Abstract
Accelerated apoptosis in skeletal muscle is increasingly recognized as a potential mechanism contributing to the development of sarcopenia of aging and disuse muscle atrophy. Given their central role in the regulation of apoptosis, mitochondria are regarded as key players in the pathogenesis of myocyte loss during aging and other atrophying conditions. Oxidative damage to mitochondrial constituents, impaired respiration and altered mitochondrial turnover have been proposed as potential triggering events for mitochondrial apoptotic signaling. In addition, iron accumulation within mitochondria may enhance the susceptibility to apoptosis during the development of sarcopenia and possibly acute muscle atrophy, likely through exacerbation of oxidative stress. Mitochondria can induce myocyte apoptosis via both caspase-dependent and independent pathways, although the apoptogenic mediators involved may be different depending on age, muscle type and specific atrophying conditions. Despite the considerable advances made, additional research is necessary to establish a definite causal link between apoptotic signaling and the development of sarcopenia and acute atrophy. Furthermore, a translational effort is required to determine the role played by apoptosis in the pathogenesis of sarcopenia and disuse-induced muscle loss in human subjects.
Keywords: Mitochondria, Iron, Sarcopenia, Muscle atrophy, Apoptosis, Caspases, Endonuclease G, Apoptosis-inducing factor
1. Introduction
Skeletal muscle atrophy and impaired muscle strength represent an important health issue and may occur as a consequence of immobilization, disuse, injury, starvation, medications, and aging. In particular, advanced age is ineluctably accompanied by the loss of muscle mass and strength [1]. This condition, known as sarcopenia of aging, has significant effects on individual health and impacts the severity of frailty [2]. Moreover, poor muscular strength is highly predictive of disability [3] and mortality [4], and general weakness often results in the loss of independent living, thereby affecting individual quality of life and imposing a high burden on healthcare expenditure [5]. Aside from aging, skeletal muscle can undergo significant atrophy following disuse [6]. Notably, the recovery from disuse muscle atrophy is impaired at old age [7].
Hypoplasia and atrophy in skeletal muscle with aging and atrophying conditions occur largely through mechanisms not clearly defined. However, in recent years, evidence has accumulated indicating that acceleration of apoptosis in skeletal muscle may be involved in the pathogenesis of sarcopenia [8–16] as well as acute atrophy [17–24].
Execution of apoptosis in skeletal muscle displays unique features due to the multinucleated nature of myofibers. Therefore, apoptosis may result in the elimination of individual myonuclei (myonuclear apoptosis) and the relative portion of sarcoplasm, without demise of the entire fiber [25]. The mitochondrion, being at the same time the repository and target of several apoptotic mediators, is considered the main center for the integration of the apoptotic signaling and induction of apoptosis [26]. Importantly, skeletal muscle, similar to the heart, contains two bioenergetically [27] and structurally [28] distinct mitochondrial subpopulations: subsarcolemmal mitochondria (SSM), located beneath the sarcolemma, and intermyofibrillar mitochondria (IMF), arranged in parallel rows between the myofibrils. These two subpopulations display different susceptibility towards apoptotic stimuli [29] and may therefore be differentially involved in the pathogenesis of sarcopenia and other muscle atrophying conditions.
Several mechanisms are thought to be involved in the execution of mitochondria-driven apoptosis in skeletal muscle [26]. However, it appears that altered mitochondrial function precedes and is required for the initiation of apoptosis both in sarcopenia of aging [30,31] and disuse muscle atrophy [23].
2. Mechanisms of muscle mitochondrial dysfunction at old age and in atrophying conditions
Mitochondria are essential for proper cellular functioning and viability, being the main sites for energy production and playing an essential role in the maintenance of redox homeostasis [32]. In addition, mitochondria are responsible for the biosynthesis of heme [33], iron–sulfur clusters (ISCs) [34] and steroids [35], as well as for the regulation of cell metabolism, signaling, growth, and the cell cycle [36]. Importantly, the mitochondrion is recognized as the center for the integration of apoptotic signaling and induction of programmed cell death [37]. With these vital responsibilities, it is readily understandable that impairment of mitochondrial integrity and function has been implicated as an important contributor to sarcopenia [26] and even as a causal factor for aging [38,39]. Derived from the well-known free radical theory of aging first pioneered by Harman in the 1950s [40], the mitochondrial theory of aging centers on mitochondrial dysfunction from oxidative damage to mitochondria DNA (mtDNA) caused by reactive oxygen species (ROS) as the key factor driving the aging process [38,39].
Because the mitochondrion is the main cellular site of ROS production, it follows that mitochondrial components are immediately susceptible to oxidative damage. In particular, mtDNA is especially prone to oxidative damage [41] due to its proximity to the electron transport chain (ETC), the lack of protective histones and a less efficient repair system compared to nuclear DNA [42]. Moreover, due to the compactness of the mitochondrial genome (i.e., lack of introns), each mutation is likely to affect gene integrity and, hence, protein function [42]. Mutations in mtDNA can lead to the synthesis of defective respiratory chain components, which may result in the impairment of oxidative phosphorylation, decreased ATP production and further ROS generation [39,40].
Several lines of evidence support the hypothesis that mtDNA damage and mutations contribute to aging and age-related diseases. Indeed, accumulation of mtDNA deletion mutations has been reported at advanced age in animal models and humans [43–48]. Furthermore, mice expressing a proofreading deficient mtDNA polymerase accumulate a high load of mtDNA mutations and display a premature aging phenotype, including severe sarcopenia, and shortened lifespan compared to wild-type littermates [49]. Accumulation of mtDNA mutations and impaired mitochondrial function have been linked with the pathogenesis of sarcopenia, as evidenced by the decreased activity of complex I and IV of the ETC observed in aged skeletal muscles of various species [47,50–53]. Notably, fibers harboring high levels of mtDNA deletions and ETC abnormalities often display morphological aberrations, including segmental atrophy, fiber splitting and breakage [47,53].
Despite these findings, the actual levels of mtDNA mutations observed during normal aging might not have a considerable impact on mitochondrial functional decline in skeletal muscle, since this can occur before a critical level of mtDNA damage is reached [54]. However, other factors can be invoked to support the involvement of mitochondrial dysfunction in the pathogenesis of sarcopenia of aging. These include decreased mitochondrial biogenesis and turnover, and oxidative damage to mitochondrial enzymes, structural proteins and membrane lipids.
Mitochondrial biogenesis has been shown to decrease with age [55,56], possibly as a consequence of the reduced expression of peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-lα) [57]. This latter is a transcriptional coactivator, central to the regulation of the transcription of genes involved in energy homeostasis and metabolism [58]. Chabi et al. [30] recently reported a 30% reduction in mitochondrial content in fast-twitch muscles of senescent rats, which was accompanied by lower PGC-1α protein levels. Furthermore, a general decrease in the expression of genes encoding several ETC subunits has been detected in aged human muscle [55]. Along with decreased mitochondrial biogenesis, advanced age is also characterized by impaired turnover of damaged mitochondria [59]. Dysfunctional mitochondria are removed by means of macroautophagy, a form of cellular “self-eating” that involves the formation of a double membrane around the damaged organelle, called an autophagosome, which then fuses with lysosomes for degradation [60]. This process, termed mitophagy, has been found to be less efficient at advanced age, especially in post-mitotic tissues, such as the skeletal muscle, possibly as a result of age-related lysosomal dysfunction [59]. Impaired clearance of damaged mitochondria and decreased biogenesis could eventually result in an overall loss of mitochondrial function, which may contribute to the loss of muscle mass and function occurring with aging [61].
Besides mtDNA, ROS generated by mitochondria can also directly damage proteins and lipids in the mitochondrial compartment. Oxidatively modified proteins within the ETC may actually result in more immediate deleterious effects (i.e., increased uncoupling and decreased efficiency of oxidative phosphorylation) than would be observed with mtDNA mutations [54]. In addition, oxidative damage to lipids of the inner mitochondrial membrane (IMM), particularly cardiolipin, can lead to disruption of membrane potential with the loss of the proton motive force [62], and affect the activities of respiratory chain complexes [63,64]. Of note, oxidized cardiolipin can directly promote the release of apoptogenic factors from the mitochondria [65,66].
As previously mentioned, altered mitochondrial function is also likely to play a role in skeletal muscle loss following chronic disuse. Adhihetty et al. [23] recently reported that mitochondrial content was reduced in the gastrocnemius muscle of rats subjected to hind limb denervation. This was concomitant with reduced expression of the mitochondrial biogenesis factors PGC-1α and transcription factor A (Tfam). In addition, denervation dramatically enhanced ROS generation in SSM, along with reduced expression of the mitochondrial superoxide dismutase (SOD) isoenzyme (MnSOD) in both IFM and SSM [23]. Using a similar experimental model, O’Leary and Hood [67] recently found that muscle denervation decreased state 3 mitochondrial oxygen consumption and increased ROS generation during state 4 respiration in both mitochondrial subpopulations.
Similar to denervation, muscle unloading has also been shown to alter mitochondrial respiration. Interestingly, SSM and IFM seem to respond differently to muscle disuse, as evidenced by reduced state 3 oxygen consumption detected in IFM, but not in SSM of hind limb suspended rats [68]. In addition, decreased mitochondrial respiration rate was only observed in the gastrocnemius muscle, whereas the soleus, extensor digitorum longus (EDL) and tibialis anterior muscles did not show significant mitochondrial dysfunction. Interestingly, impaired respiration was observed when mitochondria were energized with pyruvate–malate, a complex I electron donor, whilst respiration was not altered in the presence of succinate, a complex II electron donor [68]. This suggests that muscle unloading may negatively and selectively alter the respiration in IFM by reducing the efficiency of complex I of the ETC. Moreover, Oishi et al. [69], using a rat model of muscle unloading, documented a decreased mitochondrial mass in the soleus muscle, as evidenced by reduced levels of cytochrome c oxidase subunit IV (Cox IV). This adaptation was likely sustained by impaired mitochondrial biogenesis, as suggested by the reduced expression of PGC-1α.
Importantly, several studies have shown that muscle disuse is associated with increased levels of oxidative stress [70–74], which in turn may exacerbate the loss of muscle mass [75]. Siu et al. [73] recently reported increased levels of hydrogen peroxide, lipid peroxides and reactive nitrogen species (RNS) in the gastrocnemius muscle of hind limb suspended rats. These changes were concomitant with the reduced expression of MnSOD, whereas no alterations were detected for cytosolic Cu–ZnSOD. Interestingly, changes in oxidative stress markers and MnSOD protein content in response to hind limb unloading were exacerbated or only detectable in aged rats.
3. The emerging role of iron accumulation in age-related mitochondrial dysfunction
Iron (Fe), a trace element essential for various cellular functions, is utilized in two main functional forms: heme and Fe–sulfur clusters (ISCs), both of which are synthesized in mitochondria [33,34]. Due to the importance and potential toxicity of this vital element, cellular Fe uptake, storage, transport, export, and utilization are tightly regulated through a multitude of factors [76]. Since the mitochondrion is the site of heme and ISCs synthesis, most of the Fe taken up by the cell is transported into the mitochondrial compartment [77]. Thus, mitochondria possess their own set of Fe transport and regulatory proteins to maintain Fe homeostasis and achieve balance with cytosolic Fe levels [77]. Fe is transported and stored in the ferric (Fe3+) form; however, only the reduced ferrous (Fe2+) form is incorporated into the heme group, whereas either form can be present in ISCs [77]. While required for cellular viability, Fe plays a central role in the generation of free radicals and ROS. Reduced Fe can act as a potent catalyst in Fenton reactions resulting in the formation of the highly reactive hydroxyl radical. Since mitochondria are the major sites of oxygen consumption and ROS production, the existence of high concentrations of Fe and hydrogen peroxide within the same compartment could potentially result in elevated levels of oxidative stress and damage.
