Abstract
Neural crest cells are the primary innovation that led to evolution of the vertebrates, and transcription factors of the SoxE family (Sox8, Sox9 and Sox10) are among the central players regulating the development of these cells. In all vertebrates examined to date, one or more SoxE proteins are required for the formation of neural crest cells, the maintenance of their multipotency, and their survival. Later, SoxE proteins drive the formation of multiple neural crest derivatives including chondrocytes, melanocytes, and cells of the peripheral nervous system, particularly Schwann cells/peripheral glia. Given their multiple diverse roles in the development of the neural crest, it is important to understand how the activity of SoxE factors is controlled such that they direct the correct developmental outcome. While combinatorial control with other regulatory factors is clearly one mechanism for generating such functional versatility, modulation of SoxE activity, both by SoxD family factors and by post-translational modification, also appears to be important. Elucidating the mechanisms that control SoxE function is essential to understanding the evolutionary origin of the vertebrates, as well as a host of SoxE-linked syndromes and diseases, and may prove crucial for developing stem cell based therapies that target SoxE-regulated cell types.
Keywords: neural crest, SoxE, melanocyte, chondrocyte, PNS
Introduction
The neural crest (NC) is a population of multipotent precursor cells found at the crest of the closing neural folds in vertebrates. These cells undergo an epithelial to mesenchymal transition (EMT) and migrate extensively throughout the early embryo (Figure 1A). Ultimately, NC cells differentiate into a diverse set of derivatives that includes neurons and glia of the peripheral nervous system (PNS), skin pigment cells, endocrine cells in the adrenal and thyroid glands, craniofacial cartilage and bone, smooth muscle and subregions of the cardiovasculature, amongst others (Le Douarin and Kalcheim, 1999) (Figure 1B). A gene regulatory network controlling neural crest development has been described (Sauka-Spengler and Bronner-Fraser, 2008) and while multiple transcription factors are known to regulate this process, SoxE proteins are notable as the major class of transcriptional activators required for formation of NC precursor cells (“neural crest specifiers”), in addition to the multiple roles they play in directing the formation of distinct NC derivatives. In this review we summarize our understanding of the reiterative roles that SoxE factors play in the process of NC development, and examine the regulatory processes that may contribute to their ability to carry out such diverse functions.
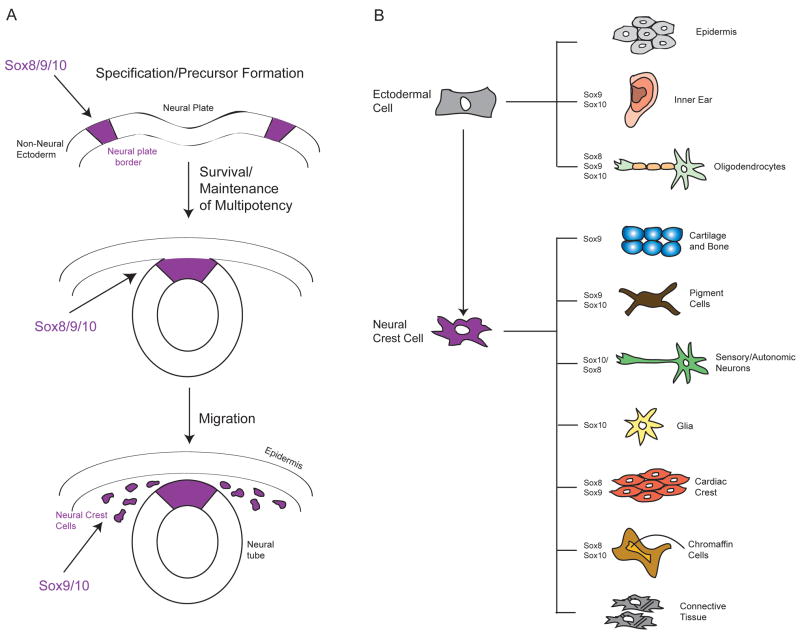
Figure 1.
Schematic overview of Neural Crest (NC) Development. (A) NC precursors with stem cell properties form at the border of the neural plate and non-neural ectoderm. Following neural tube closure, neural crest cells undergo an EMT and migrate throughout the embryo to points of differentiation. SoxE Proteins are required for the specification, precursor formation, survival and migration of NC cells throughout this process. (B) NC cells give rise to a diverse set of derivatives. SoxE proteins are required to direct the differentiation of multiple NC derivatives including glia, melanocytes and cartilage, and are also required for development of non-NC derivatives.
