Abstract
Clinical, postmortem and preclinical research strongly implicates dysregulation of glutamatergic neurotransmission in major depressive disorder (MDD). Recently, metabotropic glutamate receptors (mGluRs) have been proposed as attractive targets for discovery of novel therapeutic approaches against depression. The aim of this study was to examine mGluR2/3 protein levels in the prefrontal cortex (PFC) from depressed subjects. In addition, to test whether antidepressants influence mGluR2/3 expression we also studied levels of mGluR2/3 in fluoxetine treated monkeys. Postmortem human prefrontal samples containing Brodmann’s area 10 (BA 10) were obtained from 11 depressed and 11 psychiatrically healthy controls. Male rhesus monkeys were treated chronically with fluoxetine (dose escalated to 3mg/kg, p.o; n=7) or placebo (n=6) for 39 weeks. The mGluR2/3 immunoreactivity was investigated using Western blot method. There was a robust (+67%) increase in the expression of the mGlu2/3 protein in the PFC of depressed subjects relative to healthy controls. The expression of mGlu2/3 was unchanged in the PFC of monkeys treated with fluoxetine. Our findings provide the first evidence that mGluR2/3 is elevated in the PFC in MDD. This observation is consistent with reports showing that mGluR2/3 antagonists exhibit antidepressant-like activity in animal models and demonstrates that these receptors are promising targets for the discovery of novel antidepressants.
Keywords: prefrontal cortex, metabotropic glutamate receptor, major depression, postmortem, fluoxetine, rhesus monkey
1. Introduction
Rapidly accumulating evidence suggests that the glutamatergic system plays an important role in the neuropathology and treatment of major depressive disorder (MDD) (see Hashimoto, 2009; Sanacora 2008 for reviews). We had previously reported altered protein expression of specific subunits of the N-methyl-D-aspartate receptor (NMDAR) in the prefrontal cortex (Feyissa et al., 2009), locus coeruleus (Karolewicz et al., 2005), and amygdala (Karolewicz et al., 2009,b) from depressed subjects compared to healthy controls. These findings coincide with reports showing that NMDAR antagonists exhibit rapid and robust antidepressant effects in treatment resistant depression (Berman et al., 2000; Zarate et al., 2006).
Recent discoveries of selective agonists/antagonists to the various metabotropic glutamate receptors (mGluRs) that exhibit antidepressant-like activity in animal screening procedures provide promising avenues for the discovery of new and improved antidepressant medications (see Palucha and Pilc, 2007; Pilc et al., 2008 for reviews). To date, eight mGluR subtypes (mGluR1–mGluR8) have been described in the mammalian brain and are classified into three groups with respect to their sequence homology, neuronal signaling, and pharmacological properties (Conn and Pin 1997). Group I mGluRs (mGluR1and mGluR5) are positively linked to phospholipase C and in general function to enhance glutamate excitations. In contrast, group II (mGluR2, mGluR3) and group III (mGluR4, mGluR6, mGluR7, mGluR8) are negatively coupled to adenylyl cyclase, and thereby negatively modulate excitatory neurotransmitter efflux and neuronal excitability when activated. Group II mGluRs such as mGluR2 and mGluR3 share significant sequence homology and are widely distributed throughout the CNS, with high expression in brain regions (e.g., hippocampus, prefrontal cortex, and amygdala) implicated in MDD (Drevets, 2000). The function of mGluR2/3 in modulating glutamate neurotransmission, by sensing glutamate spillover and regulating transmitter release, makes them extremely interesting targets for antidepressant drug development (see Hashimoto, 2009; Sanacora et al., 2008; Witkin et al., 2007 for reviews). Presynaptic mGluR2/3 may also function as heteroreceptors controlling the release of γ-aminobutyric acid (GABA) and other neurotransmitters (Cartmell and Schoepp, 2000).
To our knowledge there is no previous report examining the expression of mGluR2/3 in postmortem human brain tissue from depressed subjects. Thus, the primary aim of this study was to examine protein expression of mGluR2/3 in the prefrontal cortex (PFC) from depressed subjects compared to healthy controls. Since mGluR2/3 protein levels could be influenced by antidepressants, we also examined mGluR2/3 immunoreactivity in the PFC of rhesus monkeys treated with the selective serotonin reuptake inhibitor (SSRI) fluoxetine as compared to those treated with vehicle.
2. Methods
2.1. Human subjects
Postmortem brain samples were collected at autopsy at the Cuyahoga County Coroner’s Office in Cleveland, OH from 22 subjects. Blocks of cortical tissue were dissected and frozen by submerging for 15–20 seconds in isopentane cooled by dry ice. Blocks were then buried in powdered dry ice for 20 min and then stored in a freezer at −80 °C until processed.
