Abstract
Introduction
Older adults often show sustained attention toward positive information and an improved memory for positive events. Little is known about the neural changes that may underlie these effects, although recent research has suggested that older adults may show differential recruitment of prefrontal regions during the successful encoding of emotional information. In the present study, effective connectivity analyses examined the network of regions that college-age and older adults recruited during the encoding of positive and negative images.
Methods
Participants viewed positive and negative images while undergoing a functional magnetic resonance imaging (fMRI) scan. Structural equation modeling was used to compare young and older adults’ connectivity among regions of the emotional memory network while they encoded negative or positive items.
Results
Aging did not impact the connectivity among regions engaged during the encoding of negative information, but age differences did arise during the encoding of positive information. Most notably, in older adults, the ventromedial prefrontal cortex and amygdala strongly influenced hippocampal activity during the encoding of positive information. By contrast, in young adults, a strong thalamic influence on hippocampal activity was evident during encoding.
Conclusions
These findings suggest that older adults’ “positivity effect” may arise from age-related changes in the interactions between affect-processing regions and the hippocampus during the encoding of positive information.
Keywords: aging, connectivity, emotion, fMRI, memory, structural equation modeling
1. Introduction
Though many aspects of memory change with aging, within the realm of emotional memory, one of the most intriguing effects of age has been a “positivity effect” in memory. Compared to younger adults, proportionally more of what older adults remember is positive (reviewed by Mather and Carstensen, 2005). Though there is active debate about the generality of this effect (e.g., Murphy and Isaacowitz 2008), it also is clear that there are some circumstances that reliably elicit a positivity effect (reviewed by Mather, 2006). The positivity effect generally has been interpreted within the framework of Socioemotional Selectivity Theory (Carstensen et al., 1999); this theory states that when adults view time as limited (as occurs with advancing age), they are more likely to prioritize their emotional wellbeing. The positivity effect has been hypothesized to reflect the increased importance that older adults place on emotional gratification: Older adults are more motivated than young adults to process information in a manner that will provide them with emotional fulfillment, and because of this motivational change, older adults are more likely to attend toward, and to remember, positive information (reviewed by Mather and Carstensen, 2005; Carstensen and Mikels, 2005).
The hypothesis that older adults’ positivity effect in memory is tied to motivational changes in the processing of emotional information is a plausible one, particularly because the effect is noted most readily when older adults have extensive cognitive resources available to devote toward processing emotional information (Mather and Knight, 2005). The hypothesis is also consistent with a number of studies that have suggested an age-related shift away from amygdala-based emotion processing and toward prefrontal-based processing (where this prefrontal-based processing may reflect a more deliberative, controlled type of processing; e.g., Satpute and Lieberman, 2006). Most of the studies that have revealed these age-related changes in neural activity have examined the processing of facial expressions that convey negative emotion. In these studies, older adults tend to under-activate the amygdala and to over-activate the prefrontal cortex (as compared to young adults) when processing negative facial expressions (e.g., Fischer et al., 2005; Gunning-Dixon et al., 2003; Tessitore et al., 2005; Williams et al., 2006). A recent set of studies has suggested that this shift from amygdala-driven to prefrontal-based processing may also occur when older adults view negative photographs (rather than facial expressions), and that these changes may be tied to older adults’ poor memory for negative information (St. Jacques et al., 2009; St. Jacques et al., in press; see also Mather et al., 2004).
Because these studies only examined the processing of negative information, they could not clarify whether the age-related changes in neural activity are specific to negative valence or whether they extend to positive information as well. There is reason to believe that valence may be an important factor to consider, with a few studies suggesting that older adults’ recruitment of the amygdala may be stronger during the processing of positive as compared to negative information (e.g., Mather et al., 2004; Leclerc and Kensinger, 2008) and that their prefrontal recruitment may also be heightened during the presentation of positive as compared to negative information (e.g., Leclerc and Kensinger, 2008; Leclerc and Kensinger, in press). In fact, old age sometimes results in a valence reversal: whereas young adults often activate the amygdala and medial prefrontal cortex more strongly in response to negative as compared to positive items, older adults sometimes show the opposite pattern of activity (e.g., Mather et al., 2004; Leclerc and Kensinger, 2008).
