Abstract
Reducing oxidative damage is thought to be an effective aging intervention. Açai, a fruit indigenous to the Amazon, is rich in phytochemicals that possesses high anti-oxidant activities, and has anti-inflammatory, anti-cancer and anti-cardiovascular disease properties. However, little is known about its potential anti-aging properties especially at the organismal level. Here we evaluated the effect of açai pulp on modulating lifespan in Drosophila melanogaster. We found that açai supplementation at 2% in the food increased the lifespan of female flies fed a high fat diet compared to the non-supplemented control. We measured transcript changes induced by açai for age-related genes. Although transcript levels of most genes tested were not altered, açai increased the transcript level of l(2)efl, a small heat-shock-related protein, and two detoxification genes, gstD1 and mtnA, while decreasing the transcript level of phosphoenolpyruvate carboxykinase (Pepck), a key gene involved in gluconeogenesis. Furthermore, açai increased the lifespan of oxidative stressed females caused by sod1 RNAi. This suggests that açai improves survival of flies fed a high fat diet through activation of stress response pathways and suppression of Pepck expression. Açai has the potential to antagonize the detrimental effect of fat in the diet and alleviate oxidative stress in aging.
Keywords: lifespan, aging intervention, Euterpe oleracea Mart, phytochemical, phosphoenolpyruvate carboxykinase, oxidative stress
1. Introduction
Diets rich in botanicals are known to have numerous health benefits to humans (Wright et al., 2008). Clinical trials, animal model and cell-based studies, and biochemical analyses demonstrate that plants contain a wide range of nutrients and biologically active phytochemicals that provide numerous health benefits (Bao and Fenwick, 2004; Pan et al., 2009). Phytochemicals may modulate a number of signalling pathways involved in pathological disease processes (Joseph et al., 2007; Surh, 2009). Emerging evidence indicates that phytochemicals, including flavonoids, such as proanthocyanidins and anthocyanins, can delay the aging process and increase lifespan (Joseph et al., 2007; Joseph et al., 2009; Qin et al., 2009; Schauss et al., 2006a; Wilson et al., 2006). Fruits and vegetables are rich in phytochemicals, nutrients and anti-oxidants (Liu, 2004) and their consumption may retard aging. However, due to the complexity of phytochemicals, not all plant foods necessarily contain effective anti-aging reagents. In addition, aging is a multi-factorial process and lifespan can be modulated by different molecular pathways (Kenyon, 2005). Therefore, the potential effects of fruits and vegetables on health and aging should be vigorously and individually evaluated at the molecular, cellular and organismal levels.
Açai is a fruit from the palm tree, Euterpe oleraceae Mart, indigenous to the Amazon River area in South America. It is commonly used to make beverages, and served as a food additive, and is even used in folk medicine (Baumann et al., 2009; Pacheco-Palencia et al., 2008; Plotkin and Balick, 1984; Sabbe et al., 2009). Biochemical studies reveal that açai contains numerous kinds of phytochemicals, particularly, anthocyanins, proanthocyanidins and other flavonoids (Schauss et al., 2006a). Açai pulp possesses unusually high antioxidant activity compared to other plant foods based on various antioxidant assays, particularly against the superoxide and peroxyl radicals (Jensen et al., 2008; Schauss et al., 2006b). In vitro and in vivo studies demonstrated that the pulp can protect both human erythrocytes and polymorphonuclear cells against oxidative stress (Honzel et al., 2008; Jensen et al., 2008). Polyphenolic fractions of açai pulp have been shown to reduce proliferation of HL-60 leukemia cells likely through caspase-3 activated apoptosis (Del Pozo-Insfran et al., 2006). Açai pulp can attenuate cell proliferation and size of tumors in a esophageal cancer rodent model (Stoner, 2009). An açai stone hydroalcoholic extract has been shown to induce a long-lasting vasodilation effect in the rat mesenteric vascular bed. This vasodilation effect is dependent on activation of the nitric oxide-cGMP pathway and involves the endothelium-derived hyperpolarizing factor (EDHF) (Rocha et al., 2007). In a clinical study of healthy adult humans, an açai pulp-rich juice blend has been shown to induce an increase in antioxidant capacity and an inhibition of lipid peroxidation in blood samples (Jensen et al., 2008). However, the health benefits of açai have not been extensively evaluated in vivo in animal models, nor is açai's mechanism of action understood.
Oxidative damage to macromolecules tends to accumulate in the cell with increasing age and is thought to be one of the causative factors of aging (Colavitti and Finkel, 2005; Perez et al., 2009). Strategies designed to reduce oxidative damage have been shown to extend lifespan of an organism. For example, overexpression of superoxide dismutase 1 (SOD1) can increase lifespan, while mutations of sod1 reduce lifespan in Drosophila melanogaster (Landis and Tower, 2005; Martin et al., 2009; Phillips et al., 1989). SOD1 is the major cytosolic enzyme capable of removing highly reactive and toxic superoxide radicals that are generated during metabolic processes in the cell (Fridovich, 1995). The high concentration of polyphenolics and high superoxide and peroxyl radical scavenging capacity of açai pulp suggest that açai has anti-aging properties. However, this hypothesis has not been directly tested in any animal model. Here we use D. melanogaster as the model to evaluate whether and how supplementation of açai pulp in the fly food promotes longevity.
2. Materials and methods
2.1. Materials and Media
The cornmeal medium used in this study contained cornmeal, sugar, dry yeast and agar, and was prepared according to a previously published protocol (Ashburner et al., 2005). The standard sugar-yeast (SY) medium contains 10% sugar, 10% yeast extract and 1.5% agar. To prepare the high fat diet, palmitic acid and the emulsifier Tween-80 were added to hot SY medium and mixed well. The final concentrations of palmitic acid and Tween-80 were 2% (w/v) and 1% (v/v) of the food respectively. Freeze-dried açai pulp (Abrazil LLC, Kendall Park, NJ) was generously provided by AIBMR Life Sciences Inc. (Puyallup, WA) and stored at -80 °C until it was added into fly food. The water-soluble oxygen radical absorbance capacity (ORAC) values of the açai extract, and sugar and yeast extract in the SY diet were measured by a service company, Brunswick Laboratories (Norton, MA), according to previously published methods (Huang et al., 2002; Ou et al., 2002; Ou et al., 2001). The ORAC value is expressed as micromole Trolox equivalency per gram of a sample (μmole TE/g), and reflects the scavenging capacity of antioxidants against the peroxyl radical, a common reactive oxygen species found in an organism. To make açai supplemented diets, açai was added into the SY medium with or without palmitic acid to a final concentration of 0.25%, 0.5%, 1% or 2% (w/v) of the food.
2.2. Fly strains and culture conditions
Wild type D. melanogaster strain Canton S was obtained from the Bloomington Drosophila Stock Center (Bloomington, IN), and was maintained on the cornmeal medium at 25 °C, 60% humidity and a 12-hr light/dark cycle. The mutant with reduction-of-function of sod1 was generated by using the Gal4-UAS system in combination with the RNA interference (RNAi) technique (Kirby et al., 2002). Specifically, double stranded RNA of sod1 was induced from a UAS-SOD1IR line by a ubiquitously expressed Gal4, daughterless-Gal4 (da32-Gal4). The UAS-SOD1IR stock (strain F103) was kindly provided by J. Philips (University of Guelph, Canada). The da-Gal4 stock was obtained from the Bloomington Drosophila Stock Center (Bloomington, IN).