Informed written consent was obtained from the legal next-of-kin of all subjects. Next-of-kin were interviewed and retrospective psychiatric assessments were conducted in accordance with Institutional Review Board policies as described previously (Rajkowska et al., 1999). A trained interviewer administered either the Schedule for Affective Disorders and Schizophrenia: lifetime version (SADS-L) or the Structured Clinical Interview for DSM-IV Psychiatric Disorders (SCID) to knowledgeable next-of-kin of all subjects, as previously described (Spitzer and Endicott, 1978; First et al., 1996). Diagnoses for Axis I disorders were assessed independently by a clinical psychologist and a psychiatrist, and consensus diagnosis was reached in conference, using all available information from knowledgeable informants, coroner’s office, and previous hospitalizations and doctor’s records. Research on the psychological autopsy method has revealed that diagnoses from structured clinical interviews with family members are in good agreement with diagnoses based on reviewing the subject’s medical records (Deep-Soboslay et al., 2005; Kelly and Mann, 1996). In addition, strong inter-rater concurrence has been obtained when a structured clinical interview was used to collect information from depressed patients vs. information collected from next-of-kin (McGirr et al., 2007). Eleven subjects met clinical criteria for MDD, and the other 11 subjects (termed normal controls) did not meet criteria for an Axis I diagnosis based on the Diagnostic and Statistic Manual of Mental Disorders-Revised DSM-IV (Table 1). Among 11 depressed individuals, 7 died by suicide. The average duration of depression was 6.1 ± 2.3 years. No antidepressant drugs were detected in postmortem toxicology screening (Table 1). Eleven depressed subjects and 11 controls were arranged into 11 pairs matched as closely as possible for gender, age, smoking, history of alcohol abuse, brain pH, and postmortem interval (Table 1).
Table 1.
Demographic characteristics of MDD subjects and controls
| Controls (pair no.) | Age | Sex | PMI | Brain pH | Toxicology | Cause of death |
|---|---|---|---|---|---|---|
| 1 | 30 | M | 19 | 6.98 | Clean | Heart disease |
| 2 | 33 | M | 23 | 6.86 | Clean | Heart disease |
| 3 | 37 | F | 13 | 5.93 | Clean | Viral myocarditis |
| 4 | 51 | F | 22 | 6.30 | Clean | Heart disease |
| 5 | 46 | M | 11 | 6.95 | Clean | Heart disease |
| 6 | 54 | M | 17 | 6.87 | Brompheniramine | Heart disease |
| 7 | 69 | M | 26 | 6.70 | Clean | Heart disease |
| 8 | 70 | M | 20 | 6.81 | Clean | Heart disease |
| 9 | 74 | M | 21 | 6.62 | Clean | Abdominal aortic aneurysm |
| 10 | 59 | M | 6 | 6.79 | Lidocaine | Heart disease |
| 11 | 34 | M | 24 | 6.61 | Ethanol | Thrombophlebitis |
| Major Depression (pair no.) | ||||||
| 1 | 41 | M | 19 | 6.24 | Chlorpheniramine | Heart disease |
| 2 | 30 | M | 18 | 6.91 | Ethanol | Gunshot to chest |
| 3 | 40 | F | 25 | 6.32 | Morphine, codeine, hydrocodone | Heart disease |
| 4 | 50 | F | 28 | 6.47 | Dextromethorphan | Cardiovascular disease |
| 5 | 46 | M | 17 | 6.26 | Clean | Homicide |
| 6 | 54 | M | 23 | 6.24 | Phenobarbital, phenytoin, CO | CO poisoning |
| 7 | 64 | M | 26 | 6.85 | Ethanol | Gunshot to head |
| 8 | 74 | M | 25 | 6.67 | Diazepam | Gunshot to head |
| 9 | 81 | M | 33 | 6.78 | Clean | Drowning |
| 10 | 60 | M | 20 | 6.31 | Ethanol | Gunshot to chest |
| 11 | 42 | M | 20 | 6.80 | Clean | Gunshot to chest |
M, male; F, female; PMI, postmortem interval; MDD, major depressive disorder; CO, carbon monoxide.
2.2. Rhesus monkeys
All animal use procedures were approved by the University of Mississippi Medical Center’s Animal Care and Use Committee and were in accordance with the National Research Council’s Guide for Care and Use of Laboratory Animals (1996).
Thirteen adult male rhesus monkeys (Macaca mulatta) were housed in standard stainless steel cages in a room with a 14:10 light: dark cycle, lights on at 8 AM. The monkeys were between 10 – 16.5 years of age and weighed 6.8 – 11.6 kg at the beginning of the study. The animals were fed between 105 and 290 grams of Teklad 25% Monkey Diet (Harlan/Teklad, Madison, WI), individually determined to maintain stable body weight. Water was available ad libitum. Fluoxetine HCl was added to Tang (orange-flavored, non-carbonated soft drink) for the treated group while the control group consumed Tang only. Fluoxetine (dose escalated to 3mg/kg/p.o.) was administered at the same time each day, daily, seven days/week for 39 weeks. Monkeys were euthanized 20–24 hours after their last drug or vehicle exposure. For euthanasia, monkeys were initially sedated with the combination of midazolam (Versed, 0.3 mg/kg, i.m.) and medetomidine (Domitor, 0.06 mg/kg, i.m.). They were then administered a lethal overdose of pentobarbital (75 mg/kg, i.v.) via the saphenous vein. The brain, including brain stem and cerebellum were then removed and deposited on top of ice. The cortical tissue was dissected into blocks in accordance to the Atlas of the Rhesus Monkey Brain by Saleem and Logothetis (2007) and frozen and stored as outlined above.
