Abstract
Induction of peripheral tolerance by oral administration of low-dose β-tubulin antigen may be an effective, antigen-specific method to suppress experimental autoimmune hearing loss. Five groups of mice were fed with phosphate-buffered saline (PBS), ovalbumin (OVA), 20, 30 or 200 μg of β-tubulin, respectively. All mice were then immunized by β-tubulin. Hearing thresholds were measured before and after immunization. Inner ear histology and cytokine profile were examined. Mice fed with 20 or 30 μg of β-tubulin showed less hearing loss and less inner ear damage compared to the groups treated with PBS, OVA or 200 μg of β-tubulin. Interferon-gamma (IFN-γ) was decreased while interleukin-4 (IL-4), IL-5, IL-13 and TGF-β were increased in both sera and in cell culture supernatants of the mice fed with 20 or 30 μg of β-tubulin. However, no cytokine profile change was found in the group treated with 200 μg of tubulin. These results suggest that a low dose of β-tubulin is active orally in an antigen-specific fashion and capable of inhibiting the autoimmune reactions in the inner ear by suppressingTh1 (IFN-γ) and increasing Th2 and Th3 (IL-4, IL-5, IL-13 and TGF-β) cytokines. Oral antigen tolerance may be used to treat autoimmune inner ear disease.
Key Words: Oral tolerance; β-Tubulin; Autoimmune inner ear disease, mice
Introduction
Several studies have indicated that the inner ear is not a privileged site for initial antigen stimulation [1,2,3]. It is proposed that the immune component cells initially activated in the periphery migrate into the inner ear and subsequently develop an immune response to the antigen locally. The weight of recent evidence strongly implicates immunological and/or chronic inflammatory processes as contributing to the pathogenesis of autoimmune inner ear disease. Consistent with the autoimmune mechanism, various pathological changes have been found in several animal models of autoimmune inner ear disease, including degeneration of the spiral ganglion, atrophy of Corti's organ and the endolymphatic duct epithelium, vasculitis in the cochlea, otospongiotic changes of the bone of the external meatus and otic capsule, as well as hearing loss and vestibular dysfunction [4, 5].
In autoimmune inner ear diseases, disrupted normal mechanisms of tolerance for self-proteins result in a destructive immune response against the individual's own tissue proteins. However, the specific antigen that elicits the autoimmune reactions in the inner ear is still unknown [4]. Recently, we have isolated a 55-kDa protein from the guinea pig inner ear, which has amino acid sequences similar to β-tubulin. We have also cloned the β-tubulin gene by using guinea pig inner ear cDNA library screening [6]. An immunohistological study using a monoclonal anti-tubulin antibody found positive staining in Corti's organ, spiral limb, cochlear nerve fibers and neurons in the spiral ganglions of guinea pigs, and tubulin immunization changed the immune staining pattern. However, no inner ear hydrops was found in those tubulin-immunized animals [7]. Furthermore, the antibody against β-tubulin was found in sera of about 64% of patients suffering from autoimmune inner ear diseases [6,7,8,9].
Oral tolerance is a long recognized method of inducing tolerance. It refers to the observation that if one is fed with a protein and then immunized with the same protein, a state of systemic hyporesponsiveness to the protein exists. Oral administration of an antigen isoften characterized by marked suppression of the cell-mediated immuneresponse to immunization with the same antigen. In recent years, investigators have begun to apply oral tolerance as a method to manipulate injurious immune responses, primarily in the area of autoimmune diseases. Although it was described by Wells in 1911 and Chase in 1946, oral tolerance has recently been proposed as an important mechanism to suppress the immune process in experimental models of autoimmune diseases [10].
This method has been successfully applied in autoimmune diseases such as experimental allergic encephalomyelitis, collagen-induced arthritis and type I diabetes mellitus. Oral tolerance probably induces interleukin-4 (IL-4), interleukin-6 (IL-6) and transforming growth factor beta (TGF-β) and simultaneously suppresses or inhibits the Th1a (T helper cell) cellular responses in these autoimmune diseases. Although the detailed immune mechanism in oral tolerance is not clear, both active suppression and clonal anergy or clonal deletion have been suggested [10,11,12]. While low-dose antigen treatment induces an active suppression mechanism, high-dose antigen treatment selects clonal anergy [10,11,12].