Abnormal Fe homeostasis can lead to the loss of complex IV activity and compromise the integrity of mtDNA, resulting in mitochondrial dysfunction [78,79]. Furthermore, ferrochelatase, the mitochondrial enzyme that incorporates Fe into protoporphyrin IX in the last step of heme synthesis, contains an ISC that is sensitive to oxidants [78]. Damage to ferrochelatase can therefore result in increased availability of free Fe2+ in mitochondria that is not incorporated into heme. In addition, the superoxide anion, formed during mitochondrial respiration via single-electron reduction of oxygen, can react with aconitase to release Fe2+ [80].
Interestingly, several studies have demonstrated that Fe accumulates in skeletal muscle during aging and atrophying conditions [31,74,81–84] and it has been suggested that Fe accumulation may be involved in the pathogenesis of sarcopenia and acute muscle atrophy [85]. The importance of Fe overload in muscle atrophy was first demonstrated by Kondo et al. [81], who reported mitigation of oxidative damage and attenuation of muscle mass loss following administration of the Fe chelator deferoxamine to hind limb immobilized rats. A later study by Hofer et al. [74] demonstrated that non-heme Fe levels in the rat gastrocnemius muscle increased by 233% with age and were further elevated following hind limb suspension (HS) in old, but not young rats. Importantly, free Fe accumulated in atrophied rather than normal fibers, suggesting a causal relation between Fe overload and the loss of muscle mass. Furthermore, Xu et al. [83] found that total non-heme Fe levels increased progressively with age in the rat gastrocnemius muscle and were correlated with decreased muscle mass and grip strength. Fe has also been found to accumulate in rat liver and skeletal muscle mitochondria with aging [31]. Interestingly, Seo et al. [31] found that Fe accumulated to a higher extent in SSM than in IFM of old rats, and impacted on mtRNA oxidation and mitochondrial permeability transition pore (mPTP) opening susceptibility. What is more, mitochondrial non-heme Fe levels were positively correlated with caspase-9 and caspase-3 activity. Likewise, total muscle non-heme Fe was positively correlated with the extent of apoptotic DNA fragmentation [31]. The importance of Fe accumulation in mitochondrial dysfunction is further highlighted by studies utilizing mitochondrial Fe chelation as a strategy to reverse and/or ameliorate the consequences of excess Fe in neurodegenerative disorders [86–88]. Although further research is required, the promising results of these studies together with the pioneering observations by Kondo et al. [81] suggest that mitigation of Fe overload may represent a novel strategy to attenuate mitochondrial dysfunction and muscle loss at old age and in other atrophying conditions.
4. Mechanisms of mitochondria-mediated apoptosis
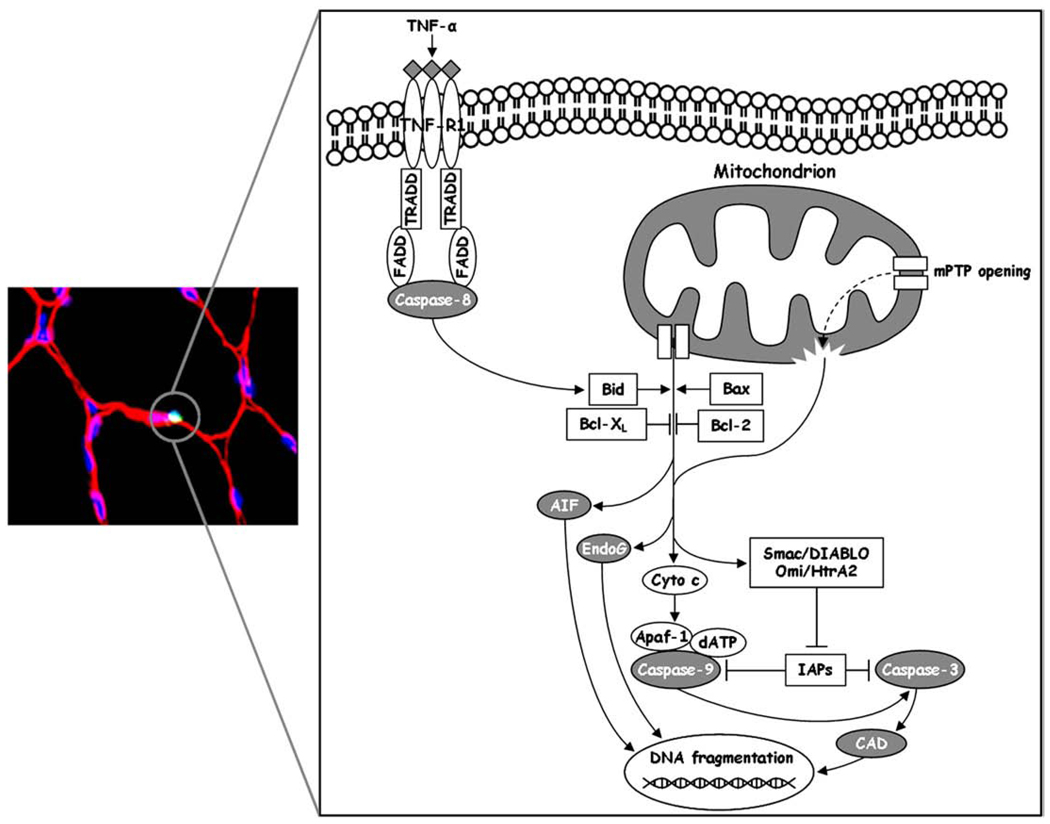
Apoptosis, a process of programmed cell death, is a highly conserved and tightly regulated systematic set of events resulting in cellular self-destruction without the induction of inflammation or damage to the surrounding tissue [89]. Apoptosis is essential for numerous biological processes including embryogenesis and development, cellular turnover, tissue homeostasis, and several immunological functions [89]. Cysteine-aspartic proteases (caspases) are the executioner enzymes that carry out the dismantling of the cell and are normally present as inactive zymogens (procaspases) [90]. Upon apoptotic stimuli, initiator caspases (i.e., caspase-8, caspase-9, caspase-12) are activated, subsequently leading to the activation of effector caspases (i.e., caspase-3, caspase-6, caspase-7) that perform the actual cellular degradation [91]. Effector caspases can be activated through extrinsic and intrinsic pathways [90]. The extrinsic apoptotic signaling is initiated by the activation of death receptors present on the cell surface, such as the Fas receptor and tumor necrosis factor receptor (TNF-R) [92]. Activation of these receptors by binding of death-stimulating ligands results in the recruitment of adaptor proteins, such as the Fas-associated death domain (FADD), which contain a death effector domain (DED) that engages procaspase-8. Active caspase-8, in turn, cleaves and activates the effector caspase-3 [92]. Intrinsic pathways of caspase activation include several internal cellular stimuli mediated by the endoplasmic reticulum or the mitochondria [90]. The sarcoplasmic reticulum (specialized endoplasmic reticulum) in skeletal muscle is essential for muscle function, by storing, releasing, and scavenging calcium ions required for excitation–contraction coupling. When intracellular calcium homeostasis is disrupted, calpain-mediated activation of caspase-12 can occur, leading to the activation of the effector caspase-3 [93]. However, the mitochondria-mediated intrinsic signaling pathway of apoptosis is probably the more prominent means of programmed cell death and has been the subject of intense scientific focus [37] (Fig. 1).
Fig. 1.
Schematic overview of mitochondrial apoptotic signaling in skeletal muscle. The left panel shows an apoptotic myonucleus (circled bright spot) identified by TUNEL staining. The right panel depicts the various molecules that may be involved in mitochondria-mediated apoptosis. Release of apoptogenic factors from the mitochondrial intermembrane space occurs as a result of an imbalance between pro- and anti-apoptotic members of the Bcl-2 family proteins and/or following mitochondrial permeability transition pore (mPTP) opening. This latter causes a sudden increase in membrane permeability, collapse of membrane potential, mitochondrial swelling and rupture of the outer membrane, with subsequent release of mitochondrial death effectors. Mitochondria-mediated apoptosis can be executed via caspase-dependent and independent pathways. The former is initiated by the release of cytochrome c (Cyto c) which associates with apoptotic protease-activating factor-1 (Apaf-1), dATP and procaspase-9. The resulting macromolecular complex (apoptosome) promotes the activation of caspase-9, followed by the engagement of caspase-3, which in turn carries out DNA fragmentation (via caspase-activated DNase, CAD) and protein breakdown. The release of the second mitochondria-derived activator of caspases/direct inhibitor of apoptosis-binding protein with low pI (Smac/Diablo) and heat requirement A2 protein (Omi/HtrA2), which block the activity of the inhibitor of apoptosis proteins (IAPs), ensures complete activation of caspases for the execution of apoptosis. Additionally, mitochondria can induce apoptosis via a caspase-independent mechanism, executed by the apoptosis-inducing factor (AIF) and endonuclease G (EndoG), both of which can cleave DNA without the participation of caspases. Finally, crosstalk between death receptor-mediated and mitochondria-driven apoptosis can occur via cleavage and activation of Bid by caspase-8.
Mitochondria play a pivotal role in the regulation of apoptosis, being the principal players in the intrinsic pathway and housing many pro- and anti-apoptotic factors involved in the modulation of apoptosis [37]. One of the most studied mediators of apoptosis is cytochrome c, a small electron carrier associated with the IMM. When an event occurs that compromises the integrity and proper functioning of the mitochondria, cytochrome c can be released into the cytoplasm where it associates with apoptotic protease-activating factor-1 (Apaf-1), dATP, and procaspase-9, forming the apoptosome [37]. Within this macromolecular complex procaspase-9 is activated via homo-oligomerization and subsequently engages the effector caspase-3 [37]. Additional pro-apoptotic factors housed in the mitochondria include the second mitochondria-derived activator of caspases/direct inhibitor of apoptosis-binding protein with low pI (Smac/DIABLO) and heat requirement A2 protein (Omi/HtrA2), both of which can block the activity of the inhibitor of apoptosis proteins (IAPs), which in turn inhibit the activity of caspases [94]. Thus, release of Smac/DIABLO and Omi/HtrA2 provides another level of regulation which ensures complete activation of caspases for the execution of apoptosis.