2.3. Lifespan assay and food intake
Flies were allowed to lay eggs on the cornmeal medium. After approximately two weeks, adult flies were collected and transferred to new bottles with the SY medium within 24 hrs of eclosion. After another 24 hrs, males and females were separated under CO2 anesthesia and placed separately in vials filled with 5 mL SY medium. Each vial had approximately 20 individual males or females. 24 hrs later at adult age of 3 days old, flies were treated with specified concentrations of açai for the rest of their life for all the experiments except that in one set of experiments for sod1 RNAi flies açai supplementation was initiated at age of 6 days old. Flies were transferred to new vials with fresh food once every 2-3 days. The number of dead flies was counted at the time of transfer. Lifespan data were recorded using Microsoft Excel® (Microsoft, Redmond, WA). 50-120 flies in 3-6 individual vials were included for each lifespan experiment.
The Capillary Feeder method (CAFÉ) as previously described (Ja et al., 2007) was employed to measure food intake in 24 hours for a group of four individually housed five-day or six-day old adult females, which were treated with the high fat diet supplemented with or without 2% açai for two days. The food intake measurement was repeated twice with the same group of flies on two different days.
2.4. Quantitative polymerase chain reaction (qPCR)
Three-day old Females flies were treated with the standard SY, the high fat, and the high fat supplemented with 2% açai diets respectively until they were 14 days old. Fly head and body were then separated using a sieve after frozen with liquid nitrogen. Total RNA was prepared from head or body using the Absolutely RNA Miniprep Kit from Stratagene (La Jolla, CA) (Cat. No. 400800). Quality and quantity of total RNA were assessed using Nanodrop 1000 from Thermo Scientific (Wilmington, DE). For qPCR, cDNA was synthesized from total RNA using Superscript® reverse transcriptase from Invitrogen (Carlsbad, CA) (Cat. No. 18064-022). qPCR was performed using the StepOnePlus real-time PCR system from Applied Biosystems (Foster City, CA) with protocols suggested by the manufacturer. The sequences of primers for genes tested in this study were designed using the Primer3 program at http://primer3.sourceforge.net/. The full list of genes and primer sequences are shown in Table 2. Each qPCR measure was repeated three times with three biologically independent samples.
Table 2.
List of genes and their primer sequences selected for measuring transcript changes induced by açai.
| Gene symbol | Annotated symbol | Gene Name | Forward primer (5′ to 3′) | Reverse primer (5′ to 3′) |
|---|---|---|---|---|
| Metabolic process | ||||
| Arc42 | CG4703 | Arc42 | gtgatggcagtagcgattcc | gcgtaggccacataatccag |
| JNK signaling pathway | ||||
| bsk | CG5680 | basket | cactcagcaggaattattcacaga | ttagagtgcagtcggccttt |
| l(2)efl | CG4533 | lethal (2) essential for life | cagacgcgtttatccaagtg | atcccaccagtcacggaac |
| gstD1 | CG10045 | Glutathione S transferase | tcgcgagtttcacaacagaa | tgagcagcttcttgttcagc |
| puc | CG7850 | puckered | cgtcatcatcaacggcaat | ggcggggtgtgtttctatatc |
| mtnA | CG9470 | Metallothionein A | aactcaatcaagatgccttgc | ttgcaggatcccttggtg |
| Ice | CG7788 | Ice | gacaagcagttggcaccag | gtacccaccgtttgatccat |
| hsp68 | CG5436 | Heat shock protein 68 | ggaggctccactcgtattcc | tctttccgccgaagaagtt |
| fer1HCH | CG2216 | Ferritin 1 heavy chain homologue | cgacgatcaaagatggtgaa | ccttggtaatctcaggaacagc |
| Insulin-like signaling pathway | ||||
| Pi3K92E | CG4141 | Pi3K92E | atccggtgcttacactttctg | gcttgatggcctgtaccaa |
| dFoxo | CG3143 | forkhead box, sub-group O | tcgagtgcaatgtcgaggag | agcggtatattgatgtccagcag |
| Pepck | CG17725 | Phosphoenolpyruvate carboxykinase | aatcccattgccatgaacac | gccctcccagaacacacc |
| ImpL2 | CG15009 | Ecdysone-inducible gene L2 | gccgataccttcgtgtatcc | tttccgtcgtcaatccaatag |
| Nutrient sensing pathway | ||||
| SNF1A | CG3051 | SNF1A/AMP-activated protein kinase | ctggcaggaagaacgtaacc | gcaggtagctccttgttgga |
| Sir2 | CG5216 | Sir2 | actggtcgagaacggcatc | tgtggtactcatccggcagtc |
| Tor | CG5092 | Target of rapamycin | gctcagaggcgagagacaag | ccagctcacggaggataaag |
| Thor | CG8846 | Thor (4E-BP) | ccagatgcccgaggtgta | agcccgctcgtagataagttt |
| S6kI I | CG17596 | RPS6-protein kinase-II | ccctaaagctctgcgatttg | caggtcatagccctgtctcttt |
Note: Gene symbol, annotation and name were cited from the flybase website, www.flybase.org.
2.5. Western blot analysis
Three-day old adult male and female sod1 RNAi (da-Gal4/sod1IR) and control (da-Gal4/+) flies were fed the standard SY medium until they reached 10 days old. Total proteins were extracted from these flies using the tissue extraction reagent (Invitrogen, Cat. No. FNN0071). Protein concentrations were measured using the BCA Protein (Cat. No.23227, Thermo Fisher Scientific, Rockford, IL). 15 μg protein from each sample was separated with NuPAGE® Novex® 10% Bis-Tris Midi Gel (Invitrogen, Cat. No. WG1203BOX) by SDS polyacrylamide gel electrophoresis (SDS-PAGE). The gel was then blotted onto a nitrocellulose membrane for 7 min using the iBlot™ Gel Transfer Device and the Regular (Nitrocellulose) iBlot™ Gel Transfer Stacks (Invitrogen, Cat. No. IB1001 and Cat. No. IB301001 respectively). The Nitrocellulose membrane was subsequently blocked with 2% ECL advance blocking agent (Cat. No. RPN418, GE Healthcare Life Sciences, Piscataway, NJ) in TBS with 0.1% Tween 20, pH 7.5 (TBST) for 1 hour at room temperature, and then incubated in a mixture of primary antibodies, rabbit anti-SOD1 (Cat. No. ab13498, Abcam, Cambridge, MA) and mouse anti-β-Actin as the loading control (Abcam, Cat. No. ab8224), at a dilution of 1:2000 for both antibodies in the blocking solution on a shaker at 4°C for overnight. After washing in TBST, the blot was incubated in a mixture of the secondary antibodies, ECL Plex goat anti-rabbit IgG-Cy3 and goat anti-mouse IgG-Cy5 (GE Healthcare Life Sciences, Cat. No. 28-9011-06 and PA45009, respectively) at a dilution of 1:2500 in TBST on a shaker for 1 h at room temperature. The membrane was then washed, dried and scanned on the Typhoon™ TRIO+ Variable Mode Imager (GE Healthcare Life Sciences, Cat. No. 63-0055-89). Imaging was performed using the red laser 633-nm with the 670BP30 filter for Cy5-conjugated antibodies and the green laser 532-nm with the 580BP30 filter for Cy3-conjugated antibodies. The images were visualized and quantified with ImageQuant™ TL software included in the Typhoon Imager. The Western blot analysis was repeated three times with three biologically independent samples.
2.6. Statistical analysis
Lifespan and quantitative PCR data were analyzed using Statview version 5.0 software (SAS, Cary, NC). For experiments with multiple samples, p values were calculated by one-way analyses of variance (ANOVA) followed by the Fisher's PLSD analysis to compare the difference between any two treatments and the Bonferroni correction to adjust multiple comparisons. For lifespan experiments with two treatments, Mantel-Cox logrank tests were performed. For qPCR with two treatments, the Student's t-test was performed. p<0.05 was considered statistically significant. The lifespan and qPCR values are presented as means ± SEM.
