Abstract
Effective oral delivery of proteins is impeded by steep pH gradients and proteolytic enzymes in the gastrointestinal tract, as well as low absorption of the proteins into the bloodstream due to their size, charge or solubility. In the present work, pH-responsive complexation hydrogels of poly(itaconic acid) with poly(ethylene glycol) grafts were synthesized for applications in oral drug delivery. These hydrogels were expected to be in collapsed configuration at low pH due to hydrogen bonding between poly(itaconic acid) carboxyl groups and poly(ethylene glycol), and to swell with increasing pH because of charge repulsion between deprotonated carboxylic acid groups. Hydrogels were prepared by UV-initiated free radical polymerization using tetraethylene glycol as the crosslinking agent and Irgacure® 2959 as the initiator. The effect of monomer ratios, crosslinking ratio and solvent amount on the properties of the hydrogels were investigated. The composition of the hydrogels was confirmed by FTIR. Equilibrium swelling studies in the pH range of 1.2 to 7 revealed that the extent of swelling increased with increasing pH up to a pH of about 6, when no further carboxylic acid deprotonation occurred. Studies in Caco-2 colorectal carcinoma cells confirmed the cytocompatibility of these materials at concentrations of up to 5 mg/ml.
Keywords: hydrogels, itaconic acid, poly(ethylene glycol), Caco-2 cells, biocompatibility
Introduction
Absorption of proteins into the bloodstream after oral delivery is impeded by a number of barriers including pH, proteolytic enzymes in the gastrointestinal fluids and endothelia, and low permeability due to size, charge, hydrophobicity, or poor solubility of the protein in gastrointestinal fluids.1 Hydrogels that respond to changes in pH have been widely investigated for applications in oral drug delivery. By engineering the composition, it is possible to tailor the trigger pH and the specific response of the hydrogel. Anionic hydrogels, containing functional groups that can ionize into negatively charged moieties, swell at pH levels above the pKa of the given functional groups as a result of charge repulsion between deprotonated moieties and increased hydrophilicity.
In the present research, anionic complexation hydrogels of poly(itaconic acid) (PIA) with poly(ethylene glycol) (PEG) tethers (henceforth designated as P(IA-g-EG)) were prepared as materials for the delivery of labile molecules through the oral route. In addition to swelling in response to a rise in pH, these hydrogels are expected to form highly compact networks at acidic pH as a result of hydrogen bonding between the carboxylic acid groups of PIA and the ether oxygen of PEG. Thus, when used in oral drug delivery systems, these materials are expected to protect therapeutic molecules from the acidity of the stomach and from the action of proteolytic enzymes in the gastrointestinal tract by impeding the diffusion of the drug out of the hydrogel or of enzymes into the hydrogel. These materials might also improve the bioavailability of the therapeutic agent by (1) only releasing it at the site of absorption in the intestine once the pH in the gastrointestinal tract has increased above the pKa of itaconic acid (IA) and the network has swollen, and (2) by increasing its residence time in the intestine through bioadhesive interactions between the hydrogel and the intestinal mucosa due to both the formation of hydrogen bonds between IA functional groups and mucosal glycoproteins, and the interpenetration of tethers into the mucus in a fashion similar to that previously reported with poly(acrylic acid) hydrogels with PEG tethers.2 Finally, as has been suggested for similar systems,3 PIA hydrogels may prevent proteolytic enzyme activity and increase the permeability of the intestinal epithelial layer due to their high capacity for cation (Ca2+) binding.4
Polymerization of IA has a number of difficulties. First, the homopolymerization of IA is hindered by allylic hydrogen atoms that act as chain transfer agents.5 Second, the rate of homopolymerization of IA has been reported to be dependent on pH and degree of ionization. Specifically, the rate of homopolymerization is approximately constant at a pH < 3.8 but rapidly drops to zero at pH greater than 4.4 The effect of pH on the copolymerization of IA may not be as radical but is still important. In the copolymerization with acrylonitrile, for example, IA was incorporated into the copolymer both at low pH and at a pH of 4.7 or even ~9, but the extent of incorporation decreased with increased pH.6 The reactivity ratios in the copolymerization of IA with other monomers are also a function of pH. As the pH increases, the reactivity ratio r1 decreases due to the increasingly difficult homopolymerization of the dianion of IA.4
Despite these difficulties, hydrogels containing IA as a comonomer have been reported in past accounts. Gamma ray irradiation has been used for the preparation of hydrogels containing IA and N-vinyl-2-pyrrolidone,7,8 N-isopropyl acrylamide,9 or 2-hydroxyethyl methacrylate.10,11 In these cases, the IA content in the hydrogels ranged only from 3 to 9 mol% and the polymerization reactions were carried out for long periods of time ranging from 16 hr to days. The preparation of hydrogels of IA by free radical polymerization using sodium persulfate, potassium persulfate or benzoyl peroxide as the initiators has also been reported. Specifically, hydrogels with IA and acrylamide12,13 or N-hydroxymethyl acrylamide14 have been investigated as pH-sensitive materials for controlled drug release or ion absorption. Hydrogels with acrylamide were prepared with up to 10 wt% IA and were investigated for their swelling ability, response to pH and ability to load and release model drugs.12,13 Those with N-hydroxymethyl acrylamide were prepared with up to 75 mol% IA and showed increased swelling with increased pH, as well as decreased swelling with increased ionic strength.14
The preparation of IA polymers by UV-initiated polymerization has only been reported in few instances. Crosslinked polymer networks of IA with ethylene glycol dimethacrylate were polymerized under UV light in the presence of 2,2-azobis-(2-methyl-propionate) as initiator for 18 hours.15 Hydrogels of IA and dimethylaminoethyl methacrylate with up to 20 mol% IA were synthesized by UV irradiation for 48 hours using 1-hydroxycyclohexyl phenyl ketone as the initiator.16 It is worth noting that in this latter preparation a reaction time of 24 hours was not sufficient for the formation of the hydrogel.
In the present work a method was developed for the UV-initiated synthesis of PIA hydrogels having PEG chains grafted throughout the structure. The effect of feed composition, crosslinking density and solvent content on the properties, swelling ability and cytocompatibility of the hydrogels was studied.
2. Materials and Methods
Materials
IA was purchased from Acros Organics (Morris Plains, NJ). Poly(ethylene glycol) methyl ether monomethacrylate (PEGMMA, molecular weight 1,100, n ~ 23) was obtained from Polysciences Inc. (Warringon, PA). The reagent 1-[4-(2-hydroxyethoxy)-phenyl]-2-hydroxy-2-methyl-1-propane-1-one (Irgacure® 2959) was purchased from Ciba Specialty Chemicals (Tarrytown, NY). Tetra(ethylene glycol) dimethacrylate (TEGDMA) was purchased from Sigma-Aldrich (Saint Louis, MO). Double distilled water was purified with a Milli-Q Plus system (Millipore, Billerica, MA). All solvents were of ACS or HPLC grade. All reagents were used as received.
Hydrogel Synthesis
Complexation hydrogels were prepared by UV-initiated free radical polymerization. IA was first dissolved in a co-solvent system of aqueous NaOH solution and ethanol in a 1:1 ratio by weight. The molar concentration of NaOH in the aqueous solution was such that there was a IA:NaOH molar ratio of 2:1. The total solvent mass was of 65 or 70% that of all monomers, crosslinker and initiator. The mixture was sonicated until IA was dissolved. The partial neutralization of IA with NaOH was performed to improve the solubility of the highly-charged IA in water. The solubility of IA in pure water is 1 g in 12 ml (0.083 g/ml),17 too low for polymerization of a homogeneous hydrogel, while in this co-solvent system the monomer was easily solubilized to 0.29 g/ml.