These studies suggest that older adults’ processing of both positive and negative information may be altered, but that the alterations may diverge for the two valences of information: The strength of older adults’ recruitment of the amygdala and of medial prefrontal regions may be stronger for positive information than for negative information. It is not clear from this prior research whether, or how, these age-related changes connect to older adults’ “positivity effect” in memory. In particular, it is widely debated whether older adults’ “positivity effect” is best characterized as a shift toward the positive or as a shift away from the negative (see Murphy and Isaacowitz 2008). For example, do older adults remember proportionally more positive information because they attend to that information and process it more deeply than young adults or because they direct less attention and devote fewer processing resources toward negative information? Neuroimaging can provide a viable way to address this issue, by examining whether aging primarily impacts the processes recruited during the encoding of positive information or if it also influences the processes recruited during the encoding of negative information.
The present study used effective connectivity analyses of fMRI data to reveal how aging affects the connections among brain regions recruited during encoding of negative and positive items. We focus on age-related connectivity changes within a network of brain regions including the amygdala, the prefrontal cortex, and the hippocampus. These regions have been linked to the successful encoding of emotional information by neuroimaging studies that have used a “subsequent memory” paradigm (reviewed by Paller and Wagner, 2002). In such paradigms, neural activity during the encoding phase is sorted based upon whether the items are later remembered or later forgotten, and regions are considered to have a link to successful encoding if their activity is greater for items that are later remembered than for those that are later forgotten. A large literature has revealed a consistent network of regions that are implicated in the successful encoding of emotional information. Some of these regions seem to be recruited specifically when information is emotional (e.g., the orbitofrontal cortex and amygdala), whereas others serve a general role in the encoding of information with or without emotional content, though their activity may be modulated by the emotional salience of information (e.g., the hippocampus; see Hamann, 2001; Kensinger, 2009; LaBar and Cabeza, 2006 for reviews)., Our primary question was how aging would affect the connections between the amygdala, the prefrontal regions, and the hippocampus, a region that is essential for the successful encoding of long-term memories (see Postle, 2009 for recent review). We were especially interested in whether age-related changes in connectivity would be comparable whenever participants were processing emotional information (regardless of its valence) or whether the age-related differences would be more pronounced for one valence of information.
2. Methods
2.1 Participants
The participants comprised 17 younger adults (12 women) between 19 and 31 years of age and 20 older adults (13 women) between 61 and 80 years of age (see Table 1 for addition participant characteristics). All participants were right-handed, native English speakers, with no history of depression or other psychiatric or neurological disorder. All participants had scores on the Mini Mental Status Examination (Folstein et al., 1975) of greater than 27. No participant was taking any medication that would affect the central nervous system. Informed consent was obtained from all the participants in a manner approved by the Boston College and Massachusetts General Hospital Institutional Review Boards. Participants were compensated $75 for their participation.
Table 1.
Participant characteristics.
| Measure | Young | Older |
|---|---|---|
| Years Education | 15.2 (.38) | 16.8 (.32) |
| Digit Symbol Substitution | 71.4 (3.3) | 49.1 (2.2) |
| Vocabulary | .82 (.02) | .89 (.02) |
| Digit Span – Forward | 5.9 (.45) | 5.3 (.25) |
| Digit Span - Backward | 5.12 (.25) | 4.65 (.33) |
Note: Digit Symbol Substitution, Digit Span Backward, & Arithmetic from WAIS-III, Vocabulary reflects the percentage correct on the Shipley (1986) measure. All values represent the mean (SE) raw, non-standardized scores.
2.2 Materials
The photo objects used in the present study were a subset of those previously rated by a separate group of young and older adults on valence and arousal dimensions, using 9-point Likert-type scales (1 = negative valence or low arousal and 9 = positive valence or high arousal). One-third of the images selected were negative and high arousal (valence ratings less than 3.5, arousal ratings higher than 5), one-third were positive and high arousal (valence higher than 5.5, arousal higher than 5), and one-third were neutral (valence ratings between 3 and 6, arousal less than 5; see Leclerc and Kensinger, 2008 for more details on the stimuli). The participants in this study also rated the stimuli for valence and arousal, and their classifications of the stimuli generally agreed with those based upon prior ratings. In the rare instances where classifications did not agree (fewer than 2% of all items), those items were not included in the analyses.
