Abstract
Cortactin is a cortex-enriched protein implicated in Arp2/3 complex-mediated actin polymerization. However, the physiological role of cortactin remains unknown. We have generated a mouse strain in which the allele of murine cortactin was disrupted by a gene trapping vector. The resulting heterozygous mice developed normally and were fertile, but embryonic fibroblasts derived from heterozygous animals displayed partial impairment in PDGF-induced membrane ruffling. No homozygous offspring or early embryos even at the two-cell stage were detected. Analysis of oocytes revealed a gradual decrease in the detection of homozygous zygotes after fertilization. In normal oocytes arrested at meiotic metaphase II (MII), cortactin immunoreactivity was detected in an apical layer that overlies the maternal chromosome and overlaps with a polarized cortex enriched with actin. The formation of the polarized cortactin layer was diminished upon treatment with latrunculin B, an actin polymerization inhibitor. After resumption of meiosis II, the majority of cortactin protein was accumulated into the second polar body. Microinjection of MII-arrested eggs with either cortactin antibody or RNA encoding a cortactin mutant deficient in Arp2/3 complex binding disrupted the integrity of the actin cap and inhibited emission of the second polar body triggered by parthenogenesis. Our data suggest that cortactin plays an important role in the mechanics of asymmetric division in oocytes.
INTRODUCTION
Asymmetric division is a fundamental mechanism to establish cell polarity necessary for differentiation and embryonic development. In mammalian oocytes, two consecutive asymmetric divisions take place before and after fertilization, resulting in two haploid minute cells or polar bodies that carry little cytoplasm, thereby allowing adequate nutrients left in the haploid oocyte for further embryo development. The mechanism for asymmetric division involves proper positioning of the meiotic spindle and subsequent formation of a membrane domain enriched with the actin filaments [1;2]. This membrane domain, which is also called the actin cap, signifies one of the earliest instance of cellular polarity and specifies a polarized location where the asymmetric division takes place [3]. Therefore, understanding the nature of the actin cap will gain insight into the mechanics of the asymmetric division.
Cortactin is an abundant intracellular protein that binds to filamentous actin (F-actin) and to Arp2/3 complex, a prominent actin nucleator that yields branched actin filaments enriched at the cell leading edge, membrane ruffles and trails of intracellular vesicles [4]. The amino acid sequence of cortactin contains three distinct motifs: an Arp2/3 binding domain at the N-terminus, a unique F-actin binding domain with six and one half 37-amino acid repeats, and an SH3 domain at the C-terminus. In the presence of cortactin, the nucleation of actin filaments mediated by the Arp2/3 complex is enhanced [5;6], and the resulting branched filaments are stabilized. Previous studies have documented that cortactin participates in a variety of signaling pathways, in particular those initiated by Src protein kinase that lead to dynamic cell shape changes [7–10]. However, the vital function of cortactin has not yet been established. Cortactin is present in both invertebrates and vertebrates, and Drosophila with null cortactin are fertile even though some defects in oogenesis were detected [11]. In the genome of mammals, cortactin is closely related to HS1, a gene that is exclusively expressed in the hematopoietic lineage [12]. Although depletion of HS1 protein in mice partially impairs the function of lymphocytes in response to antigens, HS1 knockout animals appeared to be otherwise normal in development [13]. There have been no reports describing the phenotype of a complete knockout of cortactin in mammals. However, two groups have recently generated murine cortactin null fibroblasts using the flox/Cre system and found no dramatic changes in the morphology and growth of those cells and a modest impairment in cell migration and growth factor-induced Rac activation [14;15]. These studies have implied that cortactin plays a minor role in the actin cytoskeleton reorganization and thus questioned whether cortactin is an essential gene for the mammalian organism. We have recently prepared a mouse strain in which the cortactin allele was disrupted by a gene trapping vector. Unexpectedly, we failed to obtain any homozygous mice or early embryos even at the two-cell stage. Further analysis revealed that cortactin is enriched within the actin cap of oocytes. Microinjection of cortactin antagonists into oocytes impaired the formation of the actin cap and asymmetric division of the oocytes. Our data suggest a pivotal role of cortactin and its associated actin filaments in mammalian zygotic development.
MATERIALS AND METHODS
Chemical reagents and antibodies
All the chemicals were purchased from Sigma unless otherwise indicated. Cortactin monoclonal antibody (4F11) was purchased from Millipore. Rabbit anti-cortactin polyclonal antiserum was raised against a recombinant full-length murine cortactin as described previously [16]. The antiserum was further purified with protein A Sepharose. Rabbit anti-MIM polyclonal antibody was prepared as described previously [17]. Rabbit anti-GFP antibody was raised against a recombinant enhanced green fluorescent protein (EGFP) tagged by 6 × His residues. Monoclonal antibody against GFP (3E6) was from Invitrogen. Monoclonal antibodies against Arp3 (A5979), actin (A5441) and pan-dynamin (D0438) were from Sigma. Monoclonal antibody against β-galactosidase (Z3781) was from Promega. Polyclonal Arp3 antibody was raised in rabbits against a synthesized peptide of YEEIGPSICRHNPVFGVMS, which corresponds to the C-terminal amino acid sequence of human Arp3. All the polyclonal antibodies were purified by protein A Sepharose.
Generation of cortactin knockout mice
A murine embryonic stem (ES) cell line (RRS284) carrying a gene trapping vector (pGT0lxf) was obtained from BayGenomics. Genomic PCR and DNA sequencing determined that the vector was inserted at nucleotide 1334 of the intron between exon 7 and 8. Verified ES cells were microinjected into blastocysts derived from a C57BL/6 female mouse and further transferred into a pseudo-pregnant C57BL/6 mouse. Two male chimeras derived from the injected mice were used to cross with female C57BL/6 mice, resulting in multiple cortactin heterozygous mice, which were further used to generate homozygous embryos. The genotype of the resulting offspring and embryos were determined by genomic PCR. The primer pair for the wild-type allele was: 5’-CCACCAGCCTGAAGGGAGAAG-3’/5’-ACCGAAGCCACTAGAGTAGT-3’; and the primer pair for the mutated allele was: 5’-CCACCAGCCTGAAGGGAGAAG/5’-TACTCAGTGCAGTGCAGTCAGGGGC-3’.
X-gal staining of embryos
Embryos at 10.5-dpc were fixed in 0.1 M phosphate buffer (pH 7.3) supplemented with 5 mM EGTA, 2 mM MgCl2 and 0.2% gluteraldahyde for 30 min at room temperature, washed twice (5 min each) with washing buffer containing 0.1 M phosphate buffer (pH 7.3) and 2 mM MgCl2. The washed embryos were then incubated overnight at 37°C in staining buffer containing 1 mg/ml X-gal, 0.1 M phosphate buffer (pH 7.3) supplemented with 2 mM MgCl2, 5 mM potassium ferrocyanide and 5 mM potassium ferricyanide. On the next day the stained embryos were washed twice with washing buffer and subjected to inspection under microscope.
