Abstract
Background
Fibers containing galanin (GAL) enlarge and hyperinnervate cholinergic basal forebrain (CBF) nucleus basalis (NB) neurons in late-stage Alzheimer's disease (AD), yet the physiological consequences of this phenomenon are unclear.
Objective
To determine whether GAL hyperinnervation of cholinergic NB neurons modulates the expression of genes critical to cholinergic transmission [e.g. acetylcholine (ACh) metabolism and ACh receptors] in AD.
Methods
Single-cell gene expression profiling was used to compare cholinergic mRNA levels in non-GAL-hyperinnervated NB neurons in tissue autopsied from cases classified as having no cognitive impairment (NCI) or late-stage AD (AD/GAL–) and in GAL-hyperinnervated (AD/GAL+) NB neurons from the same AD subjects.
Results
AD/GAL+ cells displayed a significant upregulation in choline acetyltransferase (ChAT) mRNA expression compared to NCI and AD/GAL– cells.
Conclusion
GAL fiber hyperinnervation of cholinergic NB neurons upregulates the expression of ChAT, the synthetic enzyme for ACh, suggesting that GAL regulates the cholinergic tone of CBF neurons in AD.
Key Words: Galanin, Alzheimer's disease, Cholinergic basal forebrain, Choline acetyltransferase
Introduction
The neuropeptide galanin (GAL) regulates the activity of cholinergic basal forebrain (CBF) neurons [1, 2] which modulate attention and memory [3]. CBF cortical projection neurons of the nucleus basalis (NB) undergo selective degeneration during the later stages of Alzheimer's disease (AD) [4]. Several groups have made the striking observation that hypertrophic GAL-containing fibers hyperinnervate surviving cholinergic NB neurons in late-stage AD [5,6,7,8]. However, the functional consequences of this GAL neuroplasticity response remain unknown. To approach this problem from a molecular profiling standpoint, we combined single-cell RNA amplification and custom-designed cDNA array analysis to compare cholinergic gene expression profiles of individual non-GAL-hyperinnervated NB neurons aspirated from subjects with no cognitive impairment (NCI) or with AD (AD/GAL–), and of GAL-hyperinnervated (AD/GAL+) NB neurons from the same AD cases.
Methods
Tissue Selection
Brain tissue from persons clinically classified before death as having NCI [n = 6; Mini Mental State Exam score = 27.8 ± 1.3 (mean ± SD)] or AD (n = 6; Mini Mental State Exam score = 4.7 ± 2.5) matched for age, postmortem interval, and education were evaluated. At autopsy, brains were cut into 0.5-cm-thick slabs and hemisected. One hemisphere was immersion-fixed in 4% paraformaldehyde, cryoprotected, and cut frozen into 40-μm-thick sections [9]. Slabs from the opposite hemisphere were frozen at −80°C. Neuropathological evaluations were performed by a neuropathologist blinded to clinical diagnosis [9].
Identification of GAL-Hyperinnervated NB Neurons
To identify GAL-immunoreactive profiles in apposition to cholinergic NB neurons, sections were dual labeled with rabbit polyclonal GAL antiserum (1:15,000; Peninsula Labs, Belmont, Calif., USA), which was visualized by nickel intensification (dark-blue reaction product), and a monoclonal antibody to the p75NTR low-affinity nerve growth factor receptor, which is an excellent marker for CBF neurons [10] and was visualized by diaminobenzidine (brown reaction product) [7, 8, 11]. RNase-free precautions were used throughout the experimental procedures.
The degree of GAL hyperinnervation upon cholinergic NB neurons was evaluated using semiquantitative methods [5,6,7,8, 11]. Each NB neuron analyzed received a score indicating both the density and thickness of GAL fibers impinging upon the neuron (1 = none to very little, 2 = moderate, and 3 = dense innervation) [8, 11]. NCI and AD/GAL– NB neurons met the criteria for a score of 1, whereas AD/GAL+ NB cells received a score of 3.