2.3. Immunoblotting
Human and monkey tissue samples were dissected from right frontal pole containing Brodmann’s area 10 (BA10). Frozen blocks were cut into 50 μm-thick sections and samples containing all six cortical layers of the gray matter were collected. Western blot experiments were performed as described previously (Feyissa et al., 2009), except that in the current study tissue samples were not heated before being subjected to gel electrophoresis. mGluR2/3 was labeled using anti-mGluR2/3 rabbit polyclonal antibody (1:500; Millipore, Temecula, CA, USA; no AB1553) and secondary anti-rabbit antibody (1:3000; Amersham Biosciences, no. NA934). The anti-mGluR2/3 antibodies have been previously used to identify mGluR2/3 in numerous postmortem studies and were demonstrated not to cross-react with other mGluRs (Aronica et al., 2003; Crook et al., 2002; Gupta et al., 2005). Actin was used as a control for transfer and loading, and was detected on each blot using an anti-actin antibody (Millipore; no MAB1501). To minimize inter-blot variability and to aid in quantifying blots, each gel was loaded with 3 concentrations of a cortical tissue standard.
2.4. Data analysis
Immunoreactive bands were analyzed using MCID Elite 7.0 (Imaging Research, St. Catherines, ON, Canada). Linear regression was used to plot a standard curve for each gel, from which relative optical density values of samples were converted to cortical standard protein units for each experimental sample for each gel. The final data are expressed in cortical standard protein units and presented as a ratio of mGluR2/3 to actin. Since the data from human samples were found to follow non-normal distribution, we used the non-parametric Wilcoxon signed ranks test and Mann Whitney test for data analysis (SPSS version 16.0; SPSS Inc., USA). The data obtained from monkey brain samples were found to be normally distributed; therefore, these data were analyzed using a two-tailed unpaired t-test (SPSS version 16.0). Linear regression analysis was performed to test for potential associations between age, pH, postmortem interval (PMI), and time in freezer and mGluR2/3 protein level (SPSS 16.0). A p value <0.05 was considered significant.
3. Results
3.1. Human study
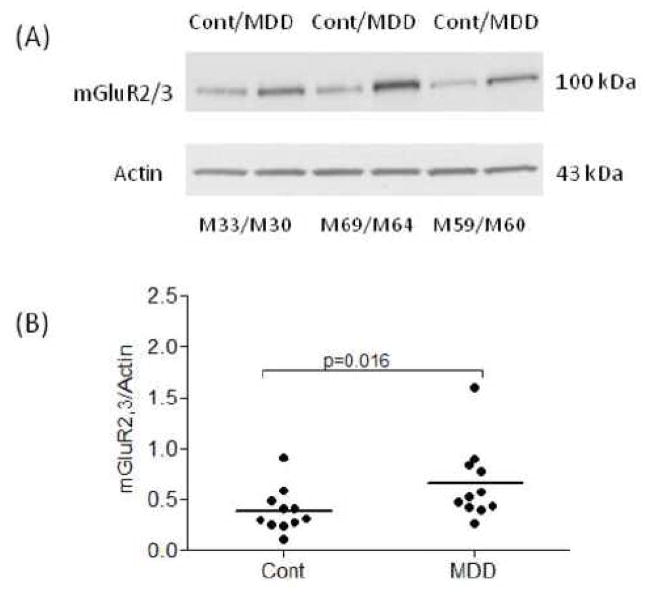
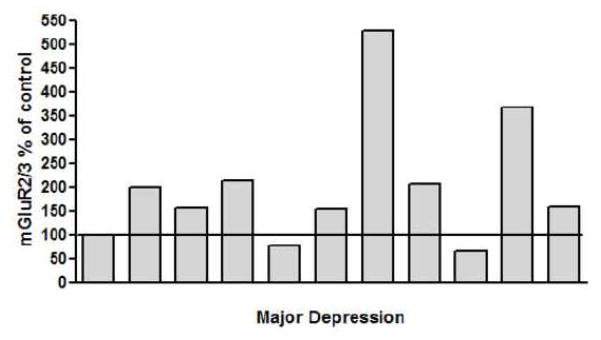
Amounts of mGluR2/3 protein were analyzed in the PFC BA10 from 11 pairs of subjects with MDD and individually matched healthy controls. Western blot analyses consistently revealed bands corresponding to the molecular mass of 100 kDa. Figure 1A shows a representative immunoblot of mGluR2/3 and actin from six subjects. The average mGluR2,3/actin ratio from subjects with MDD (0.66 ± 0.11) was significantly higher (+67 %) than that of matched controls (0.39 ± 0.06; Wilcoxon signed ranks test, Z= −2. 40, p=0.016; Fig. 1B). Linear regression analysis showed no significant correlations between the amount of mGluR2/3 immunoreactivity and age, postmortem interval, brain pH, time in freezer, or duration of depression. Figure 2 shows mGluR2/3 immunoreactivity from individual MDD subjects expressed as percentages of values from paired control subjects. The increased mGluR2/3 in MDD subjects does not appear to be related to death by suicide since there was no difference in the average mGluR2,3/actin ratio from 7 suicide MDD subjects (0.72 ± 0.16) and 4 MDD non suicide subjects (0.57 ± 0.11; Mann Whitney test, Z =−0.37, p= 0.78).