The co-monomer PEGMMA and the crosslinker TEGDMA were then added to the IA solution at specific molar ratios as shown in Table 1. See Figure 1 for the structure of all co-monomers and sample network structure. The mixture was then sonicated until all components were in solution. The pH was adjusted to 1 ± 0.5 using 37% HCl (540–750 μl depending on IA/NaOH content) and the initiator Irgacure® 2959 was added at 1% of the weight of the monomers and crosslinker. The system was briefly sonicated to assure formation of a homogeneous solution. The pre-polymer solution was purged with nitrogen for 10 minutes to remove dissolved oxygen which acts as a free radical scavenger. The solution was then rapidly transferred into four assemblies consisting of two 75 × 50mm glass slides separated by a 500 μm Teflon spacer and polymerized for 2 hours under 25 mW/cm2 ultraviolet light from a Dymax BlueWave 200 point source.
Table 1.
Hydrogel feed composition
| Sample Name | IA/PEGMMA Molar Ratio | IA Content (mol %) | TEGDMA (mol% of Monomers) | Solvent Content (Wt. %) |
|---|---|---|---|---|
| PIP-2:1 | 2.0:1.0 | 67 | 10 | 65 |
| PIP-1.5:1 | 1.5:1.0 | 60 | 10 | 65 |
| PIP-1:1 | 1.0:1.0 | 50 | 10 | 65 |
| PIP-1:1.5 | 1.0:1.5 | 40 | 10 | 65 |
| PIP-1:2 | 1.0:2.0 | 33 | 10 | 65 |
| PIP-2:1-HS | 2.0:1.0 | 67 | 10 | 70 |
| PIP-1.5:1-HS | 1.5:1.0 | 60 | 10 | 70 |
| PIP-1:1-HS | 1.0:1.0 | 50 | 10 | 70 |
| PIP-1:1.5-HS | 1.0:1.5 | 40 | 10 | 70 |
| PIP-1:2-HS | 1.0:2.0 | 33 | 10 | 70 |
| PIP-2:1-HC | 2.0:1.0 | 67 | 12.5 | 65 |
| PIP-1.5:1-HC | 1.5:1.0 | 60 | 12.5 | 65 |
| PIP-1:1-HC | 1.0:1.0 | 50 | 12.5 | 65 |
| PIP-1:1.5-HC | 1.0:1.5 | 40 | 12.5 | 65 |
| PIP-1:2-HC | 1.0:2.0 | 33 | 12.5 | 65 |
Abbreviations: HS – high solvent content, HC – high crosslinker content
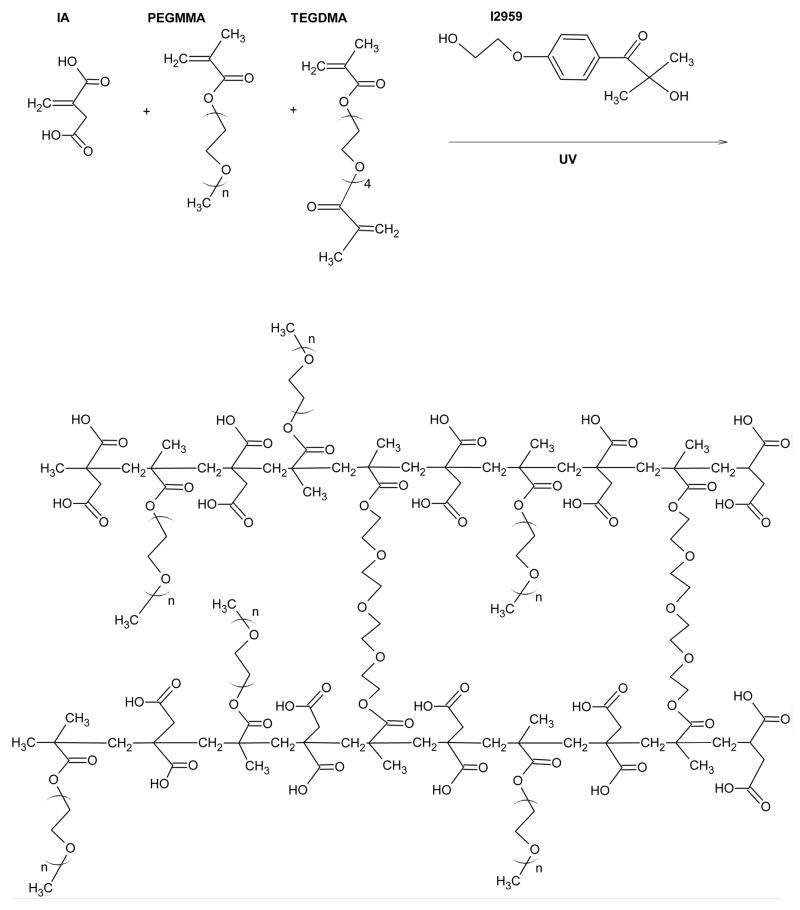
Figure 1.
Chemical synthesis of crosslinked P(IA-g-EG) hydrogels
After polymerization, samples of each hydrogel were obtained for determination of the relaxed polymer volume. Hydrogels were washed to remove residual monomer and excess reagents for seven days in 1 L of DI water, with the water being replaced twice daily. After purification, the hydrogels were cut into discs of 9 mm in diameter (aspect ratio of 18), dried for more than 4 hours at room temperature, and then dried in a vacuum oven at 30°C and 28 in Hg of vacuum for 2 days. Dried discs were stored in a vacuum desiccator for further use. Part of the polymer samples were crushed and sieved into microparticles of 53–150 μm in diameter.
Theoretical pH Dependence of Functional Group Ionization
The Henderson/Hasselbach equation (Equation (1)) was used to determine the theoretical response of the copolymers to varying pH based on the pKa values of the two carboxylic acid groups of IA (3.85 and 5.45).18 In this equation, [A−] and [HA] represent the concentrations of the deprotonated and protonated carboxylic acid groups of IA.
| (1) |
Based on the degree of ionization of these two functional groups per Equation (1) and the theoretical composition of the hydrogels (molar ratio of ionizable IA to neutral PEGMMA monomers in the feed), the percentage of charged monomers was determined as a function of pH.
Scanning Electron Microscopy
Crushed hydrogel microparticles were observed with scanning electron microscopy (SEM) to confirm their size and study their morphology. Particles were placed over carbon conductive tabs (Ted Pella Inc., Redding, CA), sputter coated with gold plasma and imaged with a Hitachi 4500 SEM to confirm their size and observe their morphology.
Fourier Transform Infrared Spectroscopy of Hydrogels
Dried hydrogel microparticles were blended at a 1:20 weight ratio with KBr, placed in a 13-mm Carver pellet die and pressed with a Carver press at a compression force of 13,000 lbs. An Infinity Gold Fourier Transform Infrared (FTIR) spectrophotometer (Thermo Mattson, Thermo Electron Corporation, Waltham, MA) with a Deuterated Triglycine Sulfate (DTGS) detector was used to study the spectrum of the hydrogel microparticles in the range of 400–4000 cm−1. Data are presented in the absorbance mode and are an average of 64 scans at a resolution of 1 cm−1.
Equilibrium Swelling Studies
Dry hydrogel discs were weighed and immersed in a buffer solution at 37 °C for 7 days. For the pH range between 3.2 and 7.0 the discs were placed in β,β-dimethylglutaric acid buffer with 0.1M NaCl, while samples at a pH of 1.2 were placed in pepsin-free simulated gastric fluid (0.1 N HCl, 2g/L NaCl, pH ~ 1.2). After the incubation period, the swollen hydrogel outer surfaces were carefully wiped and the discs were weighed. The swollen samples were weighed in hexane immediately after using a hanging basket method.
The equilibrium weight swelling ratio (q) of the hydrogels was determined as a function of pH as described by Equation (2).
| (2) |
Here Wd is the weight of a dry hydrogel disc and Ws is the weight of a swollen disc at equilibrium at given pH.