Single-Cell Microaspiration, RNA Amplification, and Array Hybridization
NB neurons selected for analysis were aspirated using a micromanipulator and vacuum source (Eppendorf, Westbury, N.Y., USA) attached to an inverted microscope [8, 9, 12]. RNA amplification from individual NB neurons was performed using terminal continuation (TC) RNA amplification methodology [13] (see http://cdr.rfmh.org/pages/ginsberglabpage.html). Hybridization probes were synthesized by in vitro transcription using T7 RNA polymerase and [33P]-UTP [12, 13]. Radiolabeled TC RNA probes were then hybridized to custom-designed cDNA arrays. Arrays were hybridized overnight at 42°C in a rotisserie oven, washed, placed in a phosphor screen for 24 h, and developed on a phosphor imager (GE Healthcare, Piscataway, N.J., USA).
Custom-Designed cDNA Array Platforms and Data Collection
The array platforms consisted of 1 μg of linearized cDNA purified from plasmid preparations adhered to high-density nitrocellulose (Hybond XL, GE Healthcare) as described previously [12, 13]. Hybridization signal intensity was quantified by subtracting background using empty vector (pBluescript). TC-amplified RNA bound to each linearized cDNA was expressed as a ratio of the total hybridization signal intensity of the array (i.e., global normalization) [12]. Data analysis generated an expression profile of relative changes in mRNA levels among the individual NB neurons.
Statistical Analysis
Expression levels of cholinergic mRNAs were clustered and displayed using a bioinformatics and graphics software package (GeneLinker Gold, Predictive Patterns, Kingston, Ont., Canada) [8, 9, 12]. Relative changes in hybridization signal intensity of individual mRNAs were analyzed by one-way ANOVA with post hoc Newman-Keuls analysis. Level of significance was set at p < 0.01.
Results
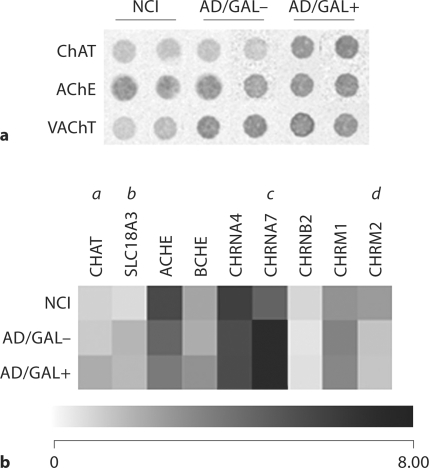
Custom-designed cDNA array analysis of NCI, AD/GAL–, and AD/GAL+ NB neurons revealed a range of hybridization signal intensities for mRNAs encoding proteins involved in the metabolism of acetylcholine (ACh) as well as muscarinic (mAChR) and nicotinic (nAChR) ACh receptors (fig. 1). Notably, the level of transcripts encoding choline acetyltransferase (ChAT) was increased by approximately 60% in AD/GAL+ cells compared to NCI and AD/GAL– cells (fig. 1a, b). In contrast, levels of the vesicular ACh transporter (VAChT, or Unigene/NCBI notation SLC18A3) were increased by approximately 70% in both AD/GAL– and AD/GAL+ cells compared to NCI NB neurons (fig. 1a, b). Acetylcholinesterase (AChE; fig. 1a, b) and butylcholinesterase (fig. 1b) levels were unchanged among the NCI and AD cells examined.
Fig. 1.
a Representative custom-designed microarray expression data showing relative hybridization signal intensities for ChAT, AChE, and VAChT in NCI, AD/GAL–, and AD/GAL+ cholinergic NB neurons. b Dendrogram illustrating relative mRNA expression levels (white to black = increasing levels) of ChAT (Unigene/NCBI notation CHAT), VAChT (SLC18A3), AChE (ACHE), butylcholinesterase (BCHE), nAChR subunits a4 (CHRNA4), a7 (CHRNA7), b2 (CHRNB2), and mAChR subtypes M1 (CHRM1) and M2 (CHRM2). a = AD/GAL+ > NCI, AD/GAL– (p < 0.01); b = AD/GAL+, AD/GAL– > NCI (p < 0.01); c = AD/GAL+, AD/GAL– > NCI (p < 0.001); d = NCI > AD/GAL–, AD/GAL+ (p < 0.01).