Figure 1.
Representative immunoblot of mGluR2/3 and actin from three male pairs of control and MDD subjects (A). Scatter plot of mGluR2/3 protein levels normalized to actin (B). The average mGluR2,3/actin ratio from subjects with MDD (n=11) was significantly higher (+67%) than that of matched controls (n=11). Normalized optical density values for the individual subjects (circles) and mean values (horizontal lines) are presented. Cont, healthy control; MDD, major depressive disorder; M, male.
Figure 2.
mGluR2/3 immunoreactivity from individual major depressive disorder subjects expressed as percentages of values from individually paired control subjects. Each bar is an average of duplicate comparisons.
3.2. Monkey study
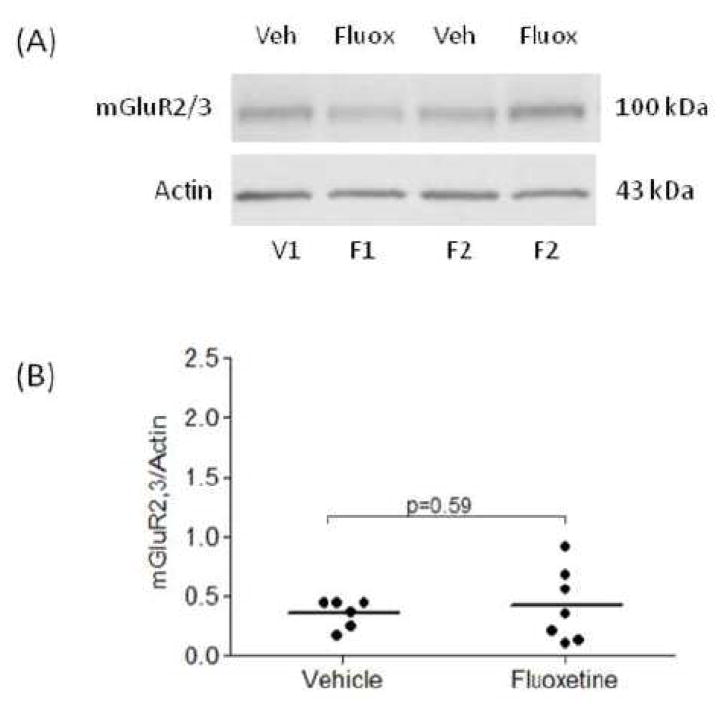
The terminal dose of fluoxetine administered to monkeys produced plasma levels of the drug in the range of 52–168 ng/ml (data not shown) that approximated clinically relevant levels (Blardi et al., 2002; Brunswick et al., 2002). Amounts of mGluR2/3 protein were analyzed in the PFC BA10 from male rhesus monkeys that were treated with fluoxetine (n=7) or placebo (n=6) for 39 weeks. Figure 3A shows a representative immunoblot of mGluR2/3 from two placebo and two fluoxetine treated monkeys. The average mGluR2,3/actin ratio from monkeys treated with fluoxetine (0.43 ± 0.11) was unchanged as compared to placebo group (0.36 ± 0.04; two-tailed t-test t=0.54, df=11, p=0.59; Fig. 3B).
Figure 3.
Representative immunoblot of mGluR2/3 and actin from two vehicle and two fluoxetine treated monkeys (A). Scatter plot of mGluR2/3 protein levels normalized to actin (B). The average mGluR2,3/actin ratio from fluoxetine treated monkeys (n=7) was unchanged compared to vehicle group (n=6). Normalized optical density values for the individual monkeys (circles) and mean values (horizontal lines) are presented. V, vehicle; F, fluoxetine.
4. Discussion
The present study demonstrates robust (+67%) increase in the level of mGluR2/3 protein in the PFC in MDD subjects as compared to controls. The increased level of mGluR2/3 in MDD is unlikely the effect of antidepressant exposure since the expression of mGluR2/3 was unchanged in the PFC of rhesus monkeys treated with fluoxetine.
