjects with KAL1, FGFR1,or GNRHR mutations did not differ after adjusting for prior degree of pubertal development. In contrast, subjects harboring DAX1 mutations showed an abnormal response to GnRH with no increase in FSH or LH consistent with the additional pituitary expression of DAX1 (fig. 2).
Fig. 2.
Gonadotropin response to GnRH stratified by genotype and degree of pubertal development. The individual graphs represent the LH and FSH (2-hour) to a single i.v. GnRH bolus on days 1 and 7 of the 8 subjects harboring a mutation in KAL1, GNRHR, FGFR1, and DAX1. The shaded region is the reference range for healthy adult males [16].
Discussion
In this study, men with isolated GnRH deficiency were utilized as a human disease model. They permit a unique investigative window into the timing and pace of pubertal development since their GnRH input can be experimentally controlled and hence quantified. Their responses to the initial exposure of 7 days of fixed doses of pulsatile GnRH differed strikingly according to the degree of prior gonadal development. In subjects with no evidence of prior puberty (Group I), FSH secretion predominates in response to GnRH. In contrast, GnRH-deficient subjects with evidence of more advanced seminiferous tubule development (Group II), as mirrored by testicular size and IB levels, exhibited predominant LH responsiveness and a significant increase in serum T. The pattern of response did not differ according to genotype with the exception of the 2 subjects harboring DAX1 mutations. In these 2 subjects, the aberrant gonadotropin responses to GnRH (fig. 2) were consistent with prior studies demonstrating a pituitary defect in some patients carrying a DAX1 mutation [33,34].
Several mechanisms have previously been proposed to explain this unique window of FSH predominant secretion seen in early puberty followed by a subsequent decline and rise in LH levels. First, gonadal steroids have been hypothesized to suppress the predominant FSH response to exogenous GnRH administration seen in normal boys [36]. This could explain some of the blunted FSH responses in men with some puberty, as there was a significant increase in serum T. E2 levels remained below the assay sensitivity (<20 pg/ml) in most subjects (data not shown). Hence a potential role of E2 in determining their relative gonadotropin response to GnRH could not be determined but is not likely, given the low levels involved. Thus, ambient sex steroid levels may account for some of the differences in gonadotrope secretion of FSH in response to GnRH.
Alternatively, varying levels of endogenous GnRH secretion were also candidates for influencing the relative gonadotropin responses. To this point, LH responses predominated in all 4 subjects with incomplete GnRH deficiency (Group II) and detectable yet apulsatile LH secretion. Further, no subjects in Group I with complete GnRH deficiency as evidenced by prepubertal testes and undetectable LH secretion exhibited a predominant LH response. Further, men in Group I with no testicular development who received prior androgen exposure demonstrated a significantly greater LH response during the first 7 days of treatment compared to T-naive men with prepubertal testes. This may suggest a role of androgens in neuronal plasticity. Cumulatively, these observations suggest that some prior gonadotropin and/or GnRH exposure might be important in determining gonadotrope responsiveness. However, the GnRH input, which was experimentally controlled in this study, could not be the explanation.
In addition, the longer half-life of FSH has been offered as an explanation for the predominant FSH secretion in early puberty [37]. However, given the known half-lives of FSH [38], steady-state concentrations of FSH should have occurred by the end of the first study day. Therefore, differences in hormone clearance cannot account for the observed differences in gonadotropin concentrations.
In our cohort, the relative and absolute FSH responses to GnRH were most clearly related to the degree of prior gonadal development and secretion of IB from the seminiferous tubules. IB is a dimeric glycoprotein secreted from Sertoli cells in response to FSH stimulation [39]. Others have previously demonstrated a ‘feed-forward’ relationship between FSH and IB during the earliest stages of pubertal development wherein FSH secretion drives IB secretion from the Sertoli cells [40]. However, once the first wave of spermatogenesis has occurred, a negative feedback loop between IB secretion and FSH is subsequently activated [40,41,42,43,44] that suppresses subsequent FSH release from the gonadotropes. Therefore, in our subjects with absent puberty, low IB levels may be responsible for the relatively unconstrained FSH responsiveness in Group I who reflect the earliest stages of pubertal development. In contrast, after the first wave of spermatogenesis had been established, as occurred in Group II with some evidence of prior gonadal activation (larger testicular size and normal serum IB), the switch to a more traditional negative feedback relationship between IB and FSH secretion would have been activated and be responsible for their relatively suppressed FSH responsiveness. This interpretation is most consistent with the data herein. It is possible that other seminiferous tubule secretory factors like IB may modulate the gonadotrope's FSH response to GnRH in these subjects.