Ooocytes and embryo collection
Female C57BL/6 mice (4–8 weeks old) were injected intraperitoneally (i.p.) with 0.1 ml water containing 5 IU of pregnant mare’s serum gonadotropin (Calbiochem). After 48 hours, the mice were injected i.p. with 0.1 ml of water containing 5 IU human chorionic gonadotropin (hCG). To collect zygotes, two injected female mice were housed together with a male mouse. The success of the mating was confirmed by inspection of vaginal plugs on the following morning. The mated female mice were humanely sacrificed 15 min before egg collection. To collect unfertilized eggs, hCG injected females were sacrificed without mating. In either case, the reproductive tract of sacrificed mice was exposed by cutting the peritoneum and moving the coils to the guts. Two oviducts were dissected and placed in 37°C pre-warmed M2 medium (Invitrogen). Eggs were collected by tearing the ampulla of the oviduct under a stereo-microscope and transferred to microdrops of M2 medium for further treatment. To remove cumulus cells, eggs were incubated for 5 min in microdrops of M2 containing 0.02% type IV-S hyaluronidase (Sigma) and further washed in M2 medium for three times. In some experiments, the egg shell or zona pellucida (ZP) was removed by treatment for 30 sec with an acidic solution containing 10 mM HEPES, pH 1.5, 1 mM NaH2PO4, 0.8 mM MgSO4, 5.4 mM KCl, and 116.4 mM NaCl, and followed by washing three times with M2 medium.
Genotyping of oocytes and zygotes
Cumulus-free eggs were collected at indicated times after hCG injection and dissolved in 10 µl lysis buffer containing 10 mM Tris-HCl (pH 8.3), 50 mM KCl, 2.5 mM MgCl2, 0.45% NP-40, 0.45% Tween-20, and 300 µg/ml proteinase K. Genomic DNA was released from the nucleus by freezing at −80°C and thawing at 25°C, followed by incubation at 56°C for 90 min and 95°C for 10 min. The treated egg lyates were analyzed by nested PCR. Briefly, 10 µl embryo lysate was combined with 40 µl PCR reaction buffer (Promega) containing four dNTP, Taq polymerase and 100 ng of primer mixture. PCR was carried out under the following condition: 95°C for 3 min, 35 cycles of 95°C for 30s, 64°C for 30s, 72°C for 1 min, and final extension at 72°C for 10 min. Aliquots (1 µl) of the first PCR product were diluted 1000 times and used as the template in the second PCR in 50 µl reaction containing the mixture of the following primers: 5’-AGGTGGCAAAGAGCAGGGTTCA-3’, 5’-CATTCTTTAGGATGACCTTGGACAC-3’, and 5’-TGAGCACCAGAGGACATCCGG-3’.
The PCR products were analyzed by 1.8% agarose gel electrophoresis followed by ethidium bromide staining.
Parthenogenesis
Cumulus-free oocytes were incubated in M2 medium in the presence of 5 µM A23178 at room temperature for 2 min, washed six times and further incubated in pre-warmed M2 medium at 37°C, 5% CO2 for 6h or overnight. Egg activation was observed based on emission of the second polar body and formation of pronucleus.
Oocyte staining
Oocytes were fixed in 4% paraformaldedyde in Whitten’s medium [18] containing 15 mM HEPES, 30 mg/ml Albumax I (egg medium) for 40 min and washed three times. For some experiments, ZP was removed before staining. Either ZP-free or intact eggs were quenched with 50 mM NH4Cl. ZP-free eggs were permeabilized by incubation in 0.5% Triton-x100 for 5 min, and ZP-intact eggs were permeabilized by 0.25% Triton-x100 for 10 min. The permeabilized eggs were washed five times with egg medium, blocked by egg medium containing 5% normal goat serum for 1h at room temperature, then incubated in the blocking medium containing purified polyclonal cortactin antibody (20 µg/ml) at 4°C overnight. On the next day, the cells were incubated in the blocking medium containing fluorescence-labeled secondary antibodies for 1h. For visualizing chromatin and F-actin, 4',6-diamidino-2-phenylindole (DAPI) (5 µg/ml) and Alexa 546-labelled phalloidin (1:200, Invitrogen) were added into the secondary antibody incubation. The stained eggs were examined with Zeiss LSM 5 Duo microscope, and the captured digital images were processed with Zeiss LSM Image Brower software.
In vitro mRNA synthesis
The templates for in vitro transcription were generated by PCR amplification of GFP tagged full length murine cortactin cDNA or cortactin mutant [CttnΔ(1–23)] with deletion of the amino acids 1 to 23. The sense primer in the PCR carried a T7 promoter sequence, and antisense primer carried 30 poly dT sequence. In vitro synthesis of capped mRNAs was performed using the mMESSAGE mMACHINE T7 kit (Ambion). After synthesis, RNAs were purified with mEGAclear kit (Ambion), dissolved in nuclease-free water, and verified with agrose gel electrophoresis.
Microinjection
MII-arrested eggs were collected at 13h post hCG treatment. After removal of cumulus cells, oocytes were recovered in M2 medium for 1 to 2h at 37°C and then were microinjected with either PBS or PBS containing 2.2 µg/µl, 3.5 µg/µl or 7.1µg/µl cortactin polyclonal antibody, or PBS containing 5 µg/µl MIM polyclonal antibody using Xenoworks microinjector and micromanipulators (Bio-Rad). After injection, the oocytes were recovered for 1–2 h at 37°C and subsequently subject to parthenogenesis. For RNA injection, synthesized RNAs were diluted at a concentration of 0.7 µg/µl in water, and were injected into the cytoplasm of MII oocytes at a constant flow rate with the microinjector. The injected oocytes were then fixed at 1–6 hours post injection and subjected to staining with phalloidin, DAPI and cortactin antibody. The stained oocytes were inspected using LSM 510 Meta Laser Scanning Microscope. The captured digital images were analyzed using LSM Image Browser. In order to detect the 2nd polar body extrusion, some injected oocytes were subjected to parthenogenesis after 2h of injection.
Plasmid preparation
pGFP-cortactin, which encodes murine cortactin tagged by EGFP, was constructed in retroviral vector MGIN as described previously [19]. To construct pGFP-Cttn(1–155), a DNA fragment corresponding to the cortactin amino acid sequence from 1 to 155 was amplified by PCR using pGFP-Cortactin as the template. The amplified DNA fragment was subcloned in frame into EcoR1 and SacII sites of MGIN vector. All the plasmids were confirmed by DNA sequencing.
