Abstract
Background
Epilepsy is a serious neurological disorder and neurocysticercosis (NCC), the central nervous system infection by the larvae of Taenia solium, is the main cause of acquired epilepsy in developing countries. NCC is becoming more frequent in industrialized countries due to immigration from endemic areas. Previously reported epilepsy incidences range from 30 to 50/100,000 people in industrialized countries and 90 to 122/100,000 people in developing countries.
Objectives
To determine the incidence of epilepsy in a cysticercosis endemic area of Peru.
Methods
A screening survey for possible seizure cases was repeated biannually in this cohort for a period of 5 years (1999–2004) using a previously validated questionnaire. All positive respondents throughout the study were examined by a trained neurologist in the field to confirm the seizure. If confirmed, they were offered treatment, serological testing, neuroimaging (CT scans and MRI) and clinical follow-up.
Results
The cohort study comprised 817 individuals. The overall epilepsy incidence rate was 162.3/100,000 person-years, and for epileptic seizures, 216.6/100,000 person-years. Out of the 8 individuals who had epileptic seizures, 4 had markers for NCC (neuroimaging and/or serology).
Conclusion
The incidence of epilepsy in this area endemic for cysticercosis is one of the highest reported worldwide.
Key Words: Epilepsy, Parasitic infection, Neurocysticercosis, Incidence study
Introduction
The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense, nor the US Government.
M.V. and S.M. are employees of the US Government. This work was prepared as part of their official duties. Title 17 USC § 105 provides that: ‘Copyright protection under this title is not available for any work of the United States Government’. Title 17 U.S.C. § 101 defines a US Government work as a work prepared by a military service member or employee of the U.S. Government as part of that person's official duties.
Epilepsy is one of the most common neurological disorders [1], and neurocysticercosis (NCC), caused by infection of the central nervous system by the helminth Taenia solium, is currently recognized as the main cause of secondary epilepsy in developing countries [2, 3]. This parasitic infection is now considered a public health problem in industrialized countries due to migration of T. solium tapeworm carriers [4,5,6].
Infection is common in endemic areas, reaching seroprevalences between 10 and 25% in the general population [7,8,9,10]. Central nervous system infection with this parasite presents a wide spectrum of symptomatology, ranging from asymptomatic to a very severe, life-threatening, subarachnoid intraventricular disease. However, by far the most common clinical manifestation of NCC is epileptic seizures [3].
Epilepsy is a frequent neurological pathology in developing countries [11], but there is only scarce data about the incidence of NCC-related epilepsy. This study aimed to assess and characterize the incidence of seizures and epilepsy in a cysticercosis endemic population on the northern coast of Peru.
Methods
Study Site
This cohort study was conducted in the rural district of Matapalo, in the Zarumilla River Valley (near the Peru-Ecuador border), between 1999 and 2005. The district of Matapalo includes 7 villages: Isla Noblecilla, Quebrada Seca, Leandro Campos, Matapalo village, Nuevo Progreso, Totora and Tutumo; in this highly commercial border region, commercial activities are the main support for many individuals and they condition the time they spend in their home region. Therefore, specific operational definitions were required (see ‘Operational definitions’).
The northern part of Peru is a known cysticercosis-endemic area. A previous seroepidemiologic survey in the study area using the enzyme-linked immunoelectrotransfer blot (EITB, Western blot) assay found 25% human seroprevalence.
Enrolment and Census
A door-to-door baseline census was carried out for demographic information as part of a large project to control of T. solium taeniasis/cysticercosis. Each record information on individuals, including full name, gender, age and residential status, as well as characteristics of the household (number of persons, number of rooms, water source, sewage, use of latrines, electricity) and presence of pigs in the household. All participants gave their informed consent. The total population in the census as of 1999 was 1,004 persons (57.2% men, 42.8% women, mean age 26.01 ± 19.46). The study was approved by the Institutional Review Boards of the Universidad Peruana Cayetano Heredia and Johns Hopkins University Bloomberg School of Public Health, and was in compliance with all applicable federal regulations governing the protection of human subjects.