3. Results
3.1. Effect of açai pulp on the lifespan of flies fed a standard diet
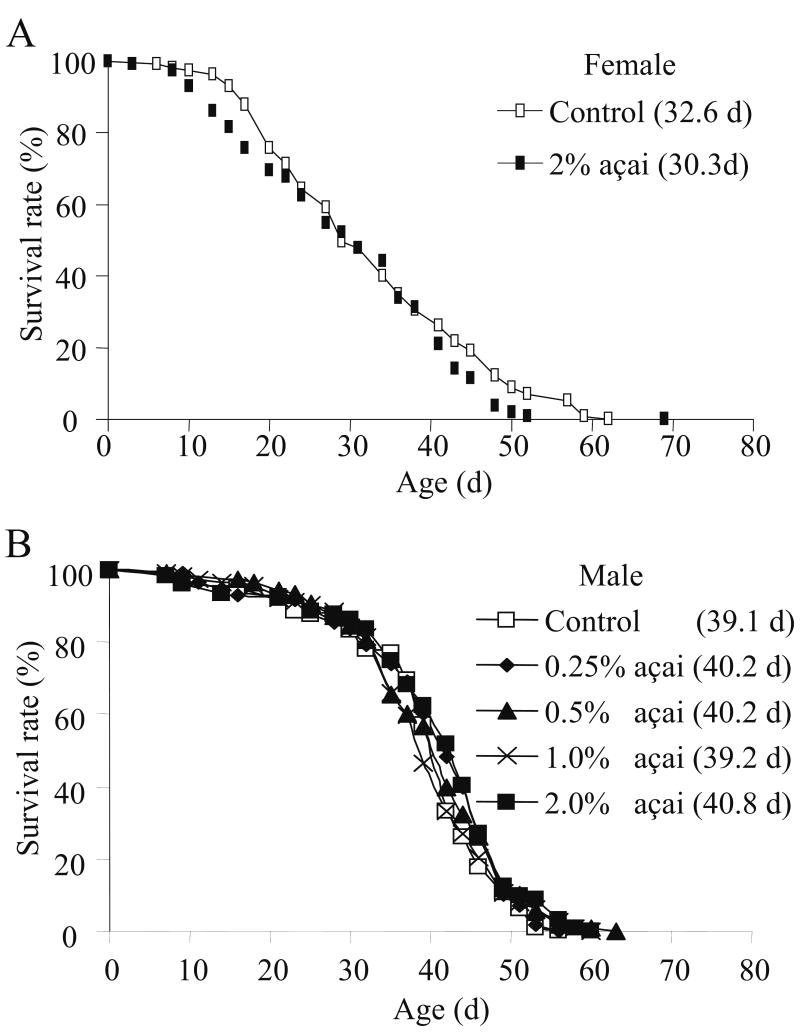
A diet containing 10% sugar and 10% yeast extract is routinely used as a standard diet for measuring lifespan in D. melanogaster. To assess the effect of açai on aging, we supplemented the standard fly diet with açai at 0.25%, 0.5%, 1% or 2%. None of these concentrations of açai were found to significantly affect the lifespan of males compared to the non-supplemented standard diet (Fig. 1A). Based on the measurements from the service company, Brunswick Laboratories, the hydrophilic oxygen radical absorbance capacity (ORAC) of the açai extract used in this study, and the two major components in the SY medium, sugar and yeast extract were 867, 0.4 and 361 μmoleTE/g respectively. Under the culture condition with the highest concentration (2%) tested in this study, açai did not change the lifespan of females, either (Fig. 1B and Table 1). Lower concentrations of açai were not evaluated in females. This indicates that açai supplementation up to 2% in the food is not sufficient to promote longevity under the culture condition using the standard fly diet. On the other hand, this also indicates that açai supplementation is not detrimental to the lifespan of flies under the current culture conditions.
Fig. 1.
Effect of açai supplementation on the lifespan of males (A) and females (B). Lifespan curves of flies fed a standard diet supplemented with various amount of açai are shown in the graph. The concentrations of açai and mean lifespan of flies in parenthesis are listed beside the lifespan curves. d refers to days for lifespan. The number of flies in each experiment is listed in Table 1.
Table 1.
Lifespan of wild type and sod1 RNAi flies.
| Strain | Gender | Diet | Conc. of açai | Number of flies | Mean lifespan (days) a | p valueb | Number of vialsc | Maximum lifespan (days)a, a | p valuee |
|---|---|---|---|---|---|---|---|---|---|
| Canton S | Female | SY | 0% | 115 | 32.9±1.2 | 6 | 53.2±2.0 | ||
| 2% | 115 | 30.3±1.2 | 0.192 | 6 | 49.3±3.7 | 0.3766 | |||
| Canton S | Male | SY | 0% | 112 | 39.1±1.0 | 6 | N.A. | ||
| 0.25% | 116 | 40.2±1.0 | 0.425 | 6 | N.A. | N.A. | |||
| 0.5% | 108 | 40.2±1.0 | 0.473 | 6 | N.A. | N.A. | |||
| 1% | 112 | 39.2±1.0 | 0.97 | 6 | N.A. | N.A. | |||
| 2% | 104 | 40.8±1.0 | 0.244 | 6 | N.A. | N.A. | |||
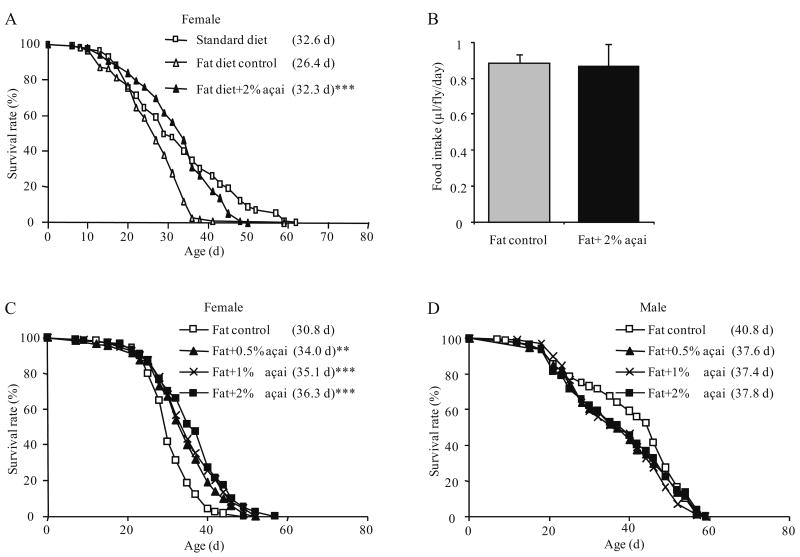
| Canton S | Female | SY+fat | 0% | 119 | 26.4±0.7 | 6 | 37.7±4.2 | ||
| (Experiment #1) | 2% | 138 | 32.3±0.9 | 0.0004* | 6 | 45.2±0.6 | 0. 0921 | ||
| Canton S | Female | SY+fat | 0% | 120 | 30.8±0.6 | 6 | 39.5±1.4 | ||
| (Experiment #2) | 0.5% | 115 | 34.0±0.8 | 0.0043* | 6 | 46.8±1.0 | 0.0023* | ||
| 1% | 113 | 35.1±0.8 | 0.0001* | 6 | 45.8±1.6 | 0.0068* | |||
| 2% | 120 | 36.3±0.8 | <0.0001* | 6 | 49.8±1.8 | 0.0002* | |||
| Canton S | Male | SY+fat | 0% | 115 | 40.8±1.2 | 6 | N.A. | ||
| 0.5% | 112 | 37.6±1.2 | 0.0636 | 6 | N.A. | N.A. | |||
| 1% | 97 | 37.4±1.2 | 0.0614 | 6 | N.A. | N.A. | |||
| 2% | 119 | 37.8±1.2 | 0.0776 | 6 | N.A. | N.A. | |||
| sod1 RNAi | Female | SY | 0% | 59 | 19.6±0.4 | 3 | N.A. | ||
| (Experiment #1) | 2% | 56 | 22.9±0.7 | <0.0001* | 3 | N.A. | N.A. | ||
| sod1 RNAi | Female | SY | 0% | 79 | 20.2±0.5 | 4 | N.A. | ||
| (Experiment #2) | 2% | 74 | 23.6±0.7 | <0.0001* | 4 | N.A. | N.A. | ||
| da-Gal4/+ | Female | SY | 0% | 60 | 32.8±0.8 | <0.0001*f | 3 | N.A. | N.A. |
| sod1 RNAi | Male | SY | 0% | 62 | 18.3±0.6 | 3 | N.A. | ||
| (Experiment #1) | 2% | 44 | 17.8±1.1 | 0.637 | 2 | N.A. | N.A. | ||
| sod1 RNAi | Male | SY | 0% | 81 | 18.0±0.5 | 4 | N.A. | ||
| (Experiment #2) | 2% | 88 | 20.4±0.6 | 0.0015* | 4 | N.A. | N.A. | ||
| da-Gal4/+ | Male | SY | 0% | 60 | 48.2±1.5 | <0.0001*f | 3 | N.A. | N.A. |
Lifespan is expressed as mean±SEM.