2.3 Procedure
While in the fMRI scanner, participants viewed 108 positive, 108 negative, and 108 neutral photo objects for 1000 ms apiece. Images from each emotion category were pseudorandomly intermixed with one another. After each image was presented, an intertrial fixation cross was presented for a variable duration, ranging between 5 and 13 seconds, to provide jitter (Dale and Buckner, 1997). The order of the images, and the length of each intertrial fixation interval, was determined using a program that optimizes for detection of the hemodynamic response associated with each image (the optseq program available from http://www.nitrc.org/projects/optseq/). While viewing the photos, participants were asked to indicate whether each object would fit inside of a file cabinet drawer. This task ensured that participants were attending to each item but did not direct them to process its emotional relevance nor to memorize the items for a later memory task (no participant indicated that they were aware that a memory assessment would follow the scan), a set of conditions that facilitates the revelation of a positivity effect (see Mather, 2006). Once participants were settled outside of the scanner (after approximately a 20 min delay), they performed a recognition memory task. Participants were presented with a series of studied items intermixed with novel items; items were presented one at a time, and for each item, participants were asked to indicate whether it had been studied. The recognition test was self-paced, so that as soon as a participant made a response, the next item was presented. The present analyses focus only on the neural activity during the encoding of items that were later recognized successfully, so that age differences in connectivity could not be attributed to differences in the proportion of items that were successfully encoded (see Leclerc and Kensinger, 2008 for presentation of the neuroimaging data for all items, regardless of later memory performance). Because successful encoding was operationalized as the encoding of items that were later recognized, it is possible that a small proportion of items were mistakenly considered to be “successfully encoded” because participants correctly guessed about those items’ presentations.
2.4 Image Acquisition and Data Analysis
Images were acquired on a 3.0 Tesla Siemens Allegra MRI scanner. Stimuli were back-projected onto a screen in the scanner bore, and the participants viewed the images through an angled mirror attached to the head coil. Detailed anatomic images were acquired using a multiplanar rapidly acquired gradient echo sequence. Functional images were acquired using a T2*-weighted echo planar imaging sequence (TR = 3000 ms, TE = 30 ms, FOV = 200 mm; flip angle = 90 degrees). Twenty-eight axial-oblique slices (3.2 mm thickness, 0.6 mm skip between slices), aligned in a plane along the axis connecting the anterior commissure and the posterior commissure, were acquired in an interleaved fashion.
All preprocessing and data analysis were conducted within SPM2 (Wellcome Department of Cognitive Neurology). Standard preprocessing was performed on the functional data, including slice-timing correction, rigid body motion correction, normalization to the Montreal Neurological Institute template (resampling at 3-mm cubic voxels), and spatial smoothing (using a 7.6-mm full-width half maximum isotropic Gaussian kernel).
2.5 Effective Connectivity Analyses
In order to examine the interactions between the amygdala and other limbic and prefrontal regions during the processing of negative and positive items, structural equation modeling (SEM) was carried out using Lisrel software (Joreskog and Sorbom, 1993). Unlike simple correlations, SEM allows for a consideration of connections across multiple nodes of a network, and it provides information about the directionality of influences between different regions. The first step of the SEM analysis was to specify the anatomical model. Regions were included in the model if they were of theoretical relevance to emotional memory (see reviews by Hamann, 2001; LaBar and Cabeza, 2006; Phelps and LeDoux, 2005) and were revealed in a whole-brain analysis comparing activity elicited during the successful encoding of positive or negative information to the activity elicited during the unsuccessful encoding of that emotional information. This analysis was conducted collapsing across the age groups, so as to define the regions in an unbiased fashion with regard to age. Because of the contrast used to define the regions, all regions included in the model were related to memory for emotional items rather than to more general emotion processing. Note, however, that we did not require regions in this model to be implicated only in memory for emotional (and not neutral) information, because we expect there should be overlap between nodes of the emotional memory network and nodes of a more general-purpose mnemonic network that is not specific to emotional information (and see Hamann, 2001; LaBar and Cabeza, 2006 for discussion). The regions selected were: ventromedial prefrontal cortex (Talairach coordinates, x y z = 0, 39, −5; Brodmann area, BA 10/32), dorsomedial prefrontal cortex (x y z = 8, 61, 15; BA 10), left orbitofrontal cortex (x y z = −36, 44, −8; spanning BA 10/11/47), left amygdala (x y z = −28, −3, −12), left hippocampus (x y z = −36, −7, −23), thalamus (x y z = −6, −20, −2), and left fusiform gyrus (x y z = −46, −48, −18; BA 37; see Figure 1 for the anatomic model). Table 2 presents additional information on the pattern of activity revealed within these regions; this information was determined by ANOVAs, computed separately for each region, which compared the maximum signal change reached (summing across 4–8 sec post-stimulus onset) as a function of subsequent memory (remembered, forgotten), valence (positive, negative, neutral), and age (young, older adult). On the basis of anatomical research in nonhumans (e.g., Patterson and Schmidt, 2003; Swanson and Petrovich, 1998), an anatomical connectivity model was created to specify the anatomically plausible connections (including multi-synaptic connections) between the specified network nodes and the potential directions of those connections (McIntosh, 1999; Addis et al., 2007).