On the other hand, this explanation does not account for the LH differences observed between these groups. The initial LH responses to GnRH administration in both groups demonstrate that releasable LH clearly exists in the gonadotropes of almost all GnRH-deficient men, even in subjects with the most profound GnRH deficit as indicated by absent puberty, small testes, cryptorchidism, and microphallus. This finding is substantiated by the immunohistochemical demonstration of LH and FSH in the pituitary of an untreated man with KS [45]. However, the significant ‘collapse’ of LH responsiveness on day 2 of GnRH administration in Group I (no prior pubertal development) suggests that their intrapituitary pool of releasable LH could be rapidly depleted and hence is likely to be small. Its subsequent recovery through the ensuing 5 days of GnRH administration reveals that continued priming of the pituitary by GnRH can rapidly replenish this releasable LH pool. Alternatively, it might reflect changes in GnRH receptor number on the gonadotrope that occurs in response to pulsatile GnRH administration [16,46].
In summary, we have demonstrated that the relative gonadotrope responsiveness of GnRH-deficient men receiving a physiologic regimen of GnRH is related to the degree of preexisting gonadal development. In prepubertal subjects, FSH release is favored, thus increasing the number of Sertoli cells and spermatogonia [unpubl. data]. This initially selective increase in FSH secretion may then cause increased IB levels, a marker of Sertoli cell number [47], to the point where rising levels of intratesticular androgens and the increasing FSH stimulation induce the first wave of spermatogenesis. In contrast, in subjects with prior evidence of partial puberty, where many of these changes have already occurred as witnessed by their testicular sizes and elevated IB levels, there is a physiologic transition from a positive ‘feed-forward’ motif to the more traditional negative feedback responses of IB secretion to FSH. Subsequently, a decrease in FSH and relative increase in LH secretion occurs in response to GnRH. Therefore, the initial period of GnRH administration to GnRH-deficient men provides a valuable model for studying the maturation of the pituitary-gonadal axis during puberty as well as providing valuable insights into the ontology of IB.
Acknowledgements
This work was supported by NIH R01 HD15788-15 and M01-RR-01066, National Institutes of Health, National Center for Research Resources, General Clinical Research Centers Program and the Eunice Kennedy Shriver NICHD/NIH through cooperative agreement U54-HD28138 as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research.
References
- 1.Boyar RM, Rosenfeld RS, Kapen S, Finkelstein JW, Roffwarg HP, Weitzman ED, Hellman L. Human puberty. Simultaneous augmented secretion of luteinizing hormone and testosterone during sleep. J Clin Invest. 1974;54:609–618. doi: 10.1172/JCI107798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prader A. Testicular size: assessment and clinical importance. Triangle. 1966;7:240–243. [PubMed] [Google Scholar]
- 3.Nielsen CT, Skakkebaek NE, Richardson DW, Darling JA, Hunter WM, Jorgensen M, Nielsen A, Ingerslev O, Keiding N, Muller J. Onset of the release of spermatozoa (spermarche) in boys in relation to age, testicular growth, pubic hair, and height. J Clin Endocrinol Metab. 1986;62:532–535. doi: 10.1210/jcem-62-3-532. [DOI] [PubMed] [Google Scholar]