The p values were calculated using lifespan of all the flies.
The number of individual vials of flies used in each lifespan experiments is indicated.
Maximum lifespan is calculated by averaging the lifespan of the longest lived fly in each vial.
The p values were calculated using lifespan of the longest lived fly in each vial.
The p values were calculated by comparing sod1 RNAi and the wild type control da-Gal4/+.
Statistically significant p values are marked after the Bonferroni correction for multiple comparisons.
N.A. : not analyzed for experiments with fewer than six vials or males.
3.2. Effect of a high fat diet on the lifespan of females
The standard fly diet contains a minimal amount of fat from yeast extract. To evaluate the effect of fat on aging, the saturated fat, palmitic acid, is routinely added to normal fly food, which contains sugar and yeast extract or sugar, yeast and cornmeal (Driver and Cosopodiotis, 1979; Driver et al., 1986). However, when using published protocols to prepare high fat diets, the fat tends to form a thin layer on the top of the food after cooling down, which makes it difficult for flies to access other nutrients in the food. The stickiness of the fat layer also causes flies to stick to the food, resulting in premature death and an underestimated lifespan (unpublished observation by Alberico and Zou).
To reliably assess the effect of fat on longevity, we modified published protocols for preparing high fat diets. We used Tween-80 as an emulsifier to evenly distribute fat in the food (Jaromin et al., 2006). Using this protocol, we found that 2% palmitic acid significantly decreased mean lifespan of females by approximately 19% compared to the control diet without the addition of fat (from 32.6 days to 26.4 days) (Fig. 2 and Table 1). The control diet contains the same amount of sugar, yeast extract and Tween-80 as the high fat diet. Maximum lifespan was also significantly decreased by palmitic acid (Table 1). In accordance with published results (Driver and Cosopodiotis, 1979; Driver et al., 1986), our findings suggest that high fat diets are detrimental to flies as is the case for humans.
Fig. 2.
Effect of fat and açai supplementation on the lifespan of females. (A) Lifespan curves of female flies fed a normal diet, a high fat diet and a high fat diet supplemented with 2% açai from experiment #1 are shown in the graph. (B) Food intake in 24 hours for females on the high fat diet control and the high fat diet supplemented with 2% açai from one experiment is shown (n=4). The similar results on food intake were observed from the other repeated experiment (data not shown). (C) Lifespan curves of female flies fed the high fat diet and the high fat supplemented with 0.5%, 1% and 2% açai from experiment #2 are shown. (D) Lifespan curves of male flies fed the high fat diet and the high fat supplemented with 0.5%, 1% and 2% açai are shown. The mean lifespan in days are listed in parenthesis. The number of flies in each experiment is listed in Table 1. ***p<0.001 by ANOVA after the Bonferroni adjustment for multiple comparisons.
3.3. Effect of açai pulp on the lifespan of females fed a high fat diet
Açai is rich in polyphenolic flavonoids, including anthocyanins (cyanidin 3-rutinoside, cyanidin 3-glucoside, cyanidin 3-sambubioside, peonidin 3-rutinoside, and peonidin 3-glucoside) and other flavonoids (chrysoeriol, orientin, homoorientin, luteolin, dihydrokaempferol, quercetin, isovitexin, vitexin) (Schauss et al., 2006a). Considering the beneficial effects of these phytochemicals and the demonstrated high antioxidant activity of açai pulp (Bao and Fenwick, 2004), we postulated that açai should reverse the detrimental effects of the high fat diet.
To test this hypothesis, flies fed the high fat diet (2% palmitic acid) were supplemented with açai pulp over their adult life. We found that supplementation of 2% açai significantly prolonged mean lifespan of female flies by approximately 22% compared to those fed the non-supplemented high fat diet (32.2 days vs 26.4 days in experimental #1), while supplementation of açai pulp at 0.5% to 2% did not change lifespan of males (Fig. 2 A and D and Table 1). Mean lifespan of female flies fed the açai supplemented high fat diet were not significantly different from that of flies fed a standard diet (Fig. 2A). We further investigated the dosage effect of açai pulp and found that 0.5% açai pulp was sufficient to significantly increased mean lifespan of females flies fed the high fat diet. The higher concentration of açai pulp in the diet was, the higher mean lifespan was observed (Fig. 2C). Maximum lifespan of females fed the high fat diet supplemented with açai pulp was found to be statistically significantly different from that of the non-supplemented control in one experiment but not the other (Table 1, Fig. 2A and C).
The prolongevity effect of açai pulp might be due to the possibility that it suppresses appetite of the flies, which can cause a DR effect and consequently lifespan extension. To test this hypothesis, we measured food intake of females using the Capillary Feeder method (CAFÉ) (Ja et al., 2007). We found that there was no statistically significant difference of food intake within 24 hours between females on the açai pulp supplemented high fat diet and the control fat diet (Fig. 2B), suggesting that açai pulp does not cause a DR effect. Taken together, these findings suggest that açai supplementation can antagonize the detrimental effects of a high fat diet on lifespan.
