Abstract
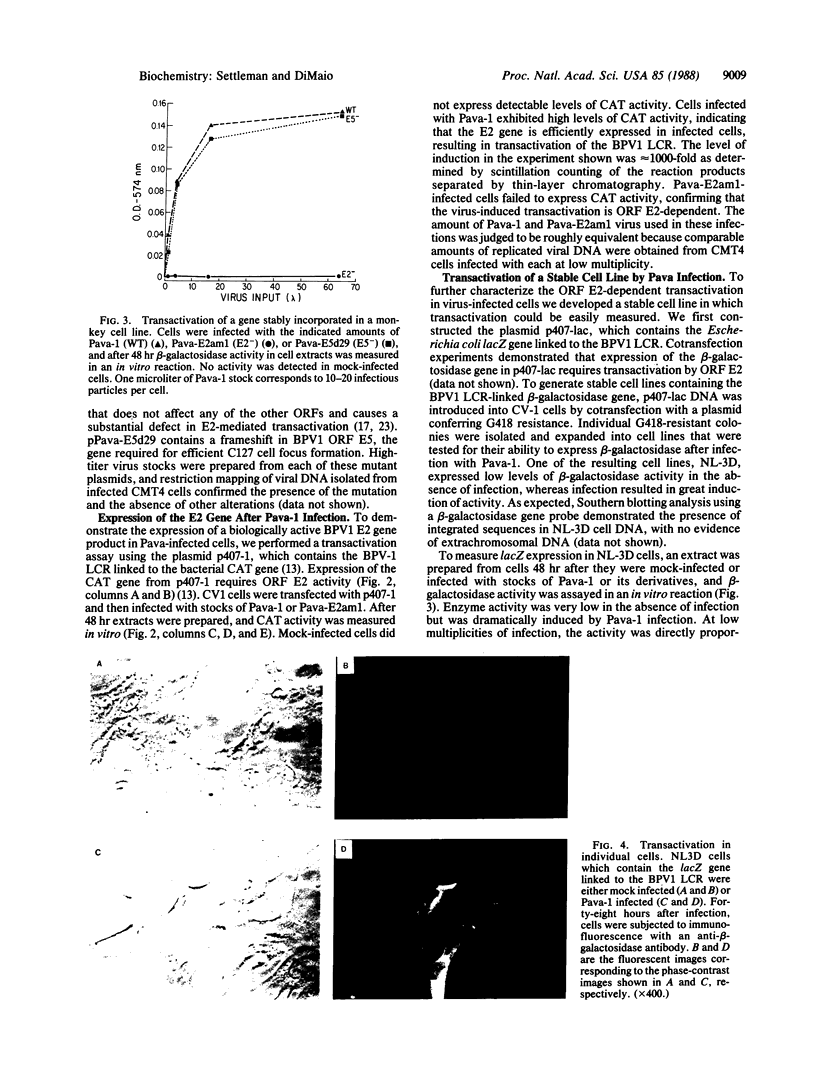
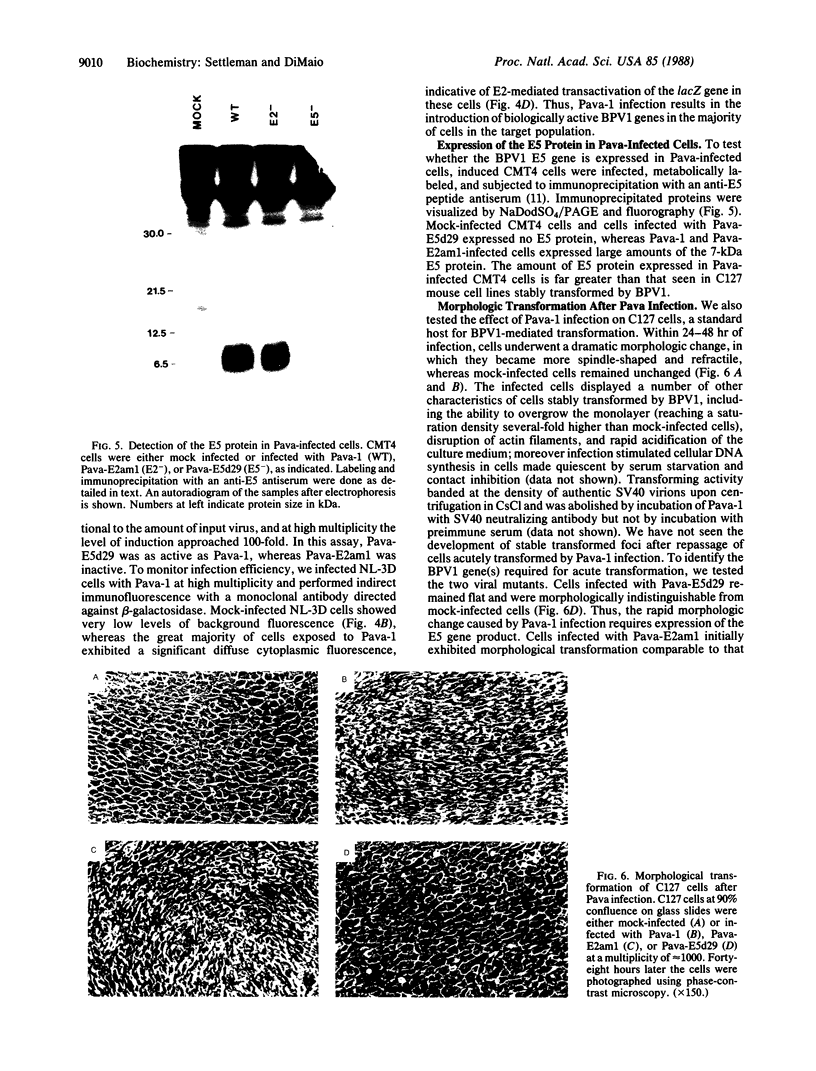
To efficiently introduce bovine papillomavirus type 1 genes into cultured cells, we constructed a hybrid viral genome in which the simian virus 40 early region is replaced with a segment of the bovine papillomavirus type 1 transforming region. High-titer stocks of simian virus 40 virions containing the recombinant genome were produced in monkey cells that express simian virus 40 large tumor antigen. Cells infected with this virus efficiently expressed the bovine papillomavirus type 1 E2 and E5 genes. Expression of the E2 gene caused transactivation of genes linked to the bovine papillomavirus type 1 control region, resulting in up to a 1000-fold induction. At high multiplicity of infection of a cell line containing an integrated reporter gene, most cells were infected and responded to transactivation. Within 48 hr of infection with wild-type virus but not with an open reading frame E5 mutant, mouse C127 cells displayed dramatic changes in morphology and growth characteristics similar to those seen in tumorigenic transformation. This system can be used to determine the acute cellular response to introduction of bovine papillomavirus type 1 transforming and regulatory genes; it can also be used to induce foreign genes stably incorporated into cultured mammalian cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergman P., Ustav M., Sedman J., Moreno-Lopéz J., Vennström B., Pettersson U. The E5 gene of bovine papillomavirus type 1 is sufficient for complete oncogenic transformation of mouse fibroblasts. Oncogene. 1988 May;2(5):453–459. [PubMed] [Google Scholar]
- Burkhardt A., DiMaio D., Schlegel R. Genetic and biochemical definition of the bovine papillomavirus E5 transforming protein. EMBO J. 1987 Aug;6(8):2381–2385. doi: 10.1002/j.1460-2075.1987.tb02515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMaio D., Guralski D., Schiller J. T. Translation of open reading frame E5 of bovine papillomavirus is required for its transforming activity. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1797–1801. doi: 10.1073/pnas.83.6.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMaio D. Nonsense mutation in open reading frame E2 of bovine papillomavirus DNA. J Virol. 1986 Feb;57(2):475–480. doi: 10.1128/jvi.57.2.475-480.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvoretzky I., Shober R., Chattopadhyay S. K., Lowy D. R. A quantitative in vitro focus assay for bovine papilloma virus. Virology. 1980 Jun;103(2):369–375. doi: 10.1016/0042-6822(80)90195-6. [DOI] [PubMed] [Google Scholar]