Figure 1.
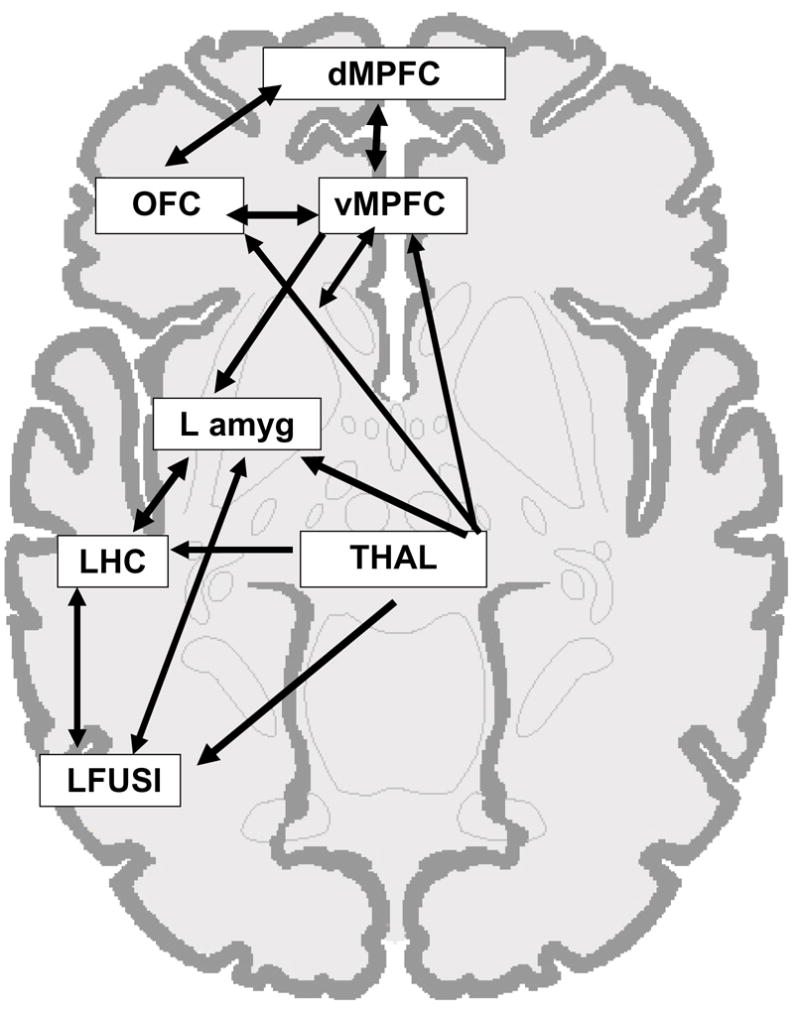
Anatomical model used in the SEM.
Table 2.