3.4. Changes in gene expression induced by açai pulp supplementation
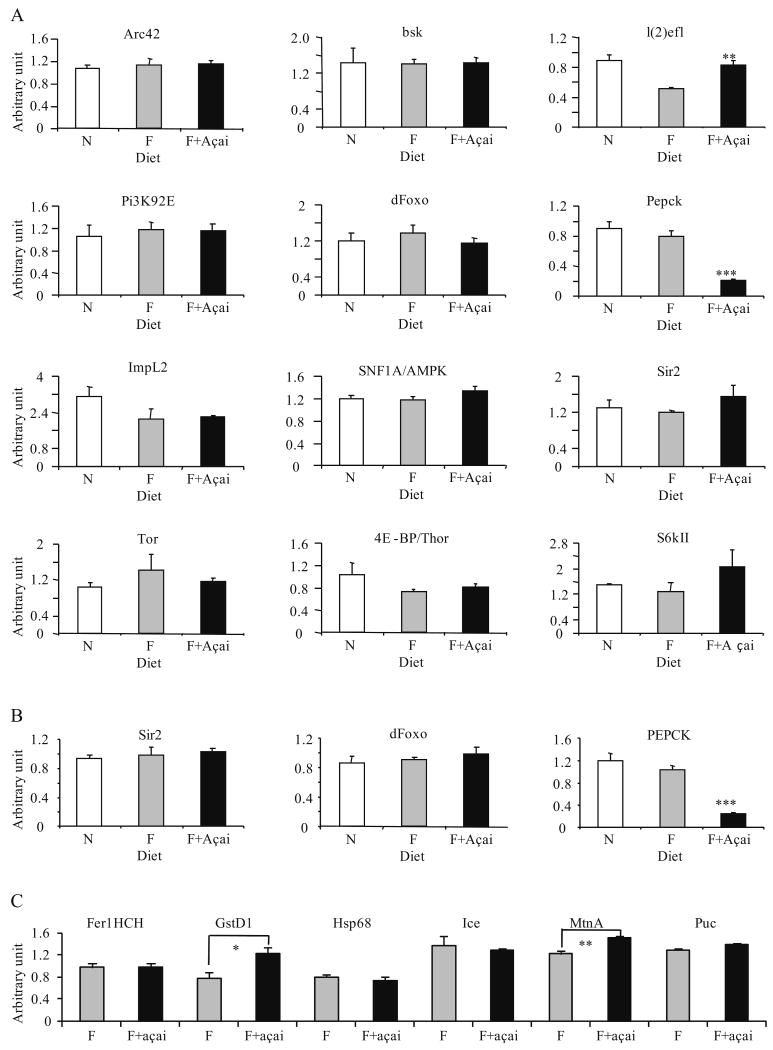
To investigate the molecular mechanisms by which açai improves survival, we measured changes in the transcript levels for more than 12 genes known to be associated with metabolic, aging and disease processes (Table 2 and data not shown) (Karpac and Jasper, 2009; Kenyon, 2005). These genes are known to be involved in nutrient sensing, Jun-N-terminal kinase (JNK) and insulin-like signalling pathways (Guarente and Picard, 2005; Karpac and Jasper, 2009; Kenyon, 2005). Specifically, we compared transcript levels of these genes among females fed a normal diet, a high fat diet and a high fat diet supplemented with 2% açai. We surveyed the expression patterns in both fly bodies, which contain predominantly muscle, and heads, which are predominantly composed of brain.
The head-specific expression patterns of 12 genes and the body-specific expression patterns of three representative genes are shown in Fig. 3A and 3B respectively. For most of these genes, including Sir2, a aging-related gene encoding a histone deacetylase, and dFoxo, a forkhead transcription factor, transcript levels in both fly bodies and heads were not significantly different among all three diets (Fig. 3A and B) (Guarente and Picard, 2005; Karpac and Jasper, 2009). Phosphoenolpyruvate carboxykinase (Pepck) encodes a key enzyme in gluconeogenesis and glyceroneogenesis, and is partially transcriptionally regulated by dFoxo (Chakravarty et al., 2005). The transcript level of Pepck was found to be reduced by açai pulp supplementation compared to the other two non-supplemented diets, but was not altered by the high fat diet compared to the standard diet in both fly bodies and heads (Fig. 3A and B).
Fig. 3.
Effect of açai supplementation on gene expression. (A) Head-specific expression levels of 12 genes were measured for females fed a normal diet (N), a high fat diet (F) and a high fat diet supplemented with 2% açai (F+ açai) (n=3). Full description of these genes and the primers for qPCR is presented in Table 1. (B) Body-specific expression levels of Sir2, dFoxo, and Pepck were measured for females fed a normal diet, a high fat diet and a high fat diet supplemented with 2% açai (n=3). (C) Head-specific expression levels of six putative JNK target genes were measured for females fed the high fat diet (F) and the high fat diet supplemented with 2% açai (F+ açai) (n=3). ***p<0.05; **p<0.01; ***p<0.001.
Lethal (2) essential for life (l(2)efl) encodes a small heat-shock-related protein and is thought to be involved in the stress response regulated by both dFoxo and JNK (Wang et al., 2005). Overexpression of l(2)efl has been shown to extend lifespan in D. melanogaster (Wang et al., 2005). The high fat diet decreased the transcript level of l(2)efl by almost 2-fold compared to the normal diet. Açai supplementation increased the transcript level of this gene by almost 2-fold compared to the non-supplemented high fat diet, which brought the transcript level of l(2)efl back to that in flies fed the standard diet. To further investigate the effect of açai on the JNK signaling pathway, we measured transcript levels of six additional putative JNK downstream targets involved in oxidative stress response, including Ferritin 1 heavy chain homologue (Fer1HCH), Glutathione S transferase D1 (GstD1), Heat shock protein 68 (Hsp68), Ice, Metallothionein A (MtnA) and Puckered (Puc) (Jasper et al., 2001, Wang et al., 2005) for the flies fed the high fat diet and 2% açai supplemented fat diet (Fig. 3C). We found that açai significantly increased the transcript levels of two JNK downstream genes, GstD1 and MtnA, both of which are involved in antioxidant function (Jasper et al., 2001) (Fig. 3C). However, açai did not significantly alter transcript levels of other four JNK downstream genes. These results suggest that açai activates a subset of JNK downstream targets. The transcript levels of other genes known to directly affect lifespan, such as Target-of-Rapamycin (TOR) (Sheaffer et al., 2008), were not altered by açai supplementation or the high fat diet (Fig. 3A). Nevertheless, these findings suggest that the prolongevity effect of açai for flies fed a high fat diet is at least partially mediated by its suppression of Pepck expression and activation of stress response pathways.
3.5. Effect of açai pulp on oxidative stressed flies
SOD1 is a major cytosolic enzyme to scavenge highly active superoxide in the cell (Fridovich, 1995). Loss-of-function and reduction-of-function mutants of sod1 have been shown to be sensitive to exogenous oxidative stress, such as that induced by paraquat, an oxidant inducing agent, shorter lifespan and higher levels of oxidative damage in the cell compared to the wild type control in D. melanogaster (Kirby et al., 2002; Missirlis et al., 2003; Phillips et al., 1989). One method to generate a reduction-of-function mutation of sod1 is to ubiquitously induce expression of double-stranded RNA of sod1 by the combination of the Gal4-UAS and RNA interference (RNAi) techniques (Kirby et al., 2002). Here we used transgenic flies with the daughterless-Gal4 (da-Gal4), a ubiquitously expressed Gal4, and a UAS construct carrying inverted repeats (IR) of part of the sod1 coding sequence to reduce the level of SOD1 by double-stranded RNA. This sod1 RNAi significantly reduced protein level of SOD1 compared to the wild type control (Fig. 4A). Comparable with published results by Wick et al. using the same sod1 RNAi flies (Wick et al., 2009), sod1 RNAi flies were found short lived and highly sensitive to paraquat compared to the wild type control da-Gal4/+ (Table 1 and data not shown).
Fig. 4.