Our purpose was to evaluate T cell responses to β-tubulin after oral administration of β-tubulin in an autoimmune hearing loss mouse model. We hypothesized that oral administration of β-tubulin to tubulin-immunized mice can induce suppression of β-tubulin T cells, which recognize specific β-tubulin peptide complexes. This suppression would produce either an altered profile of lymphokines, the majority of which are suppressive types and are capable of downregulating inflammation and the autoimmune process, or allergic and refractory to subsequent stimulation.
Materials and Methods
Animals
Female C57BL/6 4 weeks old mice were purchased from the Jackson Laboratory (Bar Harbor, Me., USA). Female mice were used in the present study because female autoimmune mice have greater hearing loss than males [13]. They were housed in the animal room of the Veterans Administration Medical Center, Memphis. The animal procedures used in this study were approved by the Institutional Animal Care and Use Committees at the University of Tennessee Health Science Center, Memphis.
β-Tubulin Protein Expression
The β-tubulin gene was expressed as a glutathione-S-transferase fusion protein by subcloning into the prokaryotic expression vector pGEX-5X-3. Briefly, the open reading frame starting with the first ATG was PCR-amplified using primers flanked with EcoRI and NotI sites (forward: CAC GTT TGG TGG TGG CGA CCA; reverse: AGA GGT TTT CAC CGT CAT CAC), respectively.
Oral Administration of β-Tubulin
Thirty mice were randomly divided into 5 groups. Two groups were used as controls: one group of mice was fed with 0.2 ml of phosphate-buffered saline (PBS)/day; the second group was fed with 1 mg of ovalbumin (OVA)/day (Sigma Chemical Co., St. Louis, Mo., USA) in 0.2 ml PBS. The other 3 groups were fed with 20, 30 or 200 μg/day of β-tubulin, respectively. All mice were fed by gastric intubation with an 18-gauge stainless-steel feeding tube (Thomas Scientific, Swedesboro, N.J., USA). Animalswere fed once a day for 5 days and immunized 2 days after the last feeding.
Immunization
All mice were immunized with 100 μg of β-tubulin emulsified with an equal volume of complete Freund's adjuvant on the lateral back. The immunization was boosted with 100 μg of β-tubulin emulsified with an equal volume of incomplete Freund's adjuvant 2 and 4 weeks later.
Auditory-Evoked Brain Stem Response Tests
Auditory function was evaluated by performing auditory-evoked brainstem response (ABR) tests. Mice were intraperitoneally anesthetized with Avertin (tribromoethanol, 500 mg/kg body weight). Body temperature was kept stable with a heating pad throughout the experiment at 37°C. Response signals were recorded with subcutaneous needle electrodes inserted at the vertex in the midline of the scalp between the external auditory canal (active), left ear postauricular bulla region (reference) and the back close to the tail (ground). Click and tone pips of 8, 16 and 32 kHz were generated using a Tucker Davis Technologies Inc. (Gainesville, Fla., USA) workstation (System II) running Siggen 32 software (Tucker Davis Technologies). Stimuli were delivered to the left external auditory meatus through a 2-mm diameter plastic tube connected to a high-frequency transducer in a controlled acoustic chamber (Industrial Acoustics Company, Bronx, N.Y., USA). The output was fed to an amplifier (HS4 Bioamp Headstage, Tucker Davis Technologies). A maximum sound pressure level (SPL) was stimulated in clicks of 85 dB and in tone pips of 90 dB. The stimulus duration was 10 ms with 1-ms rise/fall time at a repetition rate of 21/s. The band pass filters were set at 300 Hz to 3 kHz. The evoked potentials were amplified 100,000 times and averaged from a total of 500 evoked responses for the first 10-ms period following stimulation. Auditory thresholds were determined by increasing the sound intensities of the click and tone pips for each stimulus from 15 to 85 dB and from 25 to 90 dB, respectively, first at 10-dB steps and finally at 5-dB steps, until reaching the lowest sound intensity at which reproducible waves could be recognized in the response. Thresholds were verified at least twice, and the mean thresholds were calculated. If ABRs could not be obtained with the maximum SPL of 90 dB in tone pips, a nominal threshold of 95 dB was assigned.
