Abstract
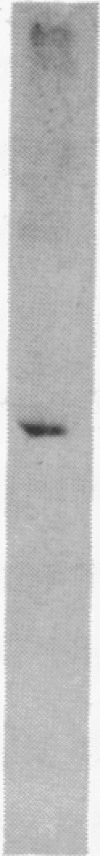
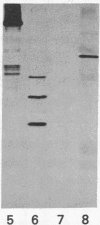
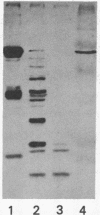
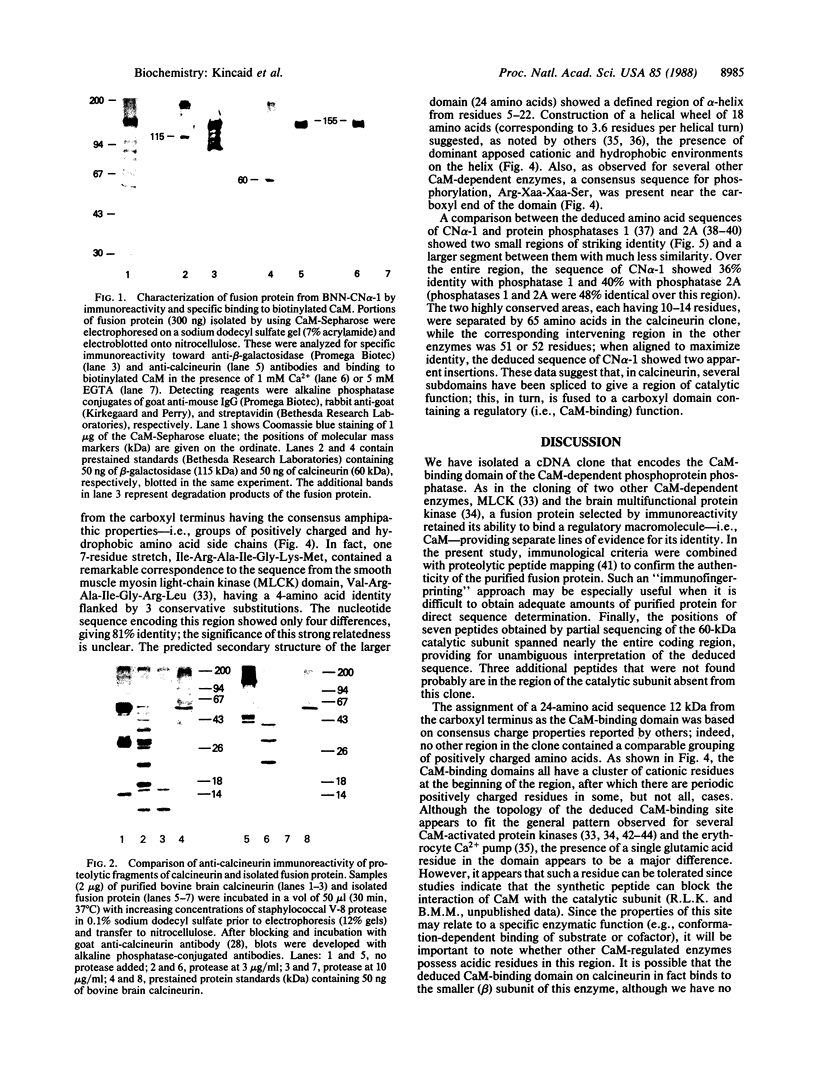
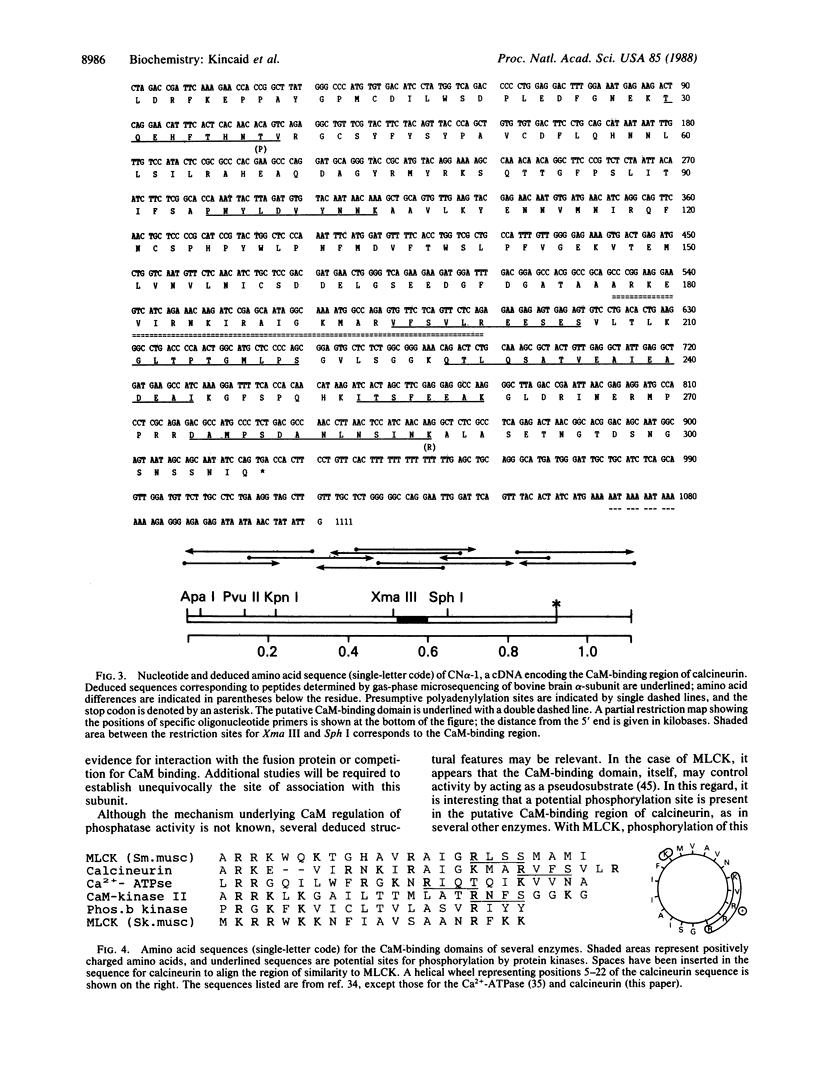
A cDNA clone corresponding to a portion of the catalytic subunit of calmodulin (CaM)-dependent phosphoprotein phosphatase (calcineurin) was isolated from a murine brain library by expression vector immunoscreening. A beta-galactosidase fusion protein that reacted on Western blots with anti-calcineurin antibodies and biotinylated CaM was purified in preparative amounts using CaM-Sepharose affinity chromatography. Partial digestion of the hybrid protein with Staphylococcus aureus V-8 protease produced several immunoreactive peptides that appeared identical to fragments generated from authentic brain calcineurin. The 1111-base-pair (bp) EcoRI insert contained an open reading frame encoding a protein of 35 kDa followed by a 190-bp 3' noncoding region; seven peptides obtained by partial amino acid sequencing of the bovine brain enzyme were found in the deduced sequence. A domain approximately 12 kDa from the carboxyl terminus was deduced to be the CaM-binding site based on consensus structural features and a sequence of seven amino acids highly related to smooth muscle myosin light-chain kinase. Two regions with identity to protein phosphatases 1 and 2A were found in the amino half of the cloned sequence; however, the intervening sequence contained apparent insertions, suggesting splicing of subdomains. Thus, the structure of calcineurin is chimeric, consisting of conserved catalytic elements and a regulatory CaM-binding domain.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aitken A., Klee C. B., Cohen P. The structure of the B subunit of calcineurin. Eur J Biochem. 1984 Mar 15;139(3):663–671. doi: 10.1111/j.1432-1033.1984.tb08055.x. [DOI] [PubMed] [Google Scholar]
- Bennett M. K., Kennedy M. B. Deduced primary structure of the beta subunit of brain type II Ca2+/calmodulin-dependent protein kinase determined by molecular cloning. Proc Natl Acad Sci U S A. 1987 Apr;84(7):1794–1798. doi: 10.1073/pnas.84.7.1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berndt N., Campbell D. G., Caudwell F. B., Cohen P., da Cruz e Silva E. F., da Cruz e Silva O. B., Cohen P. T. Isolation and sequence analysis of a cDNA clone encoding a type-1 protein phosphatase catalytic subunit: homology with protein phosphatase 2A. FEBS Lett. 1987 Nov 2;223(2):340–346. doi: 10.1016/0014-5793(87)80316-2. [DOI] [PubMed] [Google Scholar]