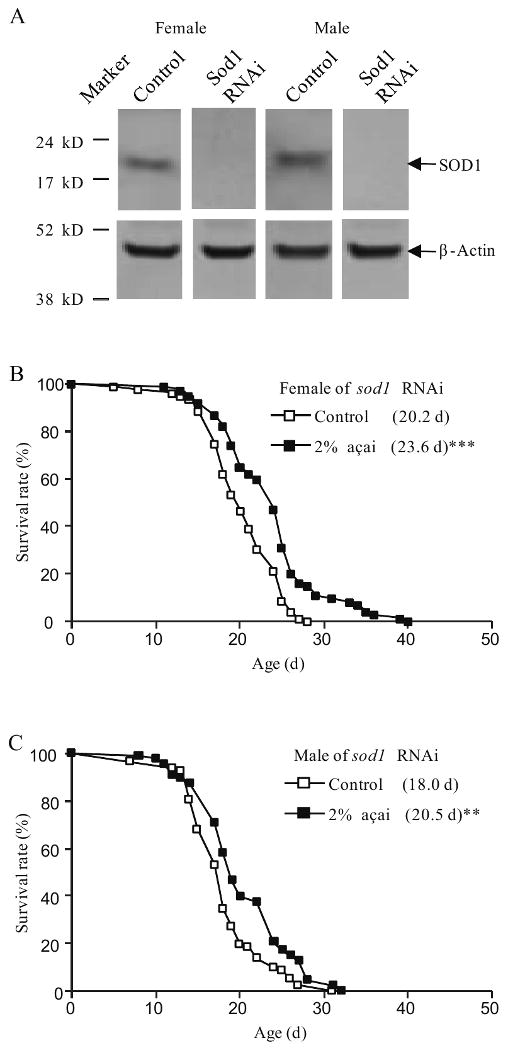
Effect of açai pulp supplementation on the lifespan of oxidative stressed female. (A) Western blot analysis of expression of SOD1 protein in the control (da-Gal4/+) and sod1 RNAi (da-Gal4/UAS-sod1IR) flies. (B) Lifespan curves of sod1 RNAi females fed the normal SY diet and the SY diet supplemented with 2% açai from experimental #2 are shown in the graph. Açai supplementation was initiated at adult age of 3 days old in experiment #2. (C) Lifespan curves of sod1 RNAi males fed the normal SY diet and the SY diet supplemented with 2% açai from experimental #2 are shown in the graph. Açai supplementation was initiated at adult age of 3 days old in experiment #2. The mean lifespan in days are listed in parenthesis. ** p<0.01;***p<0.001.
Açai pulp has high anti-oxidant activities as demonstrated by biochemical and cell-based assays (Jensen et al., 2008; Schauss et al., 2006b). As described above, açai pulp can induce expression of l(2)efl, a downstream of JNK pathway, an oxidative stress response pathway, in females fed a high fat diet. We postulated that açai pulp can improve survival of flies under oxidative stress. To test this hypothesis, we fed sod1 RNAi flies 2% açai pulp for their adult life time. We found that supplementation of 2% açai pulp initiated at adult age of 3 and 6 days old significantly increased lifespan of female flies by approximately 18% compared to the non-supplemented control in both experiments (Fig. 4B and Table 1). Mean lifespan of sod1 RNAi males was significantly increased by supplementation of 2% açai pulp initiated at adult age of 3 days old but not 6 days old (Fig. 4C and Table 1). Taken together, these results suggest that açai pulp improves survival of flies partly through alleviating oxidative stress.
4. Discussion
Supplementation of phytochemicals in human diets has been found to provide numerous health benefits (Pan et al., 2009). Açai pulp is rich in flavonoids, many of which have been shown to have potential health benefits in various test models (Schauss et al., 2006b). Here we have evaluated the anti-aging effect of açai pulp supplementation in D. melanogaster and investigated potential molecular mechanisms. By testing concentrations of açai up to 2% in the food, we have not observed any prolongevity or lifespan shortening effect of açai on flies fed a commonly used standard fly diet. Using an improved protocol to prepare high fat diets, we have found that a high fat diet containing palmitic acid, a saturated fatty acid, significantly decreases lifespan compared to a standard diet fed to controls. Açai supplementation significantly increases mean and, to a less extent, maximum lifespan of flies fed a high fat diet. This prolongevity effect appears to be associated with decreased expression of Pepck and an increase in stress response. In addition, we found that açai pulp improved survival of sod1 RNAi flies, further suggesting that açai pulp can alleviate oxidative damage in vivo at the organismal level. Interestingly the phenotype of açai supplementation resembles that of supplementation of resveratrol, a polyphenolic compound with anti-aging effects, in mice (Howitz et al., 2003). Resveratrol has been shown to prolong the lifespan of mice fed a high fat diet but not those fed a standard rodent diet (Baur et al., 2006; Pearson et al., 2008). However, resveratrol appears to improve healthspan, although not the lifespan, of mice fed the standard diet as indicated by its beneficial effects on cognitive function (Pearson et al., 2008). It remains to be determined whether açai supplementation has any effect on healthspan of flies fed a standard fly diet.
Restriction of calorie intake without causing malnutrition is an effective non-genetic approach to extend lifespan in almost every animal model (Barzilai and Bartke, 2009). A possible mechanism of the prolongevity effect of açai for flies fed the high fat diet is that it may suppress food intake and indirectly increase lifespan through calorie restriction (CR) pathways. This is not likely the scenario for the action of açai. Firstly, açai supplementation up to 2% does not increase lifespan of flies fed the normal diet. This suggests that any potential effect of açai at the levels tested in this study on food intake is not sufficient to extend lifespan. Secondly, açai supplementation does not decrease the lifespan of flies fed the normal diet, either, indicating that açai at the concentrations used in this study is not detrimental to longevity. Thirdly, a comprehensive analysis of the nutrient composition indicates that freeze-dried açai pulp contains approximately 1.3 g sugar, 8.1 g protein and 32.5 g fat per 100 gram dry mass (Schauss et al., 2006a). The high fat diet used in this study contains 10g sugar, 10g protein and 2 g fat per 100 mL food. Supplementation of açai to 2% in this diet will increase sugar, protein and fat in the food by 0.03 g, 0.16 g and 0.64 g per 100 mL food, which only slightly increases the calorie content of the diet (<7% increase). Most critically, food intake with 24 hrs was found not significantly different between female flies on the high fat control diet and the high fat diet supplemented with 2% açai. Taken together, açai supplementation has a minimal impact on calorie content of the fly diets and does not appear to promote longevity through suppressing food intake of the flies under the culture conditions used in this study.
Pepck is a key enzyme controlling gluconeogenesis and glyceroneogenesis, and known to be modulated by a number of transcription factors, including dFoxo (Chakravarty et al., 2005). dFoxo is a forkhead transcription factor and is regulated at the protein phosphorylation level by the insulin/insulinlike signalling pathway, a major pathway involved in regulating metabolism and aging (Kenyon, 2005). We have observed that açai supplementation significantly suppresses Pepck expression, but does not alter the transcript level of dFoxo. dFoxo is thought to be an transcriptional activator of Pepck (Chakravarty et al., 2005). Taken together, this suggests that açai modulates Pepck expression without changing the transcript level of dFoxo. However, we cannot rule out the possibility that açai modulates phosphorylation and nuclear entry of dFOXO protein, which in turn regulates Pepck expression in this study. It also remains to be determined whether the change of Pepck expression influences lifespan.
Our molecular analysis has revealed that açai supplementation restores the transcript level of l(2)efl in flies fed a high fat diet to that of flies fed a normal diet. l(2)efl encodes a small heat-shock-related protein and is known to be activated by the JNK signaling pathway, a major pathway that responds to various stresses, including oxidative stress (Wang et al., 2005). Oxidative damage is thought to be one of the causative factors in aging (Colavitti and Finkel, 2005; Perez et al., 2009). Activation of the JNK signaling pathway has been shown to extend lifespan in D. melanogaster, which is partially mediated by l(2)efl, a JNK target (Wang et al., 2005). Overexpression of l(2)efl in whole body or in neurons only can extend lifespan in D. melanogaster (Wang et al., 2005). Although most of the genes tested in this study, including JNK, do not show significant alterations at transcript levels, we can not rule out the possibility that their protein activities are modulated by açai supplementation. Analyses of six additional putative downstream genes of the JNK signaling pathway showed that supplementation of açai elevated expression of two of them, GstD1 and MtnA, which are involved in detoxification, compared to the high fat control. In addition, we found that açai pulp increased the lifespan of oxidative stressed flies induced by sod1 RNAi. Although l(2)efl, GstD1 and MtnA are regulated by other signaling pathways, our findings suggest açai pulp modulates lifespan at least partially through activation of the JNK signaling pathway.