Determination of Molecular Weight Between Crosslinks and Mesh Size
The molecular weight between crosslinks (M̄c) was calculated with the Peppas-Merril equation (Equation (3)) for neutral hydrogels crosslinked in the presence of a solvent19 as described previously.20
| (3) |
In Equation (3), M̄c is the average molecular weight between crosslinks, M̄n is the average molecular weight of polymer chains before crosslinking, υ is the specific volume of the polymer in dry amorphous state (1/ρpolym = Vd/Wd), V1 is the molar volume of swelling agent (18.1 ml/mol for water), χ1 is the Flory polymer-solvent interaction parameter, υ2,s is the volume fraction of the polymer in the swollen state and υ2,r is the volume fraction of the polymer in the relaxed state.
The term M̄n was experimentally determined using gel permeation chromatography (GPC) of polymers synthesized identically to the hydrogels but in the absence of TEGDMA. The GPC system consisted of a Waters HPLC with four Ultrahydrogel® columns in series; the mobile phase used was a 4:1 v/v 0.1 M NaNO3/acetonitrile. Molecular weights were determined based on poly(ethyleneglycol) standards (Fluka, Darmstadt, Germany). The average molecular weight of all formulations was used for the calculations. Flory polymer-solvent interaction parameters for IA and PEG were averaged according to their weight ratio in the feed.
The polymer volume fractions in the relaxed and swollen states (υ2,r and υ2,s) were determined by the Peppas and Barr-Howell method21 using a hanging pan balance with Equations (4) and (5) for polymers swollen in the synthesis solvent immediately after preparation (relaxed state) or in buffer (swollen state). The terms Vd, Vr and Vs are the volumes of the hydrogel in the dry, relaxed and swollen state, respectively, as calculated by Equations (6), (7) and (8).
| (4) |
| (5) |
| (6) |
| (7) |
| (8) |
An alternative method was used for estimation of M̄c that is not dependent on the value of the polymer-solvent interaction parameter. As shown in Equation (9), the theoretical molecular weight between crosslinks, M̄c,t, can be simply calculated as the molecular weight of the polymer repeat units, Mr, divided by twice the nominal crosslinking ratio, X.
| (9) |
The mesh size, ξ, or average length between crosslinks, was calculated by Equation (10) where ℓ is the bond length along polymer chain (1.54Å for carbon-carbon bonds), Cn is the characteristic ratio of the polymers weighted by feed molar content, and Mr is the molecular weight of the repeating units.
| (10) |
Table 2 shows representative values used in the determination of the molecular weight between crosslinks.
Table 2.
Variables used for determination of molecular weight between crosslinks and mesh size of PIA-g-PEG hydrogels
| Variable | Description | Value |
|---|---|---|
| V1 | Molar volume of swelling agent | 18.1 ml/mol |
| ρhex | Density of hexane | 666.7 mg/ml |
| χ1,IA | Polymer-solvent interaction parameter for poly(itaconic acid) | Not available |
| χ1,PEG | Polymer-solvent interaction parameter for poly(ethylene glycol)27 | 0.55 |
| Cn,IA | Flory characteristic ratio of poly(itaconic acid)22 | 4.63 |
| Cn,PEG | Flory characteristic ratio of poly(ethylene glycol)28 | 3.8 |
| ℓ | Carbon-carbon bond length along polymer chain | 1.54 Å |
| Mo | Molecular weight of IA repeating units | 130 g/mol |
| Mn | Number average molecular weight of the hydrogel in the absence of crosslinker (Determined experimentally) | 11,505 g/mol |
| Mr | Molecular weight of the repeating unit of the polymer | 130 g/mol |
| X | Nominal degree of crosslinking | 0.100 or 0.125 |
It is worth noting that this analysis assumes that the crosslinker concentration is negligible so that it is not considered a comonomer. Because of the relatively high crosslinker concentration used in the preparation of the hydrogels, the results provide a general sense of the behavior of the material but prevent them from being used for analysis beyond general use.
Cytocompatibility Studies
Caco-2 colorectal adenocarcionoma epithelial cells were obtained from the American Type Culture Collection (ATCC, Manassas, VA). Cells were grown in Dulbecco’s modified eagle medium (DMEM) (Mediatech, Herndon, VA) supplemented with 10% heat inactivated fetal bovine serum (Cambrex, East Rutherford, NJ), 1% non-essential amino acids (Mediatech), 100 U/ml penicillin and 100 μg/ml streptomycin (Mediatech) in a humidified incubator at 37 °C and 5% CO2 atmosphere. For cytotoxicity studies, cells were seeded at a density of 1.4 × 104 cell/cm (~ 10,000 cells/well) in three 96-well plates (Corning). Cells were fed every other day until the cells reached nearly complete confluency.
Hydrogel microparticles of various formulations in the size range of 53–150 μm were suspended in Hank’s balanced salt solution (HBSS, Mediatech) at a concentration of 10 mg/ml. The pH of the suspensions was brought up to 7.4 using microliter amounts of 1 N NaOH. Further dilutions were made with HBSS in order to have five concentrations in the range of 0.625–10 mg/ml.
One hour prior to the beginning of the study, the cell medium was removed by aspiration and replaced with 200 μl of pre-warmed HBSS to equilibrate the cell monolayers. After 1 hour of incubation, the HBSS was removed and replaced with 200 μl of the respective hydrogel microparticle formulation. Cells were incubated for 2 hours in the presence of the microparticle suspensions. After the exposure period, the hydrogel suspensions were removed and the cell monolayer was washed three times with 200 μl of HBSS. To determine the cell viability, the cells were incubated for 90 minutes with 120 μl of appropriately-diluted Cell Titer 96® Aqueous One Solution Proliferation Assay reagent (Promega, Madison, WI) and the absorbance was read at 490 nm using a Synergy HT plate reader (Bio-Tek, Winooski, VT). The Cell Titer 96 assay is based on the conversion of a tetrazolium compound (3-(4,5-dimethyl-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, MTS) into a soluble formazan colored product by metabolically active cells. Viability data are presented as a percentage of that of control cells that were maintained in HBSS only. Error bars represent the standard deviation of the results between 6 wells of cells exposed to the same batch of hydrogel microparticles.
3. Results and Discussion
Hydrogel Synthesis
Chemically crosslinked hydrogels of P(IA-g-EG) were successfully prepared by UV-initiated free-radical polymerization. In comparison to previous reports on the preparation of IA-containing hydrogels,7–13,15,16 the present preparation method permitted the incorporation of significantly higher amounts of IA in the formulation because of the increased solubility of partially-neutralized IA and the increased reactivity of this monomer at low solution pH.4
Table 3 lists observations regarding the resulting hydrogels. Qualitatively, increased IA feed content resulted in increased hydrogel strength and brittleness. Hydrogels with high PEG tether content were tacky and weaker. Increased crosslinker content also increased the strength and brittleness of the hydrogels, as expected, while increased solvent content decreased their mechanical strength.
Table 3.
Qualitative results of hydrogel synthesis
| Sample Name | Synthesis Observations |
|---|---|
| PIP-2:1 | Monomer precipitated |
| PIP-1.5:1 | Monomer precipitated |
| PIP-1:1 | Good |
| PIP-1:1.5 | Good |
| PIP-1:2 | Good |
| PIP-2:1-HS | Good |
| PIP-1.5:1-HS | Good |
| PIP-1:1-HS | Good |
| PIP-1:1.5-HS | Extremely weak and tacky |
| PIP-1:2-HS | Extremely weak and tacky |
| PIP-2:1-HC | Monomer precipitated |
| PIP-1.5:1-HC | Monomer precipitated |
| PIP-1:1-HC | Good |
| PIP-1:1.5-HC | Good |
| PIP-1:2-HC | Good |
Compared to established protocols from our laboratory for the preparation of hydrogels of methacrylic acid and PEGMMA by UV-initiated free radical polymerization (normally carried out for 20 minutes), the preparation of IA-containing hydrogels occurred at a significantly slower rate. Within 30 minutes into the polymerization, the copolymer had significantly increased in viscosity but did not form a film. After 1 hr of polymerization a film was observed and no significant changes were observed thereafter; nonetheless, all polymerizations were carried out for 2 hrs. As described previously, similar crosslinked IA polymers have been prepared by UV polymerization15,16 or by γ–irradiation7–11 in reactions lasting from 16 hours to several days.
