Abstract
Genetic studies have led to major discoveries in the pathogenesis of various neurodegenerative diseases. Ubiquitin-positive familial frontotemporal dementia was recently found to be caused by mutations in the progranulin gene (PGRN), and the major constituent of the inclusions, TDP-43, was subsequently identified. The tau gene (MAPT) causes frontotemporal dementia with parkinsonism linked to chromosome 17. In Parkinson disease, LRRK2 mutations have emerged as a major cause of both familial and sporadic forms, adding to the previously known genes SNCA, PRKN, DJ1 and PINK1. Several genes have been implicated in Alzheimer disease, including the APP gene and the PSEN genes. Recently, variants in the sortilin-related receptor 1 gene, SORL1, were associated with Alzheimer disease.
Key Words: Alzheimer disease, Dementia, Frontotemporal dementia, Frontotemporal lobar degeneration, Neurodegeneration, Parkinson disease
Introduction
Recent discoveries have advanced the understanding of the mechanisms involved in neurodegenerative disorders. The sporadic form of most of these diseases predominates; this review highlights some recent developments in the genetics of familial forms of neurodegenerative diseases.
Frontotemporal Dementia
About 50% of patients with frontotemporal dementia (FTD) report a positive family history, underlining the relevance of genetic research in this disease [1]. Although the gene responsible for FTD with parkinsonism linked to chromosome 17q21 has been known for 10 years [2], several families show linkage to the same chromosomal region, but no identifiable mutation in the tau gene (MAPT) could be found. A subset of FTD cases lacks tau pathology, displaying frontotemporal lobar dementia with ubiquitin-positive cytoplasmic and intranuclear inclusions (FTLD-U). Last year, FTLD-U was found to be caused by mutations in the progranulin gene (PGRN), located less than 2 Mb centromeric from MAPT [3]. The ubiquitinated protein in the intraneuronal inclusions was shown to be TDP-43 [4]. Mutations in MAPT and PGRN account for 50–60% of familial cases of FTD.
Carriers of PGRN mutations present in their late 50s with behavioral and language difficulties (table 1). Parkinsonism occurs later. The disease progresses to more diffuse dementia, leading to death. Although FTD patients with PGRN and MAPT mutations share similar features, clinical and pathologic findings differ (table 1).
Table 1.
Characteristics of FTD caused by MAPT and PGRN mutations
| Characteristic | FTDP-17 (MAPT) | FTLD-U (PGRN) |
|---|---|---|
| Mean onset age, years | 49 (25–76) | 59 (48–83) |
| Clinical presentation | Behavioral changes or parkinsonism, supranuclear gaze palsy, dystonia | Behavioral changes, language impairment, rarely parkinsonism, CBS, MND |
| Mean disease duration, years | 7 (2–30) | 7 (2–17) |
| Pathology | FTLD, tau-immunoreactive inclusions | FTLD, ubiquitin immuno- reactive/TDP-43-positive intranuclear and cytoplasmic inclusions |
Figures in parentheses indicate ranges. CBS = Corticobasal syndrome; FTDP-17 = frontotemporal dementia with parkinsonism linked to chromosome 17; FTLD = fronto-temporal lobar degeneration; MND = motor neuron disease.
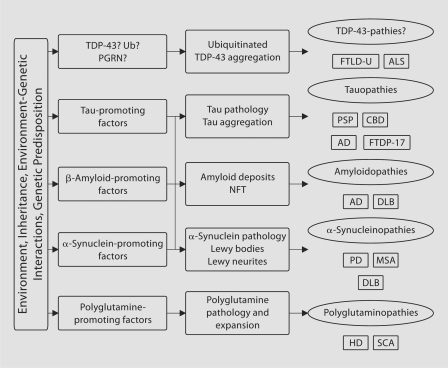
On the pathologic level, ubiquitinated TDP-43-positive inclusions are found in motor neuron disease [4] and may therefore necessitate a change in the existing classification of neurodegenerative diseases, introducing the ‘TDP-43-pathies’ (fig. 1).