Aside from their role in cytochrome c-mediated induction of cell death, mitochondria also participate in a pathway of apoptosis that does not involve caspase activation. Caspase-independent execution of apoptosis is carried out by the apoptosis-inducing factor (AIF) and endonuclease G (EndoG) [94]. AIF is a flavoprotein located within the mitochondrial intermembrane space that possesses NADH-oxidase activity and is required for the proper functioning of complex I [95]. Upon release from the mitochondria into the cytoplasm, AIF translocates to the nucleus where it binds to DNA to induce chromatin condensation [96]. AIF can also interact with cyclophilin A to form an active DNase responsible for large-scale DNA fragmentation [97]. Following an apoptotic stimulus, AIF may be released along with cytochrome c which activates the caspase cascade. However, AIF is thought to act independently of caspases, since Apaf-1 and caspase-9 knockouts still display apoptotic activity induced by AIF [98]. EndoG is a mitochondrion-specific nuclease, necessary for normal cell proliferation [99]. Under apoptotic conditions, EndoG is released from the mitochondria and enters the nucleus, where it participates in oligonucleosomal DNA fragmentation [100]. EndoG-mediated apoptosis is thought to occur independently of caspases since DNA fragmentation is still observed in caspase-activated DNase (CAD) knockouts and also in the presence of caspase inhibitors [100,101].
The critical factor for the initiation of mitochondria-mediated cell death is mitochondrial outer membrane permeabilization (MOMP), which is required for the release of pro-apoptotic factors residing within the intermembrane compartment [102]. While the exact mechanisms underlying MOMP are not fully understood, the mitochondrial apoptosis-induced channel (MAC) and the mPTP are regarded as prominent mediators [103].
The integrity of the outer mitochondrial membrane (OMM) is regulated by the Bcl-2 family of proteins which includes pro- (e.g., Bax, Bak, Bad, Bim, Bid, Puma, Noxa) and anti-apoptotic (e.g., Bcl-2, Bcl-XL, Bcl-w, Mcl-1, A1) members [104]. Under normal conditions, Bcl-2 and Bcl-XL block the activities of Bax and/or Bak [105]. In apoptotic conditions, Bax, normally present in a monomeric form in the cytosol, translocates to the mitochondria, undergoes oligomerization and inserts into the OMM in concentrated foci [106]. This forms a pore through which apoptotic factors stored in the intermembrane compartment are released [106]. Similarly, Bak, which is constitutively anchored to the OMM, can form homo-oligomeric complexes upon apoptotic stimulation [106]. Bax/Bak hetero-oligomers have also been described [107]. While there is still controversy about the identity of the pore formed by Bax and/or Bak, it is thought that this structure is functionally identical to what is known as the MAC and that the two are indeed the same entity [108]. The pro-apoptotic factors Bid and/or Bim are necessary to activate Bax and/or Bak, possibly through neutralizing Bcl-2 and Bcl-XL activity [109]. In addition, Bid allows crosstalk between extracellular and intracellular pathways of apoptosis, since it can be activated via cleavage by caspase-8 [110].
MOMP can also occur via mPTP opening [111]. The mPTP is a protein complex consisting of three main putative components: a voltage-dependent anion channel (VDAC) located in the OMM, the adenine nucleotide translocase (ANT) in the IMM, and cyclophilin D (CyPD) in the matrix [112]. Upon certain stimuli such as oxidative stress or calcium overload, CyPD becomes associated with the IMM and interacts with ANT and VDAC to form the mPTP [112]. Recent studies indicate that ANT [113] and VDAC [114] may be dispensable for the constitution of mPTP, although they may possess regulatory roles via interaction with Bcl-2 proteins [115]. On the contrary, CyPD appears to be required for permeability transition to occur [116]. Opening of the mPTP allows free diffusion of low-molecular weight (i.e., <1.5 kDa) solutes across the IMM. This results in a mitochondrial permeability transition (MPT) state where the IMM potential becomes dissipated, swelling of the mitochondria occurs, the proton motive force is lost, and subsequent uncoupling of oxidative phosphorylation and decreased ATP production ensue [117]. The OMM eventually becomes disrupted, resulting in MOMP and the release of apoptogenic factors from the intermembrane space [111].
Opening of the mPTP can be promoted by several factors, including elevated levels of oxidative [118] and nitrosative stress [119]. Furthermore, enhanced production of ROS and RNS may induce a pro-apoptotic shift in the expression pattern of Bcl-2 proteins (e.g., increased Bax-to-Bcl-2 ratio) [119,120]. Interestingly, crosstalk between mPTP and Bcl-2 proteins has been reported. In fact, Bid and Bax can promote mPTP opening [115,121], whereas Bcl-2 and Bcl-XL possess an inhibitory effect [122]. Because both MAC and mPTP can be regulated by the Bcl-2 family proteins, the interaction between the two mechanisms may allow a high level of regulation of MOMP and a more complete release of apoptotic factors to ensure cell death once appropriate signals have been triggered [108]. The involvement and importance of these pathways in apoptosis with aging and disuse in skeletal muscle remain to be determined.
5. Relevance of mitochondria-mediated apoptosis to sarcopenia
The aetiology of sarcopenia of aging is complex and not fully understood. Several factors have been identified which are thought to contribute to the development of age-related muscle loss (e.g., denervation, altered hormonal status, impaired muscle regeneration, altered protein turnover, increased levels of pro-inflammatory cytokines, oxidative damage) [1]. However, the relative impact of each contributing factor has not yet been established. Accumulating evidence suggests that enhanced activation of apoptosis takes place in aged skeletal muscle, likely contributing to the development of sarcopenia [123]. In this scenario, mitochondrial apoptotic signaling could be a central mechanism underlying the pathogenesis of age-related muscle loss [26].
As previously discussed, mitochondria-driven apoptosis can be executed with or without the participation of caspases. Studies indicate that both mechanisms may be operative during the development of sarcopenia [124], although recent evidence suggests that the caspase-independent pathway might play a more important role [14]. MOMP is required for the release of apoptogenic factors from the intermembrane space [102] and is mediated by the altered balance between pro- and anti-apoptotic members of the Bcl-2 family proteins [104] and opening of the mPTP [111]. Increased expression of Bax and reduced levels of Bcl-2 have been reported in skeletal muscles of old experimental animals [125–127]. However, other investigators demonstrated that the expression of both pro- and anti-apoptotic Bcl-2 family proteins increased in old muscles, with no changes in the Bax/Bcl-2 rheostat [14,128]. What is more, Baker and Hepple [129] found reduced levels of Bax and Bcl-2 mRNA in the plantaris muscle of old and senescent rats. These changes resulted in a 67% decline in the Bax-to-Bcl-2 ratio in the very old animals compared to younger controls. Interestingly, Rice and Blough [130] reported a different age-related pattern of Bcl-2 and Bax expression in rats depending on the muscle type. Bax content was increased at old age in the fast-twitch EDL, whereas no changes were apparent in the slow-twitch soleus. In contrast, both muscles exhibited an increased expression of Bcl-2 at advanced age. The elevation of Bcl-2 detected in aged muscles may be interpreted as a compensatory, yet imperfect action aimed at limiting myonuclei loss in the presence of strong pro-apoptotic pressure. However, increased expression of Bcl-2 in the gastrocnemius muscle of old mice was paralleled by enhanced serine-phosphorylation and subsequent inactivation of Bcl-2 [131], thereby abolishing its anti-apoptotic properties despite elevated expression [132].
Enhanced susceptibility towards mPTP opening has been demonstrated in aged skeletal muscles [30,31,133]. Notably, Seo et al. [31] observed that increased mPTP opening propensity in rat quadriceps muscle was associated with elevated mitochondrial levels of non-heme Fe and mtRNA oxidative damage. Furthermore, Marzetti et al. [14] found that mitochondrial levels of CyPD were elevated relative to ANT and VDAC in the gastrocnemius muscle of senescent rats. This finding further supports the involvement of mPTP opening in age-related MOMP, given the central role postulated for CyPD in the formation of the mPTP [116].
Following MOMP, apoptogenic factors housed in the mitochondrial intermembrane space are released, initiating the series of events that culminate in cell death [102]. Once in the cytosol, cytochrome c triggers the mitochondrial caspase-dependent apoptotic pathway through the sequential activation of caspase-9 and -3 [37]. Elevated cytosolic content of cytochrome c has been documented in skeletal muscle of old rats [128]. Accordingly, levels of Apaf-1 [10,128,134], active caspase-9 content [131,133,134], and caspase-9 proteolytic activity [128] were also found to be higher in aged muscles compared to young controls. However, other studies did not observe increases in cytosolic cytochrome c and active caspase-9 levels in skeletal muscles of aged rats [9,10]. In addition, Marzetti et al. [14] found that gastrocnemius levels of active caspase-9 were only transiently increased over the course of aging and were not correlated with the extent of apoptosis. Furthermore, Pistilli et al. [127] reported no changes in Apaf-1 and caspase-9 gene expression in the plantaris muscle of aged rats. Strikingly, it was observed that gene expression levels of Apaf-1 were even reduced in the plantaris of old and senescent rats relative to younger controls [129].
These observations question the relevance of the mitochondrial caspase-dependent mechanism of apoptosis in the pathogenesis of sarcopenia, suggesting that alternative pathways may be operative in the aging muscle. In this regard, it has been hypothesized that caspase-independent apoptosis might be particularly important in skeletal muscle, as it could allow for the elimination of individual myonuclei without subsequent dismantling of the entire fiber by caspases [25]. This idea is supported by several reports where the mitochondrial caspase-independent apoptotic pathway was activated in aged skeletal muscles [10,14,19,129]. Leeuwenburgh et al. [19], using an immunohistochemical approach, showed that EndoG co-localized with myonuclei in the soleus muscle of old rats, indicating that nuclear translocation of EndoG had occurred during aging. In addition, AIF gene expression progressively increased over the course of aging in rat plantaris muscle, such that a 50-fold increase was detected in senescent vs. young animals [129]. Furthermore, a correlation was evident between AIF gene expression and the progression of sarcopenia [129]. Similarly, an age-related increase in both cytosolic and nuclear levels of AIF and EndoG was observed in the rat gastrocnemius muscle [14]. Positive correlations were determined between the nuclear content of AIF and EndoG and the extent of DNA fragmentation. In contrast nuclear levels of both of these markers were negatively correlated with muscle mass, further suggesting a role for caspase-independent apoptosis in sarcopenia [14].
Despite the fact that mitochondrial apoptotic signaling is operative in skeletal muscle at advanced age, a direct link between myonuclear apoptosis and sarcopenia has yet to be proven. Indeed, the possibility exists that the apoptotic program may serve to eliminate dysfunctional nuclei and/or damaged muscle fibers, whose persistence would be detrimental for tissue homeostasis. Furthermore, establishing a mechanistic link between the execution of apoptosis and muscle loss is a difficult task. Indeed, virtually all of the molecules involved in the apoptotic program possess other functions in non-apoptotic conditions. This peculiarity limits the possibility of genetically manipulating apoptosis without simultaneously affecting other cellular processes. Since sarcopenia takes place over the course of a lifespan, manipulation of the expression and/or function of apoptotic mediators would result in long-term alterations of other cellular processes. Therefore, the final outcome could not be univocally attributed to changes in the apoptotic signaling rather than modifications of other processes. Hence, further research is necessary to better understand the role of myonuclear apoptosis in the pathogenesis of sarcopenia. In addition, further investigations are warranted to elucidate the relative contribution of caspase-dependent and independent mitochondrial apoptotic pathways to sarcopenia. Finally, a translational effort is required to verify whether and to what extent myonuclear apoptosis and mitochondrial apoptotic signaling are involved in the pathogenesis of sarcopenia in older persons.