As observed in Table 3, hydrogel formation with increased IA content was hindered in some instances as a result of the precipitation of IA upon re-acidification of the pre-polymer solution prior to polymerization. As mentioned in the methods section, the solubility of IA is low unless it is partially neutralized because of its highly charged nature. However, acidification of the pre-polymer solution was desired to improve the incorporation of IA into the hydrogel. As described in the introduction section, the reactivity of IA is highest when both carboxylic acid groups are protonated, i.e. when the solution pH is below the pKa of the monomer.4 As observed in preliminary experiments, polymerization without re-acidification did not result in the formation of hydrogel films for any of the compositions used in the present research. The preparation of PIA in acidified aqueous media was previously reported.22 It is also worth mentioning that as part of the present work the preparation of IA-PEGMMA hydrogels with only ethanol or methanol as the solvents and without re-acidification was attempted since the solubility of IA in these alcohols is greater than in pure water; however, these polymerizations resulted in the formation of slightly viscous solutions and not in the generation of hydrogel films. Finally, it should be noted that the preparation of P(IA-g-EG) hydrogels with lower crosslinker content (7.5 wt%) was attempted. However, although polymerization did occur, the generated material was a viscous polymer and not a film.
Scanning Electron Microscopy Characterization
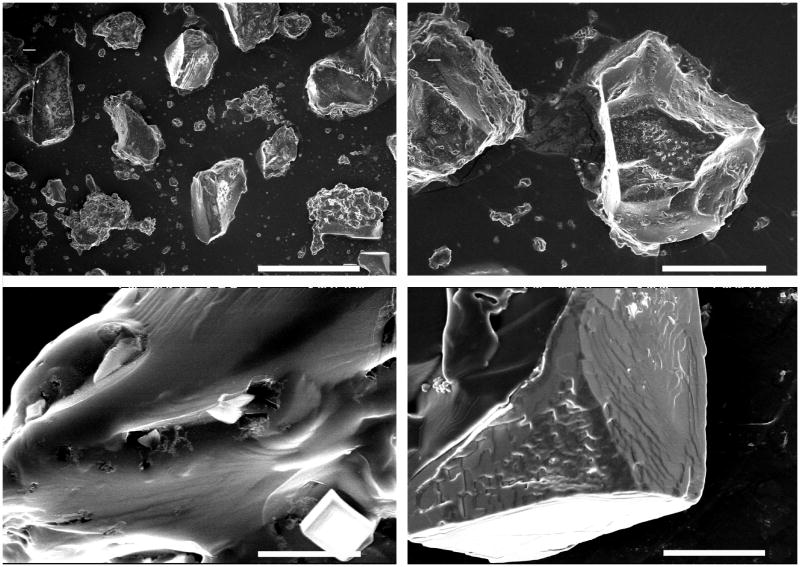
Scanning electron microscopy was used to observe the hydrogel microparticles to confirm their size and study their morphology. Figure 2 shows SEM images of the microparticles. The microparticle morphology is irregular, as expected from particles created by crushing and sieving. Particle sizes range from approximately 30 μm to up to 200 μm, although in all cases at least one of the particle sides is < 150 μm. Pores are not readily seen on the particle surfaces, as can be well noted on the close-up images in Figure 2C and D. It should be noted that white specs (cylinders on close up) observed on the particle surfaces are salt crystals that formed as the particles dried after being swollen in buffered saline.
Figure 2.
SEM images of P(IA-g-EG) hydrogels. White bar represents (A) 300, (B) 100, (C) 7 and (D) 30 μm.
Fourier Transform Infrared Spectroscopy
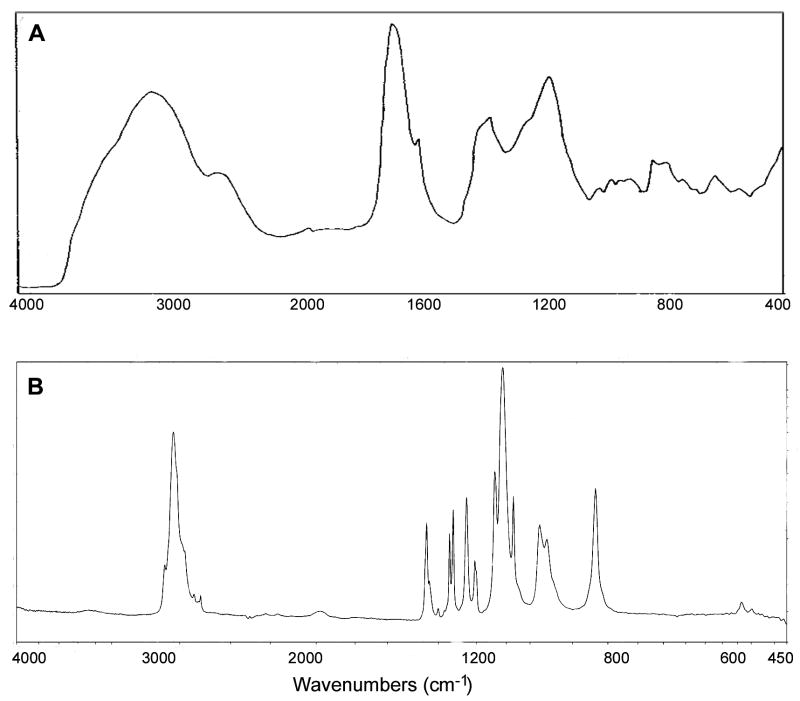
FTIR was utilized to confirm the composition of the hydrogels. Figure 3 shows the FTIR spectra of pure poly(ethylene glycol) and pure PIA, which here are used for identification of relevant functional group peaks of the P(IA-g-EG) hydrogels. Specifically, PIA shows a broad – OH stretching absorption peak in the range of 2800–3400 cm−1, a strong and narrower peak at about 1710 cm−1 corresponding to carboxylic acid C==O stretching, a peak at 1400 cm−1 corresponding to C-O-H in-plane bending, a peak at 1200 cm−1 corresponding to C—O stretching and a peak at 920 nm corresponding to O—H out-of-plane bending.23 PEG, on the other hand, shows a peak at 2890 cm−1 corresponding to asymmetric vibration of terminal methyl groups on PEG chains.
Figure 3.
FTIR absorbance spectrum of (A) poly(itaconic acid)23 and (B) poly(ethylene glycol).32
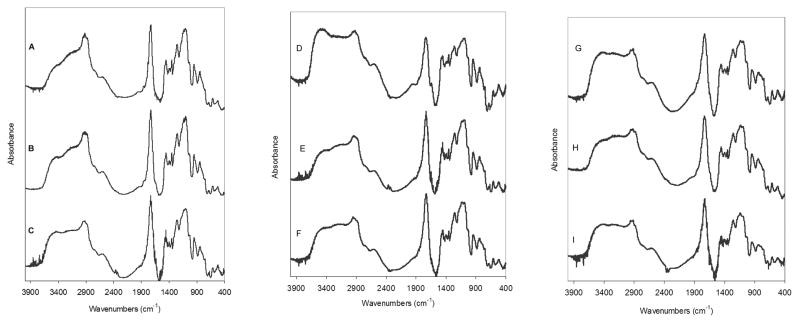
Figure 4 displays the FTIR spectra of P(IA-g-EG) hydrogels prepared with varying monomer molar ratios, crosslinker content and solvent content. The absence of a peak at 1640 cm−1, characteristic of carbon-carbon double bonds, confirms the polymerization of all monomers. All spectra exhibit peaks that can be attributed to each of the comonomers. The C==O peak at 1740 cm−1, which corresponds to the carboxylic acid groups of PIA, is shifted to a higher wavelength as a result of the presence of PEG tethers. In the absence of PEG tethers, the carboxylic acid groups of IA could potentially hydrogen bond with each other forming cyclic dimers, as has been previously described for poly(acrylic acid).24 As the concentration of PEG tethers increases, the carboxylic acid groups no longer dimerize but instead form hydrogen bonds with PEG tethers, resulting in a shifted absorption spectrum. A similar analysis has been previously applied to poly(acrylic acid) hydrogels with PEG tethers.25 The hydrogen bonding and consequent peak shift has also been previously associated with the complexation of PIA with PEG in systems created by polymer blending or polymerization of IA in the presence of PEG (template polymerization).26 Finally, the peak associated with PEG terminal methyl group vibration is readily seen at 2900 cm−1, within the broader –OH stretching peak of PIA.