Cell Culture, Proliferation and ELISA for Cytokines
Mice were deeply anesthetized at 7 weeks after the immunization, and spleen cells were harvested. For proliferation assays and cytokine analyses, lymphocytes werecultured in 96-well plates at 5 × 106 or 10 × 106 cells/ml, respectively, in an X-Vivo 20 incubator (Biowhittaker, Walkersville, Md., USA). For proliferationassays, cells were pulsed with [3H]thymidine, 72 h later; radioactivity was measured after a 16-hour incubation. Culturesupernatants were collected at 24 h for IL-2 and IL-4, at 40 h for IL-13, IL-10 and interferon-gamma (IFN-γ), and at 72 h for TGF-β. Sera collected from each mouse at 3, 5 and 7 weeks after primary immunization was used for cytokine analysis by ELISA (R&D Systems, Minneapolis, Minn., USA).
Histopathological Study
The anesthetized mice were intracardially perfused with 0.1 M PBS followed by 4% paraformaldehyde in 0.1 M PBS (pH 7.4). The cochlea was removed from the temporal bone and postfixed in the same fixative overnight. The fixed cochlea was decalcified with 10% ethylenediaminetetraacetic acid in 0.1 M PBS for 3–4 weeks with a change of solution every week. The decalcified cochlea was washed with 0.1 M PBS 3 times, then dehydrated in graded alcohols and embedded in paraffin. The paraffin-embedded cochleas were sectioned at 6 μm thickness with a microtome. Sections were mounted on slides and deparaffinized with xylene and graded ethanol; then they were stained with hematoxylin and eosin. All sections were examined with a light microscope (Carl Zeiss Axioskop 2 plus HAL 100 with final enlargement ×400). Digital images were obtained using a camera (Leica) linked to a Windows computer.
Statistical Analysis
ABR thresholds and cytokine concentrations were analyzed by ANOVA.
Results
Less Hearing Loss Was Found in the Groups with Low-Dose β-Tubulin Oral Administration
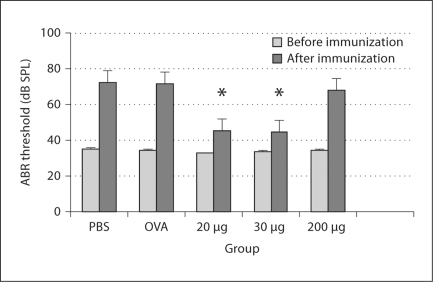
The groups fed with PBS, OVA and 200 μg β-tubulin were found to have severe hearing loss with 35.5, 35.5 and 33.5 dB SPL threshold shifts, respectively. No significant difference was found between the group fed with 200 μg β-tubulin and any control group (p > 0.05). However, the threshold shifts were only approximately 10 dB SPL in the mice fed with 20 and 30 μg β-tubulin. The differences in the threshold shifts between the mice fed with 20 or 30 μg β-tubulin and the control groups are statistically significant (p < 0.05), suggesting an effective protection to the inner ears by the low-dose oral administration of tubulin (fig. 1).
Fig. 1.
Results of the ABR test: hearing threshold changes before and after β-tubulin immunization. Significant threshold shifts are detected in the groups administered PBS, OVA and 200 μg of tubulin. However, fewer threshold shifts are found in the groups treated with 20 and 30 μg of tubulin (∗ p < 0.05).