Fig. 1.
Classification of neurodegenerative diseases, including recent discoveries. AD = Alzheimer disease; ALS = amyotrophic lateral sclerosis; CBD = corticobasal degeneration; DLB = diffuse Lewy body disease; FTDP-17 = frontotemporal dementia with parkinsonism linked to chromosome 17; HD = Huntington disease; MSA = multiple system atrophy; NFT = neurofibrillary tangles; PD = Parkinson disease; PGRN = progranulin; PSP = progressive supranuclear palsy; SCA = spinocerebellar atrophy; TDP-43 = TAR DNA-binding protein; Ub = ubi quitin.
The mechanism by which PGRN mutations cause FTLD-U remains elusive and little is known about the putative role of TDP-43. In this regard, the ongoing research effort will be critical to establish the link between the genetic defect, the neuronal inclusions and the phenotype.
Alzheimer Disease
About 100 years after the first description of Alzheimer disease (AD), much insight has been gained regarding the mechanisms leading to cerebral atrophy, neuronal loss, plaques (predominantly amyloid deposits) and neurofibrillary tangle (predominantly tau deposits) formation [5]. The most common cause of familial AD is a mutation in PSEN1, the product of which is implicated in the cleavage of the amyloid precursor protein (APP) to β-amyloid (Aβ). Mutations in PSEN1 and PSEN2 increase the ratio of Aβ42 to Aβ40[6]. Point mutations and duplications in APP, the gene coding for APP, increase plaque formation, by promoting synthesis of Aβ or its most pathogenic form, Aβ42[7, 8].
Most cases of AD are sporadic, and the most consistent and well-established genetic risk factor is the inheritance of the apolipoprotein E ∊4 allele [9]. Several other polymorphisms may alter the risk of developing AD [10]. Variants in a new gene, the sortilin-related receptor 1 gene (SORL1), have been associated with AD [11]. The SORL1 protein may play a role in the metabolism of APP: the reduction of its expressions shifts APP towards the β-secretase complex, hence increasing Aβ production.
The study of these genes has dramatically altered our understanding of the mechanisms implicated in AD. In particular, most of these mutations act by favoring amyloid deposition, indicating a tight link between the familial and the sporadic forms of AD. Based on such knowledge, new treatments will aim at modulating tau and amyloid deposition, hoping to prevent the disease or to slow its progression.
Parkinson Disease
The discovery of seven genes and numerous loci associated with Parkinson disease (PD) has identified several pathways implicated in neurodegeneration of the nigrostriatal system (table 2) [12]. As a major component of Lewy bodies, α-synuclein tends to aggregate under certain conditions, which are favored by mutations. An inverse relation between α-synuclein dose and disease onset age was found in duplications and triplications of the gene [13]. Aggregation depends on the ubiquitin-proteasome system, in which both the parkin proteinand the UCHL-1protein play a significant role. The Lrrk2 protein has several functions, including a kinase domain. Mutations may be pathogenic through a toxic gain-of-function mechanism including increased autophosphorylation [14]. In addition, DJ1, parkin and PINK1are all implicated in mitochondrial metabolism and therefore in oxidative stress. This is relevant to the etiology of PD, because several environmental neurotoxins, including MPTP, rotenone and paraquat, act on mitochondrial complex I and inflict nigrostriatal damage, with a parkinsonian phenotype similar to that of PD [15].
Table 2.
Genes associated with PD
| Gene (locus) | Inheritance | Age of onset, years | Phenotype |
|---|---|---|---|
| SNCA (PARK1/4) | AD | 20–85 (point mutations) | PD, sometimes with dementia |
| 38–65 (duplications) | (point mutations) | ||
| 24–48 (triplications) | DLBD (multiplications) | ||
| PRKN (PARK2) | AR | 16–72 (mean 30) | EOPD |
| UCHL1 (PARK5) | AD (equivocal) | 55–58 | PD |
| PINK1 (PARK6) | AR | 20–40 | EOPD |
| DJ1 (PARK7) | AR | 20–40 | EOPD |
| LRRK2 (PARK8) | AD | 32–79 | PD |
| OMI/HTRA2 (PARK13) | NA | 49–77 | PD |
AD = Autosomal dominant; AR = autosomal recessive; DLBD = diffuse Lewy body disease; EOPD = early-onset PD; NA = not applicable.