Pattern of activity in each of the regions included within the anatomical model.
| Region | Interaction between subsequent memory and age? (comments note group in which subsequent memory effect was stronger) | Interaction between subsequent memory and valence? (comments note valence(s) for which subsequent memory effect was stronger) | Valence main effect? | Interaction between valence and age? (comments note divergent effects of valence) |
|---|---|---|---|---|
| vmPFC (BA10/32) | Yes, OA > YA | Yes, pos and neg > neu | No | Yes, pos > neg in OA, neg > pos in YA |
| dmPFC (BA10) | Yes, OA > YA | Yes, pos and neg > neu | No | No |
| Left OFC (BA 10/11/47) | Yes, OA > YA | Yes, pos and neg > neu | No | Yes, pos > neg in OA, neg > pos in YA |
| Left amygdala | No | Yes, pos and neg > neu | No | No |
| Left hippocampus | Yes, YA > OA | Marginal, pos and neg > neu | No | No |
| Thalamus | No | No | No | No |
| Left fusiform gyrus (BA 37) | No | Yes, neg > pos and neu | No | No |
Note: Regions of interest were defined from a contrast analysis comparing activity to remembered and forgotten emotional items (collapsing across positive and negative valence, and collapsing across age group). R = remembered, F = forgotten, YA = young adult group, OA = older adult group. Neg = negative, Pos = positive, Neu = neutral. “Marginal” signifies significance at p<.10 and “Yes” signifies significance at p<.05.
Next, a functional model was constructed for each group. Signal change was extracted from all active voxels within a 5mm sphere (centered on the peak voxel) in each region of interest, using the MarsBar toolbox implemented within SPM2 (Brett, Anton, Valabregue, and Poline, 2002). The sum of the signal change across the 4–8sec period post-stimulus onset was extracted for remembered items only, and this signal change was extracted separately for each person, for each valence type, and for each region. We focus on the successful encoding of information (i.e., on signal change to subsequently remembered items) so that differences in the models cannot be accounted for by age differences in encoding effectiveness. Correlation matrices were then created separately for each valence and age group, revealing the correlation in signal change among the different regions of the emotional memory network (e.g., revealing how these regions were correlated in young adults during successful encoding of negative items).
Two different SEM analyses were conducted to examine whether there are age-related changes in the effective connections among regions during the successful encoding of (1) negative information and (2) positive information. For each SEM analysis, the relevant functional model (a matrix of correlations between extracted signal change values from all regions) was entered for each age-group. Estimates of path coefficients, indicating the strength and direction of the effect, were then calculated based upon these correlations. Significant differences across the groups were then assessed using the stacked-model approach (McIntosh and Gonzalez-Lima, 1994). In an omnibus test, a null model was first constructed in which the path coefficients from both age-groups were set to be equal. The fit of this null model was compared to the fit of an alternate model, in which the path coefficients were allowed to differ across the age-groups. The goodness-of-fit χ2 value was computed for each of these models, and directly compared to determine if one model was a significantly better fit than the other. If the alternate model fit better than the null model, this indicated the presence of a significant group difference. To determine which connections significantly contributed to the increased fit of the alternate model (i.e. those that decreased the p-value associated with the χ2 diff), individual connections were allowed to vary in a stepwise manner. Because the order in which connections were allowed to vary could influence those which emerged as significant, we allowed connections to vary in four different orders (beginning with anterior connections and moving posteriorly; beginning with posterior connections and moving anteriorly; beginning with subcortical connections and moving cortically, and beginning with cortical connections and moving subcortically), and we used the model that best fit the data (which was the model that began by allowing posterior connections to vary).
3. Results
3.1 Behavioral Results
Corrected recognition scores were computed for each valence type (positive, negative, neutral) by subtracting the false alarm rate for that category of items from the corresponding hit rate (e.g., subtracting the false alarm rate to negative pictures from the hit rate to negative pictures; see Table 3 for the hit, false alarm, and corrected recognition rates). An ANOVA conducted on these corrected recognition scores, with age group as a between-subjects factor and valence as a within-subjects factor, revealed a main effect of valence (F(2, 34) = 11.6, p<.001, partial eta-squared = .41) and an interaction between valence and age group (F(2, 34) = 4.7, p<.05, partial eta-squared = .22): As in previous studies using these stimuli (e.g., Kensinger, Garoff-Eaton, and Schacter, 2007), young adults remembered the negative stimuli better than the neutral or positive stimuli, whereas older adults remembered the positive and negative stimuli equally well, and better than the neutral stimuli. Note that older adults’ memory for positive items also was relatively preserved as compared to the young adults, while their memory for negative and neutral items was significantly poorer than that of the young adults.