- Billingsley M. L., Pennypacker K. R., Hoover C. G., Brigati D. J., Kincaid R. L. A rapid and sensitive method for detection and quantification of calcineurin and calmodulin-binding proteins using biotinylated calmodulin. Proc Natl Acad Sci U S A. 1985 Nov;82(22):7585–7589. doi: 10.1073/pnas.82.22.7585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenthal D. K., Takio K., Edelman A. M., Charbonneau H., Titani K., Walsh K. A., Krebs E. G. Identification of the calmodulin-binding domain of skeletal muscle myosin light chain kinase. Proc Natl Acad Sci U S A. 1985 May;82(10):3187–3191. doi: 10.1073/pnas.82.10.3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen E. Y., Seeburg P. H. Supercoil sequencing: a fast and simple method for sequencing plasmid DNA. DNA. 1985 Apr;4(2):165–170. doi: 10.1089/dna.1985.4.165. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Cohen P. The coordinated control of metabolic pathways by broad-specificity protein kinases and phosphatases. Curr Top Cell Regul. 1985;27:23–37. doi: 10.1016/b978-0-12-152827-0.50010-4. [DOI] [PubMed] [Google Scholar]
- Cohen P. The role of protein phosphorylation in neural and hormonal control of cellular activity. Nature. 1982 Apr 15;296(5858):613–620. doi: 10.1038/296613a0. [DOI] [PubMed] [Google Scholar]
- Collett M. S., Purchio A. F., Erikson R. L. Avian sarcoma virus-transforming protein, pp60src shows protein kinase activity specific for tyrosine. Nature. 1980 May 15;285(5761):167–169. doi: 10.1038/285167a0. [DOI] [PubMed] [Google Scholar]
- Conti M. A., Adelstein R. S. The relationship between calmodulin binding and phosphorylation of smooth muscle myosin kinase by the catalytic subunit of 3':5' cAMP-dependent protein kinase. J Biol Chem. 1981 Apr 10;256(7):3178–3181. [PubMed] [Google Scholar]
- Cox J. A., Comte M., Fitton J. E., DeGrado W. F. The interaction of calmodulin with amphiphilic peptides. J Biol Chem. 1985 Feb 25;260(4):2527–2534. [PubMed] [Google Scholar]
- DeLorenzo R. J. Role of calmodulin in neurotransmitter release and synaptic function. Ann N Y Acad Sci. 1980;356:92–109. doi: 10.1111/j.1749-6632.1980.tb29603.x. [DOI] [PubMed] [Google Scholar]
- Green D. D., Yang S. I., Mumby M. C. Molecular cloning and sequence analysis of the catalytic subunit of bovine type 2A protein phosphatase. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4880–4884. doi: 10.1073/pnas.84.14.4880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greengard P. Phosphorylated proteins as physiological effectors. Science. 1978 Jan 13;199(4325):146–152. doi: 10.1126/science.22932. [DOI] [PubMed] [Google Scholar]
- Guerriero V., Jr, Russo M. A., Olson N. J., Putkey J. A., Means A. R. Domain organization of chicken gizzard myosin light chain kinase deduced from a cloned cDNA. Biochemistry. 1986 Dec 30;25(26):8372–8381. doi: 10.1021/bi00374a007. [DOI] [PubMed] [Google Scholar]