SoxE function in early Neural Crest Formation
Once the neural plate border has been specified, SoxE family transcription factors are the earliest markers of the subset of these border cells competent to give rise to the definitive NC (Sauka-Spengler and Bronner-Fraser, 2008). In avians and mammals, Sox9 expression distinguishes NC precursor cells, whereas Sox8 does in Xenopus with Sox9 expression following soon after (Hong and Saint-Jeannet, 2005). Sox10 is expressed, or functions, somewhat later in NC precursors in most species and thus its predominant role may be in later regulatory events, particularly melanocyte and glia formation. At least during early stages of NC development, there appears to be considerable functional redundancy between SoxE factors; when misexpressed Sox8, Sox9, and Sox10 can all mediate the formation of ectopic NC precursor cells (Kelsh, 2006). Evidence from the chick suggests that Sox9 may also be required to confer competence for subsequent NC migration (Cheung et al., 2005). The necessity of different SoxE factors for NC precursor formation may reflect the degree of subfunctionalization that has occurred in different species, with Sox9 being required for normal precursor formation in the mouse, and all three factors required to differing degrees in Xenopus. Sox9 has been shown capable of replacing the early requirement for Sox10 in Xenopus (Taylor and LaBonne, 2005), again highlighting the overlapping functions of these factors. Once specified, NC precursor cells must be maintained in a multipotent and proliferative, or “stem cell-like” state, until the appropriate time to respond to signals directing their differentiation, and must also escape apoptosis. Evidence from multiple systems has implicated SoxE factors in each of these important regulatory steps (Hong and Saint-Jeannet, 2005; Taylor and LaBonne, 2007).
SoxE function in Neural Crest Diversification
Although it is extinguished in early migrating NC cells, Sox9 is subsequently expressed in cells that will form cartilage in most vertebrates (both NC and non NC-derived) and its role in cartilage formation has been highly studied. Mutations in human SOX9 cause the disease Campomelic Dysplasia (CD), symptoms of which include; skeletal malformation and craniofacial defects (Schafer et al., 1996). Sox9 directly regulates Type II collagen (Col2a1), one of the most important collagens in cartilage formation and it has been shown in a number of model systems that loss of Sox9 results in cartilage defects (Akiyama et al., 2002, Yan et al., 2005, Spokony et al., 2002).
In contrast to Sox9, one of the main deficiencies in Sox10 mutant embryos, such as the murine Dominant megacolon (Dom) mutant and the zebrafish colourless (cls) mutant, is in the melanocyte lineage (Wegner 2005). Consistent with a role in the specification of this NC derivative, misexpression of Sox10 results in a massive increase in pigment cells (Aoki et al., 2003). Sox10 activates the promoter of Microphthalmia-associated transcription factor (Mitf), and then acts with Mitf to control a set of genes critical for pigment cell development and pigmentation, including Dct and Tyrosinase (Ludwig et al., 2004, Murisier et al., 2007). In contrast to mammals and Xenopus, however, in fish Sox10 may only be required to turn on Mitf expression (Elworthy et al., 2003). It is important to note that although Sox10 appears to play the predominant role in regulating early melanocyte development, the melanocyte inducing activity of this factor is shared with Sox9 (Taylor and LaBonne, 2005).
SoxE factors also play a central role in the development of NC derived components of the PNS. Neural crest cells give rise to most neurons (with the exception of a subset of cranial sensory neurons) and all myelinating glia of the peripheral nervous system, and the entirety of the enteric nervous system (Le Douarin and Kalcheim, 1999). Sox10 is the major SoxE factor expressed in these cells and is required to specify the precursor population. The role of SoxE factors in PNS development has been reviewed by Stolt and Wegner elsewhere in this issue.
The many roles played by SoxE factors during neural crest formation, as well as in other cell types, highlights that the reiterative use of key regulatory factors is a common phenomenon during development, and therefore that competence and context are crucial for determining outcome. Particularly striking in the case of SoxE factors is that they are deployed both to maintain multipotency and to instruct the differentiation of multiple neural crest derivatives. Below we examine some mechanisms that may contribute to this versatility.