6. The role of mitochondria-mediated apoptosis in disuse muscle atrophy
Acute muscle atrophy following spinal cord injury, microgravity, HS or immobilization results in the rapid loss of muscle mass [6], the recovery from which is impaired at old age [7]. Despite the large number of studies on disuse muscle atrophy, the exact mechanisms underlying muscle loss remain unclear. Decreased myonuclear number has been reported in various experimental models of muscle atrophy, with myonuclear apoptosis being potentially responsible [135]. Interestingly, current evidence indicates that distinct pathways of apoptosis may be activated depending on age, muscle type and disuse model employed.
Enhanced mitochondrial apoptotic signaling has been observed in experimental models of muscle denervation and in humans affected by neuromuscular disorders. Elevated caspase-9 expression was detected in patients with muscle atrophy due to peripheral neuropathy [136]. In the same disorder, Tews et al. [137] also identified increased expression of Smac/DIABLO, which was exclusively observed in atrophied fibers. Interestingly, some atrophic fibers co-expressed Smac/DIABLO and IAPs, suggesting that an anti-apoptotic adaptation had been attempted, which was counteracted by the up-regulation of Smac/DIABLO. Furthermore, changes in the expression of pro-apoptotic Bax and anti-apoptotic Bcl-2 were detected in skeletal muscles of patients with spinal muscular atrophy [138]. In addition, altered muscle expression of Bcl-2 and Bax was found in cases of polyneuropathy and amyotrophic lateral sclerosis [139]. Altered expression of the Bcl-2 family proteins was also reported in rat hind limb muscles following the severing of the sciatic and common peroneal nerves [23]. Denervation resulted in the up-regulation of Bax by 115% and a decrease in Bcl-2 by 89%, producing a 16-fold increase in the Bax-to-Bcl-2 ratio. This adaptation was accompanied by increased whole-muscle expression of AIF and a higher frequency of apoptotic myonuclei. Similarly, Siu and Alway [20] found an increased Bax-to-Bcl-2 ratio in the rat gastrocnemius muscle 2 weeks after tibial nerve transection, as a result of ~8-fold and ~3-fold elevation in Bax and Bcl-2, respectively. This coincided with enhanced apoptotic DNA fragmentation and activation of mitochondria-dependent apoptotic signaling, as evidenced by increased cytosolic levels of cytochrome c, Smac/DIABLO and AIF. In addition, both expression levels and catalytic activity of caspase-9 and -3 were elevated following denervation [20]. The potential role of mitochondrial apoptotic signaling in denervation-induced muscle atrophy was further highlighted by a later study from the same group, which showed attenuation in the extent of muscle loss and pro-apoptotic signaling following tibial nerve transection in Bax-deficient mice relative to wild-type controls [21]. Apoptotic DNA fragmentation increased in the gastrocnemius muscle of wild-type mice, but not in Bax(−/−) littermates. Furthermore, cytosolic levels of cytochrome c were ~2-fold higher in wild-type relative to Bax-deficient mice. Accordingly, increased activity of caspase-9 and -3 was only observed in wild-type animals, whereas no increase in the nuclear content of AIF was detected in either group. Interestingly, cytosolic levels of Smac/DIABLO increased to a similar extent in both wild-type and Bax(−/−) denervated mice, suggesting that mitochondrial release of this molecule may occur through a Bax-independent mechanism.
Importantly, muscle denervation has also been associated with increased susceptibility towards mPTP opening [23,140], indicating that this mechanism may participate in the pro-apoptotic environment of atrophying muscle. Furthermore, Csukly et al. [140] reported that the enhanced propensity to mPTP opening in rat denervated hind limb muscles was associated with increased CyPD content relative to ANT and VDAC. This observation suggests that alteration in the expression of mPTP components might predispose to MPT in muscle atrophying conditions.
Evidence indicates that mitochondrial apoptotic signaling may also be involved in muscle atrophy induced by unloading and immobilization. Two recent studies provoked skeletal muscle atrophy by 14 days of HS in the soleus (slow-twitch) [19] and the gastrocnemius (predominantly fast-twitch) [128] muscles of young and aged rats. Both studies reported decreased muscle mass following HS in both age groups. However, the degree of atrophy in the soleus muscle was highest in the young animals [19], in contrast to the gastrocnemius muscle where atrophy was more severe in the old rats [128]. The activation of apoptosis was confirmed by elevated levels of DNA fragmentation in both muscle types in all HS animals [19,128]. Interestingly, the highest levels of apoptosis were observed in both the gastrocnemius [128] and the soleus [19] muscles of aged rats, suggesting a marginal role for apoptosis in soleus muscle atrophy in young animals. Investigations into the pathways contributing to the increased apoptosis observed in acute atrophy revealed some striking differences depending on the age and muscle type. Siu et al. [128] found elevated cytosolic levels of AIF in the gastrocnemius muscle of old rats following 2 weeks of HS. In contrast, no increases in AIF release were detected in young HS animals. Cytosolic levels of cytochrome c were elevated in both HS groups, with a greater increase in the young rats (41% vs. 31%) [128]. However, no differences in the levels of Apaf-1, caspase-9, caspase-3 or Smac/DIABLO were detected in either young or aged rats following HS. Interestingly, Leeuwenburgh et al. [19] reported increased caspase-3 activity in the soleus muscle of young, but not old HS rats. Moreover, the mitochondrion-specific nuclease EndoG was found to co-localize with muscle fiber nuclei after 14 days of HS in the soleus muscle of old rats, whereas the amount of nuclear-located EndoG was unchanged in the young HS animals [19]. Given the increased levels of DNA fragmentation documented in the muscles of both young and old HS rats, it may be hypothesized that distinct apoptotic pathways are operative depending on age. More specifically, it appears that the caspase-independent pathway of apoptosis mediated by EndoG or AIF (or both) predominates in aged muscle, whereas a caspase-dependent mechanism may be selectively activated at young age.
To further elucidate the dynamics of mitochondrial apoptotic signaling during acute atrophy, a recent study investigated the temporal relationship between the occurrence of apoptosis and myonuclear translocation of EndoG in the soleus muscle of young rats subjected to short-term HS [22]. A significant decline in muscle fiber cross-sectional area was observed 2 days following HS, before any measurable decrease in muscle weight could be detected. Noteworthy, evidence of apoptosis was apparent as early as 12 h following HS, reaching a maximum at 2 days, which preceded the elevation in muscle atrophy F-box (MAFbx) mRNA (a marker for protein degradation). Interestingly, co-localization of EndoG with apoptotic myonuclei occurred 12 h after the initiation of HS [22]. The localization of EndoG within a single fiber was also interesting. EndoG was observed to co-localize with apoptotic nuclei, but was often found in the vicinity of nuclei not undergoing apoptosis. The “preference” of EndoG to certain nuclei could indicate a transportation limitation, having been released from nearby mitochondria. It further suggests that not all mitochondria were releasing EndoG and/or nuclei had to be somehow susceptible (e.g., oxidative damage to the nuclear membrane) in order for EndoG to permeate the nuclear membrane. Interestingly, activated caspase-3 was expressed in interstitial cells undergoing apoptosis, suggesting that in these cells apoptosis may be executed via a caspase-dependent pathway.
The involvement of caspase-independent apoptogenic factors in the early phases of disuse atrophy has recently been confirmed by Ferreira et al. [24]. These authors detected maximal AIF expression following 24 h HS in the murine soleus muscle, temporally coinciding with the highest degree of apoptotic DNA fragmentation. In contrast, activity of caspase-8 and caspase-3 was highest at 12 h, concomitant with a decrease in muscle protein concentration. This suggests a role for the caspase cascade in protein degradation rather than apoptosis. Further confirmation for the role of mitochondria-mediated apoptosis in acute muscle atrophy arises from the observation that signaling within this pathway is activated but is then downregulated during the recovery period following immobilization [141].
The involvement of myonuclear apoptosis in disuse muscle atrophy has recently been challenged by Bruusgaard and Gundersen [142], who reported no apoptotic loss of myonuclei in murine skeletal muscles atrophied for up to 28 days. Myonuclei were transfected with green fluorescent protein containing a nuclear localization tag and tracked using in vivo time-lapse microscopy. Unexpectedly, no myonuclear loss was observed in either slow- or fast-twitch muscles, despite a significant reduction in muscle cross-sectional area. Furthermore, terminal deoxynucleotidyl transferase biotin–dUTP nick-end labeling (TUNEL) analysis of approximately 27,000 myonuclei revealed only an exiguous number to be apoptotic.
As discussed earlier with regard to sarcopenia, causality between the activation of the apoptotic program and muscle atrophy has not been definitely established. Nevertheless, the concurrent attenuation of apoptosis and muscle loss observed in Bax(−/−) mice [21] strongly suggests a causal relationship between the execution of myonuclear apoptosis and denervation-induced muscle atrophy. Furthermore, activation of mitochondrial apoptotic signaling during the early phases of disuse muscle atrophy [22,24] suggests that this may serve to achieve a new balance between muscle size and the needs of the organism. From this perspective, myonuclear apoptosis during muscle atrophy and the incorporation of new nuclei during hypertrophy may be seen as fundamental mechanisms regulating myonuclear number and, hence, muscle plasticity [143]. Clearly further research is required to better comprehend the cellular mechanisms underlying disuse muscle atrophy and the role played by apoptosis in this process. In addition, a deeper understanding of the apoptotic pathways involved at different ages and in specific muscle types is imperative to design targeted and effective interventions to rescue skeletal myofibers during periods of prolonged disuse.
7. Conclusions
Several cellular mechanisms contribute to muscle loss during aging and disuse atrophy. Among them, myonuclear apoptosis has recently emerged as a potential factor involved in the pathophysiology of both sarcopenia and disuse muscle atrophy. Due to their central position in the execution of programmed cell death, mitochondria are acknowledged as critical mediators contributing to myonuclear apoptosis. Both caspase-dependent and independent mitochondrial apoptotic pathways are activated during muscle loss, each predominating according to age, nature of the atrophying condition and muscle type. However, despite important advances in the field of myocyte apoptosis, several fundamental questions remain unanswered: is there a causal relationship between the activation of the apoptotic program and muscle loss? Are myonuclei degraded in a stochastic fashion or are they selectively targeted? Likewise, are mitochondria randomly involved in triggering apoptosis or is this process only instigated by damaged or dysfunctional mitochondria? What is the impact of apoptosis on muscle loss relative to other cellular processes (e.g., altered protein turnover, impaired satellite cell function, dysfunctional autophagy)? What is the role of apoptosis in sarcopenia and disuse muscle atrophy in humans?
Future research will attempt to elucidate these and other yet unclear aspects concerning the relevance of apoptosis to muscle loss. This knowledge will likely allow the design of more effective therapeutic tools to combat sarcopenia and disuse muscle atrophy.
Acknowledgments
This research was supported by grants to CL (NIA R01-AG17994 and AG21042), CC (NIH R01-AG024526-02), ED (NIA R01-AG028925) and by the University of Florida Institute on Aging and Claude D. Pepper Older Americans Independence Center (1 P30AG028740). The authors wish to thank Dr. Michaela Santoro for her assistance in the preparation of this manuscript.