Survey
In 1999, a prevalence study was carried out in the district of Matapalo [9]. From the 1,004 enrolled subjects, 903 complied with inclusion criteria and agreed to be part of the study. In this group, 43 individuals were found to have had epileptic seizures. The remaining 860 agreed to participate in this incidence study. A locally adapted form of the validated screening questionnaire used elsewhere [12,13,14] was given to all individuals in the prevalence study cohort. It was administered 10 times by trained field workers between 1999 and 2004. Activities were scheduled to take place every 6 months within the study timeframe (5 years); however, due to logistical reasons, although 2 activities were held every year, they did not necessarily take place at 6-month intervals. The questionnaire comprised 7 questions designed to identify all potential cases of epileptic seizures occurring after the previous survey round. After every survey round, all positive respondents were interviewed in situ by the same team neurologist (S.M.) who obtained a careful seizure history to confirm or reject the diagnosis of seizure according to the criteria of the International League Against Epilepsy [2]. In order to rule out false negatives, the survey carried out in February 2004 did not ask if the individual had presented signs or symptoms of epilepsy since the last survey round, but ever. All individuals who were confirmed as having had a seizure were taken out of the cohort and offered antiepileptic medication and follow-up by the study physicians.
Operational Definitions
Cohort. All voluntary individuals who were residents or temporary residents from the study site, and with negative screening for epileptic seizure in the cross-sectional study.
Resident. An individual who had usually slept in the village at least 2 nights per week since the previous survey.
Temporary Resident. An individual who slept in the village <2 nights per week, but more than once in a month, since the previous survey.
Epileptic Seizure. Clinical manifestation (sudden and transitory abnormal phenomena) presumed to result from the abnormal and excessive discharge of a set of neurons in the brain, perceived by the patient or an observer. It might include alterations in consciousness or motor, sensory, autonomic or psychological events [15]. Febrile seizures and eclampsia were excluded from the analyses.
Epilepsy. Two or more unprovoked epileptic seizures. An episode of status epilepticus or multiple seizures occurring in a 24-hour period were considered to be a single event [15].
Study Outcome. Occurrence of epileptic seizures or epilepsy confirmed by a neurologist.
Study Withdraws. Absence at the first 3 consecutive follow-up surveys due to any cause.
Blood Sampling/Serology
Blood samples were obtained from all individuals with clinically confirmed epileptic seizures. The EITB assay for diagnosis of human NCC using lentil lectin affinity-purified T. solium metacestode glycoprotein antigens [16] was carried out on these samples. All samples were stored at −20°C at the Cysticercosis Working Group's facilities.
Neuroimaging
All patients with a diagnosis of epileptic seizure confirmed by neurological examination were offered a brain CT scan and an MRI in nearby health facilities.
Statistical Analysis
Differences in proportions were compared by χ2 or Fisher exact tests. Incidence rate was defined as the number of new individuals with seizures in the given period over the time during which each person in the study was at risk of developing seizures, i.e. cases/100,000 person-years. All reported probability values are 2-sided; p values of <0.05 are considered to be significant. A logrank test was performed to detect differences between survival curves. A Poisson model was run to determine the covariates that were associated with epilepsy incidence. Statistical analyses were carried out using SPSS version 12.1 (SPSS Corp., Chicago, Ill., USA) and SAS version 9.1 (SAS Institute, Cary, N.C., USA).
Results
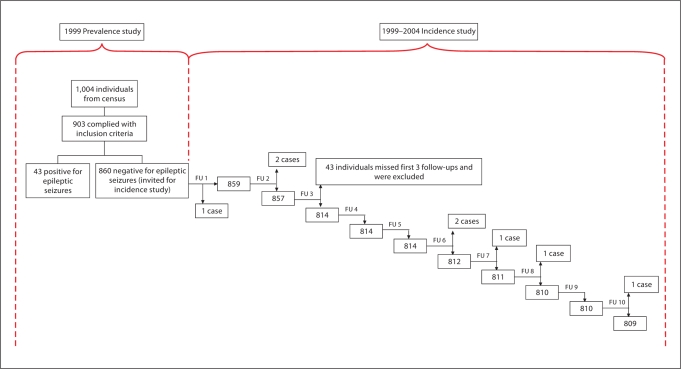
Among the 817 individuals in the cohort who responded to at least 1 of the first 3 surveys (fig. 1), there were 473 (57.9%) men and 344 (42.1%) women. Their mean age was 25.84 years (SD 19.96 years), ranging between <1 and 99 years (36.2% <15 years, 53.8% 15–55 years, 10% ≥56 years).