Figure 4.
FTIR absorbance spectrum of P(IA-g-EG) hydrogels. Spectra on the left are for hydrogels prepared with a (A) 1:2, (B) 1:1.5, or (C) 1:2 itaconic acid to PEGMMA molar ratio, 10 mol% crosslinker, and 65 wt% solvent. Spectra in the middle are for hydrogels prepared with a (D) 1:2, (E) 1:1.5, or (F) 1:1 itaconic acid to PEGMMA molar ratio, 12.5 mol% crosslinker and 65 wt% solvent. Spectra on the right are for hydrogels prepared with a (G) 2:1, (H) 1.5:1, or (I) 1:1 itaconic acid to PEGMMA molar ratio, 10 mol% crosslinker and 70 wt% solvent.
For hydrogels prepared with a given crosslinker and solvent content, the relative intensity of the PIA C==O peak at 1740 cm−1 compared to that of the PEG tethers terminal methyl groups at 2900 cm−1 suggests increased IA content in the final network with increased IA/PEG molar ratio in the feed. This is more clearly observed in the spectra of hydrogels prepared with low solvent content (Figures 4 left and center). The effect of monomer feed composition appeared to have a lesser effect on the final network composition for hydrogels made with 70 wt% solvent content judging from the relative intensities of these two peaks (Figure 4 right).
Theoretical Hydrogel Response to pH
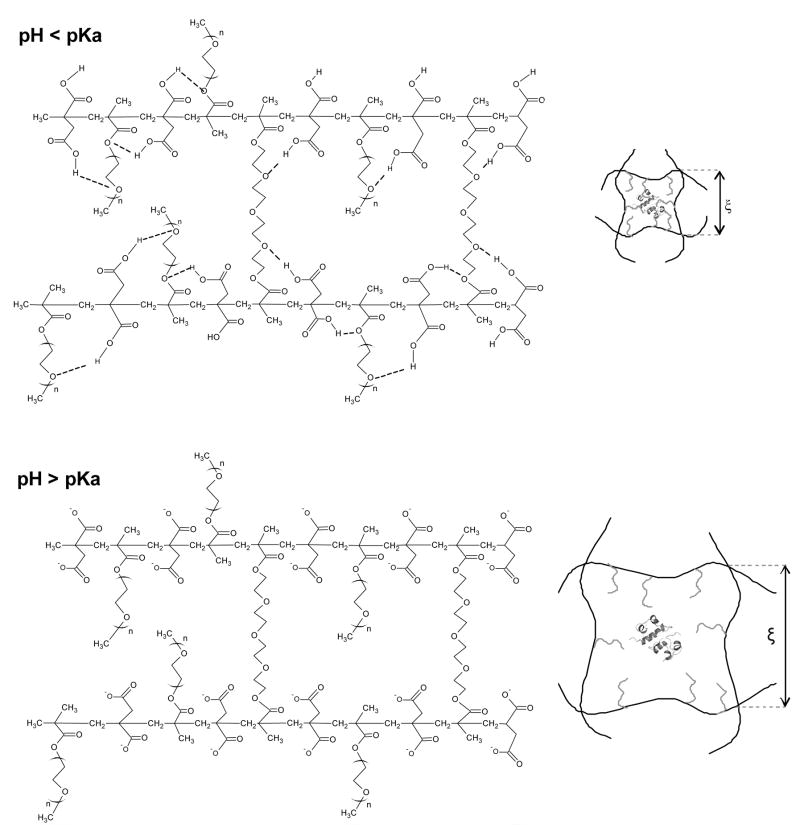
The PIA-g-PEG hydrogels were designed for use as carriers for the delivery of proteins and other acid-labile molecules to the intestinal track. As shown on Figure 5, the presence of IA and PEG within the hydrogel structure theoretically permits hydrogen bond-driven complexation and consequent hydrogel collapse at acidic conditions. A pH higher than the pKa of the acidic monomer, on the other hand, results in deprotonation of the carboxylic acid groups, termination of the hydrogen bonds, charge repulsion and increased hydrophilicity leading to hydrogel swelling and protein release.
Figure 5.
Schematic of the effect of pH on the structure of crosslinked PIA-g-PEG hydrogels
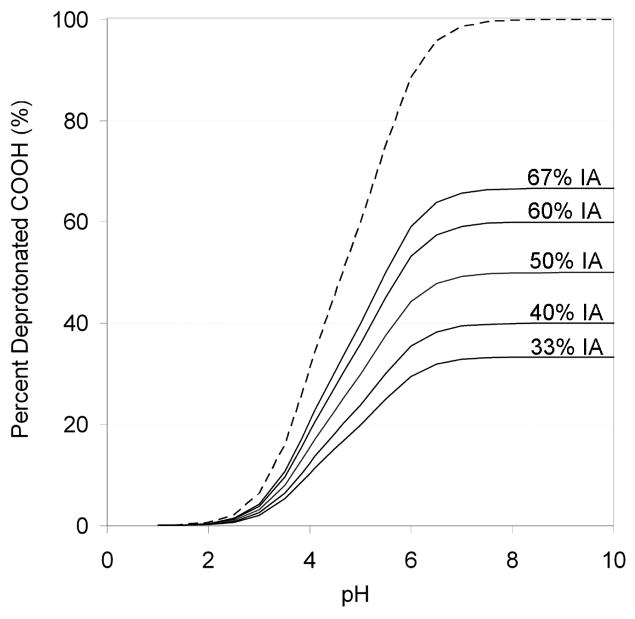
Figure 6 shows the theoretical behavior of the hydrogels with varying pH. Specifically, the dashed line shows the extent of carboxylic acid deprotonation with increasing pH of IA based on the Henderson/Hasselbach equation and the pKa of each of the carboxylic acid groups of IA. The solid lines display the percent monomer deprotonation in a hydrogel composed of IA and the neutral ethylene glycol repeat groups of PEG at the five IA/PEG feed molar ratios used in the present research. As shown, the higher the content of IA in a given hydrogel the higher the extent of monomer deprotonation and the consequent pH-driven changes in hydrogel macro-conformation.
Figure 6.
Theoretical extent of carboxylic acid deprotonation as a function of pH for itaconic acid (dashed line). Theoretical percent of charged monomer functional groups in P(IA-g-EG) hydrogels at the five feed monomer compositions used (solid lines).
Equilibrium Swelling Behavior
Equilibrium swelling studies were performed with the objective of determining the effect of pH on the swelling capacity of the PIA-g-PEG hydrogels. Specifically, the studies were designed such that the swelling of macroscopic discs would be representative of the swelling behavior of microparticles that would respond significantly more rapidly as a result of their high surface area and relative small depth for water intake. The effect of IA to PEG in the feed, the solvent content used in the hydrogel preparation, and the amount of crosslinker used were studied.