Glossary
- AIF
apoptosis-inducing factor
- ANT
adenine nucleotide translocase
- Apaf- 1
apoptotic protease-activating factor-1
- CAD
caspase-activated DNase
- Cox
cytochrome c oxidase
- CyPD
cyclophilin D
- DED
death effector domain
- EDL
extensor digitorum longus
- EndoG
endonuclease G
- ETC
electron transport chain
- FADD
Fas-associated death domain
- HS
hind limb suspension
- Omi/HtrA2
heat requirement A2 protein
- IAPs
inhibitor of apoptosis proteins
- IFM
intermyofibrillar mitochondria
- IMM
inner mitochondrial membrane
- IRPs
iron regulatory proteins
- ISCs
iron–sulfur clusters
- MAC
mitochondrial apoptosis-induced channel
- MAFbx
muscle atrophy F-box
- MOMP
mitochondrial outer membrane permeabilization
- MPT
mitochondrial permeability transition
- mPTP
mitochondrial permeability transition pore
- mtDNA
mitochondrial DNA
- mtRNA
mitochondrial RNA
- OMM
outer mitochondrial membrane
- PGC-1α
peroxisome proliferator-activated receptor-γ coactivator-1α
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
- Smac/DIABLO
second mitochondria-derived activator of caspases/direct inhibitor of apoptosis-binding protein with low pI
- SOD
superoxide dismutase
- SSM
subsarcolemmal mitochondria
- Tfam
transcription factor A
- TNF-R
tumor necrosis factor receptor
- TUNEL
terminal deoxynucleotidyl transferase biotin–dUTP nick-end labeling
- VDAC
voltage-dependent anion channel
References
- 1.Rolland Y, Czerwinski S, Van Kan AG, Morley JE, Cesari M, Onder G, Woo J, Baumgartner R, Pillard F, Boirie Y, Chumlea WM, Vellas B. Sarcopenia: its assessment, etiology, pathogenesis, consequences and future perspectives. J. Nutr. Health Aging. 2008;12:433–450. doi: 10.1007/BF02982704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roubenoff R. Sarcopenia: a major modifiable cause of frailty in the elderly. J. Nutr. Health Aging. 2000;4:140–142. [PubMed] [Google Scholar]
- 3.Rantanen T, Guralnik JM, Foley D, Masaki K, Leveille S, Curb JD, White L. Midlife hand grip strength as a predictor of old age disability. JAMA. 1999;281:558–560. doi: 10.1001/jama.281.6.558. [DOI] [PubMed] [Google Scholar]
- 4.Metter EJ, Talbot LA, Schrager M, Conwit R. Skeletal muscle strength as a predictor of all-cause mortality in healthy men. J. Gerontol. A, Biol. Sci. Med. Sci. 2002;57:B359–B365. doi: 10.1093/gerona/57.10.b359. [DOI] [PubMed] [Google Scholar]
- 5.Janssen I, Shepard DS, Katzmarzyk PT, Roubenoff R. The healthcare costs of sarcopenia in the United States. J. Am. Geriatr. Soc. 2004;52:80–85. doi: 10.1111/j.1532-5415.2004.52014.x. [DOI] [PubMed] [Google Scholar]
- 6.Edgerton VR, Roy RR, Allen DL, Monti RJ. Adaptations in skeletal muscle disuse or decreased-use atrophy. Am. J. Phys. Med. Rehabil. 2002;81:S127–S147. doi: 10.1097/00002060-200211001-00014. [DOI] [PubMed] [Google Scholar]
- 7.Zarzhevsky N, Carmeli E, Fuchs D, Coleman R, Stein H, Reznick AZ. Recovery of muscles of old rats after hindlimb immobilisation by external fixation is impaired compared with those of young rats. Exp. Gerontol. 2001;36:125–140. doi: 10.1016/s0531-5565(00)00189-3. [DOI] [PubMed] [Google Scholar]
- 8.Strasser H, Tiefenthaler M, Steinlechner M, Bartsch G, Konwalinka G. Urinary incontinence in the elderly and age-dependent apoptosis of rhabdosphincter cells. Lancet. 1999;354:918–919. doi: 10.1016/S0140-6736(99)02588-X. [DOI] [PubMed] [Google Scholar]
- 9.Dirks A, Leeuwenburgh C. Apoptosis in skeletal muscle with aging. Am. J. Physiol., Regul. Integr. Comp. Physiol. 2002;282:R519–R527. doi: 10.1152/ajpregu.00458.2001. [DOI] [PubMed] [Google Scholar]
- 10.Dirks AJ, Leeuwenburgh C. Aging and lifelong calorie restriction result in adaptations of skeletal muscle apoptosis repressor, apoptosis-inducing factor, X- linked inhibitor of apoptosis, caspase-3, and caspase-12. Free Radic. Biol. Med. 2004;36:27–39. doi: 10.1016/j.freeradbiomed.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 11.Phillips T, Leeuwenburgh C. Muscle fiber specific apoptosis and TNF-alpha signaling in sarcopenia are attenuated by life-long calorie restriction. FASEB J. 2005;19:668–670. doi: 10.1096/fj.04-2870fje. [DOI] [PubMed] [Google Scholar]
- 12.Whitman SA, Wacker MJ, Richmond SR, Godard MP. Contributions of the ubiquitin–proteasome pathway and apoptosis to human skeletal muscle wasting with age. Pflugers Arch. 2005;450:437–446. doi: 10.1007/s00424-005-1473-8. [DOI] [PubMed] [Google Scholar]
- 13.Pistilli EE, Jackson JR, Alway SE. Death receptor-associated pro-apoptotic signaling in aged skeletal muscle. Apoptosis. 2006;11:2115–2126. doi: 10.1007/s10495-006-0194-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marzetti E, Wohlgemuth SE, Lees HA, Chung HY, Giovannini S, Leeuwenburgh C. Age-related activation of mitochondrial caspase-independent apoptotic signaling in rat gastrocnemius muscle. Mech. Ageing Dev. 2008;129:542–549. doi: 10.1016/j.mad.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marzetti E, Groban L, Wohlgemuth SE, Lees HA, Lin M, obe HJ, Giovannini S, Leeuwenburgh C, Carter CS. Effects of short-term GH supplementation and treadmill exercise training on physical performance and skeletal muscle apoptosis in old rats. Am. J. Physiol., Regul. Integr. Comp. Physiol. 2008;294:R558–R567. doi: 10.1152/ajpregu.00620.2007. [DOI] [PubMed] [Google Scholar]
- 16.Marzetti E, Carter CS, Wohlgemuth SE, Lees HA, Giovannini S, Anderson B, Quinn LS, Leeuwenburgh C. Changes in IL-15 expression and death-receptor apoptotic signaling in rat gastrocnemius muscle with aging and life-long calorie restriction. Mech. Ageing Dev. 2009;130:272–280. doi: 10.1016/j.mad.2008.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jin H, Wu Z, Tian T, Gu Y. Apoptosis in atrophic skeletal muscle induced by brachial plexus injury in rats. J. Trauma. 2001;50:31–35. doi: 10.1097/00005373-200101000-00005. [DOI] [PubMed] [Google Scholar]
- 18.Alway SE, Degens H, Krishnamurthy G, Chaudhrai A. Denervation stimulates apoptosis but not Id2 expression in hindlimb muscles of aged rats. J. Gerontol. A, Biol. Sci. Med. Sci. 2003;58:687–697. doi: 10.1093/gerona/58.8.b687. [DOI] [PubMed] [Google Scholar]
- 19.Leeuwenburgh C, Gurley CM, Strotman BA, Dupont-Versteegden EE. Age-related differences in apoptosis with disuse atrophy in soleus muscle. Am. J. Physiol., Regul. Integr. Comp. Physiol. 2005;288:R1288–R1296. doi: 10.1152/ajpregu.00576.2004. [DOI] [PubMed] [Google Scholar]
- 20.Siu PM, Alway SE. Mitochondria-associated apoptotic signalling in denervated rat skeletal muscle. J. Physiol. 2005;565:309–323. doi: 10.1113/jphysiol.2004.081083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Siu PM, Alway SE. Deficiency of the Bax gene attenuates denervation-induced apoptosis. Apoptosis. 2006;11:967–981. doi: 10.1007/s10495-006-6315-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dupont-Versteegden EE, Strotman BA, Gurley CM, Gaddy D, Knox M, Fluckey JD, Peterson CA. Nuclear translocation of EndoG at the initiation of disuse muscle atrophy and apoptosis is specific to myonuclei. Am. J. Physiol., Regul. Integr. Comp. Physiol. 2006;291:R1730–R1740. doi: 10.1152/ajpregu.00176.2006. [DOI] [PubMed] [Google Scholar]
- 23.Adhihetty PJ, O’Leary MF, Chabi B, Wicks KL, Hood DA. Effect of denervation on mitochondrially mediated apoptosis in skeletal muscle. J. Appl. Physiol. 2007;102:1143–1151. doi: 10.1152/japplphysiol.00768.2006. [DOI] [PubMed] [Google Scholar]
- 24.Ferreira R, Neuparth MJ, Vitorino R, Appell HJ, Amado F, Duarte JA. Evidences of apoptosis during the early phases of soleus muscle atrophy in hindlimb suspended mice. Physiol. Res. 2008;57:601–611. doi: 10.33549/physiolres.931272. [DOI] [PubMed] [Google Scholar]
- 25.Dupont-Versteegden EE. Apoptosis in muscle atrophy: relevance to sarcopenia. Exp. Gerontol. 2005;40:473–481. doi: 10.1016/j.exger.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 26.Adhihetty PJ, O’Leary MF, Hood DA. Mitochondria in skeletal muscle: adaptable rheostats of apoptotic susceptibility. Exerc. Sport Sci. Rev. 2008;36:116–121. doi: 10.1097/JES.0b013e31817be7b7. [DOI] [PubMed] [Google Scholar]
- 27.Palmer JW, Tandler B, Hoppel CL. Biochemical properties of subsarcolemmal and interfibrillar mitochondria isolated from rat cardiac muscle. J. Biol. Chem. 1977;252:8731–8739. [PubMed] [Google Scholar]
- 28.Riva A, Tandler B, Lesnefsky EJ, Conti G, Loffredo F, Vazquez E, Hoppel CL. Structure of cristae in cardiac mitochondria of aged rat. Mech. Ageing Dev. 2006;127:917–921. doi: 10.1016/j.mad.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adhihetty PJ, Ljubicic V, Menzies KJ, Hood DA. Differential susceptibility of subsarcolemmal and intermyofibrillar mitochondria to apoptotic stimuli. Am. J. Physiol., Cell Physiol. 2005;289:C994–C1001. doi: 10.1152/ajpcell.00031.2005. [DOI] [PubMed] [Google Scholar]
- 30.Chabi B, Ljubicic V, Menzies KJ, Huang JH, Saleem A, Hood DA. Mitochondrial function and apoptotic susceptibility in aging skeletal muscle. Aging Cell. 2008;7:2–12. doi: 10.1111/j.1474-9726.2007.00347.x. [DOI] [PubMed] [Google Scholar]