Table 3.
Mean (SE) hit, false alarm (FA), and corrected recognition (CR; hit – false alarm) rates as a function of item valence and age.
| positive | negative | neutral | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Hit | FA | CR | Hit | FA | CR | Hit | FA | CR | |
| Young | .75 (.02) | .11 (.02) | .63 (.02) | .80 (.02) | .12 (.02) | .67 (.03) | .75 (.03) | .12 (.02) | .62 (.03) |
| Older | .73 (.03) | .08 (.02) | .65 (.02) | .73 (.03) | .09 (.02) | .63 (.02) | .68 (.02) | .12 (.01) | .56 (.02) |
Response time (RT) also was measured, during both encoding and retrieval. Not surprisingly, older adults were slower than young adults during both phases (average RT for older adults was 1510ms during encoding and 2570ms during retrieval; average RT for young adults was 1120ms during encoding and 1860ms during retrieval). However, there was no effect of valence on response times, and there was no interaction between valence and age group (p > .15). Thus, any neural differences revealed during the processing of the positive, negative, and neutral items should not arise from differences in RT.
3.2 Effective Connectivity Results
For the successful encoding of negative items, SEM analysis revealed that the null model could not be rejected (p>.20), suggesting that the connectivity among regions was comparable in the young and older adults. Of particular interest here, both age groups showed evidence of strong positive connections among prefrontal regions (see green arrows in bottom panel of Figure 2), and between the amygdala, hippocampus, and fusiform gyrus (see yellow arrows in bottom panel of Figure 2).
Figure 2.
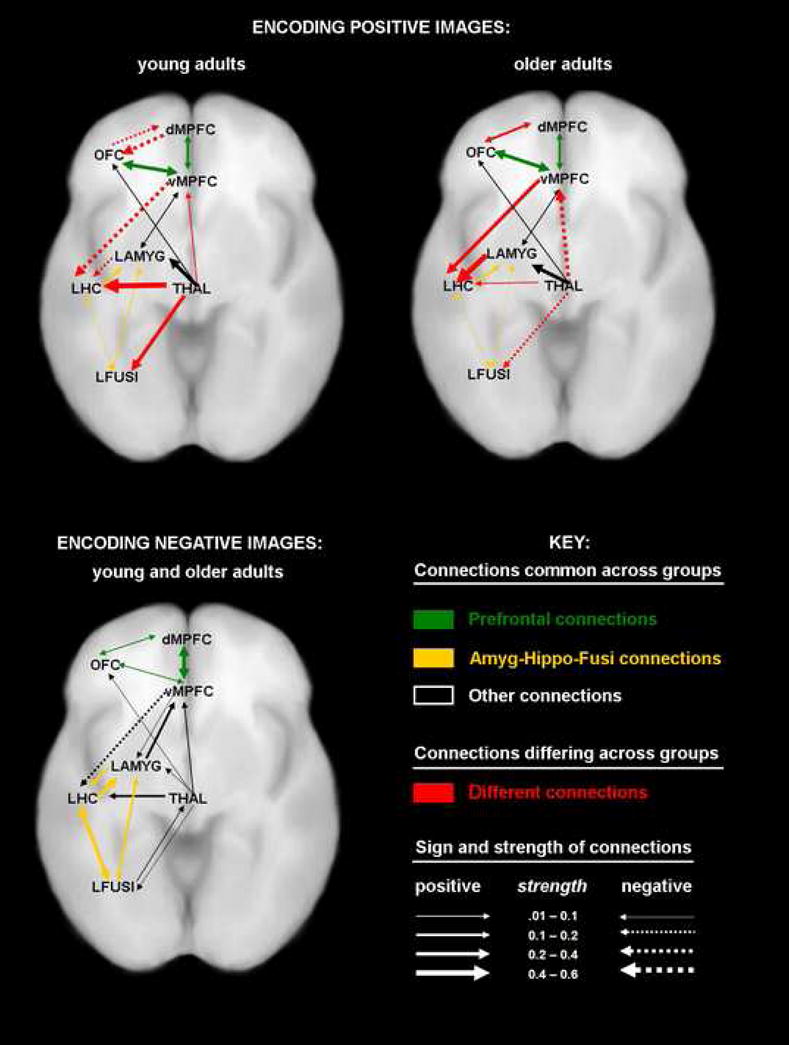
Aging influences the effective connections during the successful encoding of positive images (at top), but not during the successful encoding of negative images (at bottom)
For the positive items, SEM analysis revealed that the alternate model was a significantly better fit than the null model (p<.001). This finding reflected significant age-group differences in seven connections: the reciprocal connections between dorsomedial and orbitofrontal PFC, the influence of ventromedial PFC on the hippocampus, the influence of the amygdala on the hippocampus, and the influences the thalamus exerts on ventromedial PFC, fusiform gyrus, and the hippocampus. The top panel of Figure 2 depicts the connections for the young and the older adults, with the regions that differ between the age groups depicted in red (solid lines reveal positive connections and dotted lines signify negative connections). Of greatest interest here are the differences in hippocampal connectivity. In the young adults, the ventromedial PFC and the amygdala both have negative connections with the hippocampus, while the thalamus exhibits a strong, positive influence on the hippocampus. By contrast, in the older adults, both the ventromedial PFC and amygdala have strong, positive connections with the hippocampus during the successful encoding of positive information, suggesting strong modulation of the hippocampus by these affect-processing regions.
4. Discussion