Our findings suggest that açai pulp may be effective in prevention and control of type 2 diabetes. Chronic high fat diets have been shown to lead to insulin resistance and eventually type 2 diabetes. The effectiveness of some diabetic drugs, such as fenofibrate, appears to be mediated by their abilities to suppress Pepck expression and, in turn, gluconeogenesis and glyceroneogenesis (Chakravarty et al., 2005; Srivastava, 2009). This suppression can lower the glucose levels and improve lipid profiles in type 2 diabetic patients and animal models, which results in effective control of diabetic symptoms (Quinn and Yeagley, 2005). We have found that açai pulp reduces the transcript level of Pepck by more than four fold compared to the non-supplemented diets. This suggests that açai pulp can antagonize the detrimental effects of high fat diets as a functional food. Further animal model and clinical studies need to be conducted to investigate the effects of açai pulp consumption in prevention of diseases, including diabetes.
Acknowledgments
We thank Edward Spangler and Luc Poirier for critical reading of the manuscript and valuable comments, and thank the Natural and Medicinal Products Division of AIBMR Life Sciences, Inc. for generously providing açai samples. This work was supported by the Intramural Research Program of the National Institute on Aging, National Institutes of Health, U. S. A..
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Wright ME, Park Y, Subar AF, Freedman ND, Albanes D, Hollenbeck A, Leitzmann MF, Schatzkin A. Intakes of fruit, vegetables, and specific botanical groups in relation to lung cancer risk in the NIH-AARP Diet and Health Study. Am J Epidemiol. 2008;168:1024–34. doi: 10.1093/aje/kwn212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao Y, Fenwick R. Phytochemicals in health and disease. Marcel Dekker; New York: 2004. [Google Scholar]
- Pan MH, Lai CS, Dushenkov S, Ho CT. Modulation of inflammatory genes by natural dietary bioactive compounds. J Agric Food Chem. 2009;57:4467–77. doi: 10.1021/jf900612n. [DOI] [PubMed] [Google Scholar]
- Joseph JA, Shukitt-Hale B, Lau FC. Fruit polyphenols and their effects on neuronal signaling and behavior in senescence. Ann N Y Acad Sci. 2007;1100:470–85. doi: 10.1196/annals.1395.052. [DOI] [PubMed] [Google Scholar]
- Surh YJ. Dietary modulation of cell signaling pathways. CRC Press; Boca Raton: 2009. [Google Scholar]
- Joseph JA, Shukitt-Hale B, Willis LM. Grape juice, berries, and walnuts affect brain aging and behavior. J Nutr. 2009;139:1813S–7S. doi: 10.3945/jn.109.108266. [DOI] [PubMed] [Google Scholar]
- Qin Y, Xia M, Ma J, Hao Y, Liu J, Mou H, Cao L, Ling W. Anthocyanin supplementation improves serum LDL- and HDL-cholesterol concentrations associated with the inhibition of cholesteryl ester transfer protein in dyslipidemic subjects. Am J Clin Nutr. 2009;90:485–92. doi: 10.3945/ajcn.2009.27814. [DOI] [PubMed] [Google Scholar]
- Schauss AG, Wu X, Prior RL, Ou B, Patel D, Huang D, Kababick JP. Phytochemical and nutrient composition of the freeze-dried amazonian palm berry, Euterpe oleraceae Mart. (acai) J Agric Food Chem. 2006a;54:8598–603. doi: 10.1021/jf060976g. [DOI] [PubMed] [Google Scholar]
- Wilson MA, Shukitt-Hale B, Kalt W, Ingram DK, Joseph JA, Wolkow CA. Blueberry polyphenols increase lifespan and thermotolerance in Caenorhabditis elegans. Aging Cell. 2006;5:59–68. doi: 10.1111/j.1474-9726.2006.00192.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu RH. Potential synergy of phytochemicals in cancer prevention: mechanism of action. J Nutr. 2004;134:3479S–3485S. doi: 10.1093/jn/134.12.3479S. [DOI] [PubMed] [Google Scholar]
- Kenyon C. The plasticity of aging: insights from long-lived mutants. Cell. 2005;120:449–60. doi: 10.1016/j.cell.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Baumann L, Woolery-Lloyd H, Friedman A. “Natural” ingredients in cosmetic dermatology. J Drugs Dermatol. 2009;8:s5–9. [PubMed] [Google Scholar]
- Pacheco-Palencia LA, Mertens-Talcott S, Talcott ST. Chemical composition, antioxidant properties, and thermal stability of a phytochemical enriched oil from Acai (Euterpe oleracea Mart.) J Agric Food Chem. 2008;56:4631–6. doi: 10.1021/jf800161u. [DOI] [PubMed] [Google Scholar]
- Plotkin MJ, Balick MJ. Medicinal uses of South American palms. J Ethnopharmacol. 1984;10:157–79. doi: 10.1016/0378-8741(84)90001-1. [DOI] [PubMed] [Google Scholar]
- Sabbe S, Verbeke W, Deliza R, Matta V, Van Damme P. Effect of a health claim and personal characteristics on consumer acceptance of fruit juices with different concentrations of acai (Euterpe oleracea Mart.) Appetite. 2009;53:84–92. doi: 10.1016/j.appet.2009.05.014. [DOI] [PubMed] [Google Scholar]
- Jensen GS, Wu X, Patterson KM, Barnes J, Carter SG, Scherwitz L, Beaman R, Endres JR, Schauss AG. In vitro and in vivo antioxidant and anti-inflammatory capacities of an antioxidant-rich fruit and berry juice blend. Results of a pilot and randomized, double-blinded, placebo-controlled, crossover study. J Agric Food Chem. 2008;56:8326–33. doi: 10.1021/jf8016157. [DOI] [PubMed] [Google Scholar]
- Schauss AG, Wu X, Prior RL, Ou B, Huang D, Owens J, Agarwal A, Jensen GS, Hart AN, Shanbrom E. Antioxidant capacity and other bioactivities of the freeze-dried Amazonian palm berry, Euterpe oleraceae Mart. (acai) J Agric Food Chem. 2006b;54:8604–10. doi: 10.1021/jf0609779. [DOI] [PubMed] [Google Scholar]
- Honzel D, Carter SG, Redman KA, Schauss AG, Endres JR, Jensen GS. Comparison of chemical and cell-based antioxidant methods for evaluation of foods and natural products: generating multifaceted data by parallel testing using erythrocytes and polymorphonuclear cells. J Agric Food Chem. 2008;56:8319–25. doi: 10.1021/jf800401d. [DOI] [PubMed] [Google Scholar]
- Del Pozo-Insfran D, Percival SS, Talcott ST. Acai (Euterpe oleracea Mart.) polyphenolics in their glycoside and aglycone forms induce apoptosis of HL-60 leukemia cells. J Agric Food Chem. 2006;54:1222–9. doi: 10.1021/jf052132n. [DOI] [PubMed] [Google Scholar]
- Stoner GD. Foodstuffs for preventing cancer: the preclinical and clinical development of berries. Cancer Prev Res (Phila Pa) 2009;2:187–94. doi: 10.1158/1940-6207.CAPR-08-0226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha AP, Carvalho LC, Sousa MA, Madeira SV, Sousa PJ, Tano T, Schini-Kerth VB, Resende AC, Soares de Moura R. Endothelium-dependent vasodilator effect of Euterpe oleracea Mart. (Acai) extracts in mesenteric vascular bed of the rat. Vascul Pharmacol. 2007;46:97–104. doi: 10.1016/j.vph.2006.08.411. [DOI] [PubMed] [Google Scholar]
- Colavitti R, Finkel T. Reactive oxygen species as mediators of cellular senescence. IUBMB Life. 2005;57:277–81. doi: 10.1080/15216540500091890. [DOI] [PubMed] [Google Scholar]