Fig. 1.
Tree diagram: cohort evolution. Analyses were performed using 817 individuals since 43/860 missed the first 3 follow-ups, and were therefore not included in the incidence calculations. FU = Follow-up.
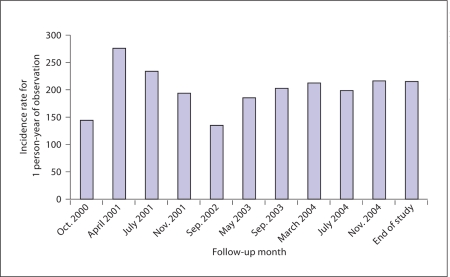
Throughout the 10 survey rounds (from October 1999 to October 2004), we identified 75 individuals with positive responses to possible seizures, 7 of them were confirmed to have had epileptic seizures by the neurologist. One individual presented an initial seizure during survey round number 10 (October 2004) and was confirmed at the neurologist's visit (April 2005). During this visit, this individual asked to be examined, and was included into the epileptic seizures positive group. Therefore, a total of 8 individuals were identified as positive for epileptic seizures in the 5-year period, giving an overall incidence rate of 216.6/100,000 person-years (95% CI: 93.5–427.3; table 1). Among these, 6 had a second seizure, fulfilling the diagnosis of epilepsy (incidence rate: 162.4/100,000 person-years, 95% CI: 59.5–353.6). There was a fairly even distribution of epileptic seizure incidence rates throughout each survey round, as can be seen in figure 2.
Table 1.
Computation of incidence for each period
| Time interval | Positive responders | Epileptic seizure events | Epilepsy cases | Total person- days | Epileptic seizures incidence rate (per 100,000 person-years) | Epilepsy cases incidence rate (per 100,000 person-years) |
|---|---|---|---|---|---|---|
| October 1999–December 2000 | 4 | 2 | 1 | 323,969 | 225.5 | 112.7 |
| January–December 2001 | 6 | 1 | 1 | 284,262 | 128.5 | 128.5 |
| January–December 2002 | 1 | 1 | 1 | 278,954 | 130.9 | 130.9 |
| January–December 2003 | 2 | 3 | 3 | 267,279 | 410.0 | 410.0 |
| January–December 2004 (includes to April 2005) | 62 | 1 | 0 | 194,583 | 187.7 | 0 |
| Overall incidence rate | 75 | 8 | 6 | 1,349,047 | 216.6 | 162.3 |
Fig. 2.
Cumulative incidence rates throughout the follow-up period.
Although few incident cases were identified, the difference in mean ages between the positive group and the negative group was significant (19.4 vs. 25.9, respectively; p < 0.05; table 2). All of the 8 cases occurred between 10 and 29 years of age, with a significant association between age and occurrence of epileptic seizures (p = 0.002, Fisher's exact test). Other nonsignificant variables are shown in table 2.
Table 2.
Residency, age, sex, Western blot results, number of follow-ups and days in the study for epileptics vs. nonepileptics
| Proportion of residents (vs. temporary residents), % | Age, years | Males, % | Western blot positive, % | Follow- up visits, n | Time in the Study, days | |
|---|---|---|---|---|---|---|
| Persons diagnosed with epileptic seizures (n = 8) | 87.5 | 19.4 ± 5.32∗ | 25.0 | 25.0 | 5.3 (3.53)∗ | 1,251.6 (693.25) |
| Nonepileptic persons (n = 809) | 97.4 | 25.9 ± 20.05∗ | 58.2 | 76.1 | 8.2 (2.29)∗ | 1,618.6 (405.90) |
Figures in parentheses are SD.
p = 0.05, between-groups difference.