- 4.Burr IM, Sizonenko PC, Kaplan SL, Grumbach MM. Hormonal changes in puberty. I. Correlation of serum luteinizing hormone and follicle stimulating hormone with stages of puberty, testicular size, and bone age in normal boys. Pediatr Res. 1970;4:25–35. doi: 10.1203/00006450-197001000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Jakacki RI, Kelch RP, Sauder SE, Lloyd JS, Hopwood NJ, Marshall JC. Pulsatile secretion of luteinizing hormone in children. J Clin Endocrinol Metab. 1982;55:453–458. doi: 10.1210/jcem-55-3-453. [DOI] [PubMed] [Google Scholar]
- 6.Dunkel L, Alfthan H, Stenman UH, Selstam G, Rosberg S, Albertsson-Wikland K. Developmental changes in 24-hour profiles of luteinizing hormone and follicle-stimulating hormone from prepuberty to midstages of puberty in boys. J Clin Endocrinol Metab. 1992;74:890–897. doi: 10.1210/jcem.74.4.1548356. [DOI] [PubMed] [Google Scholar]
- 7.Winter JS. Analysis of clinical studies with LH-RH in children and adolescents. Am J Dis Child. 1976;130:590–592. doi: 10.1001/archpedi.1976.02120070016002. [DOI] [PubMed] [Google Scholar]
- 8.Marshall JC, Case GD, Valk TW, Corley KP, Sauder SE, Kelch RP. Selective inhibition of follicle-stimulating hormone secretion by estradiol. Mechanism for modulation of gonadotropin responses to low-dose pulses of gonadotropin-releasing hormone. J Clin Invest. 1983;71:248–257. doi: 10.1172/JCI110765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oerter KE, Uriarte MM, Rose SR, Barnes KM, Cutler GB., Jr Gonadotropin secretory dynamics during puberty in normal girls and boys. J Clin Endocrinol Metab. 1990;71:1251–1258. doi: 10.1210/jcem-71-5-1251. [DOI] [PubMed] [Google Scholar]
- 10.Manasco PK, Umbach DM, Muly SM, Godwin DC, Negro-Vilar A, Culler MD, Underwood LE. Ontogeny of gonadotropin, testosterone, and inhibin secretion in normal boys through puberty based on overnight serial sampling. J Clin Endocrinol Metab. 1995;80:2046–2052. doi: 10.1210/jcem.80.7.7608253. [DOI] [PubMed] [Google Scholar]
- 11.Albertsson-Wikland K, Rosberg S, Lannering B, Dunkel L, Selstam G, Norjavaara E. Twenty-four-hour profiles of luteinizing hormone, follicle-stimulating hormone, testosterone, and estradiol levels: a semilongitudinal study throughout puberty in healthy boys. J Clin Endocrinol Metab. 1997;82:541–549. doi: 10.1210/jcem.82.2.3778. [DOI] [PubMed] [Google Scholar]
- 12.Seminara SB, Hayes FJ, Crowley WF., Jr Gonadotropin-releasing hormone deficiency in the human (idiopathic hypogonadotropic hypogonadism and Kallmann's syndrome): pathophysiological and genetic considerations. Endocr Rev. 1998;19:521–539. doi: 10.1210/edrv.19.5.0344. [DOI] [PubMed] [Google Scholar]
- 13.Hoffman AR, Crowley WF., Jr Induction of puberty in men by long-term pulsatile administration of low-dose gonadotropin-releasing hormone. N Engl J Med. 1982;307:1237–1241. doi: 10.1056/NEJM198211113072003. [DOI] [PubMed] [Google Scholar]
- 14.Spratt DI, Finkelstein JS, O'Dea LS, Badger TM, Rao PN, Campbell JD, Crowley WF., Jr Long-term administration of gonadotropin-releasing hormone in men with idiopathic hypogonadotropic hypogonadism. A model for studies of the hormone's physiologic effects. Ann Intern Med. 1986;105:848–855. doi: 10.7326/0003-4819-105-6-848. [DOI] [PubMed] [Google Scholar]