Changes in Cytokine Levels after β-Tubulin Oral Administration and Immunization
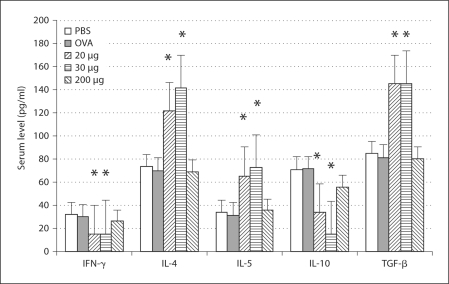
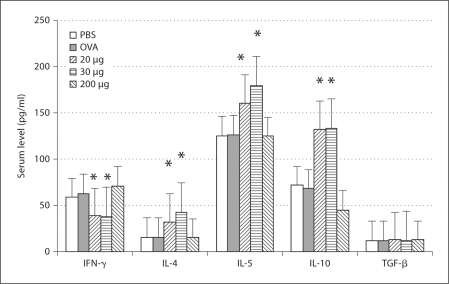
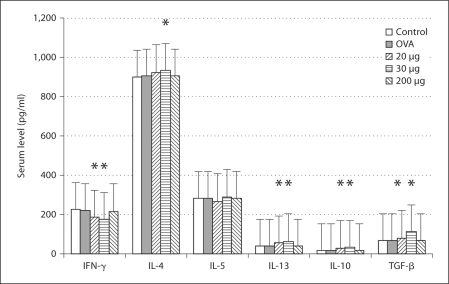
Three weeks after the primary immunization, the profile of the sera's cytokine production showed that levels of IFN-γ and IL-10 decreased, but levels of IL-4, IL-5, IL-13 and TGF-β increased in the mice fed with 20 and 30 μg of β-tubulin (fig. 2). Five weeks after the primary immunization, the profile of the sera's cytokine production showed that the level of IFN-γ decreased, but levels of IL-4, IL-5 and IL-10 increased in the mice fed with 20 and 30 μg of β-tubulin (fig. 3). Seven weeks after the primary immunization, the cytokine profile of the lymphocyte culture supernatant showed that the level of IFN-γ decreased, but levels of IL-4, IL-13, IL-10 and TGF-β increased in the mice fed with 20 and 30 μg of β-tubulin (fig. 4).
Fig. 2.
Profile of serum cytokine production at 3 weeks after the primary immunization. Levels of IFN-γ and IL-10 decreased while levels of IL-4, IL-5, IL-13 and TGF-β increased in the mice fed with 20 and 30μ g of β-tubulin. ∗ p < 0.05. No significant changes of the cytokine levels are found in the group fed with 200 μg of β-tubulin.
Fig. 3.
Profile of serum cytokine production at 5 weeks after the primary immunization. The level of IFN-γ decreased while levels of IL-4, IL-5 and IL-10 increased in the mice fed with 20 and 30 μg of β-tubulin. ∗ p < 0.05. No significant changes of the cytokine levels are found in the group treated with 200 μg of β-tubulin.
Fig. 4.
Profile of serum cytokine production at 7 weeks after the primary immunization. The level of IFN-γ decreased while levels of IL-4, IL-13, IL-10 and TGF-β increased in the mice fed with 20 and 30 μg of β-tubulin. ∗ p < 0.05. No significant changes of the cytokine levels are found in the group administered 200 μg of β-tubulin.
These results demonstrate that orally administered β-tubulinin β-tubulin-immunized animals suppressedTh1 (IFN-γ) and increased Th2 and Th3 (IL-4, IL-5, IL-13 and TGF-β). However, the IL-10 level decreased 3 weeks after immunization, but significantly increased at 5 and 7 weeks, suggesting a rebounding effect after an initial inhibition.
However, it is still unclear why oral administration of 200 μg tubulin did not change any cytokine's level at all 3 time points tested in the present study. Furthermore, levels of cytokines, for example the value of IL-4, were fluctuating at 3 time points. This fluctuation is thought to be caused by the amount of the antibody coated in the ELISA. However, this fluctuation does not affect the analysis since all samples from a time point were tested at the same time.
Less Inner Ear Damage Was Found in Low-Dose Beta-Tubulin Administration Groups
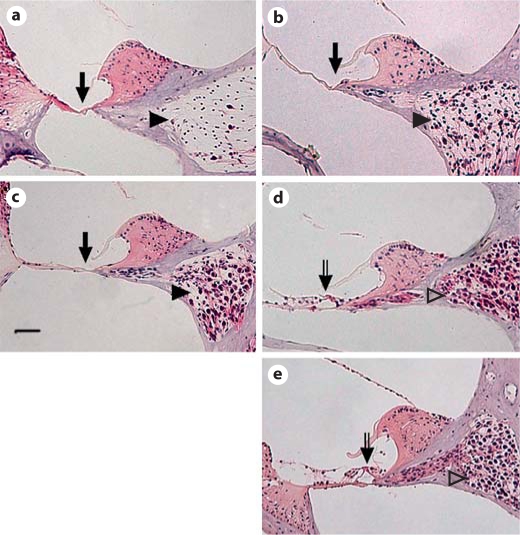
Histopathology of the cochleas was examined 7 weeks after primary immunization. The organ of Corti was missing at the basal turns of the cochleas in the groups treated with PBS, OVA and 200 μg β-tubulin (arrows in fig. 5a–c). Most of the neurons in the spiral ganglion are missing in the PBS and OVA groups (arrowheads in fig. 5a and b). However, most of the neurons in the spiral ganglion are preserved in the group fed with 200 μg β-tubulin (arrowhead in fig. 5c). In contrast, the organ of Corti (open arrows in fig. 5d and e) and neuron in the spiral ganglion (open arrowheads in fig. 5d and e) are preserved in the groups fed with 20 and 30 μg of β-tubulin. The results demonstrate a protection by low-dose oral administration of tubulin to the cochlea and the spiral ganglions.