There were no differences in transcript levels of mAChRs or nAChRs between AD/GAL– and AD/GAL+ NB neurons. However, transcript levels of the M2 mAChR were reduced by approximately 50% in both AD/GAL– and AD/GAL+ cells relative to NCI cells (fig. 1b). In addition, a7 nAChR expression was increased by approximately 50% in both AD/GAL– and AD/GAL+ cells relative to NCI cells (fig. 1b), similar to previous observations in mild/moderate AD [14].
Discussion
The physiological consequences of GAL fiber plasticity upon cholinergic NB neurons in late-stage AD are controversial. For instance, GAL inhibits ACh release in rodent hippocampal preparations and disrupts cognitive performance in animals [1, 15], suggesting that GAL overexpression may exacerbate the cholinergic deficit seen in late-stage AD. On the other hand, GAL was found to elicit excitatory actions in rodent CBF septal neurons [2] and to protect these cells from amyloid toxicity [16], raising the possibility that GAL upregulation promotes cholinergic activity and/or neuronal survival in the AD brain. The present findings show that GAL hyperinnervation increases ChAT mRNA levels in cholinergic NB neurons, suggesting an increase in the cholinergic tone of AD/GAL+ cells. We have previously shown that GAL hyperinnervation prevents the decreased production of protein phosphatase 1 subtype mRNAs (PP1α and PP1γ) in cholinergic NB neurons [17], potentially attenuating phosphorylation events involved in neurofibrillary tangle formation [18]. Taken together, these molecular observations lend support for a neuroprotective role of GAL within selected NB neurons in AD. Interestingly, NB neurons express all three known GAL receptor subtypes [8]. If increased ChAT message results in increased ACh production and release in AD/GAL+ cells relative to AD/GAL– cells, then this would provide a rationale for the continued development of GAL receptor subtype-specific ligands [16] for the pharmacotherapeutic augmentation of basocortical cholinergic transmission in AD.
Acknowledgements
We thank M. Nadeem and S. Dar for expert technical assistance. This work was supported by the National Institute of Health grants AG14449, AG16088, AG09466 AG10161, AG17617, NS48447, AG26032, and the Illinois Department of Public Health.
References
- 1.Fisone G, Wu CF, Consolo S, Nordstrom O, Brynne N, Bartfai T, Melander T, Hokfelt T. Galanin inhibits acetylcholine release in the ventral hippocampus of the rat: histochemical, autoradiographic, in vivo, and in vitro studies. Proc Natl Acad Sci USA. 1987;84:7339–7343. doi: 10.1073/pnas.84.20.7339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jhamandas JH, Harris KH, MacTavish D, Jassar BS. Novel excitatory actions of galanin on rat cholinergic basal forebrain neurons: implications for its role in Alzheimer's disease. J Neurophysiol. 2002;87:696–704. doi: 10.1152/jn.00416.2001. [DOI] [PubMed] [Google Scholar]
- 3.Bartus RT. On neurodegenerative diseases, models, and treatment strategies: lessons learned and lessons forgotten a generation following the cholinergic hypothesis. Exp Neurol. 2000;163:495–529. doi: 10.1006/exnr.2000.7397. [DOI] [PubMed] [Google Scholar]
- 4.Davies P, Maloney AJ. Selective loss of central cholinergic neurons in Alzheimer's disease. Lancet. 1976;ii:1403. doi: 10.1016/s0140-6736(76)91936-x. [DOI] [PubMed] [Google Scholar]