- Hanley R. M., Means A. R., Ono T., Kemp B. E., Burgin K. E., Waxham N., Kelly P. T. Functional analysis of a complementary DNA for the 50-kilodalton subunit of calmodulin kinase II. Science. 1987 Jul 17;237(4812):293–297. doi: 10.1126/science.3037704. [DOI] [PubMed] [Google Scholar]
- Hunter T., Sefton B. M. Transforming gene product of Rous sarcoma virus phosphorylates tyrosine. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1311–1315. doi: 10.1073/pnas.77.3.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingebritsen T. S., Cohen P. Protein phosphatases: properties and role in cellular regulation. Science. 1983 Jul 22;221(4608):331–338. doi: 10.1126/science.6306765. [DOI] [PubMed] [Google Scholar]
- Ingebritsen T. S., Cohen P. The protein phosphatases involved in cellular regulation. 1. Classification and substrate specificities. Eur J Biochem. 1983 May 2;132(2):255–261. doi: 10.1111/j.1432-1033.1983.tb07357.x. [DOI] [PubMed] [Google Scholar]
- James P., Maeda M., Fischer R., Verma A. K., Krebs J., Penniston J. T., Carafoli E. Identification and primary structure of a calmodulin binding domain of the Ca2+ pump of human erythrocytes. J Biol Chem. 1988 Feb 25;263(6):2905–2910. [PubMed] [Google Scholar]
- Kelly P. T., McGuinness T. L., Greengard P. Evidence that the major postsynaptic density protein is a component of a Ca2+/calmodulin-dependent protein kinase. Proc Natl Acad Sci U S A. 1984 Feb;81(3):945–949. doi: 10.1073/pnas.81.3.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp B. E., Pearson R. B., Guerriero V., Jr, Bagchi I. C., Means A. R. The calmodulin binding domain of chicken smooth muscle myosin light chain kinase contains a pseudosubstrate sequence. J Biol Chem. 1987 Feb 25;262(6):2542–2548. [PubMed] [Google Scholar]
- Kennedy M. B., Bennett M. K., Erondu N. E. Biochemical and immunochemical evidence that the "major postsynaptic density protein" is a subunit of a calmodulin-dependent protein kinase. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7357–7361. doi: 10.1073/pnas.80.23.7357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kincaid R. L., Balaban C. D., Billingsley M. L. Differential localization of calmodulin-dependent enzymes in rat brain: evidence for selective expression of cyclic nucleotide phosphodiesterase in specific neurons. Proc Natl Acad Sci U S A. 1987 Feb;84(4):1118–1122. doi: 10.1073/pnas.84.4.1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kincaid R. L., Manganiello V. C., Odya C. E., Osborne J. C., Jr, Stith-Coleman I. E., Danello M. A., Vaughan M. Purification and properties of calmodulin-stimulated phosphodiesterase from mammalian brain. J Biol Chem. 1984 Apr 25;259(8):5158–5166. [PubMed] [Google Scholar]