LRRK2 mutations and variants have emerged as a major cause of both familial and sporadic PD. The LRRK2 gene accounts for 1–2% of sporadic cases in the Caucasian population, whereas occurrence is lower in Asia and much higher in other populations (Ashkenazi Jews, North African Arabs). LRRK2 mutations are found in about 10% of families with autosomal dominant parkinsonism [16]. The most common mutation is G2019S in exon 41. In Europe, the frequency of this mutation has been reported to be similar among sporadic (1.9%) [17] and familial (2.9%) [18] cases. Penetrance depends on age, but the reported lifetime prevalence varies from 32% [19] to 85% [20]. The penetrance may be influenced by other factors, including yet undiscovered new genes.
Aside from mutations causing mendelian inherited parkinsonism, several variants in SNCA and LRRK2 have been associated with PD. Of special interest is LRRK2 G2385A, a common variant associated with PD in Asia [21]. In the ethnic Chinese population, this variant has been observed in 5% of otherwise normal individuals, in 10% of sporadic PD cases and in 20% of familial PD cases [22]. Given the size of the Chinese population, this observation may represent the strongest genetic risk factor for PD identified so far.
Acknowledgements
Editing, proofreading, and reference verification were provided by the Section of Scientific Publications, Mayo Clinic. C.W. received support from the Swiss National Science Foundation, the Swiss Parkinson's Disease Foundation, and the Robert and Clarice Smith Fellowship program. Z.K.W. is supported by the Morris K. Udall NIH/NINDS Parkinson Disease Center of Excellence Grant awarded to the Mayo Clinic Jacksonville (P01 NS0256).
References
- 1.Rosso SM, Donker KL, Baks T, et al. Frontotemporal dementia in the Netherlands: patient characteristics and prevalence estimates from a population-based study. Brain. 2003;126:2016–2022. doi: 10.1093/brain/awg204. [DOI] [PubMed] [Google Scholar]
- 2.Hutton M, Lendon CL, Rizzu P, et al. Association of missense and 5′-splice-site mutations in tau with the inherited dementia FTDP-17. Nature. 1998;393:702–705. doi: 10.1038/31508. [DOI] [PubMed] [Google Scholar]
- 3.Baker M, Mackenzie IR, Pickering-Brown SM, et al. Mutations in progranulin cause tau-negative frontotemporal dementia linked to chromosome 17. Nature. 2006;442:916–919. doi: 10.1038/nature05016. [DOI] [PubMed] [Google Scholar]
- 4.Neumann M, Sampathu DM, Kwong LK, et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314:130–133. doi: 10.1126/science.1134108. [DOI] [PubMed] [Google Scholar]
- 5.Goedert M, Spillantini MG. A century of Alzheimer's disease. Science. 2006;314:777–781. doi: 10.1126/science.1132814. [DOI] [PubMed] [Google Scholar]
- 6.Levy-Lahad E, Wasco W, Poorkaj P, et al. Candidate gene for the chromosome 1 familial Alzheimer's disease locus. Science. 1995;269:973–977. doi: 10.1126/science.7638622. [DOI] [PubMed] [Google Scholar]
- 7.Goate A, Chartier-Harlin MC, Mullan M, et al. Segregation of a missense mutation in the amyloid precursor protein gene with familial Alzheimer's disease. Nature. 1991;349:704–706. doi: 10.1038/349704a0. [DOI] [PubMed] [Google Scholar]