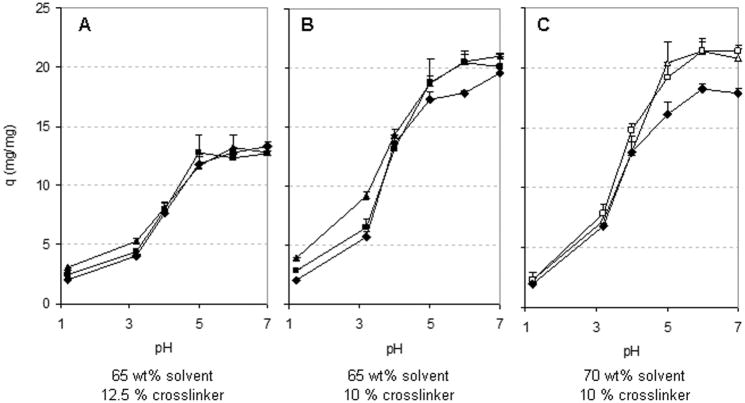
Figure 7 shows the effect of monomer composition in the feed on the equilibrium weight swelling ratio (q). Specifically, the three plots show q for hydrogels made with 65 wt% solvent and 12.5% crosslinker (A), 65 wt% solvent and 10% crosslinker (B), and 70 wt% solvent and 10% crosslinker (C). In all cases, increased pH led to increased swelling, as expected, with the hydrogels reaching maximum swelling at a pH of about 5–6. As discussed before and shown on Figure 6, the Henderson-Hasselbach equation predicted such pH-dependent behavior as a result of the pH-mediated deprotonation of the carboxylic acid groups of IA and the consequent termination of hydrogen bonds and charge repulsion. On the other hand, no major differences were observed in the swelling of the network with varying feed itaconic acid to PEG repeating unit molar ratios. These results could be explained in two ways. First, they suggest that, despite the addition of increased amounts of IA relative to PEGMMA in the polymerization, the increase in IA actually incorporated within the polymer network was not comparable. This could be explained by the difficulties with polymerization of IA as described in the introduction section. On the other hand, it is important to note that increased PEG content also contributes to increased network hydrophilicity. Consequently, both pH-controlled deprotonation of IA and the hydrophilicity of the system control the maximum degree of swelling of a particular hydrogel.
Figure 7.
Effect of monomer composition on equilibrium weight swelling ratio, q, of PIA-g-PEG hydrogels. Molar ratio of itaconic acid to PEG repeating units in the feed: △ 2:1, □ 1.5:1, ◆ 1:1, ■ 1:1.5, ▲ 1:2.
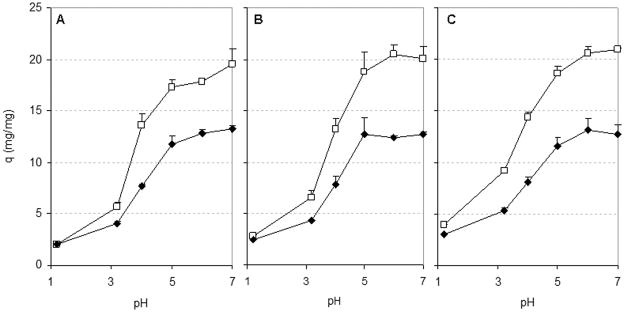
Figure 8 shows that the amount of solvent used during polymerization of the hydrogels results in insignificant differences in their equilibrium swelling despite the qualitative differences in hydrogel mechanical properties immediately after polymerization. Since the difference in solvent content between these samples is of only 5 wt%, it is possible that any difference was minimal on the conformation of the networks after hydrogel washing and drying. It is worth noting, however, that while increased solvent allowed the incorporation of higher amounts of IA in the polymerization, it also resulted in the formation of hydrogels with decreased strength and increased tackiness when compared to hydrogels made with less solvent but equivalent monomer compositions.
Figure 8.
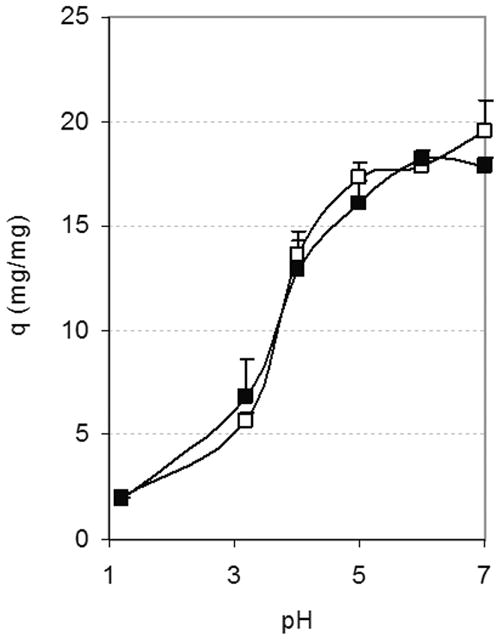
Effect of solvent content during polymerization on equilibrium weight swelling ratio, q, of PIA-g-PEG hydrogels. Data shown for hydrogels made with a 1:1 molar ratio of itaconic acid to PEG repeating units with (□) 65 wt% or (■) 70 wt% solvent.
Figure 9 shows the effect of the amount of crosslinker in the equilibrium swelling of hydrogels. As shown, significantly higher swelling was observed in hydrogels made with lower crosslinker content for all pH values greater than 1.2 and for all monomer compositions tested in the present work. The crosslinking ratio limits the extent of maximal inter-chain repulsion at any given pH, consequently controlling the maximal extent of hydrogel swelling.
Figure 9.
Effect of crosslinker content —(□) 10% vs (■) 12.5% by mol of all monomers — on the equilibrium weight swelling ratio, q, of PIA-g-PEG hydrogels. Data shown are for hydrogels prepared with a (A) 1:1, (B) 1:1.5, and (C) 1:2 itaconic acid to PEG repeating unit molar ratio.
Determination of Network Mesh Size
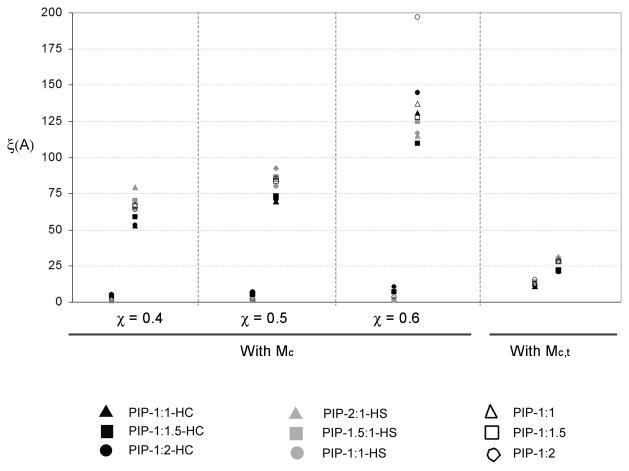
The hydrogel network mesh size was studied as a function of hydrogel feed composition and pH using the Peppas-Merril equation for neutral hydrogels crosslinked in the presence of a solvent (Equation (3)) as well as an approximation based on the nominal crosslinking ratio (Equation (9)). These analyses were developed for systems with low extent of crosslinking in which the crosslinker is not considered a co-monomer; however, the P(IA-g-EG) hydrogels developed in the present research required high levels of crosslinker (> 10%) for the preparation of structurally sound networks that could be manually handled. Consequently, the present analysis provides only a general estimate of the distance in between crosslinks. In addition, in the absence of quantitative values for the χ polymer-solvent interaction parameter for PIA systems, overall mesh size ranges are provided for likely χ value ranges. It is worth noting that the mesh size, when calculated based on the theoretical molecular weight between crosslinks M̄c,t, not a function of χ.
Figure 10 shows the range of mesh sizes, ξ, as determined for a range of values of χ for PIA from pH 1.2 to pH 7.0 using both the theoretical and experimentally determined molecular weight between crosslink values, M̄c,t and M̄c. As expected, at the low pH of 1.2 the hydrogel network is in its collapsed form and presents a small ξ on the order of <10 Å or 11–16 Å when determined using the experimental or theoretical M̄c respectively. At neutral pH, the hydrogel network swells and ξ increases significantly to a range between 50 Å and 198 Å depending on the composition of the hydrogel and the χ value used for the experimentally determined M̄c, or to 21–30 Å with the theoretical M̄c,t.
Figure 10.