- 31.Seo AY, Xu J, Servais S, Hofer T, Marzetti E, Wohlgemuth SE, Knutson MD, Chung HY, Leeuwenburgh C. Mitochondrial iron accumulation with age and functional consequences. Aging Cell. 2008;7:706–716. doi: 10.1111/j.1474-9726.2008.00418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jezek P, Hlavata L. Mitochondria in homeostasis of reactive oxygen species in cell, tissues, and organism. Int. J. Biochem. Cell Biol. 2005;37:2478–2503. doi: 10.1016/j.biocel.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 33.Ajioka RS, Phillips JD, Kushner JP. Biosynthesis of heme in mammals. Biochim. Biophys. Acta. 2006;1763:723–736. doi: 10.1016/j.bbamcr.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 34.Lili R, Diekert K, Kaut A, Lange H, Pelzer W, Prohl C, Kispal G. The essential role of mitochondria in the biogenesis of cellular iron–sulfur proteins. Biol. Chem. 1999;380:1157–1166. doi: 10.1515/BC.1999.147. [DOI] [PubMed] [Google Scholar]
- 35.Miller WL. Mitochondrial specificity of the early steps in steroidogenesis. J. Steroid Biochem. Mol. Biol. 1995;55:607–616. doi: 10.1016/0960-0760(95)00212-x. [DOI] [PubMed] [Google Scholar]
- 36.McBride HM, Neuspiel M, Wasiak S. Mitochondria: more than just a powerhouse. Curr. Biol. 2006;16:R551–R560. doi: 10.1016/j.cub.2006.06.054. [DOI] [PubMed] [Google Scholar]
- 37.Jeong SY, Seol DW. The role of mitochondria in apoptosis. BMB Rep. 2008;41:11–22. doi: 10.5483/bmbrep.2008.41.1.011. [DOI] [PubMed] [Google Scholar]
- 38.Harman D. The biologic clock: the mitochondria? J. Am. Geriatr. Soc. 1972;20:145–147. doi: 10.1111/j.1532-5415.1972.tb00787.x. [DOI] [PubMed] [Google Scholar]
- 39.Miquel J, Economos AC, Fleming J, Johnson JE., Jr Mitochondrial role in cell aging. Exp. Gerontol. 1980;15:575–591. doi: 10.1016/0531-5565(80)90010-8. [DOI] [PubMed] [Google Scholar]
- 40.Harman D. Aging: a theory based on free radical and radiation chemistry. J. Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 41.Yakes FM, Van HB. Mitochondrial DNA damage is more extensive and persists longer than nuclear DNA damage in human cells following oxidative stress. Proc. Nad. Acad. Sci. U. S. A. 1997;94:514–519. doi: 10.1073/pnas.94.2.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wei YH, Lee HC. Oxidative stress, mitochondrial DNA mutation, and impairment of antioxidant enzymes in aging. Exp. Biol. Med. (Maywood) 2002;227:671–682. doi: 10.1177/153537020222700901. [DOI] [PubMed] [Google Scholar]
- 43.Piko L, Hougham AJ, Bulpitt KJ. Studies of sequence heterogeneity of mitochondrial DNA from rat and mouse tissues: evidence for an increased frequency of deletions/additions with aging. Mech. Ageing Dev. 1988;43:279–293. doi: 10.1016/0047-6374(88)90037-1. [DOI] [PubMed] [Google Scholar]
- 44.Cortopassi GA, Arnheim N. Detection of a specific mitochondrial DNA deletion in tissues of older humans. Nucleic Acids Res. 1990;18:6927–6933. doi: 10.1093/nar/18.23.6927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yen TC, Su JH, King KL, Wei YH. Ageing-associated 5 kb deletion in human liver mitochondrial DNA. Biochem. Biophys. Res. Commun. 1991;178:124–131. doi: 10.1016/0006-291x(91)91788-e. [DOI] [PubMed] [Google Scholar]
- 46.Corral-Debrinski M, Horton T, Lott MT, Shoffner JM, Beal MF, Wallace DC. Mitochondrial DNA deletions in human brain: regional variability and increase with advanced age. Nat. Genet. 1992;2:324–329. doi: 10.1038/ng1292-324. [DOI] [PubMed] [Google Scholar]
- 47.Wanagat J, Cao Z, Pathare P, Aiken JM. Mitochondrial DNA deletion mutations colocalize with segmental electron transport system abnormalities, muscle fiber atrophy, fiber splitting, and oxidative damage in sarcopenia. FASEB J. 2001;15:322–332. doi: 10.1096/fj.00-0320com. [DOI] [PubMed] [Google Scholar]
- 48.Bua E, Johnson J, Herbst A, Delong B, McKenzie D, Salamat S, Aiken JM. Mitochondrial DNA-deletion mutations accumulate intracellularly to detrimental levels in aged human skeletal muscle fibers. Am. J. Hum. Genet. 2006;79:469–480. doi: 10.1086/507132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kujoth GC, Hiona A, Pugh TD, Someya S, Panzer K, Wohlgemuth SE, Hofer T, Seo AY, Sullivan R, Jobling WA, Morrow JD, Van Remmen H, Sedivy JM, Yamasoba T, Tanokura M, Weindruch R, Leeuwenburgh C, Proila TA. Mitochondrial DNA mutations, oxidative stress, and apoptosis in mammalian aging. Science. 2005;309:481–484. doi: 10.1126/science.1112125. [DOI] [PubMed] [Google Scholar]
- 50.Muller-Hocker J, Schneiderbanger K, Stefani FH, Kadenbach B. Progressive loss of cytochrome c oxidase in the human extraocular muscles in ageing—a cytochemical-immunohistochemical study. Mutat. Res. 1992;275:115–124. doi: 10.1016/0921-8734(92)90016-i. [DOI] [PubMed] [Google Scholar]
- 51.Aspnes LE, Lee CM, Weindruch R, Chung SS, Roecker EB, Aiken JM. Caloric restriction reduces fiber loss and mitochondrial abnormalities in aged rat muscle. FASEB J. 1997;11:573–581. doi: 10.1096/fasebj.11.7.9212081. [DOI] [PubMed] [Google Scholar]
- 52.Lee CM, Lopez ME, Weindruch R, Aiken JM. Association of age-related mitochondrial abnormalities with skeletal muscle fiber atrophy. Free Radic. Biol. Med. 1998;25:964–972. doi: 10.1016/s0891-5849(98)00185-3. [DOI] [PubMed] [Google Scholar]
- 53.Cao Z, Wanagat J, McKiernan SH, Aiken JM. Mitochondrial DNA deletion mutations are concomitant with ragged red regions of individual, aged muscle fibers: analysis by laser-capture microdissection. Nucleic Acids Res. 2001;29:4502–4508. doi: 10.1093/nar/29.21.4502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Conley KE, Marcinek DJ, Villarin J. Mitochondrial dysfunction and age. Curr. Opin. Clin. Nutr. Metab. Care. 2007;10:688–692. doi: 10.1097/MCO.0b013e3282f0dbfb. [DOI] [PubMed] [Google Scholar]
- 55.Zahn JM, Sonu R, Vogel H, Crane E, Mazan-Mamczarz K, Rabkin R, Davis RW, Becker KG, Owen AB, Kim SK. Transcriptional profiling of aging in human muscle reveals a common aging signature. PLoS Genet. 2006;2:e115. doi: 10.1371/journal.pgen.0020115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Reznick RM, Zong H, Li J, Morino K, Moore IK, Yu HJ, Liu ZX, Dong J, Mustard KJ, Hawley SA, Befroy D, Pypaert M, Hardie DG, Young LH, Shulman GI. Aging-associated reductions in AMP-activated protein kinase activity and mitochondrial biogenesis. Cell Metab. 2007;5:151–156. doi: 10.1016/j.cmet.2007.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Anderson R, Prolla T. PGC-1alpha in aging and anti-aging interventions. Biochim. Biophys. Acta. 2009 doi: 10.1016/j.bbagen.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wu Z, Puigserver P, Andersson U, Zhang C, Adelmant G, Mootha V, Troy A, Cinti S, Lowell B, Scarpulla RC, Spiegelman BM. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell. 1999;98:115–124. doi: 10.1016/S0092-8674(00)80611-X. [DOI] [PubMed] [Google Scholar]
- 59.Brunk UT, Terman A. The mitochondrial–lysosomal axis theory of aging: accumulation of damaged mitochondria as a result of imperfect autophagocy- tosis. Eur. J. Biochem. 2002;269:1996–2002. doi: 10.1046/j.1432-1033.2002.02869.x. [DOI] [PubMed] [Google Scholar]
- 60.Levine B, Klionsky DJ. Development by self-digestion: molecular mechanisms and biological functions of autophagy. Dev. Cell. 2004;6:463–477. doi: 10.1016/s1534-5807(04)00099-1. [DOI] [PubMed] [Google Scholar]
- 61.Dirks AJ, Hofer T, Marzetti E, Pahor M, Leeuwenburgh C. Mitochondrial DNA mutations, energy metabolism and apoptosis in aging muscle. Ageing Res. Rev. 2006;5:179–195. doi: 10.1016/j.arr.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 62.Gonzalvez F, Gottlieb E. Cardiolipin: setting the beat of apoptosis. Apoptosis. 2007;12:877–885. doi: 10.1007/s10495-007-0718-8. [DOI] [PubMed] [Google Scholar]
- 63.Paradies G, Petrosillo G, Pistolese M, Ruggiero FM. Reactive oxygen species affect mitochondrial electron transport complex I activity through oxidative cardiolipin damage. Gene. 2002;286:135–141. doi: 10.1016/s0378-1119(01)00814-9. [DOI] [PubMed] [Google Scholar]
- 64.Petrosillo G, Ruggiero FM, Di Venosa N, Paradies G. Decreased complex III activity in mitochondria isolated from rat heart subjected to ischemia and reperfusion: role of reactive oxygen species and cardiolipin. FASEB J. 2003;17:714–716. doi: 10.1096/fj.02-0729fje. [DOI] [PubMed] [Google Scholar]
- 65.Shidoji Y, Hayashi K, Komura S, Ohishi N, Yagi K. Loss of molecular interaction between cytochrome c and cardiolipin due to lipid peroxidation. Biochem. Biophys. Res. Commun. 1999;264:343–347. doi: 10.1006/bbrc.1999.1410. [DOI] [PubMed] [Google Scholar]
- 66.Petrosillo G, Ruggiero FM, Paradies G. Role of reactive oxygen species and cardiolipin in the release of cytochrome c from mitochondria. FASEB J. 2003;17:2202–2208. doi: 10.1096/fj.03-0012com. [DOI] [PubMed] [Google Scholar]
- 67.O’Leary MF, Hood DA. Effect of prior chronic contractile activity on mitochondrial function and apoptotic protein expression in denervated muscle. J. Appl. Physiol. 2008;105:114–120. doi: 10.1152/japplphysiol.00724.2007. [DOI] [PubMed] [Google Scholar]
- 68.Yajid F, Mercier JG, Mercier BM, Dubouchaud H, Prefaut C. Effects of 4 wk of hindlimb suspension on skeletal muscle mitochondrial respiration in rats. J. Appl. Physiol. 1998;84:479–485. doi: 10.1152/jappl.1998.84.2.479. [DOI] [PubMed] [Google Scholar]
- 69.Oishi Y, Ogata T, Yamamoto KI, Terada M, Ohira T, Ohira Y, Taniguchi K, Roy RR. Cellular adaptations in soleus muscle during recovery after hindlimb unloading. Acta Physiol. (Oxf) 2008;192:381–395. doi: 10.1111/j.1748-1716.2007.01747.x. [DOI] [PubMed] [Google Scholar]