- 8.Rovelet-Lecrux A, Hannequin D, Raux G, et al. APP locus duplication causes autosomal dominant early-onset Alzheimer disease with cerebral amyloid angiopathy. Nat Genet. 2006;38:24–26. doi: 10.1038/ng1718. [DOI] [PubMed] [Google Scholar]
- 9.Farrer LA, Cupples LA, Haines JL, et al. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. JAMA. 1997;278:1349–1356. [PubMed] [Google Scholar]
- 10.Bertram L, McQueen MB, Mullin K, Blacker D, Tanzi RE. Systematic meta-analyses of Alzheimer disease genetic association studies: the AlzGene database. Nat Genet. 2007;39:17–23. doi: 10.1038/ng1934. [DOI] [PubMed] [Google Scholar]
- 11.Rogaeva E, Meng Y, Lee JH, et al. The neuronal sortilin-related receptor SORL1 is genetically associated with Alzheimer disease. Nat Genet. 2007;39:168–177. doi: 10.1038/ng1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Farrer MJ. Genetics of Parkinson disease: paradigm shifts and future prospects. Nat Rev Genet. 2006;7:306–318. doi: 10.1038/nrg1831. [DOI] [PubMed] [Google Scholar]
- 13.Ibanez P, Bonnet AM, Debarges B, et al. Causal relation between alpha-synuclein gene duplication and familial Parkinson's disease. Lancet. 2004;364:1169–1171. doi: 10.1016/S0140-6736(04)17104-3. [DOI] [PubMed] [Google Scholar]
- 14.West AB, Moore DJ, Biskup S, et al. Parkinson's disease-associated mutations in leucine-rich repeat kinase 2 augment kinase activity. Proc Natl Acad Sci USA. 2005;102:16842–16847. doi: 10.1073/pnas.0507360102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abou-Sleiman PM, Muqit MM, Wood NW. Expanding insights of mitochondrial dysfunction in Parkinson's disease. Nat Rev Neurosci. 2006;7:207–219. doi: 10.1038/nrn1868. [DOI] [PubMed] [Google Scholar]
- 16.Di FA, Tassorelli C, De MM, et al. Comprehensive analysis of the LRRK2 gene in sixty families with Parkinson's disease. Eur J Hum Genet. 2006;14:322–331. doi: 10.1038/sj.ejhg.5201539. [DOI] [PubMed] [Google Scholar]
- 17.Lesage S, Janin S, Lohmann E, et al. LRRK2 exon 41 mutations in sporadic Parkinson disease in Europeans. Arch Neurol. 2007;64:425–430. doi: 10.1001/archneur.64.3.425. [DOI] [PubMed] [Google Scholar]
- 18.Lesage S, Ibanez P, Lohmann E, et al. G2019S LRRK2 mutation in French and North African families with Parkinson's disease. Ann Neurol. 2005;58:784–787. doi: 10.1002/ana.20636. [DOI] [PubMed] [Google Scholar]
- 19.Goldwurm S, Zini M, Mariani L, et al. Evaluation of LRRK2 G2019S penetrance: relevance for genetic counseling in Parkinson disease. Neurology. 2007;68:1141–1143. doi: 10.1212/01.wnl.0000254483.19854.ef. [DOI] [PubMed] [Google Scholar]
- 20.Kachergus J, Mata IF, Hulihan M, et al. Identification of a novel LRRK2 mutation linked to autosomal dominant parkinsonism: evidence of a common founder across European populations. Am J Hum Genet. 2005;76:672–680. doi: 10.1086/429256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Di FA, Wu-Chou YH, Lu CS, et al. A common missense variant in the LRRK2 gene, Gly2385Arg, associated with Parkinson's disease risk in Taiwan. Neurogenetics. 2006;7:133–138. doi: 10.1007/s10048-006-0041-5. [DOI] [PubMed] [Google Scholar]
- 22.Ross OA, Farrer MJ, Wu RM. Common variants in Parkinson's disease. Mov Disord. 2007;22:899–900. doi: 10.1002/mds.21463. [DOI] [PubMed] [Google Scholar]