- Gerard R. D., Gluzman Y. New host cell system for regulated simian virus 40 DNA replication. Mol Cell Biol. 1985 Nov;5(11):3231–3240. doi: 10.1128/mcb.5.11.3231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groff D. E., Lancaster W. D. Genetic analysis of the 3' early region transformation and replication functions of bovine papillomavirus type 1. Virology. 1986 Apr 15;150(1):221–230. doi: 10.1016/0042-6822(86)90281-3. [DOI] [PubMed] [Google Scholar]
- Haugen T. H., Cripe T. P., Ginder G. D., Karin M., Turek L. P. Trans-activation of an upstream early gene promoter of bovine papilloma virus-1 by a product of the viral E2 gene. EMBO J. 1987 Jan;6(1):145–152. doi: 10.1002/j.1460-2075.1987.tb04732.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz B. H., Burkhardt A. L., Schlegel R., DiMaio D. 44-amino-acid E5 transforming protein of bovine papillomavirus requires a hydrophobic core and specific carboxyl-terminal amino acids. Mol Cell Biol. 1988 Oct;8(10):4071–4078. doi: 10.1128/mcb.8.10.4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaskulski D., Kaczmarek L., DiMaio D. Stimulation of cellular DNA synthesis by wild type and mutant bovine papillomavirus DNA. Biochem Biophys Res Commun. 1987 Oct 14;148(1):86–91. doi: 10.1016/0006-291x(87)91079-5. [DOI] [PubMed] [Google Scholar]
- Kriegler M., Perez C. F., Hardy C., Botchan M. Transformation mediated by the SV40 T antigens: separation of the overlapping SV40 early genes with a retroviral vector. Cell. 1984 Sep;38(2):483–491. doi: 10.1016/0092-8674(84)90503-8. [DOI] [PubMed] [Google Scholar]
- Lambert P. F., Spalholz B. A., Howley P. M. A transcriptional repressor encoded by BPV-1 shares a common carboxy-terminal domain with the E2 transactivator. Cell. 1987 Jul 3;50(1):69–78. doi: 10.1016/0092-8674(87)90663-5. [DOI] [PubMed] [Google Scholar]
- Lancaster W. D., Olson C. Animal papillomaviruses. Microbiol Rev. 1982 Jun;46(2):191–207. doi: 10.1128/mr.46.2.191-207.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law M. F., Lowy D. R., Dvoretzky I., Howley P. M. Mouse cells transformed by bovine papillomavirus contain only extrachromosomal viral DNA sequences. Proc Natl Acad Sci U S A. 1981 May;78(5):2727–2731. doi: 10.1073/pnas.78.5.2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowy D. R., Dvoretzky I., Shober R., Law M. F., Engel L., Howley P. M. In vitro tumorigenic transformation by a defined sub-genomic fragment of bovine papilloma virus DNA. Nature. 1980 Sep 4;287(5777):72–74. doi: 10.1038/287072a0. [DOI] [PubMed] [Google Scholar]