- 70.Lawler JM, Song W, Demaree SR. Hindlimb unloading increases oxidative stress and disrupts antioxidant capacity in skeletal muscle. Free Radic. Biol. Med. 2003;35:9–16. doi: 10.1016/s0891-5849(03)00186-2. [DOI] [PubMed] [Google Scholar]
- 71.Liu MJ, Li JX, Lee KM, Oin L, Chan KM. Oxidative stress after muscle damage from immobilization and remobilization occurs locally and systemically. Clin. Orthop. Relat. Res. 2005:246–250. doi: 10.1097/01.blo.0000150464.29883.ca. [DOI] [PubMed] [Google Scholar]
- 72.Servais S, Letexier D, Favier R, Duchamp C, Desplanches D. Prevention of unloading-induced atrophy by vitamin E supplementation: links between oxidative stress and soleus muscle proteolysis? Free Radic. Biol. Med. 2007;42:627–635. doi: 10.1016/j.freeradbiomed.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Siu PM, Pistilli EE, Alway SE. Age-dependent increase in oxidative stress in gastrocnemius muscle with unloading. J. Appl. Physiol. 2008;105:1695–1705. doi: 10.1152/japplphysiol.90800.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hofer T, Marzetti E, Xu J, Seo AY, Gulec S, Knutson MD, Leeuwenburgh C, Dupont-Versteegden EE. Increased iron content and RNA oxidative damage in skeletal muscle with aging and disuse atrophy. Exp. Gerontol. 2008;43:563–570. doi: 10.1016/j.exger.2008.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Powers SK, Kavazis AN, McClung JM. Oxidative stress and disuse muscle atrophy. J. Appl. Physiol. 2007;102:2389–2397. doi: 10.1152/japplphysiol.01202.2006. [DOI] [PubMed] [Google Scholar]
- 76.Chua AC, Graham RM, Trinder D, Olynyk JK. The regulation of cellular iron metabolism. Crit. Rev. Clin. Lab. Sci. 2007;44:413–459. doi: 10.1080/10408360701428257. [DOI] [PubMed] [Google Scholar]
- 77.Levi S, Rovida E. The role of iron in mitochondrial function. Biochim. Biophys. Acta. 2008 doi: 10.1016/j.bbagen.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 78.Atamna H, Walter PB, Ames BN. The role of heme and iron-sulfur clusters in mitochondrial biogenesis, maintenance, and decay with age. Arch. Biochem. Biophys. 2002;397:345–353. doi: 10.1006/abbi.2001.2671. [DOI] [PubMed] [Google Scholar]
- 79.Ames BN, Liu J. Delaying the mitochondrial decay of aging with acetylcarnitine. Ann. N.Y. Acad. Sci. 2004;1033:108–116. doi: 10.1196/annals.1320.010. [DOI] [PubMed] [Google Scholar]
- 80.Vasquez-Vivar J, Kalyanaraman B, Kennedy MC. Mitochondrial aconitase is a source of hydroxyl radical. An electron spin resonance investigation. J. Biol. Chem. 2000;275:14064–14069. doi: 10.1074/jbc.275.19.14064. [DOI] [PubMed] [Google Scholar]
- 81.Kondo H, Miura M, Kodama J, Ahmed SM, Itokawa Y. Role of iron in oxidative stress in skeletal muscle atrophied by immobilization. Pflugers Arch. 1992;421:295–297. doi: 10.1007/BF00374844. [DOI] [PubMed] [Google Scholar]
- 82.Altun M, Edstrom E, Spooner E, Flores-Moralez A, Bergman E, Tollet-Egnell P, Norstedt G, Kessler BM, Ulfhake B. Iron load and redox stress in skeletal muscle of aged rats. Muscle Nerve. 2007;36:223–233. doi: 10.1002/mus.20808. [DOI] [PubMed] [Google Scholar]
- 83.Xu J, Knutson MD, Carter CS, Leeuwenburgh C. Iron accumulation with age, oxidative stress and functional decline. PLoS ONE 3. 2008:e2865. doi: 10.1371/journal.pone.0002865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jung SH, DeRuisseau LR, Kavazis AN, Deruisseau KC. Plantaris muscle of aged rats demonstrates iron accumulation and altered expression of iron regulation proteins. Exp. Physiol. 2008;93:407–414. doi: 10.1113/expphysiol.2007.039453. [DOI] [PubMed] [Google Scholar]
- 85.Marzetti E, Lees HA, Wohlgemuth SE, Leeuwenburgh C. Sarcopenia of aging: underlying cellular mechanisms and protection by calorie restriction. BioFactors. 2009;35:28–35. doi: 10.1002/biof.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Liang LP, Jarrett SG, Patel M. Chelation of mitochondrial iron prevents seizure-induced mitochondrial dysfunction and neuronal injury. J. Neurosci. 2008;28:11550–11556. doi: 10.1523/JNEUROSCI.3016-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kakhlon O, Manning H, Breuer W, Melamed-Book N, Lu C, Cortopassi G, Munnich A, Cabantchik ZI. Cell functions impaired by frataxin deficiency are restored by drug-mediated iron relocation. Blood. 2008;112:5219–5227. doi: 10.1182/blood-2008-06-161919. [DOI] [PubMed] [Google Scholar]
- 88.Whitnall M, Rahmanto YS, Sutak R, Xu X, Becker EM, Mikhael MR, Ponka P, Richardson DR. The MCK mouse heart model of Friedreich’s ataxia: alterations in iron-regulated proteins and cardiac hypertrophy are limited by iron chelation. Proc. Natl. Acad. Sci. U. S. A. 2008;105:9757–9762. doi: 10.1073/pnas.0804261105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br. J. Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Danial NN, Korsmeyer SJ. Cell death: critical control points. Cell. 2004;116:205–219. doi: 10.1016/s0092-8674(04)00046-7. [DOI] [PubMed] [Google Scholar]
- 91.Hengartner MO. The biochemistry of apoptosis. Nature. 2000;407:770–776. doi: 10.1038/35037710. [DOI] [PubMed] [Google Scholar]
- 92.Wang ZB, Liu YQ, Cui YF. Pathways to caspase activation. Cell Biol. Int. 2005;29:489–496. doi: 10.1016/j.cellbi.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 93.Nakagawa T, Yuan J. Cross-talk between two cysteine protease families. Activation of caspase-12 by calpain in apoptosis. J. Cell Biol. 2000;150:887–894. doi: 10.1083/jcb.150.4.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.van Loo G, Saelens X, van Gurp M, MacFarlane M, Martin SJ, Vandenabeele P. The role of mitochondrial factors in apoptosis: a Russian roulette with more than one bullet. Cell Death Differ. 2002;9:1031–1042. doi: 10.1038/sj.cdd.4401088. [DOI] [PubMed] [Google Scholar]
- 95.Vahsen N, Cande C, Briere JJ, Benit P, Joza N, Larochette N, Mastroberardino PG, Pequignot MO, Casares N, Lazar V, Feraud O, Debili N, Wissing S, Engelhardt S, Madeo F, Piacentini M, Penninger JM, Schagger H, Rustin P, Kroemer G. AIF deficiency compromises oxidative phosphorylation. EMBO J. 2004;23:4679–4689. doi: 10.1038/sj.emboj.7600461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Daugas E, Nochy D, Ravagnan L, Loeffler M, Susin SA, Zamzami N, Kroemer G. Apoptosis-inducing factor (AIF): a ubiquitous mitochondrial oxidoreductase involved in apoptosis. FEBS Lett. 2000;476:118–123. doi: 10.1016/s0014-5793(00)01731-2. [DOI] [PubMed] [Google Scholar]
- 97.Cande C, Vahsen N, Kouranti I, Schmitt E, Daugas E, Spahr C, Luban J, Kroemer RT, Giordanetto F, Garrido C, Penninger JM, Kroemer G. AIF and cyclophilin A cooperate in apoptosis-associated chromatinolysis. Oncogene. 2004;23:1514–1521. doi: 10.1038/sj.onc.1207279. [DOI] [PubMed] [Google Scholar]
- 98.Joza N, Susin SA, Daugas E, Stanford WL, Cho SK, Li CY, Sasaki T, Elia AJ, Cheng HY, Ravagnan L, Ferri KF, Zamzami N, Wakeham A, Hakem R, Yoshida H, Kong YY, Mak TW, Zuniga-Pflucker JC, Kroemer G, Penninger JM. Essential role of the mitochondrial apoptosis-inducing factor in programmed cell death. Nature. 2001;410:549–554. doi: 10.1038/35069004. [DOI] [PubMed] [Google Scholar]
- 99.Huang KJ, Ku CC, Lehman IR. Endonuclease G: a role for the enzyme in recombination and cellular proliferation. Proc. Natl. Acad. Sci. U. S. A. 2006;103:8995–9000. doi: 10.1073/pnas.0603445103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Li LY, Luo X, Wang X. Endonuclease G is an apoptotic DNase when released from mitochondria. Nature. 2001;412:95–99. doi: 10.1038/35083620. [DOI] [PubMed] [Google Scholar]
- 101.van Loo G, Schotte P, van Gurp M, Demol H, Hoorelbeke B, Gevaert K, Rodriguez I, Ruiz-Carrillo A, Vandekerckhove J, Declercq W, Beyaert R, Vandenabeele P. Endonuclease G: a mitochondrial protein released in apoptosis and involved in caspase-independent DNA degradation. Cell Death Differ. 2001;8:1136–1142. doi: 10.1038/sj.cdd.4400944. [DOI] [PubMed] [Google Scholar]
- 102.Chipuk JE, Bouchier-Hayes L, Green DR. Mitochondrial outer membrane permeabilization during apoptosis: the innocent bystander scenario. Cell Death Differ. 2006;13:1396–1402. doi: 10.1038/sj.cdd.4401963. [DOI] [PubMed] [Google Scholar]
- 103.Gogvadze V, Orrenius S, Zhivotovsky B. Multiple pathways of cytochrome c release from mitochondria in apoptosis. Biochim. Biophys. Acta. 2006;1757:639–647. doi: 10.1016/j.bbabio.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 104.Chao DT, Korsmeyer SJ. BCL-2 family: regulators of cell death. Annu. Rev. Immunol. 1998;16:395–419. doi: 10.1146/annurev.immunol.16.1.395. [DOI] [PubMed] [Google Scholar]
- 105.Antonsson B, Conti F, Ciavatta A, Montessuit S, Lewis S, Martinou I, Bernasconi L, Bernard A, Mermod JJ, Mazzei G, Maundrell K, Gambale F, Sadoul R, Martinou JC. Inhibition of Bax channel-forming activity by Bcl-2. Science. 1997;277:370–372. doi: 10.1126/science.277.5324.370. [DOI] [PubMed] [Google Scholar]
- 106.van Delft MF, Huang DC. How the Bcl-2 family of proteins interact to regulate apoptosis. Cell Res. 2006;16:203–213. doi: 10.1038/sj.cr.7310028. [DOI] [PubMed] [Google Scholar]
- 107.Zhou L, Chang DC. Dynamics and structure of the Bax–Bak complex responsible for releasing mitochondrial proteins during apoptosis. J. Cell Sci. 2008;121:2186–2196. doi: 10.1242/jcs.024703. [DOI] [PubMed] [Google Scholar]
- 108.Kinnally KW, Antonsson B. A tale of two mitochondrial channels, MAC and PTP, in apoptosis. Apoptosis. 2007;12:857–868. doi: 10.1007/s10495-007-0722-z. [DOI] [PubMed] [Google Scholar]