- Kincaid R. L., Martensen T. M., Vaughan M. Modulation of calcineurin phosphotyrosyl protein phosphatase activity by calmodulin and protease treatment. Biochem Biophys Res Commun. 1986 Oct 15;140(1):320–328. doi: 10.1016/0006-291x(86)91093-4. [DOI] [PubMed] [Google Scholar]
- Kincaid R. L. Preparation, characterization, and properties of affinity-purified antibodies to calmodulin-dependent cyclic nucleotide phosphodiesterase and the protein phosphatase calcineurin. Methods Enzymol. 1988;159:627–652. doi: 10.1016/0076-6879(88)59059-6. [DOI] [PubMed] [Google Scholar]
- Kincaid R. L. The use of melittin-sepharose chromatography for gram-preparative purification of calmodulin. Methods Enzymol. 1987;139:3–19. doi: 10.1016/0076-6879(87)39070-6. [DOI] [PubMed] [Google Scholar]
- Kincaid R. L., Vaughan M. Sequential adsorption-electrophoresis: combined procedure for purification of calcium-dependent cyclic nucleotide phosphodiesterase. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4903–4907. doi: 10.1073/pnas.76.10.4903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King M. M., Huang C. Y. Activation of calcineurin by nickel ions. Biochem Biophys Res Commun. 1983 Aug 12;114(3):955–961. doi: 10.1016/0006-291x(83)90653-8. [DOI] [PubMed] [Google Scholar]
- King M. M., Huang C. Y. The calmodulin-dependent activation and deactivation of the phosphoprotein phosphatase, calcineurin, and the effect of nucleotides, pyrophosphate, and divalent metal ions. Identification of calcineurin as a Zn and Fe metalloenzyme. J Biol Chem. 1984 Jul 25;259(14):8847–8856. [PubMed] [Google Scholar]
- Klee C. B., Crouch T. H., Krinks M. H. Calcineurin: a calcium- and calmodulin-binding protein of the nervous system. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6270–6273. doi: 10.1073/pnas.76.12.6270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs E. G., Beavo J. A. Phosphorylation-dephosphorylation of enzymes. Annu Rev Biochem. 1979;48:923–959. doi: 10.1146/annurev.bi.48.070179.004423. [DOI] [PubMed] [Google Scholar]
- Li H. C. Activation of brain calcineurin phosphatase towards nonprotein phosphoesters by Ca2+, calmodulin, and Mg2+. J Biol Chem. 1984 Jul 25;259(14):8801–8807. [PubMed] [Google Scholar]
- Lukas T. J., Burgess W. H., Prendergast F. G., Lau W., Watterson D. M. Calmodulin binding domains: characterization of a phosphorylation and calmodulin binding site from myosin light chain kinase. Biochemistry. 1986 Mar 25;25(6):1458–1464. doi: 10.1021/bi00354a041. [DOI] [PubMed] [Google Scholar]