Actin Polymerization
Polymerization of G-actin (10% pyrene-labeled, rabbit skeletal muscle actin from Cytoskeleton Inc.) was performed as described previously [20]. Briefly, Ca2+-ATP-G-actin in G-actin buffer (5 mM Tris-HCl, pH 8.0, 0.2mM CaCl2, 0.2 mM ATP, and 0.5 mM DTT) was mixed with one-tenth volume of 10 × exchange buffer (2 mM EGTA, 1 mM MgCl2) for 3 min at 25°C to convert to Mg2+-ATP-G-actin. Polymerization was initiated by adding 60 µl of Mg2+-ATP-G-actin (7.5 µM) to 240 µl of 1.25 × polymerization buffer (62.5 mM KCl, 2.5 mM MgCl2, 12.5 mM imidazole, pH 7.3, 1.25 mM EGTA, 0.125 mM CaCl2, 0.625 mM DTT, 0.3125 mM ATP, and 3.75 mM NaN3) containing 10 nM bovine Arp2/3 complex, 5 nM GST-VCA, 100 nM GST-cortactin and anti-cortactin antibody at concentrations as indicated. The kinetics of actin polymerization was monitored by measuring the increase in pyrene fluorescence detected by LS50B spectrophotometer (Perkin Elmer Life Sciences) with filters for excitation at 365 nm and emission at 407 nm.
Analysis of cells expressing GFP-Cttn(1–155)
NIH 3T3 cells were transiently transfected with plasmids MGIN (the vector that encodes GFP only), pGFP-cortactin and pGFP-Cttn(1–155), respectively. The transfected cells were plated on fibronectin coated slips, serum-starved for two days and treated with 30 ng/ml PDGF. The treated cells were fixed with 4% paraformaldehyde, stained with Alexa 546-phalloidin and DAPI and inspected by Zeiss LSM 5 Duo microscope as described above. To analyze apoptosis, the cells were inspected by Nikon TE2000U fluorescent microscope. Apoptotic cells were characterized as those having degraded nucleus as revealed by DAPI staining.
RESULTS
Cortactin heterozygous embryonic fibroblasts displayed partial impairment in actin dynamics
To obtain insight into the physiological function of cortactin, we prepared cortactin knockout mice using an embryonic stem (ES) cell line that carries a gene trapping vector (pGT0lxf) at the intron between the 7th and the 8th exons (Fig. 1A). Microinjection of ES cells into blastocysts and subsequent transfer into a pseudo-pregnant recipient mouse resulted in two chimeras, both of which were able to transmit ES cells to germ cells and then further used to generate heterozygous mice. The presence of the mutated cortactin allele in heterozygous mice was confirmed by staining of embryos for expression of β-gal, which was carried by the trapping vector and controlled by the endogenous cortactin promoter in the targeted allele (Fig. 1A). The β-gal staining of 10.5-dpc heterozygous embryos demonstrated that cortactin is nearly ubiquitously expressed (Fig. 1B), a result that is consistent with the previous observation of a wide expression profile in adult and fetal tissues [21;22]. Western blot analysis demonstrated approximately 50% reduction in cortactin expression in mouse embryonic fibroblasts (MEF) derived from heterozygous animals (Fig. 1C). While no apparent gross difference in the morphology was found between heterozygous and wild type MEFs, it appears that many heterozygous MEFs cultured in serum-containing media on fibronectin contained more prominent stress fibers as compared to wild type MEFs (Fig. 1D and Supplementary Fig. S1A and B). Quantification of phalloidin stained cells indicated that nearly 50% heterozygous MEFs exhibited strong stress fiber staining while only 25% wild type MEFs did so (Fig. 1D). Heterozygous MEFs also showed less membrane ruffles at the periphery (Fig. 1D). To confirm the activity of cortactin for ruffle formation, we analyzed the morphological response of MEFs to PDGF, a growth factor that is known to induce profound dorsal ruffling on fibroblasts [23;24]. Indeed, the PDGF treatment induced dorsal ruffles, which were enriched with both cortactin and F-actin, in 25% wild type MEFs within 10 minutes (Fig. 1E). However, only 15% heterozygous MEF developed dorsal ruffles after PDGF treatment (Fig. 1E). Confocal microscopy also revealed less F-actin staining in the ruffles associated with heterozygous MEFs than that with wild type MEFs (Supplementary Fig. S1C and D), indicative of a partial impairment in the actin dynamics. This observation is in agreement with a recent study that reported nearly 60% reduction in the PDGF-induced ruffling with cortactin null fibroblasts [14].
Fig. 1. Disruption of a cortactin allele caused a modest alteration in the actin cytoskeleton of MEF.

(A) Schematic presentation of cortactin protein, cDNA and the genomic structure. Murine cortactin exons and their relative positions in relation to the protein structure and the genome are indicated. The intron between exon 7 and 8 is magnified to illustrate the insertion site of the targeting vector pGT0lXf. The β-gal and neo gene (β-geo) was not drawn to the scale. The numbers shown under the vector refer to the nucleotide positions for the insert of the targeted intron. N, the NH2-terminal domain; neo, neomycin; PRD, proline rich domain; R, cortactin repeated domain; and SH3, Src homology 3. (B) Whole mount X-gal staining of wild type and heterozygous embryos at 10.5-dpc. (C) Western blot analysis of cortactin expression in Cttn(+/+) and Cttn(−/+) MEF using a monoclonal cortactin antibody (4F11). (D) Cttn(+/+) (a and b) and Cttn(−/+) MEF (c and d) were grown on a fibronectin pre-coated coverslip in a serum-containing medium. After fixation and permeabilization, cells were co-stained with polyclonal cortactin antibody (a and c) and phalloidin (b and d) and inspected under a 60 × lens of fluorescent microscope. A representative membrane ruffle enriched with both cortactin and F-actin was indicated by arrow heads. Cttn(−/+) MEFs were also scored based on an arbitrarily defined strength of stress fiber staining. The chart on the right illustrates the percentage of cells with prominent stress fiber staining based on three independent experiments (mean ± SD). (E) Cttn(+/+) (a–d) and Cttn(−/+) (e–h) MEF were placed on a fibronectin coated coverslip and starved in a serum-free medium. After 24 h, cells were treated with PDGF at 30 ng/ml. Treated (c, d, g, and h) and untreated (a, b, e, and f) cells were co-stained with cortactin antibody and phalloidin as in D. The chart on the right illustrates quantification of cells with apparent dorsal ruffles measured based on three independent experiments (mean ± SD). The p values were calculated using the Student’s t test, referring to the difference between the two groups in each plot.
Knockout of cortactin in mice affects zygotic development
In spite of the partial impairment in membrane ruffling in vitro, heterozygous mice developed normally and were fertile. However, we have not been able to obtain any homozygous offspring (Supplementary S-Table 1). Further analysis also failed to identify homozygous embryos at stages from 1.5-dpc to 14.5-dpc. At 0.5-dpc when most embryos remained as single-cell zygotes, we found only one homozygous zygote out of 100 fertilized eggs. We considered several reasons for the absence of cortactin-null embryos. One possibility might be that heterozygous mice did not generate sufficient active gametes carrying the mutated allele. By genotyping of unfertilized oocytes collected from heterozygous female mice, we found the nearly same number of oocytes carrying the wild type cortactin allele as that of mutated oocytes (Supplementary S-Table 2). Furthermore, intercross between heterozygous mice produced approximately twice the number of heterozygous offspring as that of wild type offspring (Table 1). These observations indicated a normal Mendelian splitting during meiosis of the oocytes from heterozygous mice. It was also possible that gametes carrying the mutated cortactin allele were not properly developed and thereby unable to undergo normal fertilization or membrane fusion. To examine this possibility, male Cttn(−/+) were intercrossed with female Cttn(+/+) and vice verse. Both matting procedures gave rise to a normal birth rate with approximately 10 pups per litter, and nearly 50% of the pups derived from either mating were heterozygous (Table 1). Again, this genetic data fitted with the prediction based on Mendelian inheritance. Thus, cortactin null gametes, either sperms or oocytes, derived from heterozygous mice were able to interact with wide type gametes in vivo and to generate fully functional mice. This data also indicated that null cortactin caused a failure in the zygotic development after fertilization but before entering into the two-cell stage.