- 15.Crowley WF, Jr, Whitcomb RW, Jameson JL, Weiss J, Finkelstein JS, O'Dea LS. Neuroendocrine control of human reproduction in the male. Recent Prog Horm Res. 1991;47:27–67. doi: 10.1016/b978-0-12-571147-0.50006-8. [DOI] [PubMed] [Google Scholar]
- 16.Spratt DI, O'Dea LS, Schoenfeld D, Butler J, Rao PN, Crowley WF., Jr Neuroendocrine-gonadal axis in men: frequent sampling of, LH, FSH, and testosterone. Am J Physiol. 1988;254:E658. doi: 10.1152/ajpendo.1988.254.5.E658. [DOI] [PubMed] [Google Scholar]
- 17.Pitteloud N, Hayes FJ, Dwyer A, Boepple PA, Lee H, Crowley WF., Jr Predictors of outcome of long-term GnRH therapy in men with idiopathic hypogonadotropic hypogonadism. J Clin Endocrinol Metab. 2002;87:4128–4136. doi: 10.1210/jc.2002-020518. [DOI] [PubMed] [Google Scholar]
- 18.Doty RL, Applebaum S, Zusho H, Settle RG. Sex differences in odor identification ability: a cross-cultural analysis. Neuropsychologia. 1985;23:667–672. doi: 10.1016/0028-3932(85)90067-3. [DOI] [PubMed] [Google Scholar]
- 19.De Roux N, Young J, Misrahi M, Genet R, Chanson P, Schaison G, Milgrom E. A family with hypogonadotropic hypogonadism and mutations in the gonadotropin-releasing hormone receptor. N Engl J Med. 1997;337:1597–1602. doi: 10.1056/NEJM199711273372205. [DOI] [PubMed] [Google Scholar]
- 20.Oliveira LM, Seminara SB, Beranova M, Hayes FJ, Valkenburgh SB, Schipani E, Costa EM, Latronico AC, Crowley WF, Jr, Vallejo M. The importance of autosomal genes in Kallmann syndrome: genotype-phenotype correlations and neuroendocrine characteristics. J Clin Endocrinol Metab. 2001;86:1532–1538. doi: 10.1210/jcem.86.4.7420. [DOI] [PubMed] [Google Scholar]
- 21.Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS, Jr, Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, Zahn D, Dixon J, Kaiser UB, Slaugenhaupt SA, Gusella JF, O'Rahilly S, Carlton MB, Crowley WF, Jr, Aparicio SA, Colledge WH. The GPR54 gene as a regulator of puberty. N Engl J Med. 2003;349:1614–1627. doi: 10.1056/NEJMoa035322. [DOI] [PubMed] [Google Scholar]
- 22.Achermann JC, Gu WX, Kotlar TJ, Meeks JJ, Sabacan LP, Seminara SB, Habiby RL, Hindmarsh PC, Bick DP, Sherins RJ, Crowley WF, Jr, Layman LC, Jameson JL. Mutational analysis of DAX1 in patients with hypogonadotropic hypogonadism or pubertal delay. J Clin Endocrinol Metab. 1999;84:4497–4500. doi: 10.1210/jcem.84.12.6269. [DOI] [PubMed] [Google Scholar]
- 23.Pitteloud N, Acierno JS, Jr, Meysing AU, Dwyer AA, Hayes FJ, Crowley WF., Jr Reversible Kallmann syndrome, delayed puberty, and isolated anosmia occurring in a single family with a mutation in the fibroblast growth factor receptor 1 gene. J Clin Endocrinol Metab. 2005;90:1317–1322. doi: 10.1210/jc.2004-1361. [DOI] [PubMed] [Google Scholar]
- 24.Yoshiura K, Leysens NJ, Chang J, Ward D, Murray JC, Muenke M. Genomic structure, sequence, and mapping of human FGF8 with no evidence for its role in craniosynostosis/limb defect syndromes. Am J Med Genet. 1997;72:354–362. [PubMed] [Google Scholar]