When the Poisson model was applied, males were found to have a lower incidence of epilepsy than females (incidence rate ratio = 0.21); a positive Western blot was associated with 160% higher risk for epileptic seizures, although this was not significant. The association between age and incidence showed a curvilinear pattern, with a higher incidence in young ages up to approximately 20 years, which then decreased (table 3).
Table 3.
Poisson model (exposure offset)
| Parameter | Estimate | IRR | p value | 95% CI | |
|---|---|---|---|---|---|
| Intercept | −13.5940 | 0.001 | −21.8033 | −5.3847 | |
| Males | −1.5705 | 0.2080 | 0.05 | −3.1998 | 0.0589 |
| Temporary resident | 0.3029 | 1.3537 | 0.71 | −1.3077 | 1.9135 |
| Positive WB | 0.9556 | 2.6002 | 0.41 | −1.2992 | 3.2104 |
| Age | 0.8879 | 2.4301 | 0.02 | 0.1166 | 1.6592 |
| Age × age | −0.0219 | 0.9783 | 0.01 | −0.0395 | −0.0044 |
IRR = Incidence rate ratio; WB = Western blot.
Serology
From the 8 individuals who presented epileptic seizures, 2 had a positive Western blot at baseline. A third individual had turned positive by the time he had his first seizure. During the serological follow-up that was carried out on these 8 patients, 1 of the seropositive participants turned negative.
Neuroimaging
The 8 individuals who developed seizures had MRI performed, but only 7 had CT scans (the eighth was a pregnant woman). Three were considered suggestive of NCC: a patient had 2 calcifications in the left frontal lobe, another had 2 calcifications in the left frontal lobe and 1 in the right frontal lobe, and the third individual had 1 calcification in the right temporal lobe. A fourth positive CT was not considered suggestive of NCC because the individual had brain calcifications near the falx, which could have been physiological calcifications.
Discussion
A previous study carried out in 1999 described Matapalo as a high prevalence area for epilepsy and for neurocysticercosis. The district had a prevalence of epilepsy of 32.1/1,000, and a prevalence of active epilepsy of 16.6/ 1,000; the seroprevalence was 24.2% and an estimated NCC-attributable fraction of epilepsy of between 25 and 30% was calculated [9].
The present study adds to a short list of publications related to epilepsy incidence in nonindustrialized countries, and even a shorter list of epilepsy incidence related to NCC.
There is a considerable heterogeneity in the rates of seizures and epilepsy between different publications, both between countries and different cities of the same country, due to etiologic, methodological and sociocultural reasons. However, despite this heterogeneity and the wide confidence intervals, the incidences we report are higher than those of other developing countries. For example, in the Mariana Islands, an annual incidence between 30 and 47.3 per 100,000 was found [2, 17, 18]; in Salamá, Honduras, the incidence was between 30.9 and 92.7 per 100,000 [19], and in the northern Ecuadorian Andean region the incidence was 122 per 100,000/person-years [20]. Industrialized countries have age-adjusted incidences of epilepsy from 24 to 53 per 100,000 person-years [18,21,22,23,24,25].
This study was not designed to evaluate the association between neurocysticercosis and epilepsy, mainly due to the huge sample size required to test associations in an incidence study. However, 4 out of the 8 new cases had markers of NCC (either compatible brain CT findings or positive serology), and the age distribution showed all new cases occurring in age strata compatible with secondary epilepsies. In population surveys in rural areas, serology and imaging for NCC are frequently discrepant. The majority of NCC cases are individuals with calcified brain lesions only, as previously reported in Guatemala [26], Honduras [27], Ecuador [12] and Mexico [28], and a significant proportion of them may have negative serology because of the antibody reaction wanes months or years after the parasites die. This would agree with a short report by Garcia et al. [29], which showed that approximately 40% of seropositive individuals turn seronegative in about 1 year. On the other hand, positive serology (as for most infectious diseases) does not necessarily represent established infection. Thus, T. solium is a dynamic infection, and many newly exposed or infected people will develop only a transient serologic antibody reaction. Nevertheless, although 2 of our 8 participants with seizures were seropositive at the beginning of the study (which correlates with the 24% previously described in the area [9]), there was a third participant who turned seropositive by the time of the seizures debut; this means that at a point in the timeframe of the study, the seroprevalence was 37.5%; however, given the number of cases, no further conclusions can be made related to this.