The present findings coincide with reports showing that mGluR2/3 antagonists MGS0039 and LY341495 exhibit antidepressant-like effects in animal screening procedures (Chaki et al., 2004; Yasuhara et al., 2006; Yoshimizu et al., 2006). The mechanism by which mGluR2/3 antagonists act as antidepressants is thought to be related to an increased presynaptic glutamate release and involves direct downstream consequences of the enhancement of glutamate transmission at α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate receptors (AMPARs) in brain regions involved in depression (Karasawa et al., 2005; Valentine and Sanacora, 2008; Witkin et al., 2007). Thus, the antidepressant effects of mGlu2/3 antagonists may be secondary to promotion of excitatory activity in critical neuronal circuits. There is preliminary evidence that mGluR2/3 is reduced in the hippocampus in animal models of depression such as the olfactory bulbectomy in mice (Wierońska et al., 2008), and in spontaneously depressed flinders sensitive line rats as compared to controls (Matrisciano et al., 2008). Because both animal models show reduced mGluR2/3 expression in the hippocampus, further studies to elucidate mGluR2/3 expression in the hippocampus from subjects diagnosed with MDD will be necessary to ascertain if parallel changes occur in humans.
At the present time, in light of insufficient data, we can only speculate about mechanisms that account for the robust elevation of mGluR2/3 protein levels in the PFC in MDD. The increased mGluR2/3 protein expression may represent a biological trait associated with increased risk of depression, possibly due to genetic factors. Alternatively, increased mGluR2/3 protein levels may be due to the influence of repeated stress, increased glutamate activity, and/or hormonal changes (e.g., glucocorticoids) in depression. Given that mGluR2/3 is known to be involved in modulation of glutamatergic neurotransmission by sensing glutamate spillover and reducing transmitter release, the increased mGluR2/3 protein expression observed herein could be a consequence of altered glutamate levels in brains of depressed subjects. Along this line, some studies have reported elevated levels of glutamate in depression (Hashimoto et al., 2007; Sanacora et al., 2004). While others (Auer et al., 2000; Hasler et al., 2007) report reduced glutamate or glutamate/glutamine (Glx) levels in depressed patients.
Previously we have reported reduced level of mGluR5 protein in the PFC (Karolewicz et al., 2009a) and this observation is in line with novel neuroimaging findings showing markedly reduced binding to mGluR5 in the PFC in living depressed patients (Hasler, 2009). Now, in the same cohort of depressed subjects, we have found robust increase in the mGluR2/3 protein level. Taken together these data indicate that cortical metabotropic glutamate receptors are differentially regulated in the course of depressive disorder. These distinct MDD-associated abnormalities in glutamate receptors could be due to their diverse involvements in the regulation of glutamate neurotransmission and/or their different cellular distribution. Implications of these findings are that glutamate receptors are well-suited as potential biomarkers for depression, and are attractive targets for the discovery of novel antidepressant medications.
Several potential limitations of this study are worth mentioning. First, we measured mGluR2/3 immunoreactivity in cortical tissue homogenates collected from BA10 across all six cortical layers containing a mixture of many different cell types. Given that mGluR2/3 are expressed by both neurons and glia (Crook et al., 2002; Ohishi et al., 1998; Petralia et al., 1996), the functional consequences of glutamate receptors pathology depend on which specific population (or populations) of cells are affected. Thus, the mGluR2/3 pathology in MDD should be explored in individual cortical cell-types in future postmortem studies. The next apparent limitation is the fact that both group II receptors (2 and 3) were investigated as a total pool of mGluR2/3 immunoreactivity due to their sequence homology and the unavailability of selective antibodies. Likewise, most of the group II ligands with reported antidepressant-like activity are equipotent at mGlu2 and mGlu3 receptors, indicating that the pharmacology of these receptors is very similar (Kingston et al., 1998; Palucha and Pilc, 2007). Nevertheless, we cannot rule out subtype specific receptor changes due to receptor selective mechanisms. Another possible limitation of this study is the confounding effect of antidepressant medication. In fact, antidepressants may affect the expression of mGluR2/3 (Matrisciano et al., 2002) and mask possible changes associated with disease pathology. All of our MDD subjects were antidepressant free at the time of death as revealed by postmortem toxicology screening (Table 1); however, three subjects with MDD had prescriptions for SSRIs within 4 weeks prior to death (Table 2). Nevertheless, the absence of any significant difference in mGluR2/3 levels between fluoxetine-treated and non-treated rhesus monkeys in our study, albeit using a small sample size, suggests that treatment with SSRIs is unlikely to contribute to the changes found in the mGluR2/3 level in depression. Finally, given that 7 out of 11 depressed subjects committed suicide it is possible that behaviors related to suicide, but distinct from depressive symptoms associated with depressive disorder studied might contribute to altered mGluR2/3 protein. However, it is worth noting that the mGluR2/3 level detected in suicide MDD subjects was not different than that detected in non suicide MDD subjects. Therefore, even though the sample size for our study is small, the present findings are suggestive of altered mGluR2/3 protein in depressive disorder.
Table 2.