- 25.Pitteloud N, Zhang C, Pignatelli D, Li JD, Raivio T, Cole LW, Plummer L, Jacobson-Dickman EE, Mellon PL, Zhou QY, Crowley WF., Jr Loss-of-function mutation in the prokineticin 2 gene causes Kallmann syndrome and normosmic idiopathic hypogonadotropic hypogonadism. Proc Natl Acad Sci USA. 2007;104:17447–17452. doi: 10.1073/pnas.0707173104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pitteloud N, Hayes FJ, Boepple PA, DeCruz S, Seminara SB, MacLaughlin DT, Crowley WF., Jr The role of prior pubertal development, biochemical markers of testicular maturation, and genetics in elucidating the phenotypic heterogeneity of idiopathic hypogonadotropic hypogonadism. J Clin Endocrinol Metab. 2002;87:152–160. doi: 10.1210/jcem.87.1.8131. [DOI] [PubMed] [Google Scholar]
- 27.Raivio T, Falardeau J, Dwyer A, Quinton R, Hayes FJ, Hughes VA, Cole LW, Pearce SH, Lee H, Boepple P, Crowley WF, Jr, Pitteloud N. Reversal of idiopathic hypogonadotropic hypogonadism. N Engl J Med. 2007;357:863–873. doi: 10.1056/NEJMoa066494. [DOI] [PubMed] [Google Scholar]
- 28.Antonarakis SE. Recommendations for a nomenclature system for human gene mutations. Nomenclature Working Group. Hum Mutat. 1998;11:1–3. doi: 10.1002/(SICI)1098-1004(1998)11:1<1::AID-HUMU1>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 29.Groome NP, Illingworth PJ, O'Brien M, Pai R, Rodger FE, Mather JP, McNeilly AS. Measurement of dimeric inhibin B throughout the human menstrual cycle. J Clin Endocrinol Metab. 1996;81:1401–1405. doi: 10.1210/jcem.81.4.8636341. [DOI] [PubMed] [Google Scholar]
- 30.Hayes FJ, McNicholl DJ, Schoenfeld D, Marsh EE, Hall JE. Free α-subunit is superior to luteinizing hormone as a marker of gonadotropin-releasing hormone despite desensitization at fast pulse frequencies. J Clin Endocrinol Metab. 1999;1999:1028–1036. doi: 10.1210/jcem.84.3.5579. [DOI] [PubMed] [Google Scholar]
- 31.Santen RJ, Bardin CW. Episodic luteinizing hormone secretion in man. Pulse analysis, clinical interpretation, physiologic mechanisms. J Clin Invest. 1973;52:2617–2628. doi: 10.1172/JCI107454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pitteloud N, Meysing A, Quinton R, Acierno JS, Jr, Dwyer AA, Plummer L, Fliers E, Boepple P, Hayes F, Seminara S, Hughes VA, Ma J, Bouloux P, Mohammadi M, Crowley WF., Jr Mutations in fibroblast growth factor receptor 1 cause Kallmann syndrome with a wide spectrum of reproductive phenotypes. Mol Cell Endocrinol. 2006;254–255:60–69. doi: 10.1016/j.mce.2006.04.021. [DOI] [PubMed] [Google Scholar]
- 33.Seminara SB, Achermann JC, Genel M, Jameson JL, Crowley WF., Jr X-linked adrenal hypoplasia congenita: a mutation in DAX1 expands the phenotypic spectrum in males and females. J Clin Endocrinol Metabol. 1999;84:4501–4509. doi: 10.1210/jcem.84.12.6172. [DOI] [PubMed] [Google Scholar]
- 34.Habiby RL, Boepple P, Nachtigall L, Sluss PM, Crowley WF, Jr, Jameson JL. Adrenal hypoplasia congenita with hypogonadotropic hypogonadism: evidence that DAX-1 mutations lead to combined hypothalmic and pituitary defects in gonadotropin production. J Clin Invest. 1996;98:1055–1062. doi: 10.1172/JCI118866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pitteloud N, Boepple PA, DeCruz S, Valkenburgh SB, Crowley WF, Jr, Hayes FJ. The fertile eunuch variant of idiopathic hypogonadotropic hypogonadism: spontaneous reversal associated with a homozygous mutation in the gonadotropin-releasing hormone receptor. J Clin Endocrinol Metab. 2001;86:2470–2475. doi: 10.1210/jcem.86.6.7542. [DOI] [PubMed] [Google Scholar]