Mechanisms for Modulating SoxE Activity
One means of modulating SoxE function appears to involve the Sox Group D factors Sox5 (L-Sox5) and Sox6. These three factors form a complex on the Col2a1 enhancer in chondrocytes (Zhou et al., 1998, Lefebvre et al., 1998). L-Sox5 and Sox6 preferentially bind HMG-like consensus sites in the Col2A1 enhancer as homodimers, and cooperatively enhance the activation of Col2A1 by Sox9 (Lefebvre et al., 1998). Consistent with an essential role for these factors, Sox5/Sox6 double mutant mice show a virtual absence of all cartilage (Smits et al., 2001) and expression of both SoxD factors appears to be under the control of Sox9 (Akiyama et al., 2002).
SoxD proteins are likely to modulate SoxE function during other aspects of NC development as well. L-Sox5 is expressed in early migrating NC cells and co-localizes with Sox10 and Mitf in the melanocyte lineage (Stolt et al., 2008). Although Sox5−/− embryos do not show melanocyte defects, loss of Sox5 in heterozygous-mutant Sox10 mice partially relieves the Sox10 phenotype. L-Sox5 appears to compete with Sox10 for binding to the Mitf and Dct promoters, resulting in inhibition of Sox10-dependent transcription. This may be mediated, in part, by L-Sox5 dependent recruitment of co-repressors such as HDAC1 and CtBP2 (Stolt et al., 2008). These effects contrast greatly with what occurs on the Col2a1 promoter, where there is a cooperative recruitment of co-activators by L-Sox5 and Sox9 (Hattori et al., 2008), further highlighting the importance of context in determining outcome.
Interestingly, Sox5 is also expressed in the PNS, in the NC-derived trigeminal ganglion and differentiating neurons of the cranial ganglia. It is co-expressed with Sox10 in the satellite glial cells of the cranial ganglia (Morales et al., 2007) and in Schwann cells (Perez-Alcala et al., 2004). Little is known about the function of Sox5 in the PNS, and it will therefore be important to determine how SoxD factors modulate SoxE activity in these cell types.
In addition to regulation by SoxD factors, the diverse activities displayed by SoxE factors may also be controlled by post-translational modification. For example, phosphorylation of Sox9 at two consensus cAMP-dependent protein kinase A (PKA) sites has been shown to increase both its DNA binding and its ability to transactivate the Col2a1 promoter (Huang et al., 2000), and may also regulate nuclear localization (Malki et al., 2005). Significantly, neither of these two PKA phosphorylation sites is conserved in Sox10, and only one is apparent in Sox8 (C. E. Haldin and C. LaBonne, unpublished). Thus, phosphorylation at these sites represents a potential means of differentially regulating the activity of different SoxE family members.
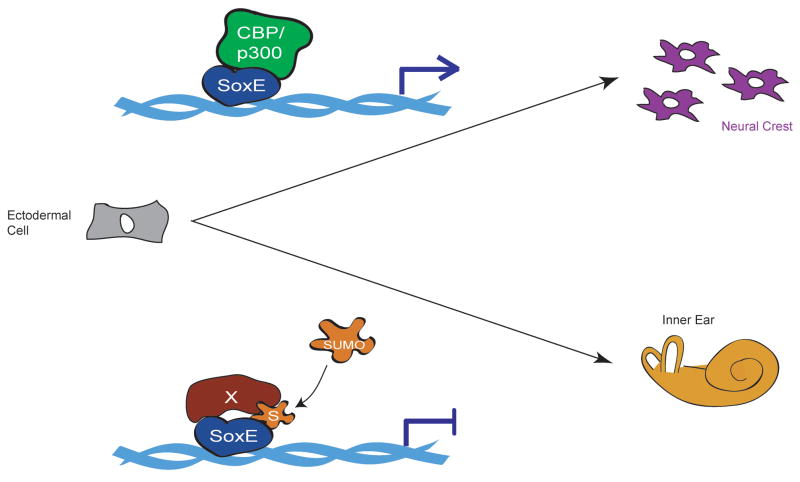
Growing evidence suggests that reversible modification of proteins by the small ubiquitin like modifier SUMO plays a major role in the regulation of gene expression (Lyst and Stancheva, 2007). The effects of SUMOylation vary from substrate to substrate, and include changes in subcellular localization, DNA binding, protein-protein interactions and transcriptional activity, and for DNA binding proteins, SUMO modification is most frequently associated with the promotion of transcriptional repression (Gill, 2004). It was recently shown that SUMO modification can alter the function of SoxE transcription factors in ways that can have profound effects on developmental cell fate decisions (Taylor and LaBonne, 2005). Here SUMO modification of Sox9 or Sox10 (and presumably Sox8) inhibits NC formation and promotes inner ear formation whereas mutant SoxE factors on which the SUMO acceptor sites have been mutated to block SUMOylation showed enhanced NC inducing activity and antagonized ear formation (Figure 2). SUMOylated Sox10 has also been shown to inhibit activation of MITF (Girard and Goossens, 2006). Recent work indicates that SUMOylation converts SoxE factors to transcriptional repressors by mediating the recruitment of Grg4 (PC Lee and C LaBonne, unpublished) and highlights the importance of SUMOylation as a versatile modification contributing to the ability of a limited pool of transcription factors to regulate a diversity of gene expression programs.