Fig. 5.
Illustration of hematoxylin-eosin staining in the cochlear basal turn. The inner ear histology illustrates that the cochleas of the PBS (a), OVA (b) and 200-μg groups (c) had the organ of Corti missing (arrows) and neuron loss in the spiral ganglion (arrowheads). However, hair cells in the organ of Corti (open arrows in d, e) and neurons in the spiral ganglion (open arrowheads in d, e) are preserved in groups orally fed with 20 and 30 μg of β-tubulin (d, e). Scale bar = 50 μm.
Discussion
Peripheral tolerance by oral antigen administration has been demonstrated in several autoimmune animal models, including proteolipid protein-induced experimental allergic encephalomyelitis, the diabetic mouse and adjuvant arthritis. In all 3 of these models, autoimmunity to the tolerance does not appear to initiate but suppress the disease when the tolerance is given orally [14,15,16,17,18,19]. This phenomenon is also found in an experimental model of asthma [20]. In the present study, low-dose oral administration of tubulin (20 or 30 μg) is effective in preventing inner ear damage and hearing loss, which are associated with autoimmune inner ear diseases. These results have demonstrated that oral tolerance may be used to treat autoimmune inner ear diseases.
Orally administered antigens can induce tolerance by a numberof mechanisms including anergy, deletion and the induction of regulatory cells that secrete IL-4, IL-10 and TGF-β [21,22,23]. However, the mechanisms of immune tolerance are associated with the dose of antigen fed, whereas higher antigen doses induce anergy or deletion.
Upregulation of IL-4, IL-5, IL-13 and TGF-β and downregulation of IFN-γ have been detected throughout the experimental period (up to 7 weeks) after the administration of the low dose of tubulin. Downregulation of IFN-γ is also found in oral tolerance in the T cell receptor transgenic mice [24, 25] and in murine gut-associated lymphoid tissue [26]. Low antigen doses also favor the induction of Th2-type regulatory T cells that secrete IL-4 and IL-13 and Th3-type regulatory cells that secrete TGF-β and IL-10 [21,22,23, 27, 28], which is supported by our observation. Furthermore, studies using knockout animals have demonstrated that low doses of antigen induceactive suppression, probably via induction of CD4–CD8+ suppressorT cells or mediated by CD4+CD8+ T cells [29]. In the present study, IL-10 is not upregulated until 5 weeks after the primary immunization, suggesting a non-antigen-specific downregulation of this cytokine [30], while secretion of TGF-β is antigen specific [24]. Furthermore, secretion of TGF-β is generally dissociated with IL-10/IL-4 [31].
High-dose-induced tolerance has been found in various animal models. Animalsfed high doses of antigen secrete more IL-4 and less TGF-β [32,33,34,35]. High doses of orally administered antigen resulted in anergy/deletion of specific T cells in the gut and in systemic antigen presentation after antigen has passed through the gut. Presentation of high doses of antigen would induce unresponsiveness of T cell function, primarily via anergy/deletion [32, 33]. In the present study, however, no tolerance has been found in the administration of high doses of tubulin, which is demonstrated by more hearing loss and severer inner ear damage in the mice treated with the high dose of tubulin. Consistent with this observation, no significant cytokine profile changes were observed in the high-dose (200 μg) administration of tubulin. This phenomenon may be related to the dose of tubulin used in the present study, which is about 10 times higher than the low dose (20–30 μg). A relatively lower dose of tubulin, for example 100 μg, has to be tested in future.