Mesh size, ξ, of P(IA-g-EG) hydrogel networks as a function of pH, feed monomer molar ratios, and polymer–solvent interaction factor, χ, of poly(itaconic acid). Values calculated from the experimental M̄c or theoretical M̄c,t (not a function of χ).
Although the use of the theoretical M̄c,t based on the nominal crosslinker content used for the polymerization gives valuable information regarding the behavior of the network, the experimentally determined M̄c more accurately describes the response of the hydrogels to changes in pH. Because of the relatively high extent of crosslinker used in the preparation of the P(IA-g-EG) hydrogels, the use of the theoretical M̄c,t predicts a low degree of chain freedom which restricts both the collapse and swelling of the hydrogel chains. This restraint results in a moderately high calculated mesh size at acidic pH, and a relatively small mesh size at neutral pH that is only about twice the size of the network at the acidic pH, as seen in Figure 10. The experimentally-determined M̄c, on the other hand, is based on the observed water imbibition but is also a function of the unknown χ factor. Although no data are available on the χ factor of PIA or other similar polymers (those with monomers such as the unsaturated dicarboxylic acids 2-methylidene propanedioic acid, maleic acid, fumaric acid, mesaconic acid, citaconic acid; or unsaturated carboxylic acids such as 3-butenoic acid or isopropenyl acetic acid), the value of the χ factor for poly(methacrylic acid) is 0.587727 and that of most tabulated polymers at low polymer concentration ranges between 0.4 and 0.6.28 This χ range was used to study its effect on the mesh size of the hydrogels.
As shown on Figure 10, the mesh size calculated with the experimental M̄c increases with increasing values of χIA. Nonetheless, changes in pH have the most significant impact in the hydrogel mesh size. This analysis provides a range of mesh sizes that can be used for tailoring the system for the entrapment of therapeutic molecules of various sizes.
In Vitro Cytotoxicity Studies
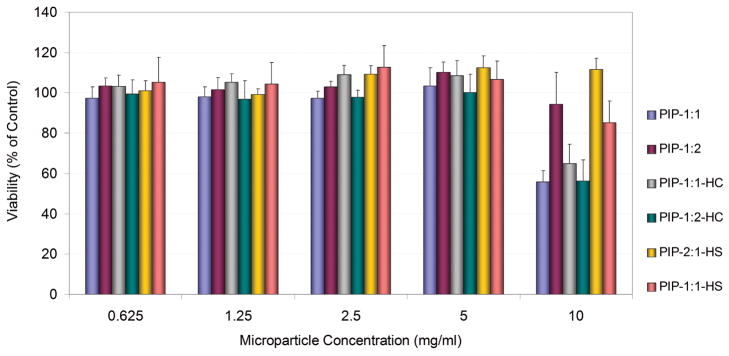
The effect of cell exposure to microparticles of various compositions was studied as a function of dose. Cells were exposed to microparticles suspended in Hank’s balanced salt solution for 2 hours, a time period representative of the expected in vivo time residence of these type of particles in the intestine.
No reduction in viability or metabolic activity was observed in Caco-2 cells exposed to P(IA-g-EG) microparticles at concentrations of up to 5 mg/ml regardless of the polymer composition, as can be observed in Figure 11. Some of the microparticle formulations, on the other hand, caused cytotoxicity when cells were exposed to the high microparticle concentration of 10 mg/ml. Most notably, microparticle formulations with high crosslinker content (12.5 % of all monomer moles) reduced the cell viability to approximately 56–65% of control regardless of the IA to PEG monomer ratio (no statistically significant difference between these). Also, microparticles prepared with 10% crosslinker and 50% IA monomer composition reduced the viability to 56% while those with the same crosslinker amount but only 33% IA showed no statistically significant reduction in cell viability.
Figure 11.
Viability of Caco-2 cells after exposure to P(IA-g-EG) microparticles. Data are presented as a percentage of control. Vertical error bars represent standard deviation (n=6).
Results agree with previous accounts of the general cytocompatibility of hydrogel microparticles of similar composition and sizes, such as those of poly(methacrylic acid-g-ethylene glycol) previously reported by our group.29–31 The lower metabolic activity observed at high microparticle concentrations could be associated with complete cell surface coverage by swollen microparticles as was observed by optical microscopy during the study. Because of their size and density, the particles settled on top of the cell monolayer, possibly restricting the transport of nutrients or even of the reagent used for determination of cell viability from the media to the cells, as well as affecting the pH of the cell microenvironment. In fact, previous research has shown that microparticle concentrations of 10 mg/ml cause Caco-2 cytotoxicity regardless of the hydrogel composition with P(MAA-g-EG) hydrogels.30 Increased hydrogel crosslinking could more severely isolate the cell monolayer from the continuous cell media as a result of decreased diffusion across the deposited microparticle layer. In addition, deprotonation of IA and its consequent buffering capacity can result in increased localized acidity at the cell-microparticle interface, potentially decreasing cell viability. This would be more significant in the case of hydrogels containing higher IA monomer, such as was the case for cells exposed to microparticles with 10% crosslinking and 50% IA versus equivalent particles with only 33% IA. Similar findings were reported for P(MAA-g-EG) hydrogels with higher ionic to neutral (4:1) monomer molar feed ratio.31 Finally, particles prepared in the presence of higher solvent volume did not decrease cell viability as significantly, possibly because the less compact network provides less of a barrier between the cells and the medium and the more loosely packed ionized groups are not as effective at buffering the pH of the local medium.
Testing of the biocompatibility of polymeric biomaterials is an important step in the development of systems for drug delivery and other biomedical applications. Frequently, toxicity caused by polymeric biomaterials is associated with residual monomer, initiator and solvents, and not with the polymer network itself. It is for this reason that the synthesized hydrogel films were washed for 7 days in water, with the water being changed twice daily. In addition, Williams et al.31 have previously shown that the photoinitiator used in the present research (Irgacure® 2959) caused significantly less toxicity if at all to various cell types after direct administration and in conjunction with UV light exposure in comparison to other photoinitiators commonly used for the preparation of hydrogels. In the case of the hydrogels here prepared, the photoinitiator is polymerized into the chains of the hydrogel and is consequently no longer available for further UV-initiated generation of free radicals.
Conclusions
The present work describes methods for the preparation of a novel type of pH responsive hydrogel materials with potential applications in oral drug delivery. Specifically, anionic hydrogels of PIA containing PEG tethers were developed. The effect of monomer composition, crosslinker content and solvent content in the feed on the resulting properties of the hydrogel was investigated. The hydrogels maintained a collapsed configuration at acidic pH as a result of hydrogen bonding interactions between the carboxylic acid groups of PIA and the ether groups of PEG. At more neutral pH, these hydrogels swelled significantly as the carboxylic acids of PIA became deprotonated. The experimental results of network swelling were as expected according to the extent of deprotonation predicted by the Henderson/Hasselbach equation. Although quantitative determination of the hydrogel network mesh size was prevented by the lack of published data on the polymer-solvent interaction parameter for PIA, a range of likely mesh sizes were determined as a function of pH which can be used for tailoring the networks for the loading and delivery of therapeutic molecules of specific sizes. In vitro studies in a colorectal adenocarcinoma model of the intestinal epithelium confirmed the cytocompatibility of the hydrogels at a concentration of up to 5 mg/ml, although toxicity was observed at 10 mg/ml.
Acknowledgments
This work was supported in part by a grant from the National Institutes of Health (EB-000246).