Figure 2.
Model for regulation of SoxE protein function by SUMO during inductive events in the early ectoderm. In the absence of SUMO modification SoxE factors function as transcriptional activators and direct NC development at the expense of otic placode (ear). SUMOylation serves as a switch causing these proteins to inhibit NC formation and promote ear formation, most likely through the displacement of co-activators and the recruitment of new co-factors. (CBP/p300, activator protein; S, SUMO moiety; X, repressor protein; arrow on DNA indicates transcriptional activation of target genes, blunt arrow indicates transcriptional repression).
Another mechanism via which SoxE factors could direct distinct developmental outcomes may involve context dependent interactions with key signal transduction pathways. Wnt signals, for example, though essential for neural crest induction and formation of neural crest derived melanocytes, must be inactivated in order to promote chondrogenesis (Guo et al., 2004, Hartmann and Tabin, 2001). Intriguingly, Sox9 has been shown to physically interact with β-catenin, the downstream effector of canonical Wnt signals, and appears to play an active role in down-regulating its activity (Akiyama et al., 2004, Topol et al., 2009). Two mechanisms seem to be at play here. First, the Sox9 c-terminus directly binds β-catenin, and this inhibits the activity of β-catenin in reporter assays (Akiyama et al., 2004, Topol et al., 2009). Second, regions outside the c-terminus can also bind β-catenin, and additionally bind members of the β-catenin destruction complex that targets it to the proteasome for degradation. This binding promotes nuclear localization of the complex, and increased casein kinase (CKIα) dependent phosphorylation of β-catenin, thereby promoting its degradation (Topol et al., 2009). Similar activity has yet to be shown for Sox8 and Sox10, although other non-GroupE Sox proteins have also been shown to interact with β-catenin. In contrast to cartilage formation, canonical Wnt signals are required for some SoxE-dependent inductive events, including NC precursor and melanocyte formation (Taylor and LaBonne, 2007). If Sox8 and Sox10 can also bind β-catenin and inhibit Wnt signaling, this raises the question of how this activity is regulated such that SoxE factors and β-catenin sometimes function cooperatively and other times antagonistically. It is possible, for example, that SoxE protein levels may help determine the timing and perdurance of Wnt-dependent transcriptional responses in some contexts.
Conclusions and Perspectives
SoxE factors play multiple essential roles in NC formation, including conferring the competence for cells at the neural plate border to become NC precursors, maintaining the stem-cell like state of these cells, and promoting NC survival (Figures 1A). These proteins are further involved in directing the formation of multiple NC derivatives including melanocytes, chondrocytes, and glia (Figure 1B). This is not a surprising feature of SoxE proteins, as the myriad of cell fate decisions that take place during embryonic development are orchestrated by a comparatively small number of transcription factors. Nevertheless, it highlights the need to understand how the activity of developmental regulatory factors such as SoxE factors are modulated to allow such functional versatility. In the case of SoxE factors many mechanisms may be employed, including post-translational modification by phosphorylation and SUMOylation, and context dependent interactions with factors such as SoxD proteins and β-catenin. Understanding the consequences of these regulatory events will be necessary for understanding the development and evolution of the NC, as well as a number of SoxE-linked syndromes and diseases, including Campomelic Dysplasia.