- Prakash S. S., Horwitz B. H., Zibello T., Settleman J., DiMaio D. Bovine papillomavirus E2 gene regulates expression of the viral E5 transforming gene. J Virol. 1988 Oct;62(10):3608–3613. doi: 10.1128/jvi.62.10.3608-3613.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabson M. S., Yee C., Yang Y. C., Howley P. M. Bovine papillomavirus type 1 3' early region transformation and plasmid maintenance functions. J Virol. 1986 Nov;60(2):626–634. doi: 10.1128/jvi.60.2.626-634.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarver N., Byrne J. C., Howley P. M. Transformation and replication in mouse cells of a bovine papillomavirus--pML2 plasmid vector that can be rescued in bacteria. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7147–7151. doi: 10.1073/pnas.79.23.7147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller J. T., Vass W. C., Lowy D. R. Identification of a second transforming region in bovine papillomavirus DNA. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7880–7884. doi: 10.1073/pnas.81.24.7880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller J. T., Vass W. C., Vousden K. H., Lowy D. R. E5 open reading frame of bovine papillomavirus type 1 encodes a transforming gene. J Virol. 1986 Jan;57(1):1–6. doi: 10.1128/jvi.57.1.1-6.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlegel R., Wade-Glass M., Rabson M. S., Yang Y. C. The E5 transforming gene of bovine papillomavirus encodes a small, hydrophobic polypeptide. Science. 1986 Jul 25;233(4762):464–467. doi: 10.1126/science.3014660. [DOI] [PubMed] [Google Scholar]
- Spalholz B. A., Lambert P. F., Yee C. L., Howley P. M. Bovine papillomavirus transcriptional regulation: localization of the E2-responsive elements of the long control region. J Virol. 1987 Jul;61(7):2128–2137. doi: 10.1128/jvi.61.7.2128-2137.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spalholz B. A., Yang Y. C., Howley P. M. Transactivation of a bovine papilloma virus transcriptional regulatory element by the E2 gene product. Cell. 1985 Aug;42(1):183–191. doi: 10.1016/s0092-8674(85)80114-8. [DOI] [PubMed] [Google Scholar]
- Tornow J., Cole C. N. Intracistronic complementation in the simian virus 40 A gene. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6312–6316. doi: 10.1073/pnas.80.20.6312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y. C., Okayama H., Howley P. M. Bovine papillomavirus contains multiple transforming genes. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1030–1034. doi: 10.1073/pnas.82.4.1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y. C., Spalholz B. A., Rabson M. S., Howley P. M. Dissociation of transforming and trans-activation functions for bovine papillomavirus type 1. Nature. 1985 Dec 12;318(6046):575–577. doi: 10.1038/318575a0. [DOI] [PubMed] [Google Scholar]