Population studies in rural areas of developing countries have shown that most individuals present the onset of seizures in the first 2 decades of life [23, 25, 30, 31]. This observation was confirmed in Matapalo. In the study period, no individual was younger than 10 years old, nor older than 29 years old at the onset of epilepsy. While seizures in older ages may decrease due to increased mortality in elders, it was remarkable that the age range of all incident seizure cases was 10–29 years, and not at earlier ages. Most studies report no differences between genders, or a slightly higher prevalence and incidence in men [9, 32]. In our study area, women had a higher incidence of epileptic seizures, as per the previously calculated prevalence [9].
The incidence rate for epileptic seizures throughout the follow-up was fairly even, which reassures us that the methodology was properly applied (fig. 2). Most (approx. 85%) of the global burden of epilepsy resides in the developing world [33]. This is not only due to a higher population denominator, but also to increased rates. The coexistence of increased rates and neurocysticercosis supports a contributory role for NCC in the etiology of epilepsy at a population level [11].
Acknowledgements
This study was partially funded by research grants numbers P01 AI51976, U01 AI35894 and D43 TW001140 from the National Institute of Allergy and Infectious Diseases, NIH, USA. Research grants from the Wellcome Trust, the Bill and Melinda Gates Foundation, the National Institute of Neurological Diseases and Stroke, and the Fogarty Center, NIH, USA, support other research by the authors.
References
- 1.Scott RA, Lhatoo SD, Sander JW. The treatment of epilepsy in developing countries: where do we go from here? Bull World Health Organ. 2001;79:344–351. [PMC free article] [PubMed] [Google Scholar]
- 2.Relationship between epilepsy and tropical diseases. Commission on Tropical Diseases of the International League Against Epilepsy. Epilepsia. 1994;35:89–93. [PubMed] [Google Scholar]
- 3.Garcia HH, Gonzalez AE, Evans CA, Gilman RH. Taenia solium cysticercosis. Lancet. 2003;362:547–556. doi: 10.1016/S0140-6736(03)14117-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garcia HH, Araoz R, Gilman RH, Valdez J, Gonzalez AE, Gavidia C, Bravo ML, Tsang VC. Increased prevalence of cysticercosis and taeniasis among professional fried pork vendors and the general population of a village in the Peruvian highlands. Cysticercosis Working Group in Peru. Am J Trop Med Hyg. 1998;59:902–905. doi: 10.4269/ajtmh.1998.59.902. [DOI] [PubMed] [Google Scholar]
- 5.Schantz PM, Wilkins P, Tsang VC. Immigrants, imaging and immunoblots: the emergence of neurocysticercosis as a significant public health problem. In: Scheld WM, Craig WA, Hughes JM, editors. Emerging Infections. Washington: American Society for Microbiology; 1998. pp. 213–241. [Google Scholar]
- 6.White AC., Jr Neurocysticercosis: updates on epidemiology, pathogenesis, diagnosis, and management. Annu Rev Med. 2000;51:187–206. doi: 10.1146/annurev.med.51.1.187. [DOI] [PubMed] [Google Scholar]