Th1 cytokines (IL-2 and IFN-γ) activate macrophages, NK cells and cell-mediated immunity which may play a crucial role in immune-mediated inner ear diseases [36]. Expression of IFN-γ following secondary immune reaction in the endolymphatic sac can then upregulate intercellular adhesion molecule 1 expression and induce cell infiltration [37]; then it may cause inflammatory cell damage in the inner ear. On the other hand, Th2 cytokines favor isotype switching in the humoral immune response and depress macrophage activation and cell-mediated immunity, which antagonize the Th1 cytokines. In the present study, oral low-dose tubulin administration inhibits Th1 cytokines and upregulates Th2 cytokines. Rebalanced Th1 and Th2 cytokines in the inner ear do not favor inflammatory cell infiltration and further protect the hair cells and prevent hearing loss.
In summary, our present study has demonstrated that β-tubulin is orally active in an antigen-specific fashion and capable of inhibiting the autoimmune reactions in the inner ear by suppressing Th1 (IFN-γ) and increasing Th2 and Th3 (IL-4, IL-5, IL-13 and TGF-β) cytokines. Oral antigen tolerance may be used to treat autoimmune inner ear disease in future.
Acknowledgements
We would like to thank Dr. David L. Armbruster (PhD, Scientific Publication Consultations, Health Science Center, University of Tennessee) for reading and commenting on the manuscript. This work was supported by National Institutes of Health grant R01-R073375065.
References
- 1.Satoh H, Firestein GS, Billings PB, Harris JP, Keithley EM. Tumor necrosis factor-α, an initiator, and etanercept, an inhibitor of cochlear inflammation. Laryngoscope. 2002;112:1627–1634. doi: 10.1097/00005537-200209000-00019. [DOI] [PubMed] [Google Scholar]
- 2.Harris JP, Ryan AF. Fundamental immune mechanisms of the brain and inner ear. Otolaryngol Head Neck Surg. 1995;112:639–653. doi: 10.1016/s0194-5998(95)70170-2. [DOI] [PubMed] [Google Scholar]
- 3.Ryan AF, Keithley EM, Harris JP. Autoimmune inner ear disorders. Curr Opin Neurol. 2001;14:35–40. doi: 10.1097/00019052-200102000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Yoo TJ, Stuart JM, Kang AH, Townes AS, Tomoda K, Dixit S. Type II collagen autoimmunity in otosclerosis and Ménière's disease. Science. 1982;217:1153–1155. doi: 10.1126/science.7112122. [DOI] [PubMed] [Google Scholar]
- 5.Suzuki M, Yazawa Y, Yoo TJ, Cheng KC, Krug MS, Bernstein J. Antibodies against inner ear proteins in the sera of patients with inner ear disease. ORL. 1997;59:10–17. doi: 10.1159/000276899. [DOI] [PubMed] [Google Scholar]
- 6.Yoo TJ, Tanaka H, Kwon SS, Krug M, Yazawa Y, Suzuki M, Kitajima K. β-Tubulin as an autoantigen for autoimmune inner ear disease. In: Sterkers O, Ferrary E, Dauman R, Sauvage JP, Tran Bahuy P, editors. Ménière's Disease. The Hague: Kugler; 2000. pp. 529–535. [Google Scholar]
- 7.Du X, Yoo TJ, Mora R. Distribution of beta-tubulin in the guinea pig inner ear. ORL. 2003;65:7–16. doi: 10.1159/000068654. [DOI] [PubMed] [Google Scholar]
- 8.Yoo TJ, Shea J, Jr, Ge X, Kwon SS, Yazawa Y, Sener O, Mora F, Mora R, Mora M, Barbieri M, Du X. Presence of autoantibodies in the sera of Ménière's disease. Ann Otol Rhinol Laryngol. 2001;110:425–429. doi: 10.1177/000348940111000506. [DOI] [PubMed] [Google Scholar]
- 9.Suzuki M, Krug MS, Cheng KC, Yazawa Y, Bernstein J, Yoo TJ. Antibodies against inner ear proteins in the sera of patients with inner ear disease. ORL J Otorhinolaryngol Relat Spec. 1997;59:10–17. doi: 10.1159/000276899. [DOI] [PubMed] [Google Scholar]