Table 1.
analysis of the transmission of gametes of Cttn(−/+) mice
| Genotype | ||
|---|---|---|
| Cross | Wild type | Heterozygous |
| ♂ Cttn(−/+) × ♀ Cttn(+/+) | 52 | 58 |
| ♀ Cttn(−/+) × ♂ Cttn(+/+) | 130 | 134 |
Male heterozygous mice were mated with wild type C57BL/6 female mice or vice verse. The genotype of the resulting postnatal offspring was determined by genomic PCR.
To determine the possible developmental step affected by null cortactin, we analyzed the genotype of the fertilized oocytes at different time points after superovulation induced by human chorionic gonadotropin (hCG). HCG treatment induced the release of oocytes after 12 hours into the oviduct where fertilization takes place. At 13 hours after hCG treatment, the time that corresponded to approximately 1 hour after fertilization, we detected nearly 21% of the zygotes with the homozygous genotype. However, detection of homozygous zygotes became gradually difficult in late stages, with 9% at 17 hours, 4.8% at 19 hours, and 1% at 22 hours after hCG (Figure 2A). This result indicated a broad role of cortactin in the egg activation, in particular, during a stage after 17 hours post hCG when the most eggs had entered into the second meiotic division as indicated by forming the secondary polar body. Morphological analysis revealed that the most wild type and heterozygous zygotes were normal (Fig. 2B) and extruded the second polar body after 17 hours of hCG (Fig. 2A). In contrast, the zygotes that were later identified as homozygous displayed either no second polar body or abnormal morphology such as significant cellular fragmentation (Fig. 2B). Abnormal morphology was also found with some wild type and heterozygous zygotes. Quantitative analysis showed that about 30% wild type and 24% heterozygous eggs displayed certain degrees of abnormality. The slight difference between wild type and heterozygous embryos was not statistically significant (p=0.12). In contrast, nearly 75% homozygous zygotes exhibited abnormal shapes, and the difference as compared to wild type or heterozygous was significant (p<0.03). There were also many isolated zygotes that failed to give a result in the genotyping. Interestingly, 76% of those zygotes displayed abnormal morphology (Fig. 2C), suggestive of DNA degradation in these embryos. This may also suggest that null cortactin zygotes could have become fragile and undergone apoptosis either in vivo or in an in vitro process.
Fig. 2. Morphological analysis of homozygous zygotes.
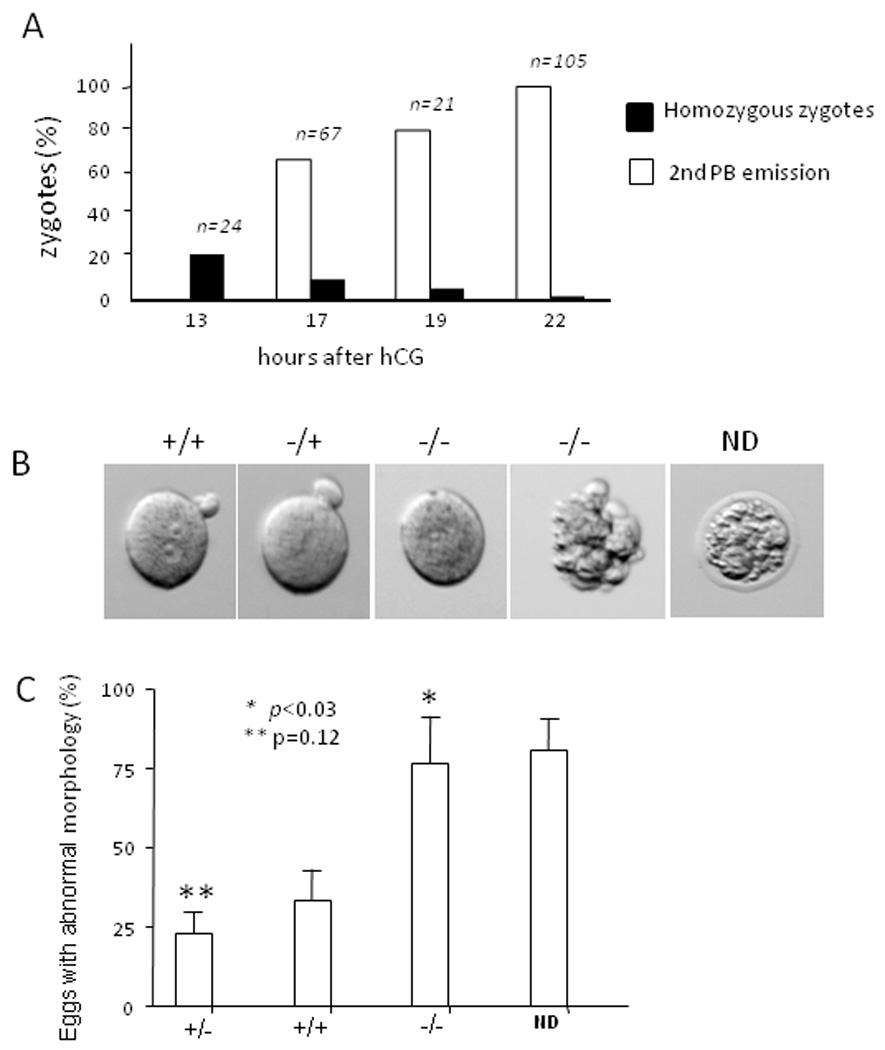
(A) Zygotes were collected from the oviduct of superovulated heterozygous mice at different times after hCG and subjected to removal of ZP. The morphology of the individual ZP-free embryos was recorded by digital photograph. Genotype of the zygotes was determined by single-cell genomic PCR. The percentage of the eggs with the homozygous genotype and those with an apparent secondary polar body emission was plotted as a function of time after hCG treatment. (B) Images shown were the morphology of representative ZP-free zygotes with different genotypes. A ZP-coated egg without defined genotype was also presented (ND). (C) All the analyzed zygotes were grouped based on morphology and genotype. The percentage of the embryos with abnormal morphology was plotted based on three independent experiments (mean ± SD). The p values, which were calculated by the Student’s t test, refer to the difference between wild type and homozygous (*), and between wild type and heterozygous embryos (**).