- 7.Bern C, Garcia HH, Evans C, Gonzalez AE, Verastegui M, Tsang VC, Gilman RH. Magnitude of the disease burden from neurocysticercosis in a developing country. Clin Infect Dis. 1999;29:1203–1209. doi: 10.1086/313470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garcia HH, Gilman RH, Gonzalez AE, Verastegui M, Rodriguez S, Gavidia C, Tsang VC, Falcon N, Lescano AG, Moulton LH, Bernal T, Tovar M. Hyperendemic human and porcine Taenia solium infection in Peru. Am J Trop Med Hyg. 2003;68:268–275. [PubMed] [Google Scholar]
- 9.Montano SM, Villaran MV, Ylquimiche L, Figueroa JJ, Rodriguez S, Bautista CT, Gonzalez AE, Tsang VC, Gilman RH, Garcia HH. Neurocysticercosis: association between seizures, serology, and brain CT in rural Peru. Neurology. 2005;65:229–233. doi: 10.1212/01.wnl.0000168828.83461.09. [DOI] [PubMed] [Google Scholar]
- 10.Garcia HH, Parkhouse RM, Gilman RH, et al. Serum antigen detection in the diagnosis, treatment, and follow-up of neurocysticercosis patients. Trans R Soc Trop Med Hyg. 2000;94:673–676. doi: 10.1016/s0035-9203(00)90228-1. [DOI] [PubMed] [Google Scholar]
- 11.Zarrelli MM, Beghi E, Rocca WA, Hauser WA. Incidence of epileptic syndromes in Rochester, Minnesota: 1980–1984. Epilepsia. 1999;40:1708–1714. doi: 10.1111/j.1528-1157.1999.tb01587.x. [DOI] [PubMed] [Google Scholar]
- 12.Del Brutto OH, Santibanez R, Idrovo L, Rodriguez S, Diaz-Calderon E, Navas C, Gilman RH, Cuesta F, Mosquera A, Gonzalez AE, Tsang VC, Garcia HH. Epilepsy and neurocysticercosis in Atahualpa: a door-to-door survey in rural coastal Ecuador. Epilepsia. 2005;46:583–587. doi: 10.1111/j.0013-9580.2005.36504.x. [DOI] [PubMed] [Google Scholar]
- 13.Placencia M, Sander JW, Shorvon SD, Ellison RH, Cascante SM. Validation of a screening questionnaire for the detection of epileptic seizures in epidemiological studies. Brain. 1992;115(part 3):783–794. doi: 10.1093/brain/115.3.783. [DOI] [PubMed] [Google Scholar]
- 14.Placencia M, Suarez J, Crespo F, Sander JW, Shorvon SD, Ellison RH, Cascante SM. A large-scale study of epilepsy in Ecuador: methodological aspects. Neuroepidemiology. 1992;11:74–84. doi: 10.1159/000110915. [DOI] [PubMed] [Google Scholar]
- 15.Guidelines for epidemiologic studies on epilepsy. Commission on Epidemiology and Prognosis, International League Against Epilepsy. Epilepsia. 1993;34:592–596. doi: 10.1111/j.1528-1157.1993.tb00433.x. [DOI] [PubMed] [Google Scholar]
- 16.Tsang VC, Brand JA, Boyer AE. An enzyme-linked immunoelectrotransfer blot assay and glycoprotein antigens for diagnosing human cysticercosis (Taenia solium) J Infect Dis. 1989;159:50–59. doi: 10.1093/infdis/159.1.50. [DOI] [PubMed] [Google Scholar]
- 17.Grill J, Rakotomalala W, Andriantsimahavandy A, Boisier P, Guyon P, Roux J, Esterre P. High prevalence of serological markers of cysticercosis among epileptic Malagasy children. Ann Trop Paediatr. 1996;16:185–191. doi: 10.1080/02724936.1996.11747824. [DOI] [PubMed] [Google Scholar]
- 18.Rajshekhar V. Etiology and management of single small CT lesions in patients with seizures: understanding a controversy. Acta Neurol Scand. 1991;84:465–470. doi: 10.1111/j.1600-0404.1991.tb04996.x. [DOI] [PubMed] [Google Scholar]
- 19.Sotelo J, Guerrero V, Rubio F. Neurocysticercosis: a new classification based on active and inactive forms: a study of 753 cases. Arch Intern Med. 1985;145:442–445. [PubMed] [Google Scholar]