- 36.Valk TW, Corley KP, Kelch RP, Marshall JC. Pulsatile gonadotropin-releasing hormone in gonadotropin-deficient and normal men: suppression of follicle-stimulating hormone responses by testosterone. J Clin Endocrinol Metab. 1981;53:184–191. doi: 10.1210/jcem-53-1-184. [DOI] [PubMed] [Google Scholar]
- 37.Stuenkel CA, Dudley RE, Yen SS. Sublingual administration of testosterone-hydroxypropyl-β-cyclodextrin inclusion complex simulates episodic androgen release in hypogonadal men. J Clin Endocrinol Metab. 1991;72:1054–1059. doi: 10.1210/jcem-72-5-1054. [DOI] [PubMed] [Google Scholar]
- 38.Miller C, Ulloa-Aguirre A, Hyland L, Chappel S. Pituitary follicle-stimulating hormone heterogeneity: assessment of biologic activities of each follicle-stimulating hormone form. Fertil Steril. 1983;40:242–247. doi: 10.1016/s0015-0282(16)47244-4. [DOI] [PubMed] [Google Scholar]
- 39.O'Connor AE, De Kretser DM. Inhibins in normal male physiology. Semin Reprod Med. 2004;22:177–185. doi: 10.1055/s-2004-831893. [DOI] [PubMed] [Google Scholar]
- 40.Andersson AM, Juul A, Petersen JH, Muller J, Groome NP, Skakkebaek NE. Serum inhibin B in healthy pubertal and adolescent boys: relation to age, stage of puberty, and follicle-stimulating hormone, luteinizing hormone, testosterone, and estradiol levels. J Clin Endocrinol Metab. 1997;82:3976–3981. doi: 10.1210/jcem.82.12.4449. [DOI] [PubMed] [Google Scholar]
- 41.Illingworth PJ, Groome NP, Byrd W, Rainey WE, McNeilly AS, Mather JP, Bremner WJ. Inhibin-B: a likely candidate for the physiologically important form of inhibin in men. J Clin Endocrinol Metab. 1996;81:1321–1325. doi: 10.1210/jcem.81.4.8636325. [DOI] [PubMed] [Google Scholar]
- 42.Rivier C, Cajander S, Vaughan J, Hsueh AJ, Vale W. Age-dependent changes in physiological action, content, and immunostaining of inhibin in male rats. Endocrinology. 1988;123:120–126. doi: 10.1210/endo-123-1-120. [DOI] [PubMed] [Google Scholar]
- 43.Anawalt BD, Bebb RA, Matsumoto AM, Groome NP, Illingworth PJ, McNeilly AS, Bremner WJ. Serum inhibin B levels reflect Sertoli cell function in normal men and men with testicular dysfunction. J Clin Endocrinol Metab. 1996;81:3341–3345. doi: 10.1210/jcem.81.9.8784094. [DOI] [PubMed] [Google Scholar]
- 44.Lambert-Messerlian GM, Hall JE, Sluss PM, Taylor AE, Martin KA, Groome NP, Crowley WF, Jr, Schneyer AL. Relatively low levels of dimeric inhibin circulate in men and women with polycystic ovarian syndrome using a specific two-site enzyme-linked immunosorbent assay. J Clin Endocrinol Metab. 1994;79:45–50. doi: 10.1210/jcem.79.1.8027251. [DOI] [PubMed] [Google Scholar]
- 45.Kovacs K, Sheehan HL. Pituitary changes in Kallmann's syndrome: a histologic, immunocytologic, ultrastructural, and immunoelectron microscopic study. Fertil Steril. 1982;37:83–89. doi: 10.1016/s0015-0282(16)45982-0. [DOI] [PubMed] [Google Scholar]
- 46.Bédécarrats GY, Kaiser UB. Differential regulation of gonadotropin subunit gene promoter activity by pulsatile gonadotropin-releasing hormone in perifused L β T2 cells: role of GnRH receptor concentration. Endocrinology. 2003;144:1802–1811. doi: 10.1210/en.2002-221140. [DOI] [PubMed] [Google Scholar]
- 47.Pierik FH, Vreeburg JT, Stijnen T, De Jong FH, Weber RF. Serum inhibin B as a marker of spermatogenesis. J Clin Endocrinol Metab. 1998;83:3110–3114. doi: 10.1210/jcem.83.9.5121. [DOI] [PubMed] [Google Scholar]