Cortactin protein accumulates in a polarized cortical structure of oocytes and overlaps with the actin cap
The above observations strongly suggested a role of cortactin in zygotic development and thus prompted us to examine the subcellular distribution of cortactin in oocytes. We first stained unfertilized normal oocytes arrested at meiotic metaphase II (MII) with a purified polyclonal antibody against murine cortactin. The specificity of the antibody was confirmed by Western blot and immunostaining of MEFs. In Western blot, the antibody detected two characteristic cortactin bands at 80 and 85 kDa, respectively, and few cross-reactivity proteins were detected even after a prolonged exposure (Supplementary Fig.S2A). When the antibody was used in immunostaining, it revealed a typical cortactin distribution as characterized by staining at the cell cortex and numerous punctate structures in the cytoplasm (Fig. 1D, Supplementary Fig. S2D and E). Furthermore, the strength of the staining was significantly less with cortactin heterozygous MEF or human cells treated with a cortactin siRNA than control cells (Supplementary Fig. S2B–E), indicative of a high specificity of the antibody. We then used the antibody to stain oocytes after fixation. The stained oocytes were initially analyzed by two-dimensional confocal microscopy, which detected cortactin immunoreactivity in the cortex, in particular, the area near the position of the maternal chromosome (Fig. 3A and C). No apparent immunoreactivity was detected with eggs stained with either a polyclonal GFP antibody or the secondary antibody only (data not shown). To analyze the relationship between the cortactin immunoreactivity and the actin network, the embryo was also co-stained with phalloidin, which stained F-actin in the nearly same area as the cortactin immunoreactivity (Fig. 3B and C). More detailed analysis using three-dimensional confocal microscopy demonstrated that both cortactin and F-actin staining formed a thin layer or cap with more than 80% overlapping expression (Fig. 3D–F), indicating that cortactin is a component of the actin cap.
Fig. 3. Cortactin forms an apical layer overlapping with the actin cap.

(A–C) A MII-arrested oocyte was stained with cortactin antibody (red), phalloidin (green) and DAPI (blue). The position corresponding to the maternal chromosome was indicated by an arrow (C). (D–F) The same oocyte was analyzed by 3-D confocal microscopy. Two small cells labeled with C as shown in F were the cumulus cells attached to the oocyte. (G–I) Cortactin distribution was analyzed by 3-D microscopy in a MII-arrested oocyte (G), a zygote collected at 18h (H), and a zygote at 22h after hCG (I). Maternal DNA (MD), pronucleuses (PN) and polar body (PB) were indicated by arrows. (J) A zygote was treated latrunculin B for 1h followed by staining with cortactin antibody and DAPI. Maternal DNA (MD) and sperm DNA (SD) were indicated by arrows.
We next analyzed zygotes collected from fertilized wild type females. In a zygote that was collected after 18 hours of hCG treatment, cortactin staining was mainly found in the cortex surrounding the second polar body (Fig. 3H). In a zygote that was collected at 22 hours after hCG and showed a late stage of maturation as indicated by more closely positioned pronuclei (PN), cortactin was concentrated within the zygotic polar body (Fig. 3I). Quantification of cortactin staining on a Z-series estimated approximately 20% cellular cortactin was accumulated in the polar body, which is about 1/100 volume of the oocyte. Such uneven distribution into a non-functional polar body would likely leave fewer cortactin proteins in oocytes and may cause abnormal behavior of homozygous zygotes in vitro or in vivo.
To analyze whether the polarized distribution of cortactin depended on actin polymerization, we examined zygotes that were treated with latrunculin B, an actin filament disrupting toxin. As shown in Fig. 3J, the latrunculin B treatment diminished the formation of polarized cortactin. Instead, the cortactin staining appeared to be scattered all over the cortex as multiple patches with different sizes. Thus, actin polymerization is necessary for the formation of the cortactin cap.
Evidence for the role of cortactin in the formation of the actin cap and asymmetric division of MII oocytes
We wanted to examine a possible role of cortactin in the asymmetric division with homozygous embryos. Unfortunately, extensive characterization of these embryos was not possible because identification of these embryos by genotyping requires cell destruction, leaving no material to investigate. Thus, we examined the normal MII-oocytes injected with a polyclonal cortactin antibody raised against full-length recombinant cortactin. To examine whether the antibody has a neutralization activity, we used the antibody to immunoprecipitate the lysate of cells overexpressing GFP-cortactin. As a control, a polyclonal GFP antibody was used in a parallel experiment. While the GFP antibody precipitated less GFP-cortactin protein than the cortactin antibody, it was able to co-precipitate effectively several cortactin associated proteins, including actin, Arp3 and dynamin [5;25;26] (Fig 4A). In contrast, the cortactin antibody precipitated much less these proteins. Previous studies had shown that cortactin was able to enhance the actin nucleation mediated by Arp2/3 complex[5]. Thus, we also analyzed the effect of the antibody on the actin polymerization and found that the antibody, but not a control antibody, inhibited effectively and specifically the cortactin mediated actin polymerization in a dose dependent manner (supplementary Fig. S3). These results indicate that the antibody has a strong neutralization activity specifically toward cortactin.
Fig. 4. Cortactin antibody inhibited the second polar body extrusion.

(A) Analysis of the inhibitory activity of cortactin polyclonal antibody. MDA-MB-231 cells overexpressing GFP-cortactin were lysed and subjected to immunoprecipitation using cortactin and GFP polyclonal antibodies, respectively. The precipitates were immuoblotted with monoclonal antibodies against actin, Arp3 and pan-dynamin, respectively. (B) MII-arrested oocytes were microinjected with either PBS or PBS containing polyclonal cortactin antibody at the concentrations of 2.2 µg/µl, 3.5 µg/µl and 7.0 µg/µl, respectively. As a control, eggs were also injected with a control antibody (polyclonal MIM antibody) at the concentration of 5 µg/µl. The injected eggs were subsequently subjected to parthenogenesis to induce the second polar body extrusion. The number of eggs with observed polar body extrusion was countered at 12h post parthenogenesis. The p value, as calculated based on the Student’s t-test, refers to the difference between the injection with the control antibody at 5 µg/µl and that with the cortactin antibody at 3.5 µg/µl. (C) A MII-oocyte injected with cortactin antibody at 3.5 µg/µl (a and b) and a MII-oocyte injected with the control antibody at 5 µg/µl (c and d) was stained with phalloidin (red) and DAPI (blue). The stained eggs were inspected by confocal (a and c) and phase contrast (b and d) microscopy. Actin cap (ac) was indicated by an arrow.