- 20.Carpio A, Escobar A, Hauser WA. Cysticercosis and epilepsy: a critical review. Epilepsia. 1998;39:1025–1040. doi: 10.1111/j.1528-1157.1998.tb01287.x. [DOI] [PubMed] [Google Scholar]
- 21.Hauser WA, Hesdorffer D. Epilepsy: frequency, causes and consequences. New York: Demos Medical; 1990. pp. 93–118. [Google Scholar]
- 22.Kammerman S, Wasserman L. Seizure disorders. 1. Classification and diagnosis. West J Med. 2001;175:99–103. doi: 10.1136/ewjm.175.2.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Medina MT, Duron RM, Martinez L, Osorio JR, Estrada AL, Zuniga C, Cartagena D, Collins JS, Holden KR. Prevalence, incidence, and etiology of epilepsies in rural Honduras: the Salama Study. Epilepsia. 2005;46:124–131. doi: 10.1111/j.0013-9580.2005.11704.x. [DOI] [PubMed] [Google Scholar]
- 24.Rajshekhar V, Chandy MJ. Comparative study of CT and MRI in patients with seizures and a solitary cerebral cysticercus granuloma. Neuroradiology. 1996;38:542–546. doi: 10.1007/BF00626094. [DOI] [PubMed] [Google Scholar]
- 25.Shorvon SD. Epidemiology, classification, natural history, and genetics of epilepsy. Lancet. 1990;336:93–96. doi: 10.1016/0140-6736(90)91603-8. [DOI] [PubMed] [Google Scholar]
- 26.Garcia-Noval J, Moreno E, de Mata F, Soto de Alfaro H, Fletes C, Craig PS, Allan JC. An epidemiological study of epilepsy and epileptic seizures in two rural Guatemalan communities. Ann Trop Med Parasitol. 2001;95:167–175. doi: 10.1080/00034980120050260. [DOI] [PubMed] [Google Scholar]
- 27.Sanchez AL, Lindback J, Schantz PM, Sone M, Sakai H, Medina MT, Ljungstrom I. A population-based, case-control study of Taenia solium taeniasis and cysticercosis. Ann Trop Med Parasitol. 1999;93:247–258. [PubMed] [Google Scholar]
- 28.Fleury A, Gomez T, Alvarez I, Meza D, Huerta M, Chavarria A, Carrillo Mezo RA, Lloyd C, Dessein A, Preux PM, Dumas M, Larralde C, Sciutto E, Fragoso G. High prevalence of calcified silent neurocysticercosis in a rural village of Mexico. Neuroepidemiology. 2003;22:139–145. doi: 10.1159/000068748. [DOI] [PubMed] [Google Scholar]
- 29.Garcia HH, Gonzalez AE, Gilman RH, Palacios LG, Jimenez I, Rodriguez S, Verastegui M, Wilkins P, Tsang VC. Short report: transient antibody response in Taenia solium infection in field conditions – a major contributor to high seroprevalence. Am J Trop Med Hyg. 2001;65:31–32. doi: 10.4269/ajtmh.2001.65.31. [DOI] [PubMed] [Google Scholar]
- 30.Hauser WA, Hesdorffer D. Epidemiology of epilepsy. In: Schoenberg B, Anderson DW, Schoenberg DG, editors. Neuroepidemiology: A Tribute to Bruce Schoenberg. Boca Raton: CRC Press; 1991. pp. 97–119. [Google Scholar]
- 31.Senanayake N, Roman GC. Epidemiology of epilepsy in developing countries. Bull World Health Organ. 1993;71:247–258. [PMC free article] [PubMed] [Google Scholar]
- 32.Hauser WA, Annegers JF, Rocca WA. Descriptive epidemiology of epilepsy: contributions of population-based studies from Rochester, Minnesota. Mayo Clin Proc. 1996;71:576–586. doi: 10.4065/71.6.576. [DOI] [PubMed] [Google Scholar]
- 33.Burneo JG, Tellez-Zenteno J, Wiebe S. Understanding the burden of epilepsy in Latin America: a systematic review of its prevalence and incidence. Epilepsy Res. 2005;66:63–74. doi: 10.1016/j.eplepsyres.2005.07.002. [DOI] [PubMed] [Google Scholar]