- 5.Bowser R, Kordower JH, Mufson EJ. A confocal microscopic analysis of galaninergic hyperinnervation of cholinergic basal forebrain neurons in Alzheimer's disease. Brain Pathol. 1997;7:723–730. doi: 10.1111/j.1750-3639.1997.tb01058.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan-Palay V. Galanin hyperinnervates surviving neurons of the human basal nucleus of Meynert in dementias of Alzheimer's and Parkinson's disease: a hypothesis for the role of galanin in accentuating cholinergic dysfunction in dementia. J Comp Neurol. 1988;273:543–557. doi: 10.1002/cne.902730409. [DOI] [PubMed] [Google Scholar]
- 7.Mufson EJ, Cochran E, Benzing W, Kordower JH. Galaninergic innervation of the cholinergic vertical limb of the diagonal band (Ch2) and bed nucleus of the stria terminalis in aging, Alzheimer's disease and Down's syndrome. Dementia. 1993;4:237–250. doi: 10.1159/000107329. [DOI] [PubMed] [Google Scholar]
- 8.Counts SE, Chen EY, Che S, Ikonomovic MD, Wuu J, Ginsberg SD, Dekosky ST, Mufson EJ. Galanin fiber hypertrophy within the cholinergic nucleus basalis during the progression of Alzheimer's disease. Dement Geriatr Cogn Disord. 2006;21:205–214. doi: 10.1159/000090906. [DOI] [PubMed] [Google Scholar]
- 9.Mufson EJ, Counts SE, Ginsberg SD. Gene expression profiles of cholinergic nucleus basalis neurons in Alzheimer's disease. Neurochem Res. 2002;27:1035–1048. doi: 10.1023/a:1020952704398. [DOI] [PubMed] [Google Scholar]
- 10.Mufson EJ, Bothwell M, Hersh LB, Kordower JH. Nerve growth factor receptor immunoreactive profiles in the normal, aged human basal forebrain: colocalization with cholinergic neurons. J Comp Neurol. 1989;285:196–217. doi: 10.1002/cne.902850204. [DOI] [PubMed] [Google Scholar]
- 11.Sendera TJ, Ma SY, Jaffar S, Kozlowski PB, Kordower JH, Mawal Y, Saragovi HU, Mufson EJ. Reduction in TrkA-immunoreactive neurons is not associated with an overexpression of galaninergic fibers within the nucleus basalis in Down's syndrome. J Neurochem. 2000;74:1185–1196. doi: 10.1046/j.1471-4159.2000.741185.x. [DOI] [PubMed] [Google Scholar]
- 12.Ginsberg SD, Che S, Wuu J, Counts SE, Mufson EJ. Downregulation of trk but not p75NTR gene expression in single cholinergic basal forebrain neurons mark the progression of Alzheimer's disease. J Neurochem. 2006;97:475–487. doi: 10.1111/j.1471-4159.2006.03764.x. [DOI] [PubMed] [Google Scholar]
- 13.Che S, Ginsberg SD. Amplification of RNA transcripts using terminal continuation. Lab Invest. 2004;84:131–137. doi: 10.1038/labinvest.3700005. [DOI] [PubMed] [Google Scholar]
- 14.Counts SE, He B, Che S, Ikonomovic MD, Dekosky ST, Ginsberg SD, Mufson EJ: Upregulation of alpha-7 nicotinic receptors in cholinergic basal forebrain neurons in Alzheimer's disease. Arch Neurol; in press. [DOI] [PubMed]
- 15.McDonald MP, Willard LB, Wenk GL, Crawley JN. Coadministration of galanin antagonist M40 with a muscarinic M1 agonist improves delayed nonmatching to position choice accuracy in rats with cholinergic lesions. J Neurosci. 1998;18:5078–5085. doi: 10.1523/JNEUROSCI.18-13-05078.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ding X, MacTavish D, Kar S, Jhamandas JH. Galanin attenuates beta-amyloid (Abeta) toxicity in rat cholinergic basal forebrain neurons. Neurobiol Dis. 2006;21:413–420. doi: 10.1016/j.nbd.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 17.Counts SE, Perez SE, Ginsberg SD, De Lacalle S, Mufson EJ. Galanin in Alzheimer disease. Mol Interv. 2003;3:137–156. doi: 10.1124/mi.3.3.137. [DOI] [PubMed] [Google Scholar]
- 18.Trojanowski JQ, Lee VM. Phosphorylation of paired helical filament tau in Alzheimer's disease neurofibrillary lesions: focusing on phosphatases. FASEB J. 1995;9:1570–1576. doi: 10.1096/fasebj.9.15.8529836. [DOI] [PubMed] [Google Scholar]