Next, we microinjected the cortactin neutralization antibody at different doses followed by parthenogenesis, a condition that triggers effectively the second polar body extrusion in vitro in a controllable manner. Injection of oocytes with either PBS or a polyclonal antibody against MIM, a cortactin binding protein that is not expressed in eggs (data not shown), showed little effect on polar body extrusion (Fig. 4B). In contrast, injection with the cortactin antibody blocked the polar body emission in a dose dependent manner. At the injected concentration of 3.5 µg/µl, 38% of the injected oocytes showed the extruded second polar body; at 7.5 µg/µl only 12% oocytes did so. Because polar body extrusion requires actin polymerization [27], we were interested in whether cortactin was required for the establishment of the actin cap. Therefore, injected eggs were also stained with phalloidin. As shown in Fig. 4C, injection with the cortactin antibody (Fig. 4C-a), but not the control antibody (Fig. 4C-c), reduced the actin cap to a small ring structure distal to the maternal DNA (MD). This distortion was not due to a global change with the injected oocytes as they appeared to maintain a normal morphology after injection (Fig. 4C-b and d).
To verify the result obtained with the cortactin antibody, we also attempted to use a cortactin antagonist in the RNA form, which is used more widely in analyzing the function of a particular gene in oocytes [28;29]. Our previous study had shown that a cortactin mutant with deletion of the first 23 amino acids for Arp2/3 complex binding exhibited an inhibitory activity to the cortactin mediated actin polymerization in vitro and was able to block recruitment of the endogenous cortactin into the endothelial cell periphery in response to an extracellular signal [30]. Thus, we prepared RNAs that encoded either GFP-CttnΔ(1–23) or GFP-cortactin in vitro and injected the synthesized RNAs into oocytes. Expression of injected RNAs in live oocytes was confirmed by confocal microscopic analysis three hours after injection. The GFP-cortactin protein appeared to be diffused in the cytoplasm but more concentrated on the polarized cortex (Fig. 5A-a). On the other hand, the mutant CttnΔ(1–23) was found in many punctate structures in the center of the oocyte (Fig. 5A-b). To analyze the effect of injection on the formation of the actin cap, the injected oocytes were stained with phalloidin. As shown in Fig. 5A-c, injection of wild type cortactin had no apparent effect on the actin cap. However, the cortical actin cap over the chromosome was apparently diminished in the oocytes injected with the mutant GFP-CttnΔ(1–23) (Fig. 5A-d). In some eggs, the chromosome appeared to be dislocated from the polar body (Fig. 5A-e). Next, we analyzed the RNA injected oocytes under parthenogenesis. While 91% of the oocytes injected with GFP-cortactin showed the second polar body extrusion, only 35% of the oocytes injected with GFP-CttnΔ(1–23) did so (Fig. 5C). This result agrees well with that obtained with the antibody injection.
Fig. 5. A cortactin mutant RNA inhibited the actin cap formation and polar body extrusion.

(A) MII-oocytes were injected with in vitro synthesized RNAs encoding GFP-Cttn (a and c) and GFP-CttnΔ (1–23) (b, d and e), respectively. After three hours, live injected oocytes were inspected by confocal microscopy (a and b). Some injected eggs were fixed and further stained with phalloidin (red) and DAPI (blue) (c, d and e). All stained cells were analyzed by confocal microscopy. The positions of the first polar body (PB) and maternal DNA (MD) were indicated. (B) MII-oocytes were injected with RNAs and subjected to parthenogenesis. The eggs with an apparent second polar body extrusion were scored. Error bars (standard derivation) were calculated based on three independent experiments.
DISCUSSION
The presented study demonstrates an important role of cortactin in early murine embryonic development during zygote maturation. This finding was unexpected in light of two recent reports about little or only a modest defect in the actin cytoskeleton reorganization in cortactin null MEFs [14;15]. In particularly, a cortactin null MEF cell line generated by the Okabe’s group was successfully isolated from an embryo after intercrossing Cttn(−/+) mice [15], implying that cortactin may not be required for early embryonic development. One possible explanation for the discrepancy is that different targeting systems were used to target the cortactin allele. The mice from Okabe’s group were derived from a CMV-Cre/loxp system, while ours were from an ES cell line carrying a gene trapping vector that was inserted in the intron between exon 7 and exon 8 (Fig. 1A). Sequence analysis of a putative fusion transcript between cortactin exon 7 and the gene trapping vector predicts generation of a truncated protein that contains a cortactin N-terminal sequence from 1 to 157 amino acids. Thus, it is possible that the phenotype manifested by our cortactin knockout mice might be due to a dominant negative effect of this putative truncated protein. Although we have not yet been able to demonstrate the presence of the truncated protein product in Cttn(−/+) MEF using a cortactin polyclonal antibody against whole recombinant cortactin (data not shown), we prepared a GFP-Cttn(1–155) construct and analyzed whether this N-terminal truncated protein has any significant impact on NIH3T3 cells. Microscopic analysis of the transfectants revealed no significant alteration in either the actin cytoskeleton or apoptosis (Supplementary Fig. S4 and Fig. S5), suggesting that the truncated protein has little dominant negative effect on cell growth. The insertion of the gene trapping vector may also create additional dominant negative protein products. Inspection of the murine cortactin genome sequence indicates fusion of three exones preceding exon 7 with the vector would generate in-frame transcripts. Indeed, a Western blot analysis of Cttn(−/+) MEF lysate using a β-galactosidase antibody revealed a protein band with an apparent molecular weight in the range of 155 to 160 KDa (data not shown). While we have not yet defined the precise form of this protein product, its gel motility agrees with a possible fusion between the β-neo gene and exon 3, which encodes the N-terminal 29 amino acids, a motif for binding to the Arp2/3 complex. We have previously shown that the function of this domain requires the whole F-actin binding motif to achieve an optimal binding to Arp2/3 complex [20]. Since we did not observe any defect in the offspring production from intercrossing between Cttn(−/+) mice, which harbored a single copy of the mutated allele, this fusion protein product would likely have a minimal impact in vivo. However, we could not exclude the possibility that the fusion protein may affect zygote development in the context where no normal cortactin alleles are present. Because currently there is no cortactin homozygous mouse available, a final resolving of this issue would be beyond the limit of the system employed in this study.
We also noticed different genetic backgrounds involved in these studies, which may influence the final observed phenotype [31]. The heterozygous mice generated by Okabe’s group were apparently a mixture of 129/sv-C57BL/6-Balb/c based on the information described in the paper. In contrast, the mice used in this study have a genetic background of 129/sv-C57BL/6. Whether a Balb/c background could compromise the defect caused by null cortactin in zygotic development will be tested in the future. Another explanation for the unexpected phenotype is that the gene trapping vector in our mice may disrupt an allele for another essential gene of which the identity is unknown. If this is the case, one would expect that this allele has to be in a position very close to that of cortactin so that it would be co-segregated with the cortactin allele all the time. So far, we have not been able to detect multiple copies of the vector in the genome of the mice based on a quantitative PCR analysis (data not shown). Future study using fluorescence in situ hybridization may be required to vigorously rule out this possibility.