- 109.Chipuk JE, Green DR. How do BCL-2 proteins induce mitochondrial outer membrane permeabilization? Trends Cell Biol. 2008;18:157–164. doi: 10.1016/j.tcb.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Li H, Zhu H, Xu CJ, Yuan J. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell. 1998;94:491–501. doi: 10.1016/s0092-8674(00)81590-1. [DOI] [PubMed] [Google Scholar]
- 111.Crompton M. The mitochondrial permeability transition pore and its role in cell death. Biochem. J. 1999;341(Pt 2):233–249. [PMC free article] [PubMed] [Google Scholar]
- 112.Crompton M, Virji S, Ward JM. Cyclophilin-D binds strongly to complexes of the voltage-dependent anion channel and the adenine nucleotide translocase to form the permeability transition pore. Eur. J. Biochem. 1998;258:729–735. doi: 10.1046/j.1432-1327.1998.2580729.x. [DOI] [PubMed] [Google Scholar]
- 113.Kokoszka JE, Waymire KG, Levy SE, Sligh JE, Cai J, Jones DP, MacGregor GR, Wallace DC. The ADP/ATP translocator is not essential for the mitochondrial permeability transition pore. Nature. 2004;427:461–465. doi: 10.1038/nature02229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Baines CP, Kaiser RA, Sheiko T, Craigen WJ, Molkentin JD. Voltage-dependent anion channels are dispensable for mitochondrial-dependent cell death. Nat. Cell Biol. 2007;9:550–555. doi: 10.1038/ncb1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zamzami N, El Hamel C, Maisse C, Brenner C, Munoz-Pinedo C, Belzacq AS, Costantini P, Vieira H, Loeffler M, Molle G, Kroemer G. Bid acts on the permeability transition pore complex to induce apoptosis. Oncogene. 2000;19:6342–6350. doi: 10.1038/sj.onc.1204030. [DOI] [PubMed] [Google Scholar]
- 116.Baines CP, Kaiser RA, Purcell NH, Blair NS, Osinska H, Hambleton MA, Brunskill EW, Sayen MR, Gottlieb RA, Dorn GW, Robbins J, Molkentin JD. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature. 2005;434:658–662. doi: 10.1038/nature03434. [DOI] [PubMed] [Google Scholar]
- 117.Tsujimoto Y, Shimizu S. Role of the mitochondrial membrane permeability transition in cell death. Apoptosis. 2007;12:835–840. doi: 10.1007/s10495-006-0525-7. [DOI] [PubMed] [Google Scholar]
- 118.Rajesh KG, Sasaguri S, Suzuki R, Maeda H. Antioxidant MCI-186 inhibits mitochondrial permeability transition pore and upregulates Bcl-2 expression. Am. J. Physiol., Heart Circ. Physiol. 2003;285:H2171–H2178. doi: 10.1152/ajpheart.00143.2003. [DOI] [PubMed] [Google Scholar]
- 119.Marriott HM, Ali F, Read RC, Mitchell TJ, Whyte MK, Dockrell DH. Nitric oxide levels regulate macrophage commitment to apoptosis or necrosis during pneumococcal infection. FASEB J. 2004;18:1126–1128. doi: 10.1096/fj.03-1450fje. [DOI] [PubMed] [Google Scholar]
- 120.Mishra OP, Randis T, Ashraf QM, Delivoria-Papadopoulos M. Hypoxia-induced Bax and Bcl-2 protein expression, caspase-9 activation, DNA fragmentation, and lipid peroxidation in mitochondria of the cerebral cortex of newborn piglets: the role of nitric oxide. Neuroscience. 2006;141:1339–1349. doi: 10.1016/j.neuroscience.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 121.Marzo I, Brenner C, Zamzami N, Jurgensmeier JM, Susin SA, Vieira HL, Prevost MC, Xie Z, Matsuyama S, Reed JC, Kroemer G. Bax and adenine nucleotide translocator cooperate in the mitochondrial control of apoptosis. Science. 1998;281:2027–2031. doi: 10.1126/science.281.5385.2027. [DOI] [PubMed] [Google Scholar]
- 122.Zamzami N, Brenner C, Marzo I, Susin SA, Kroemer G. Subcellular and submitochondrial mode of action of Bcl-2-like oncoproteins. Oncogene. 1998;16:2265–2282. doi: 10.1038/sj.onc.1201989. [DOI] [PubMed] [Google Scholar]
- 123.Marzetti E, Leeuwenburgh C. Skeletal muscle apoptosis, sarcopenia and frailty at old age. Exp. Gerontol. 2006;41:1234–1238. doi: 10.1016/j.exger.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 124.Marzetti E, Lawler JM, Hiona A, Manini T, Seo AY, Leeuwenburgh C. Modulation of age-induced apoptotic signaling and cellular remodeling by exercise and calorie restriction in skeletal muscle. Free Radic. Biol. Med. 2008;44:160–168. doi: 10.1016/j.freeradbiomed.2007.05.028. [DOI] [PubMed] [Google Scholar]
- 125.Alway SE, Degens H, Krishnamurthy G, Smith CA. Potential role for Id myogenic repressors in apoptosis and attenuation of hypertrophy in muscles of aged rats. Am. J. Physiol., Cell Physiol. 2002;283:C66–C76. doi: 10.1152/ajpcell.00598.2001. [DOI] [PubMed] [Google Scholar]
- 126.Song W, Kwak HB, Lawler JM. Exercise training attenuates age-induced changes in apoptotic signaling in rat skeletal muscle. Antioxid. Redox Signal. 2006;8:517–528. doi: 10.1089/ars.2006.8.517. [DOI] [PubMed] [Google Scholar]
- 127.Pistilli EE, Siu PM, Alway SE. Molecular regulation of apoptosis in fast plantaris muscles of aged rats. J. Gerontol. A, Biol. Sci. Med. Sci. 2006;61:245–255. doi: 10.1093/gerona/61.3.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Siu PM, Pistilli EE, Alway SE. Apoptotic responses to hindlimb suspension in gastrocnemius muscles from young adult and aged rats. Am. J. Physiol., Regul. Integr. Comp. Physiol. 2005;289:R1015–R1026. doi: 10.1152/ajpregu.00198.2005. [DOI] [PubMed] [Google Scholar]
- 129.Baker DJ, Hepple RT. Elevated caspase and AIF gene expression correlate with progression of sarcopenia during aging in male F344BN rats. Exp. Gerontol. 2006;41:1149–1156. doi: 10.1016/j.exger.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 130.Rice KM, Blough ER. Sarcopenia-related apoptosis is regulated differently in fast and slow-twitch muscles of the aging F344/N x BN rat model. Mech. Ageing Dev. 2006;127:670–679. doi: 10.1016/j.mad.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 131.Braga M, Sinha Hikim AP, Datta S, Ferrini MG, Brown D, Kovacheva EL, Gonzalez-Cadavid NF, Sinha-Hikim I. Involvement of oxidative stress and caspase 2- mediated intrinsic pathway signaling in age-related increase in muscle cell apoptosis in mice. Apoptosis. 2008;13:822–832. doi: 10.1007/s10495-008-0216-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Rajah R, Lee KW, Cohen P. Insulin-like growth factor binding protein-3 mediates tumor necrosis factor-alpha-induced apoptosis: role of Bcl-2 phosphorylation. Cell Growth Differ. 2002;13:163–171. [PubMed] [Google Scholar]
- 133.Tamilselvan J, Jayaraman G, Sivarajan K, Panneerselvam C. Age-dependent upregulation of p53 and cytochrome c release and susceptibility to apoptosis in skeletal muscle fiber of aged rats: role of carnitine and lipoic acid. Free Radic. Biol. Med. 2007;43:1656–1669. doi: 10.1016/j.freeradbiomed.2007.08.028. [DOI] [PubMed] [Google Scholar]
- 134.Chung L, Ng YC. Age-related alterations in expression of apoptosis regulatory proteins and heat shock proteins in rat skeletal muscle. Biochim. Biophys. Acta. 2006;1762:103–109. doi: 10.1016/j.bbadis.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 135.Allen DL, Linderman JK, Roy RR, Bigbee AJ, Grindeland RE, Mukku V, Edgerton VR. Apoptosis: a mechanism contributing to remodeling of skeletal muscle in response to hindlimb unweighting. Am. J. Physiol. 1997;273:C579–C587. doi: 10.1152/ajpcell.1997.273.2.C579. [DOI] [PubMed] [Google Scholar]
- 136.Tews DS, Behrhof W, Schindler S. Expression patterns of initiator and effector caspases in denervated human skeletal muscle. Muscle Nerve. 2005;31:175–181. doi: 10.1002/mus.20253. [DOI] [PubMed] [Google Scholar]
- 137.Tews DS, Behrhof W, Schindler S. SMAC-expression in denervated human skeletal muscle as a potential inhibitor of coexpressed inhibitor-of-apoptosis proteins. Appl. Immunohistochem. Mol. Morphol. 2008;16:66–70. doi: 10.1097/PAI.0b013e318030b32e. [DOI] [PubMed] [Google Scholar]
- 138.Tews DS, Goebel HH. Apoptosis-related proteins in skeletal muscle fibers of spinal muscular atrophy. J. Neuropathol. Exp. Neurol. 1997;56:150–156. doi: 10.1097/00005072-199702000-00005. [DOI] [PubMed] [Google Scholar]
- 139.Tews DS, Goebel HH. DNA fragmentation and BCL-2 expression in infantile spinal muscular atrophy. Neuromuscul. Disord. 1996;6:265–273. doi: 10.1016/0960-8966(96)00018-1. [DOI] [PubMed] [Google Scholar]
- 140.Csukly K, Ascah A, Matas J, Gardiner PF, Fontaine E, Burelle Y. Muscle denervation promotes opening of the permeability transition pore and increases the expression of cyclophilin D. J. Physiol. 2006;574:319–327. doi: 10.1113/jphysiol.2006.109702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Vazeille E, Codran A, Claustre A, Averous J, Listrat A, Bechet D, Taillandier D, Dardevet D, Attaix D, Combaret L. The ubiquitin–proteasome and the mitochondria-associated apoptotic pathways are sequentially downregulated during recovery after immobilization-induced muscle atrophy. Am. J. Physiol., Endocrinol. Metab. 2008;295:E1181–E1190. doi: 10.1152/ajpendo.90532.2008. [DOI] [PubMed] [Google Scholar]
- 142.Bruusgaard JC, Gundersen K. In vivo time-lapse microscopy reveals no loss of murine myonuclei during weeks of muscle atrophy. J. Clin. Invest. 2008;118:1450–1457. doi: 10.1172/JCI34022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Allen DL, Monke SR, Talmadge RJ, Roy RR, Edgerton VR. Plasticity of myonuclear number in hypertrophied and atrophied mammalian skeletal muscle fibers. J. Appl. Physiol. 1995;78:1969–1976. doi: 10.1152/jappl.1995.78.5.1969. [DOI] [PubMed] [Google Scholar]