- Manalan A. S., Klee C. B. Activation of calcineurin by limited proteolysis. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4291–4295. doi: 10.1073/pnas.80.14.4291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merat D. L., Hu Z. Y., Carter T. E., Cheung W. Y. Bovine brain calmodulin-dependent protein phosphatase. Regulation of subunit A activity by calmodulin and subunit B. J Biol Chem. 1985 Sep 15;260(20):11053–11059. [PubMed] [Google Scholar]
- Pallen C. J., Wang J. H. Regulation of calcineurin by metal ions. Mechanism of activation by Ni2+ and an enhanced response to Ca2+/calmodulin. J Biol Chem. 1984 May 25;259(10):6134–6141. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibley D. R., Peters J. R., Nambi P., Caron M. G., Lefkowitz R. J. Desensitization of turkey erythrocyte adenylate cyclase. Beta-adrenergic receptor phosphorylation is correlated with attenuation of adenylate cyclase activity. J Biol Chem. 1984 Aug 10;259(15):9742–9749. [PubMed] [Google Scholar]
- Stewart A. A., Ingebritsen T. S., Manalan A., Klee C. B., Cohen P. Discovery of a Ca2+- and calmodulin-dependent protein phosphatase: probable identity with calcineurin (CaM-BP80). FEBS Lett. 1982 Jan 11;137(1):80–84. doi: 10.1016/0014-5793(82)80319-0. [DOI] [PubMed] [Google Scholar]
- Stone S. R., Hofsteenge J., Hemmings B. A. Molecular cloning of cDNAs encoding two isoforms of the catalytic subunit of protein phosphatase 2A. Biochemistry. 1987 Nov 17;26(23):7215–7220. doi: 10.1021/bi00397a003. [DOI] [PubMed] [Google Scholar]
- Tallant E. A., Cheung W. Y. Activation of bovine brain calmodulin-dependent protein phosphatase by limited trypsinization. Biochemistry. 1984 Feb 28;23(5):973–979. doi: 10.1021/bi00300a027. [DOI] [PubMed] [Google Scholar]
- Tallant E. A., Cheung W. Y. Characterization of bovine brain calmodulin-dependent protein phosphatase. Arch Biochem Biophys. 1984 Jul;232(1):269–279. doi: 10.1016/0003-9861(84)90543-5. [DOI] [PubMed] [Google Scholar]
- Winkler M. A., Merat D. L., Tallant E. A., Hawkins S., Cheung W. Y. Catalytic site of calmodulin-dependent protein phosphatase from bovine brain resides in subunit A. Proc Natl Acad Sci U S A. 1984 May;81(10):3054–3058. doi: 10.1073/pnas.81.10.3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Efficient isolation of genes by using antibody probes. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1194–1198. doi: 10.1073/pnas.80.5.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Cruz e Silva O. B., Alemany S., Campbell D. G., Cohen P. T. Isolation and sequence analysis of a cDNA clone encoding the entire catalytic subunit of a type-2A protein phosphatase. FEBS Lett. 1987 Sep 14;221(2):415–422. doi: 10.1016/0014-5793(87)80966-3. [DOI] [PubMed] [Google Scholar]