- 10.Teitelbaum D, Arnon R, Sela M. Immunomodulation of experimental autoimmune encephalomyelitis by oral administration of copolymer 1. Proc Natl Acad Sci USA. 1999;96:3842–3848. doi: 10.1073/pnas.96.7.3842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maron R, Slavin AJ, Hoffmann E, Komagata Y. Oral tolerance to copolymer 1 in myelin basic protein (MBP) TCR transgenic mice: cross-reactivity with MBP-specific TCR and differential induction of anti-inflammatory cytokines. Int Immunol. 2002;14:131–138. doi: 10.1093/intimm/14.2.131. [DOI] [PubMed] [Google Scholar]
- 12.Benson JM, Stuckman SS, Cox KL. Oral administration of myelin basic protein is superior to myelin in suppressing established relapsing experimental autoimmune encephalomyelitis. J Immunol. 1999;162:6247. [PubMed] [Google Scholar]
- 13.Trune DR, Kempton JB. Female MRL. MpJ-Faslpr autoimmune mice have greater hearing loss than males. Hear Res. 2002;167:170–174. doi: 10.1016/s0378-5955(02)00384-2. [DOI] [PubMed] [Google Scholar]
- 14.Gonnella PA, Kodali D, Weiner HL. Induction of low dose oral tolerance in monocyte chemoattractant protein-1- and CCR2-deficient mice. J Immunol. 2003;170:2316–2322. doi: 10.4049/jimmunol.170.5.2316. [DOI] [PubMed] [Google Scholar]
- 15.Zhang ZJ, Davidson L, Eisenbarth G, Weiner HL. Suppression of diabetes in nonobese diabetic mice by oral administration of porcine insulin. Proc Natl Acad Sci USA. 1991;88:10252–10258. doi: 10.1073/pnas.88.22.10252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim N, Cheng KC, Kwon SS, Mora R, Barbieri M, Yoo TJ. Oral administration of collagen conjugated with cholera toxin induces tolerance to type II collagen and suppresses chondritis in an animal model of autoimmune ear disease. Ann Otol Rhinol Laryngol. 2001;110:646–654. doi: 10.1177/000348940111000710. [DOI] [PubMed] [Google Scholar]
- 17.Nagler-Anderson C, Bober LA, Robinson ME, Siskind GW, Thorbecke GJ. Suppression of type II collagen-induced arthritis by intragastric administration of soluble type II collagen. Proc Natl Acad Sci USA. 1986;83:7443–7446. doi: 10.1073/pnas.83.19.7443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thompson HS, Staines NA. Gastric administration of type II collagen delays the onset and severity of collagen-induced arthritis in rats. Clin Exp Immunol. 1986;64:581–586. [PMC free article] [PubMed] [Google Scholar]
- 19.Chen Y, Inobe J, Weiner HL. Induction of oral tolerance to myelin basic protein in CD8-depleted mice: both CD4+ and CD8+ cells mediate active suppression. J Immunol. 1995;155:910–916. [PubMed] [Google Scholar]
- 20.Nagatani K, Dohi M, To Y, Tanaka R, Okunishi K, Nakagome K, Sagawa K, Tanno Y, Komagata Y, Yamamoto K. Splenic dendritic cells induced by oral antigen administration are important for the transfer of oral tolerance in an experimental model of asthma. J Immunol. 2006;176:1481–1489. doi: 10.4049/jimmunol.176.3.1481. [DOI] [PubMed] [Google Scholar]
- 21.Ke Y, Kapp JA. Oral antigen inhibits priming of CD8+ CTL, CD4+ T cells, and antibody responses while activating CD8+ suppressor T cells. J Immunol. 1996;156:916–921. [PubMed] [Google Scholar]
- 22.Chen Y, Inobe J, Weiner HL. Induction of oral tolerance to myelin basic protein in CD8-depleted mice: both CD4+ and CD8+ cells mediate active suppression. J Immunol. 1995;155:910–916. [PubMed] [Google Scholar]