Summary of demographic characteristics of subjects
| Parameter | Controls (n=11) | Major Depression (n=11) |
|---|---|---|
| Age (years)* | 51 ± 4.8 | 53 ± 4.7 |
| PMI (hours)* | 18 ± 1.8 | 23 ± 1.5 |
| pH* | 6.7 ± 0.09 | 6.5 ± 0.08 |
| Gender (F/M) | 2/9 | 2/9 |
| Time in freezer (mo)* | 141 ± 6 | 142 ± 13 |
| Medication history a | none | n=3 (sertraline, n=2; fluoxetine, n=1) |
| Comorbid diagnosis | History of alcohol dependence, n=2 History of alcohol abuse, n=1 |
Alcohol abuse, n=2 History of alcohol abuse, n=1 |
| Smoking | Smokers, n=3 History of smoking, n=2 |
Smokers, n=3 |
Prescriptions for antidepressants within 4 weeks prior to death;
mean ± S.E.M; PMI, postmortem interval; F, female; M, male; mo, month.
The unique pharmacology of mGlu2/3 receptors, their localization in key cortical/thalamic/amygdaloid circuits, and the promise of subtle modulation of glutamatergic neurotransmission by sensing glutamate spillover and regulating transmitter release make these receptors intriguing targets for the development of improved medication against depression. Our findings further reinforce the evidence that basal or compensatory changes in excitatory neurotransmission play roles in the pathophysiology of MDD.
Acknowledgments
We gratefully acknowledge the assistance of Drs James C Overholser, George Jurjus, Herbert Y Meltzer, Lisa Konick, Lesa Dieter and Nicole Herbst in the establishment of retrospective psychiatric diagnoses and in tissue collection in the postmortem study. We acknowledge the invaluable contributions made by the families consenting to donate brain tissue and be interviewed. The excellent assistance of the Cuyahoga County Coroner’s Office, Cleveland, OH, USA, is gratefully acknowledged. These studies were supported by grant from the IDeA Program of the National Center of Research Resources (RR17701), and NARSAD (BK).
Abbreviations
- MDD
major depressive disorder
- mGluR2/3
metabotropic glutamate receptor 2/3
- PFC
prefrontal cortex
- BA 10
Brodmann’s area 10
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aronica E, Gorter JA, Ijlst-Keizers H, Rozemuller AJ, Yankaya B, Leenstra S, et al. Expression and functional role of mGluR3 and mGluR5 in human astrocytes and glioma cells: opposite regulation of glutamate transporter proteins. Eur J Neurosci. 2003;17:2106–18. doi: 10.1046/j.1460-9568.2003.02657.x. [DOI] [PubMed] [Google Scholar]
- Auer DP, Pütz B, Kraft E, Lipinski B, Schill J, Holsboer F. Reduced glutamate in the anterior cingulate cortex in depression: an in vivo proton magnetic resonance spectroscopy study. Biol Psychiatry. 2000;47:305–13. doi: 10.1016/s0006-3223(99)00159-6. [DOI] [PubMed] [Google Scholar]
- Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47:351–4. doi: 10.1016/s0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- Blardi P, De Lalla A, Leo A, Auteri A, Iapichino S, Di Muro A, et al. Serotonin and fluoxetine levels in plasma and platelets after fluoxetine treatment in depressive patients. J Clin Psychopharmacol. 2002;22:131–6. doi: 10.1097/00004714-200204000-00005. [DOI] [PubMed] [Google Scholar]
- Brunswick DJ, Amsterdam JD, Fawcett J, Quitkin FM, Reimherr FW, Rosenbaum JF, et al. Fluoxetine and norfluoxetine plasma concentrations during relapse-prevention treatment. J Affect Disord. 2002;68:243–9. doi: 10.1016/s0165-0327(00)00333-5. [DOI] [PubMed] [Google Scholar]
- Cartmell J, Schoepp DD. Regulation of neurotransmitter release by metabotropic glutamate receptors. J Neurochem. 2000;75:889–907. doi: 10.1046/j.1471-4159.2000.0750889.x. [DOI] [PubMed] [Google Scholar]
- Chaki S, Yoshikawa R, Hirota S, Shimazaki T, Maeda M, Kawashima N, et al. MGS0039: a potent and selective group II metabotropic glutamate receptor antagonist with antidepressant-like activity. Neuropharmacology. 2004;46:457–67. doi: 10.1016/j.neuropharm.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Conn PJ, Pin JP. Pharmacology and functions of metabotropic glutamate receptors. Annu Rev Pharmacol Toxicol. 1997;37:205–37. doi: 10.1146/annurev.pharmtox.37.1.205. [DOI] [PubMed] [Google Scholar]