- Perez VI, Bokov A, Remmen HV, Mele J, Ran Q, Ikeno Y, Richardson A. Is the oxidative stress theory of aging dead. Biochim Biophys Acta. 2009 doi: 10.1016/j.bbagen.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landis GN, Tower J. Superoxide dismutase evolution and life span regulation. Mech Ageing Dev. 2005;126:365–79. doi: 10.1016/j.mad.2004.08.012. [DOI] [PubMed] [Google Scholar]
- Martin I, Jones MA, Grotewiel M. Manipulation of Sod1 expression ubiquitously, but not in the nervous system or muscle, impacts age-related parameters in Drosophila. FEBS Lett. 2009;583:2308–14. doi: 10.1016/j.febslet.2009.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips JP, Campbell SD, Michaud D, Charbonneau M, Hilliker AJ. Null mutation of copper/zinc superoxide dismutase in Drosophila confers hypersensitivity to paraquat and reduced longevity. Proc Natl Acad Sci U S A. 1989;86:2761–5. doi: 10.1073/pnas.86.8.2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridovich I. Superoxide radical and superoxide dismutases. Annu Rev Biochem. 1995;64:97–112. doi: 10.1146/annurev.bi.64.070195.000525. [DOI] [PubMed] [Google Scholar]
- Ashburner M, Golic KG, Hawley RS, editors. Drosophila: A Laboratory Handbook. 2nd. Cold Spring Harbor Laboratory Press; 2005. [Google Scholar]
- Huang D, Ou B, Hampsch-Woodill M, Flanagan JA, Deemer EK. Development and validation of oxygen radical absorbance capacity assay for lipophilic antioxidants using randomly methylated beta-cyclodextrin as the solubility enhancer. J Agric Food Chem. 2002;50:1815–21. doi: 10.1021/jf0113732. [DOI] [PubMed] [Google Scholar]
- Ou B, Hampsch-Woodill M, Flanagan J, Deemer EK, Prior RL, Huang D. Novel fluorometric assay for hydroxyl radical prevention capacity using fluorescein as the probe. J Agric Food Chem. 2002;50:2772–7. doi: 10.1021/jf011480w. [DOI] [PubMed] [Google Scholar]
- Ou B, Hampsch-Woodill M, Prior RL. Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescent probe. J Agric Food Chem. 2001;49:4619–26. doi: 10.1021/jf010586o. [DOI] [PubMed] [Google Scholar]
- Ja WW, Carvalho GB, Mak EM, de la Rosa NN, Fang AY, Liong JC, Brummel T, Benzer S. Prandiology of Drosophila and the CAFE assay. Proc Natl Acad Sci U S A. 2007;104:8253–6. doi: 10.1073/pnas.0702726104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driver CJ, Cosopodiotis G. The effect of dietary fat on longevity of Drosophila melanogaster. Exp Gerontol. 1979;14:95–100. doi: 10.1016/0531-5565(79)90023-8. [DOI] [PubMed] [Google Scholar]
- Driver CJ, Wallis R, Cosopodiotis G, Ettershank G. Is a fat metabolite the major diet dependent accelerator of aging? Exp Gerontol. 1986;21:497–507. doi: 10.1016/0531-5565(86)90002-1. [DOI] [PubMed] [Google Scholar]
- Jaromin A, Zarnowski R, Kozubek A. Emulsions of oil from Adenanthera pavonina L. seeds and their protective effect. Cell Mol Biol Lett. 2006;11:438–48. doi: 10.2478/s11658-006-0037-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpac J, Jasper H. Insulin and JNK: optimizing metabolic homeostasis and lifespan. Trends Endocrinol Metab. 2009;20:100–6. doi: 10.1016/j.tem.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarente L, Picard F. Calorie restriction--the SIR2 connection. Cell. 2005;120:473–82. doi: 10.1016/j.cell.2005.01.029. [DOI] [PubMed] [Google Scholar]
- Chakravarty K, Cassuto H, Reshef L, Hanson RW. Factors that control the tissue-specific transcription of the gene for phosphoenolpyruvate carboxykinase-C. Crit Rev Biochem Mol Biol. 2005;40:129–54. doi: 10.1080/10409230590935479. [DOI] [PubMed] [Google Scholar]
- Jasper H, Benes V, Schwager C, Sauer S, Clauder-Munster S, Ansorge W, Bohmann D. The genomic response of the Drosophila embryo to JNK signaling. Dev Cell. 2001;1:579–86. doi: 10.1016/s1534-5807(01)00045-4. [DOI] [PubMed] [Google Scholar]
- Wang MC, Bohmann D, Jasper H. JNK extends life span and limits growth by antagonizing cellular and organism-wide responses to insulin signaling. Cell. 2005;121:115–25. doi: 10.1016/j.cell.2005.02.030. [DOI] [PubMed] [Google Scholar]
- Sheaffer KL, Updike DL, Mango SE. The Target of Rapamycin pathway antagonizes pha-4/FoxA to control development and aging. Curr Biol. 2008;18:1355–64. doi: 10.1016/j.cub.2008.07.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby K, Hu J, Hilliker AJ, Phillips JP. RNA interference-mediated silencing of Sod2 in Drosophila leads to early adult-onset mortality and elevated endogenous oxidative stress. Proc Natl Acad Sci U S A. 2002;99:16162–7. doi: 10.1073/pnas.252342899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missirlis F, Hu J, Kirby K, Hilliker AJ, Rouault TA, Phillips JP. Compartment-specific protection of iron-sulfur proteins by superoxide dismutase. J Biol Chem. 2003;278:47365–9. doi: 10.1074/jbc.M307700200. [DOI] [PubMed] [Google Scholar]
- Wicks S, Bain N, Duttaroy A, Hilliker AJ, Phillips JP. Hypoxia rescues early mortality conferred by superoxide dismutase deficiency. Free Radic Biol Med. 2009;46:176–81. doi: 10.1016/j.freeradbiomed.2008.09.036. [DOI] [PubMed] [Google Scholar]
- Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang LL, Scherer B, Sinclair DA. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425:191–6. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, Pistell PJ, Poosala S, Becker KG, Boss O, Gwinn D, Wang M, Ramaswamy S, Fishbein KW, Spencer RG, Lakatta EG, Le Couteur D, Shaw RJ, Navas P, Puigserver P, Ingram DK, de Cabo R, Sinclair DA. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–42. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson KJ, Baur JA, Lewis KN, Peshkin L, Price NL, Labinskyy N, Swindell WR, Kamara D, Minor RK, Perez E, Jamieson HA, Zhang Y, Dunn SR, Sharma K, Pleshko N, Woollett LA, Csiszar A, Ikeno Y, Le Couteur D, Elliott PJ, Becker KG, Navas P, Ingram DK, Wolf NS, Ungvari Z, Sinclair DA, de Cabo R. Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metab. 2008;8:157–68. doi: 10.1016/j.cmet.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barzilai N, Bartke A. Biological approaches to mechanistically understand the healthy life span extension achieved by calorie restriction and modulation of hormones. J Gerontol A Biol Sci Med Sci. 2009;64:187–91. doi: 10.1093/gerona/gln061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava RA. Fenofibrate ameliorates diabetic and dyslipidemic profiles in KKAy mice partly via down-regulation of 11beta-HSD1, PEPCK and DGAT2. Comparison of PPARalpha, PPARgamma, and liver x receptor agonists. Eur J Pharmacol. 2009;607:258–63. doi: 10.1016/j.ejphar.2009.02.024. [DOI] [PubMed] [Google Scholar]
- Quinn PG, Yeagley D. Insulin regulation of PEPCK gene expression: a model for rapid and reversible modulation. Curr Drug Targets Immune Endocr Metabol Disord. 2005;5:423–37. doi: 10.2174/156800805774912962. [DOI] [PubMed] [Google Scholar]