- 23.Miller A, Lider O, Roberts AB, Sporn MB, Weiner HL. Suppressor T cells generated by oral tolerization to myelin basic protein suppress both in vitro and in vivo immune responses by the release of TGF-β following antigen specific triggering. Proc Natl Acad Sci USA. 1992;89:421–425. doi: 10.1073/pnas.89.1.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen Y, Inobe J, Weiner HL. Inductive events in oral tolerance in the TCR transgenic adoptive transfer model. Cell Immunol. 1997;178:62–68. doi: 10.1006/cimm.1997.1119. [DOI] [PubMed] [Google Scholar]
- 25.Marth T, Strober W, Kelsall BL. High dose oral tolerance in ovalbumin TCR-transgenic mice: systemic neutralization of IL-12 augments TGF-beta secretion and T cell apoptosis. J Immunol. 1996;157:2348–2357. [PubMed] [Google Scholar]
- 26.Spiekermann GM, Nagler-Anderson C. Oral administration of the bacterial superantigen staphylococcal enterotoxin B induces activation and cytokine production by T cells in murine gut-associated lymphoid tissue. J Immunol. 1998;161:5825–5831. [PubMed] [Google Scholar]
- 27.Chen Y, Kuchroo VK, Inobe JI, Hafler DA, Weiner HL. Regulatory T cell clones induced by oral tolerance: suppression of autoimmune encephalomyelitis. Science. 1994;265:1237–1240. doi: 10.1126/science.7520605. [DOI] [PubMed] [Google Scholar]
- 28.Melamed D, Friedman A. Direct evidence for anergy in T lymphocytes tolerized by oral administration of ovalbumin. Eur J Immunol. 1993;23:935–942. doi: 10.1002/eji.1830230426. [DOI] [PubMed] [Google Scholar]
- 29.Smith KM, Eaton AD, Finlayson LM, Garside P. Oral tolerance. Am J Respir Crit Care Med. 2000;162:175–178. doi: 10.1164/ajrccm.162.supplement_3.15tac7. [DOI] [PubMed] [Google Scholar]
- 30.Faria AM, Weiner HL. Oral tolerance: mechanism and therapeutic applications. Adv Immunol. 1999;73:153–264. doi: 10.1016/s0065-2776(08)60787-7. [DOI] [PubMed] [Google Scholar]
- 31.Weiner HL. Induction and mechanism of action of transforming growth factor-β secreting Th3 regulatory cells. Immunol Rev. 2001;182:207–214. doi: 10.1034/j.1600-065x.2001.1820117.x. [DOI] [PubMed] [Google Scholar]
- 32.Whitacre CC, Gienapp IE, Orosz CG, Bitar DM. Oral tolerance in experimental autoimmune encephalomyelitis. III. Evidence for clonal anergy. J Immunol. 1991;147:2155–2163. [PubMed] [Google Scholar]
- 33.Friedman A, Weiner HL. Induction of anergy or active suppression following oral tolerance is determined by antigen dosage. Proc Natl Acad Sci USA. 1994;91:6688–6692. doi: 10.1073/pnas.91.14.6688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miller A, Lider O, Roberts AB, Sporn MB, Weiner HL. Suppressor T cells generated by oral tolerization to myelin basic protein suppress both in vivo and in vitro immune response by the release of transforming growth factor beta after antigen-specific triggering. Proc Natl Acad Sci USA. 1992;89:421–425. doi: 10.1073/pnas.89.1.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen Y, Inobe JI, Marks R, Gonnella P, Kuchroo VK, Weiner HL. Peripheral deletion of antigen-reactive T cells following oral tolerance. Nature. 1995;376:177–180. doi: 10.1038/376177a0. [DOI] [PubMed] [Google Scholar]
- 36.Pawankar R, Tomiyama S, Ikezono T, Nonaka M, Jinnouchi K, Yagi T. Interferon-gamma expression in the inner ear of rats following secondary immune reaction in the endolymphatic sac. Acta Otolaryngol Suppl. 2004;553:6–12. doi: 10.1080/03655230410017580. [DOI] [PubMed] [Google Scholar]
- 37.Tomiyama S. Th1: mediator lymphocytes in experimental autoimmune labyrinthitis. Acta Otolaryngol. 2001;121:673–678. doi: 10.1080/00016480152583601. [DOI] [PubMed] [Google Scholar]