References
- 1.Mustata G, Dinh SM. Approaches to oral drug delivery for challenging molecules. Critical Reviews in Therapeutic Drug Carrier Systems. 2006;23(2):111–135. doi: 10.1615/critrevtherdrugcarriersyst.v23.i2.20. [DOI] [PubMed] [Google Scholar]
- 2.Serra L, Domenech J, Peppas NA. Design of poly(ethylene glycol)-tethered copolymers as novel mucoadhesive drug delivery systems. Eur J Pharm Biopharm. 2006;63(1):11–8. doi: 10.1016/j.ejpb.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 3.Madsen F, Peppas NA. Complexation graft copolymer networks: swelling properties, calcium binding and proteolytic enzyme inhibition. Biomaterials. 1999;20:1701–1708. doi: 10.1016/s0142-9612(99)00071-x. [DOI] [PubMed] [Google Scholar]
- 4.Tate BE. Polymerization of itaconic acid and derivatives. Advanced Polymer Science. 1967;5:214–232. [Google Scholar]
- 5.Marvel CS, Shepherd TH. Polymerization reactions of itaconic acid and some of its derivatives. J Org Chem. 1959;24(5):599–605. [Google Scholar]
- 6.Nagai S. Polymerization and polymers of itaconic acid derivatives. V. The copolymerization reactivity of itaconic acid in an aqueous solution. Bull Chem Soc Japan. 1963;36(11):1459–1463. [Google Scholar]
- 7.Şen M, Yakar A. Controlled release of antifungal drug terbinafine hydrochloride from poly(N-vinyl 2-pyrrolidone/itaconic acid) hydrogels. International Journal of Pharmaceutics. 2001;228:33–41. doi: 10.1016/s0378-5173(01)00804-3. [DOI] [PubMed] [Google Scholar]
- 8.Şen M, Güven O. Dynamic deswelling studies of poly(N-vinyl-2-pyrrolidone/itaconic acid) hydrogels swollen in water and terbinafine hydrochloride solutions. European Polymer Journal. 2002;38:751–757. [Google Scholar]
- 9.Taşdelen B, Kayaman-Apohan N, Güven O, Baysal BM. Preparation of poly(N-isopropylacrylamide/itaconic acid) copolymeric hydrogels and their drug release behavior. International Journal of Pharmaceutics. 2004;278:343–351. doi: 10.1016/j.ijpharm.2004.03.017. [DOI] [PubMed] [Google Scholar]
- 10.Tomić S, Mićić MM, Filipović J, Suljovrujić EH. Swelling and thermodynamic studies of temperature responsive 2-hydroxyethyl methacrylate/itaconic acid copolymeric hydrogels prepared via gamma radiation. Radiation Physics and Chemistry. 2007;76:1390–1394. [Google Scholar]
- 11.Tomić S, Mićić MM, Filipović JM, Suljovrujić EH. Swelling and drug release behavior of poly(2-hydroxyethyl methacrylate/itaconic acid) copolymeric hydrogels obtained by gamma irradiation. Radiation Physics and Chemistry. 2007;76:801–810. [Google Scholar]
- 12.El-Hamshary H. Synthesis and water sorption studies of pH sensitive poly(acrylamide-co-itaconic acid) hydrogels. European Polymer Journal. 2007;43:4830–4838. [Google Scholar]
- 13.Stanojević M, Krušić MK, Filipović J, Parojčić J, Stupar M. An investigation into the influence of hydrogel composition on swelling behavior and drug release from poly(acrylamide-co-itaconic acid) hydrogels in various media. Drug Deliv. 2006;13(1):1–7. doi: 10.1080/10717540500313034. [DOI] [PubMed] [Google Scholar]
- 14.Jeria-Orell M, Pizarro GdC, Marambio OG, Huerta M, Geckeler KE. Synthesis of N-hydroxymethyl acrylamide with β-methyl hydrogen itaconate and itaconic acid hydrogels: effects of the pH, composition, and ionic strength on the swelling behavior. Journal of Applied Polymer Science. 2006;100(3):1735–1741. [Google Scholar]
- 15.Suedee R, Songkram C, Petmoreekul A, Sangkunakup S, Sankasa S, Kongyarit N. Direct Enantioseparation of adrenergic drugs via thin layer chromatography using molecularly imprinted polymers. Journal of Pharmaceutical and Biomedical Analysis. 1999;19:519–527. doi: 10.1016/s0731-7085(98)00248-9. [DOI] [PubMed] [Google Scholar]
- 16.Kakinoki S, Kaetsu I, Nakayama M, Sutani K, Uchida K, Yukutake K. Temperature and pH responsiveness of poly-(DMAA-co-unsaturated carboxylic acid) hydrogels synthesized by UV-irradiation. Radiation Physics and Chemistry. 2003;67:685–693. [Google Scholar]
- 17.The Merck Index. Rahway, NJ: Merck & Co., Inc; 1989. [Google Scholar]
- 18.Weast R, Astle MJ. Handbook of Chemistry and Physics. Cleveland, OH: CRC Press; 1981–1982. [Google Scholar]
- 19.Peppas NA, Merrill EW. PVA hydrogels: reinforcement of radiation-crosslinked networks by crystallization. Journal of Polymer Science, Part A: Polymer Chemistry. 1976;14:441–457. [Google Scholar]
- 20.Peppas NA, Wood KM, Thomas JB. Membranes in Controlled Release. In: Peinemann K-V, Pereira Nunes S, editors. Membranes for the Life Sciences. Weinheim: Wiley-VCH; 2008. [Google Scholar]
- 21.Peppas NA, Barr-Howell BD. Characterization of. the crosslinked structure of hydrogels. In: Peppas NA, editor. Hydrogels in Medicine and Pharmacy. Boca Raton, FL: CRC Press; 1986. p. 1986.p. 180. [Google Scholar]
- 22.Veličković J, Filipović J, Djakov DP. The synthesis and characterization of poly(itaconic acid) Polymer Bulletin. 1994;32:169–172. [Google Scholar]
- 23.Kayaman N, Hamurcu EEG, Uyanik N, Baysal BM. Interpenetrating hydrogel networks based on polyacrylamide and poly(itaconic acid): synthesis and characterization. Macromol Chem Phys. 1999;200:231–238. [Google Scholar]
- 24.Jian D, Yukihiro O, Kenichi N. Infrared, raman, and near-infrared spectroscopic evidence for the coexistence of various hydrogen-bond forms in poly(acrylic acid) Macromolecules. 1997;30(4):1111–1117. [Google Scholar]
- 25.Thomas JB, Tingsanchali JH, Rosales AM, Creecy CM, McGinity JW, Peppas NA. Dynamics of poly(ethylene glycol)-tethered, pH responsive networrks. Polymer. 2007;48(17):5042–5048. doi: 10.1016/j.polymer.2007.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tomic S, Filipovic JM. Synthesis and characterization of complexes between poly(itaconic acid) and poly(ethylene glycol) Polymer Bulletin. 2004;52(5):355–364. [Google Scholar]
- 27.Barton AFM. Handbook of polymer-liquid interaction parameters and solubility parameters. Boca Raton: CRC Press, Inc; 1990. [Google Scholar]
- 28.Brandup J, Immergut EH, Grulke EA, editors. Polymer Handbook. 4. Hoboken, NJ: John Wiley & Sons; 1999. [Google Scholar]
- 29.Ichikawa H, Peppas NA. Novel complexation hydrogels for oral peptide delivery: in vitro evaluation of their cytocompatibility and insulin-transport enhancing effects using Caco-2 cell monolayers. J Biomed Mater Res A. 2003;67(2):609–17. doi: 10.1002/jbm.a.10128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Torres-Lugo M, Garcia M, Record R, Peppas NA. Physicochemical behavior and cytotoxic effects of p(methacrylic acid-g-ethylene glycol) nanospheres for oral delivery of proteins. J Control Release. 2002;80(1–3):197–205. doi: 10.1016/s0168-3659(02)00027-5. [DOI] [PubMed] [Google Scholar]
- 31.Foss AC, Peppas NA. Investigation of the cytotoxicity and insulin transport of acrylic-based copolymer protein delivery systems in contact with Caco-2 cultures. Eur J Pharm Biopharm. 2004;57(3):447–55. doi: 10.1016/j.ejpb.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 32.Keller RJ, Company SC. Sigma Library of FT-IR Spectra. St. Louis, MO: 1986. p. 2894. [Google Scholar]