- Crook JM, Akil M, Law BC, Hyde TM, Kleinman JE. Comparative analysis of group II metabotropic glutamate receptor immunoreactivity in Brodmann’s area 46 of the dorsolateral prefrontal cortex from patients with schizophrenia and normal subjects. Mol Psychiatry. 2002;7:157–64. doi: 10.1038/sj.mp.4000966. [DOI] [PubMed] [Google Scholar]
- Deep-Soboslay A, Akil M, Martin C, Bigelow L, Herman M, Hyde T, et al. Reliability of psychiatric diagnosis in postmortem research. Biol Psychiatry. 2005;57:96–101. doi: 10.1016/j.biopsych.2004.10.016. [DOI] [PubMed] [Google Scholar]
- Drevets WC. Neuroimaging studies of mood disorders. Biol Psychiatry. 2000;48:813–29. doi: 10.1016/s0006-3223(00)01020-9. [DOI] [PubMed] [Google Scholar]
- Feyissa AM, Chandran A, Stockmeier CA, Karolewicz B. Reduced levels of NR2A and NR2B subunits of NMDA receptor and PSD-95 in the prefrontal cortex in major depression. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:70–5. doi: 10.1016/j.pnpbp.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Donovan S, Frances A. Nosology of chronic mood disorders. Psychiatr Clin North Am. 1996;19:29–39. doi: 10.1016/s0193-953x(05)70271-9. [DOI] [PubMed] [Google Scholar]
- Gupta DS, McCullumsmith RE, Beneyto M, Haroutunian V, Davis KL, Meador-Woodruff JH. Metabotropic glutamate receptor protein expression in the prefrontal cortex and striatum in schizophrenia. Synapse. 2005;57:123–31. doi: 10.1002/syn.20164. [DOI] [PubMed] [Google Scholar]
- Hashimoto K. Emerging role of glutamate in the pathophysiology of major depressive disorder. Brain Res Rev. 2009;61:105–23. doi: 10.1016/j.brainresrev.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Sawa A, Iyo M. Increased levels of glutamate in brains from patients with mood disorders. Biol Psychiatry. 2007;62:1310–6. doi: 10.1016/j.biopsych.2007.03.017. [DOI] [PubMed] [Google Scholar]
- Hasler G. Abnormal prefrontal glutamatergic and GABAeric systems in mood and anxiety disorders. Biol Psychiatry. 2009;65 (Suppl):176S–177S. [Google Scholar]
- Hasler G, van der Veen JW, Tumonis T, Meyers N, Shen J, Drevets WC. Reduced prefrontal glutamate/glutamine and gamma-aminobutyric acid levels in major depression determined using proton magnetic resonance spectroscopy. Arch Gen Psychiatry. 2007;64:193–200. doi: 10.1001/archpsyc.64.2.193. [DOI] [PubMed] [Google Scholar]
- Karasawa J, Shimazaki T, Kawashima N, Chaki S. AMPA receptor stimulation mediates the antidepressant-like effect of a group II metabotropic glutamate receptor antagonist. Brain Res. 2005;1042:92–8. doi: 10.1016/j.brainres.2005.02.032. [DOI] [PubMed] [Google Scholar]
- Karolewicz B, Feyissa AM, Chandran A, Legutko B, Ordway GA, Rajkowska G, et al. Glutamate receptors expression in postmortem brain from depressed subjects. Biol Psychiatry. 2009a;65 (Suppl):177S. [Google Scholar]
- Karolewicz B, Stockmeier CA, Ordway GA. Elevated levels of the NR2C subunit of the NMDA receptor in the locus coeruleus in depression. Neuropsychopharmacology. 2005;30:1557–67. doi: 10.1038/sj.npp.1300781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karolewicz B, Szebeni K, Gilmore T, Maciag D, Stockmeier CA, Ordway GA. Elevated levels of NR2A and PSD-95 in the lateral amygdala in depression. Int JNeuropsychopharmacol. 2009b;12:143–53. doi: 10.1017/S1461145708008985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly TM, Mann JJ. Validity of DSM-III-R diagnosis by psychological autopsy: A comparison with clinician ante-mortem diagnosis. Acta Psychiatr Scand. 1996;94:337–43. doi: 10.1111/j.1600-0447.1996.tb09869.x. [DOI] [PubMed] [Google Scholar]
- Kingston AE, Ornstein PL, Wright RA, Johnson BG, Mayne NG, Burnett JP, et al. LY341495 is a nanomolar potent and selective antagonist of group II metabotropic glutamate receptors. Neuropharmacology. 1998;37:1–12. doi: 10.1016/s0028-3908(97)00191-3. [DOI] [PubMed] [Google Scholar]
- Matrisciano F, Caruso A, Orlando R, Marchiafava M, Bruno V, Battaglia G, Gruber SH, Melchiorri D, Tatarelli R, Girardi P, Mathè AA, Nicoletti F. Defective group-II metaboropic glutamate receptors in the hippocampus of spontaneously depressed rats. Neuropharmacology. 2008;55:525–31. doi: 10.1016/j.neuropharm.2008.05.014. [DOI] [PubMed] [Google Scholar]