Acknowledgments
We apologize to colleagues whose work was not cited due to space constraints. We thank members of the lab for helpful suggestions, and Pei-Chih Lee and Kimberly Taylor for help with figures. Work in the authors’ laboratory was supported by RO1CA114058.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akiyama H, Chaboissier MC, Behringer RR, Rowitch DH, Schedl A, Epstein JA, de Crombrugghe B. Essential role of Sox9 in the pathway that controls formation of cardiac valves and septa. Proc Natl Acad Sci U S A. 2004;101:6502–6507. doi: 10.1073/pnas.0401711101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama H, Chaboissier MC, Martin JF, Schedl A, de Crombrugghe B. The transcription factor Sox9 has essential roles in successive steps of the chondrocyte differentiation pathway and is required for expression of Sox5 and Sox6. Genes Dev. 2002;16:2813–2828. doi: 10.1101/gad.1017802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki Y, Saint-Germain N, Gyda M, Magner-Fink E, Lee YH, Credidio C, Saint-Jeannet JP. Sox10 regulates the development of neural crest-derived melanocytes in Xenopus. Dev Biol. 2003;259:19–33. doi: 10.1016/s0012-1606(03)00161-1. [DOI] [PubMed] [Google Scholar]
- Cheung M, Chaboissier MC, Mynett A, Hirst E, Schedl A, Briscoe J. The transcriptional control of trunk neural crest induction, survival, and delamination. Dev Cell. 2005;8:179–192. doi: 10.1016/j.devcel.2004.12.010. [DOI] [PubMed] [Google Scholar]
- Elworthy S, Lister JA, Carney TJ, Raible DW, Kelsh RN. Transcriptional regulation of mitfa accounts for the sox10 requirement in zebrafish melanophore development. Development. 2003;130:2809–2818. doi: 10.1242/dev.00461. [DOI] [PubMed] [Google Scholar]
- Gill G. SUMO and ubiquitin in the nucleus: different functions, similar mechanisms? Genes Dev. 2004;18:2046–2059. doi: 10.1101/gad.1214604. [DOI] [PubMed] [Google Scholar]
- Girard M, Goossens M. Sumoylation of the SOX10 transcription factor regulates its transcriptional activity. FEBS Lett. 2006;580:1635–1641. doi: 10.1016/j.febslet.2006.02.011. [DOI] [PubMed] [Google Scholar]
- Guo X, Day TF, Jiang X, Garrett-Beal L, Topol L, Yang Y. Wnt/beta-catenin signaling is sufficient and necessary for synovial joint formation. Genes Dev. 2004;18:2404–2417. doi: 10.1101/gad.1230704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann C, Tabin CJ. Wnt-14 plays a pivotal role in inducing synovial joint formation in the developing appendicular skeleton. Cell. 2001;104:341–351. doi: 10.1016/s0092-8674(01)00222-7. [DOI] [PubMed] [Google Scholar]
- Hattori T, Coustry F, Stephens S, Eberspaecher H, Takigawa M, Yasuda H, de Crombrugghe B. Transcriptional regulation of chondrogenesis by coactivator Tip60 via chromatin association with Sox9 and Sox5. Nucleic Acids Res. 2008;36:3011–3024. doi: 10.1093/nar/gkn150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong CS, Saint-Jeannet JP. Sox proteins and neural crest development. Semin Cell Dev Biol. 2005;16(6):694–703. doi: 10.1016/j.semcdb.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Huang W, Zhou X, Lefebvre V, de Crombrugghe B. Phosphorylation of SOX9 by cyclic AMP-dependent protein kinase A enhances SOX9’s ability to transactivate a Col2a1 chondrocyte-specific enhancer. Mol Cell Biol. 2000;20:4149–4158. doi: 10.1128/mcb.20.11.4149-4158.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelsh RN. Sorting out Sox10 functions in neural crest development. Bioessays. 2006;28(8):788–98. doi: 10.1002/bies.20445. [DOI] [PubMed] [Google Scholar]
- Le Douarin N, Kalcheim C. The Neural Crest, second edition. Cambridge: Cambridge University Press; 1999. [Google Scholar]
- Lefebvre V, Li P, de Crombrugghe B. A new long form of Sox5 (L-Sox5), Sox6 and Sox9 are coexpressed in chondrogenesis and cooperatively activate the type II collagen gene. EMBO J. 1998;17:5718–5733. doi: 10.1093/emboj/17.19.5718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig A, Rehberg S, Wegner M. Melanocyte-specific expression of dopachrome tautomerase is dependent on synergistic gene activation by the Sox10 and Mitf transcription factors. FEBS Lett. 2004;556:236–244. doi: 10.1016/s0014-5793(03)01446-7. [DOI] [PubMed] [Google Scholar]