We conclude that cortactin plays a more vital role in oocytes than somatic cells. There is another case for a paramount role of actin cytoskeleton proteins in oocytes. Complete knockout of formin-2, which is an actin nucleator, did not affect mice development but influenced the maturation of MI-arrested oocytes [32]. Formin-2-deficient oocytes failed to extrude the first polar body and were impaired in the migration of meiotic spindle toward the polarized cortex at the first meiosis stage. However, formin-2 deficient oocytes retained the ability to form the actin cap [33], indicating that the formation of the actin cap is a formin-2 independent event. We provided evidence here that cortactin-mediated actin polymerization is responsible for the actin cap formation. First, we observed that cortactin overlaps well with the actin cap (Fig. 2). Furthermore, a chemical inhibiting actin polymerization abolished the polarization of cortactin. Also, microinjection of two types of cortactin antagonists inhibited the formation of the actin cap (Fig. 4 and 5). The finding for the role of cortactin in the actin cap implies that different types of actin filaments execute distinct functions in the asymmetric division. It is well established that formin proteins direct formation of linear actin filaments in somatic cells [34]. In oocytes, formin-2 has been thought to be responsible for the formation of actin clusters that are transiently organized into stress fibers [35;36]. In contrast, cortactin participates in the Arp2/3 mediated-actin polymerization, which is characterized by branched actin filaments. Thus, our data strongly suggests that the actin cap is made of branched actin filaments. Enrichment of branched actin filaments in the actin cap may provide a structural explanation for a previously well-described observation that the membrane domain for the actin cap is normally devoid of microvilli [2;37], a type of membrane protrusion that is primarily filled with straight actin bundles. The exact function of the actin cap in oocytes remains not fully defined. In addition to the asymmetric division, it has been also suggested that the actin cap is involved in the direction of migrating chromosomes during the first meiosis [1;2]. In the present study we have only looked at MII-oocytes where the chromosome has already been located to the proximity of the cortex where the first polar body is extruded. Therefore, the role of cortactin in the chromosome migration is not clear, and we will address this issue with MI-oocytes in the future. However, in the analysis of MII-oocytes injected with a cortactin mutant deficient in Arp2/3 binding, we have found a dislocation of the maternal chromosome in relation to the polar body (Fig. 5 A-e). This suggests that cortactin may be required for the stabilization of the chromosome in the cortex of MII-oocytes.
Our microinjection experiment using cortactin antagonists indicated a role for cortactin in the asymmetric division. While we could not rule out the possibility that these antagonists may have some unexpected side-effects, cortactin could play other roles in egg activation. In fact, we have observed a gradual decrease in the detection of homozygous zygotes after fertilization. The precise reason for the gradual loss of homozygous oocytes is unknown. Since the majority of homozygous eggs displayed certain morphological abnormality such as defragmentation and many eggs with the abnormal morphology failed to give results in the PCR based genotyping, it was quite possible that many cortactin homozygous embryos had become fragile or undergone apoptosis during either in vivo or in vitro process. How the loss of cortactin could make oocytes more fragile is unknown. Because of the difficulty to obtain homozygous embryos and the presence of parent proteins, the effort to characterize in more detail the function of cortactin in the egg activation will be made with other animal models such as conditional knockout mice.
Supplementary Material
ACKNOWLEDGEMENTS
We appreciate greatly Valerie Stewart and Dennis Wilson for the help in the generation of cortactin knockout mice and microinjection of oocytes. We also thank Jeff Winkles for a critical reading.
Abbreviations
- Cttn
Cortactin
- dpc
days post coitum (dpc)
- ES
embryonic stem
- F-actin
filamentous actin
- hCG
human chorionic gonadotropin
- ZP
zona pellucida
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Longo FJ. Fine structure of the mammalian egg cortex. Am.J.Anat. 1985;174:303–315. doi: 10.1002/aja.1001740310. [DOI] [PubMed] [Google Scholar]
- 2.Maro B, Johnson MH, Webb M, Flach G. Mechanism of polar body formation in the mouse oocyte: an interaction between the chromosomes, the cytoskeleton and the plasma membrane. J.Embryol.Exp.Morphol. 1986;92:11–32. [PubMed] [Google Scholar]
- 3.Verlhac MH, Dumont J. Interactions between chromosomes, microfilaments and microtubules revealed by the study of small GTPases in a big cell, the vertebrate oocyte. Mol.Cell Endocrinol. 2008;282:12–17. doi: 10.1016/j.mce.2007.11.018. [DOI] [PubMed] [Google Scholar]
- 4.Borisy GG, Svitkina TM. Actin machinery: pushing the envelope. Curr.Opin.Cell Biol. 2000;12:104–112. doi: 10.1016/s0955-0674(99)00063-0. [DOI] [PubMed] [Google Scholar]
- 5.Uruno T, Liu J, Zhang P, Fan Y, Egile C, Li R, Mueller SC, Zhan X. Activation of Arp2/3 complex-mediated actin polymerization by cortactin. Nat.Cell Biol. 2001;3:259–266. doi: 10.1038/35060051. [DOI] [PubMed] [Google Scholar]
- 6.Weaver AM, Karginov AV, Kinley AW, Weed SA, Li Y, Parsons JT, Cooper JA. Cortactin promotes and stabilizes Arp2/3-induced actin filament network formation. Curr.Biol. 2001;11:370–374. doi: 10.1016/s0960-9822(01)00098-7. [DOI] [PubMed] [Google Scholar]
- 7.Ammer AG, Weed SA. Cortactin branches out: roles in regulating protrusive actin dynamics. Cell Motil.Cytoskeleton. 2008;65:687–707. doi: 10.1002/cm.20296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weaver AM. Cortactin in tumor invasiveness. Cancer Lett. 2008;265:157–166. doi: 10.1016/j.canlet.2008.02.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Daly RJ. Cortactin signalling and dynamic actin networks. Biochem.J. 2004;382:13–25. doi: 10.1042/BJ20040737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schuuring E. The involvement of the chromosome 11q13 region in human malignancies: cyclin D1 and EMS1 are two new candidate oncogenes--a review. Gene. 1995;159:83–96. doi: 10.1016/0378-1119(94)00562-7. [DOI] [PubMed] [Google Scholar]