- Matrisciano F, Storto M, Ngomba RT, Cappuccio I, Caricasole A, Scaccianoce S, et al. Imipramine treatment up-regulates the expression and function of mGlu2/3 metabotropic glutamate receptors in the rat hippocampus. Neuropharmacology. 2002;42:1008–15. doi: 10.1016/s0028-3908(02)00057-6. [DOI] [PubMed] [Google Scholar]
- McGirr A, Renaud J, Seguin M, Alda M, Benkelfat C, Lesage A, et al. An examination of DSM-IV depressive symptoms and risk for suicide completion in major depressive disorder: a psychological autopsy study. J Affect Disord. 2007;97:203–209. doi: 10.1016/j.jad.2006.06.016. [DOI] [PubMed] [Google Scholar]
- Ohishi H, Neki A, Mizuno N. Distribution of a metabotropic glutamate receptor, mGluR2, in the central nervous system of the rat and mouse: an immunohistochemical study with a monoclonal antibody. Neurosci Res. 1998;30:65–82. doi: 10.1016/s0168-0102(97)00120-x. [DOI] [PubMed] [Google Scholar]
- Palucha A, Pilc A. Metabotropic glutamate receptor ligands as possible anxiolytic and antidepressant drugs. Pharmacol Ther. 2007;115:116–47. doi: 10.1016/j.pharmthera.2007.04.007. [DOI] [PubMed] [Google Scholar]
- Petralia RS, Wang YX, Niedzielski S, Wenthold RJ. The metabotropic glutamate receptors, mGluR2 and mGluR3, show unique postsynaptic, presynaptic and glial localizations. Neuroscience. 1996;71:949–76. doi: 10.1016/0306-4522(95)00533-1. [DOI] [PubMed] [Google Scholar]
- Pilc A, Chaki S, Nowak G, Witkin JM. Mood disorders: regulation by metabotropic glutamate receptors. Biochem Pharmacol. 2008;75:997–1006. doi: 10.1016/j.bcp.2007.09.021. [DOI] [PubMed] [Google Scholar]
- Rajkowska G, Miguel-Hidalgo JJ, Wei J, Dilley G, Pittman SD, Meltzer HY, et al. Morphometric evidence for neuronal and glial prefrontal cell pathology in major depression. Biol Psychiatry. 1999;45:1085–98. doi: 10.1016/s0006-3223(99)00041-4. [DOI] [PubMed] [Google Scholar]
- Saleem KS, Logothetis NK. Atlas of the Rhesus Monkey Brain. 1. Academic Press; London: 2007. [Google Scholar]
- Sanacora G, Gueorguieva R, Epperson CN, Wu YT, Appel M, Rothman DL, Krystal JH, Mason GF. Subtype-specific alterations of gamma-aminobutyric acid and glutamate in patients with major depression. Arch Gen Psychiatry. 2004;61:705–13. doi: 10.1001/archpsyc.61.7.705. [DOI] [PubMed] [Google Scholar]
- Sanacora G, Zarate CA, Krystal JH, Manji HK. Targeting the glutamatergic system to develop novel, improved therapeutics for mood disorders. Nat Rev Drug Discov. 2008;7:426–37. doi: 10.1038/nrd2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer RL, Endicott J. Schedule for Affective Disorders and Schizophrenia (SADS) 3. New York: New York State Psychiatric Institute; 1978. [Google Scholar]
- Valentine GW, Sanacora G. Targeting glial physiology and glutamate cycling in the treatment of depression. Biochem Pharmacol. 2009;78:431–39. doi: 10.1016/j.bcp.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierońska JM, Legutko B, Dudys D, Pilc A. Olfactory bulbectomy and amitriptyline treatment influences mGlu receptors expression in the mouse brain hippocampus. Pharmacol Rep. 2008;60:844–55. [PubMed] [Google Scholar]
- Witkin JM, Marek GJ, Johnson BG, Schoepp DD. Metabotropic glutamate receptors in the control of mood disorders. CNS Neurol Disord Drug Targets. 2007;6:87–100. doi: 10.2174/187152707780363302. [DOI] [PubMed] [Google Scholar]
- Yasuhara A, Nakamura M, Sakagami K, Shimazaki T, Yoshikawa R, Chaki S, et al. Prodrugs of 3-(3,4-dichlorobenzyloxy)-2-amino-6-fluorobicyclo[3.1.0]hexane-2,6-dicarboxylic acid (MGS0039): a potent and orally active group II mGluR antagonist with antidepressant-like potential. Bioorg Med Chem. 2006;14:4193–207. doi: 10.1016/j.bmc.2006.01.060. [DOI] [PubMed] [Google Scholar]
- Yoshimizu T, Shimazaki T, Ito A, Chaki S. An mGluR2/3 antagonist, MGS0039, exerts antidepressant and anxiolytic effects in behavioral models in rats. Psychopharmacology (Berl) 2006;186:587–93. doi: 10.1007/s00213-006-0390-7. [DOI] [PubMed] [Google Scholar]
- Zarate CA, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63:856–64. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]