- Lyst MJ, Stancheva I. A role for SUMO modification in transcriptional repression and activation. Biochem Soc Trans. 2007;35:1389–1392. doi: 10.1042/BST0351389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malki S, Nef S, Notarnicola C, Thevenet L, Gasca S, Mejean C, Berta P, Poulat F, Boizet-Bonhoure B. Prostaglandin D2 induces nuclear import of the sex-determining factor SOX9 via its cAMP-PKA phosphorylation. EMBO J. 2005;24:1798–1809. doi: 10.1038/sj.emboj.7600660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales AV, Perez-Alcala S, Barbas JA. Dynamic Sox5 protein expression during cranial ganglia development. Dev Dyn. 2007;236:2702–2707. doi: 10.1002/dvdy.21282. [DOI] [PubMed] [Google Scholar]
- Murisier F, Guichard S, Beermann F. The tyrosinase enhancer is activated by Sox10 and Mitf in mouse melanocytes. Pigment Cell Res. 2007;20:173–184. doi: 10.1111/j.1600-0749.2007.00368.x. [DOI] [PubMed] [Google Scholar]
- Perez-Alcala S, Nieto MA, Barbas JA. LSox5 regulates RhoB expression in the neural tube and promotes generation of the neural crest. Development. 2004;131:4455–4465. doi: 10.1242/dev.01329. [DOI] [PubMed] [Google Scholar]
- Sauka-Spengler T, Bronner-Fraser M. A gene regulatory network orchestrates neural crest formation. Nat Rev Mol Cell Biol. 2008;9:557–568. doi: 10.1038/nrm2428. [DOI] [PubMed] [Google Scholar]
- Schafer AJ, Foster JW, Kwok C, Weller PA, Guioli S, Goodfellow PN. Campomelic dysplasia with XY sex reversal: diverse phenotypes resulting from mutations in a single gene. Ann N Y Acad Sci. 1996;785:137–49. doi: 10.1111/j.1749-6632.1996.tb56252.x. [DOI] [PubMed] [Google Scholar]
- Smits P, Li P, Mandel J, Zhang Z, Deng JM, Behringer RR, de Crombrugghe B, Lefebvre V. The transcription factors L-Sox5 and Sox6 are essential for cartilage formation. Dev Cell. 2001;1:277–290. doi: 10.1016/s1534-5807(01)00003-x. [DOI] [PubMed] [Google Scholar]
- Spokony RF, Aoki Y, Saint-Germain N, Magner-Fink E, Saint-Jeannet JP. The transcription factor Sox9 is required for cranial neural crest development in Xenopus. Development. 2002;129:421–432. doi: 10.1242/dev.129.2.421. [DOI] [PubMed] [Google Scholar]
- Stolt CC, Lommes P, Hillgartner S, Wegner M. The transcription factor Sox5 modulates Sox10 function during melanocyte development. Nucleic Acids Res. 2008;36:5427–5440. doi: 10.1093/nar/gkn527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor KM, Labonne C. SoxE factors function equivalently during neural crest and inner ear development and their activity is regulated by SUMOylation. Dev Cell. 2005;9:593–603. doi: 10.1016/j.devcel.2005.09.016. [DOI] [PubMed] [Google Scholar]
- Taylor KM, LaBonne C. Modulating the activity of neural crest regulatory factors. Curr Opin Genet Dev. 2007;17:326–331. doi: 10.1016/j.gde.2007.05.012. [DOI] [PubMed] [Google Scholar]
- Topol L, Chen W, Song H, Day TF, Yang Y. Sox9 inhibits Wnt signaling by promoting beta-catenin phosphorylation in the nucleus. J Biol Chem. 2009;284:3323–3333. doi: 10.1074/jbc.M808048200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegner M. Secrets to a healthy Sox life: lessons for melanocytes. Pigment Cell Res. 2005;18(2):74–85. doi: 10.1111/j.1600-0749.2005.00218.x. [DOI] [PubMed] [Google Scholar]
- Yan YL, Willoughby J, Liu D, Crump JG, Wilson C, Miller CT, Singer A, Kimmel C, Westerfield M, Postlethwait JH. A pair of Sox: distinct and overlapping functions of zebrafish sox9 co-orthologs in craniofacial and pectoral fin development. Development. 2005;132:1069–1083. doi: 10.1242/dev.01674. [DOI] [PubMed] [Google Scholar]
- Zhou G, Lefebvre V, Zhang Z, Eberspaecher H, de Crombrugghe B. Three high mobility group-like sequences within a 48-base pair enhancer of the Col2a1 gene are required for cartilage-specific expression in vivo. J Biol Chem. 1998;273:14989–14997. doi: 10.1074/jbc.273.24.14989. [DOI] [PubMed] [Google Scholar]