- 11.Somogyi K, Rorth P. Cortactin modulates cell migration and ring canal morphogenesis during Drosophila oogenesis. Mech.Dev. 2004;121:57–64. doi: 10.1016/j.mod.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 12.Kitamura D, Kaneko H, Miyagoe Y, Ariyasu T, Watanabe T. Isolation and characterization of a novel human gene expressed specifically in the cells of hematopoietic lineage. Nucleic Acids Res. 1989;17:9367–9379. [PMC free article] [PubMed] [Google Scholar]
- 13.Taniuchi I, Kitamura D, Maekawa Y, Fukuda T, Kishi H, Watanabe T. Antigen-receptor induced clonal expansion and deletion of lymphocytes are impaired in mice lacking HS1 protein, a substrate of the antigen-receptor-coupled tyrosine kinases. EMBO J. 1995;14:3664–3678. doi: 10.1002/j.1460-2075.1995.tb00036.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lai FP, Szczodrak M, Oelkers JM, Ladwein M, Acconcia F, Benesch S, Auinger S, Faix J, Small JV, Polo S, Stradal TE, Rottner K. Cortactin Promotes Migration and PDGF-induced Actin Reorganization by Signaling to Rho-GTPases. Mol.Biol.Cell. 2009;20:3209–3223. doi: 10.1091/mbc.E08-12-1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tanaka S, Kunii M, Harada A, Okabe S. Generation of cortactin floxed mice and cellular analysis of motility in fibroblasts. Genesis. 2009;47:638–646. doi: 10.1002/dvg.20544. [DOI] [PubMed] [Google Scholar]
- 16.Zhan X, Hu X, Hampton B, Burgess WH, Friesel R, Maciag T. Murine cortactin is phosphorylated in response to fibroblast growth factor-1 on tyrosine residues late in the G1 phase of the BALB/c 3T3 cell cycle. J.Biol.Chem. 1993;268:24427–24431. [PubMed] [Google Scholar]
- 17.Wang Y, Liu J, Smith E, Zhou K, Liao J, Yang GY, Tan M, Zhan X. Downregulation of missing in metastasis gene (MIM) is associated with the progression of bladder transitional carcinomas. Cancer Invest. 2007;25:79–86. doi: 10.1080/07357900701205457. [DOI] [PubMed] [Google Scholar]
- 18.Whitten WK. Neutrient requirement for the culture of preimplantation mouse embryos in vitro. Advances of the Biosciences. 1971;6:129–139. [Google Scholar]
- 19.Li Y, Liu J, Zhan X. Tyrosine phosphorylation of cortactin is required for H2O2-mediated injury of human endothelial cells. J.Biol.Chem. 2000;275:37187–37193. doi: 10.1074/jbc.M005301200. [DOI] [PubMed] [Google Scholar]
- 20.Uruno T, Liu J, Li Y, Smith N, Zhan X. Sequential interaction of actin-related proteins 2 and 3 (Arp2/3) complex with neural Wiscott-Aldrich syndrome protein (N-WASP) and cortactin during branched actin filament network formation. J.Biol.Chem. 2003;278:26086–26093. doi: 10.1074/jbc.M301997200. [DOI] [PubMed] [Google Scholar]
- 21.Zhan X, Haudenschild CC, Ni Y, Smith E, Huang C. Upregulation of cortactin expression during the maturation of megakaryocytes. Blood. 1997;89:457–464. [PubMed] [Google Scholar]
- 22.Wu H, Montone KT. Cortactin localization in actin-containing adult and fetal tissues. J.Histochem.Cytochem. 1998;46:1189–1191. doi: 10.1177/002215549804601011. [DOI] [PubMed] [Google Scholar]
- 23.Hedberg KM, Bengtsson T, Safiejko-Mroczka B, Bell PB, Lindroth M. PDGF and neomycin induce similar changes in the actin cytoskeleton in human fibroblasts. Cell Motil.Cytoskeleton. 1993;24:139–149. doi: 10.1002/cm.970240207. [DOI] [PubMed] [Google Scholar]
- 24.Dharmawardhane S, Sanders LC, Martin SS, Daniels RH, Bokoch GM. Localization of p21-activated kinase 1 (PAK1) to pinocytic vesicles and cortical actin structures in stimulated cells. J.Cell Biol. 1997;138:1265–1278. doi: 10.1083/jcb.138.6.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McNiven MA, Kim L, Krueger EW, Orth JD, Cao H, Wong TW. Regulated interactions between dynamin and the actin-binding protein cortactin modulate cell shape. J.Cell Biol. 2000;151:187–198. doi: 10.1083/jcb.151.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu H, Parsons JT. Cortactin, an 80/85-kilodalton pp60src substrate, is a filamentous actin-binding protein enriched in the cell cortex. J.Cell Biol. 1993;120:1417–1426. doi: 10.1083/jcb.120.6.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maro B, Johnson MH, Pickering SJ, Flach G. Changes in actin distribution during fertilization of the mouse egg. J.Embryol.Exp.Morphol. 1984;81:211–237. [PubMed] [Google Scholar]
- 28.Gurdon JB. Attmepts to analyse the biochemical basis of regional differences in animal eggs. Ciba Found.Symp. 1975;0:223–239. doi: 10.1002/9780470720110.ch11. [DOI] [PubMed] [Google Scholar]
- 29.Motlik J, Kubelka M. Cell-cycle aspects of growth and maturation of mammalian oocytes. Mol.Reprod.Dev. 1990;27:366–375. doi: 10.1002/mrd.1080270411. [DOI] [PubMed] [Google Scholar]
- 30.Li Y, Uruno T, Haudenschild C, Dudek SM, Garcia JG, Zhan X. Interaction of cortactin and Arp2/3 complex is required for sphingosine-1-phosphate-induced endothelial cell remodeling. Exp.Cell Res. 2004;298:107–121. doi: 10.1016/j.yexcr.2004.03.023. [DOI] [PubMed] [Google Scholar]
- 31.Wolfer DP, Crusio WE, Lipp HP. Knockout mice: simple solutions to the problems of genetic background and flanking genes. Trends Neurosci. 2002;25:336–340. doi: 10.1016/s0166-2236(02)02192-6. [DOI] [PubMed] [Google Scholar]
- 32.Leader B, Lim H, Carabatsos MJ, Harrington A, Ecsedy J, Pellman D, Maas R, Leder P. Formin-2, polyploidy, hypofertility and positioning of the meiotic spindle in mouse oocytes. Nat.Cell Biol. 2002;4:921–928. doi: 10.1038/ncb880. [DOI] [PubMed] [Google Scholar]
- 33.Dumont J, Million K, Sunderland K, Rassinier P, Lim H, Leader B, Verlhac MH. Formin-2 is required for spindle migration and for the late steps of cytokinesis in mouse oocytes. Dev.Biol. 2007;301:254–265. doi: 10.1016/j.ydbio.2006.08.044. [DOI] [PubMed] [Google Scholar]
- 34.Goode BL, Eck MJ. Mechanism and function of formins in the control of actin assembly. Annu.Rev.Biochem. 2007;76:593–627. doi: 10.1146/annurev.biochem.75.103004.142647. [DOI] [PubMed] [Google Scholar]
- 35.Azoury J, Lee KW, Georget V, Rassinier P, Leader B, Verlhac MH. Spindle positioning in mouse oocytes relies on a dynamic meshwork of actin filaments. Curr.Biol. 2008;18:1514–1519. doi: 10.1016/j.cub.2008.08.044. [DOI] [PubMed] [Google Scholar]
- 36.Deng M, Suraneni P, Schultz RM, Li R. The Ran GTPase mediates chromatin signaling to control cortical polarity during polar body extrusion in mouse oocytes. Dev.Cell. 2007;12:301–308. doi: 10.1016/j.devcel.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 37.Longo FJ. Actin-plasma membrane associations in mouse eggs and oocytes. J.Exp.Zool. 1987;243:299–309. doi: 10.1002/jez.1402430215. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