Though older adults often show a “positivity effect” in memory (Mather and Carstensen, 2005), it has been unclear what neural changes may underlie this shift toward the positive. Of particular relevance to the current findings, there has been debate about whether the “positivity effect” primarily represents a change in how negative information is processed or whether it can also be driven by changes in the processing of positive information. The present study revealed fundamental changes in regional effective connectivity as young and older adults encoded positive items. In older adults, hippocampal activity was strongly and positively modulated by activity in affect-processing regions including the amygdala and the ventromedial PFC. In contrast, hippocampal activity in young adults was negatively influenced by these regions, and instead hippocampal activity during successful encoding in young adults was modulated by the thalamus. There were no significant age-related changes in effective connectivity during the encoding of negative items; both age groups showed strong connectivity between the hippocampus, amygdala, and fusiform gyrus and between the dorsomedial, ventromedial, and orbital prefrontal cortex.
Affect-related modulation of mnemonic processes has long been proposed to be a critical contributor to the memory enhancement enjoyed by emotional stimuli (see recent reviews by Phelps, 2004; LaBar and Cabeza, 2006). Young adults often show enhanced connectivity between the hippocampus and the amygdala as they encode negative information (e.g., Kilpatrick et al., 2003), and the strength of connectivity between these regions can be a strong predictor of subsequent memory for emotional information (e.g., Kensinger and Corkin, 2004). The present results indicate that older adults benefit from this affective modulation of hippocampal processes as well. This finding does not necessitate that older adults should remember negative information as well as younger adults - because of changes in the efficiency or efficacy of older adults’ hippocampal binding (see Dennis et al., 2008; Duverne et al., in press), it is plausible that the same patterns of hippocampal connectivity in young and older adults could nevertheless lead to superior levels of memory performance for negative items in the young adults. This point is an important one, because it indicates that even when older adults have poorer memory for negative information than young adults, this does not necessitate that there is a disruption of the connectivity between affect processing and mnemonic regions.
Although some prior discussions of age-related changes in emotion processing and emotional memory have focused on older adults’ inability to recruit the amygdala while processing negative information (e.g., Mather et al., 2004), or to their weakened connections between the amygdala and the hippocampus during the encoding of negative information (e.g., St. Jacques et al., 2009), the present results suggest that there are instances in which older adults’ affective modulation of hippocampal processes is not mitigated for negative information. Because of the dearth of data examining the neural processes supporting emotional memory in an older population, it is difficult to know what variables may account for the divergent findings, but one possible difference relates to whether the encoding task directs participants’ attention to the affective nature of the stimuli. The studies that have revealed reduced activity in the amygdala during older adults’ processing of negative photographs have required the older adults to explicitly judge the valence or the arousal of the stimuli (e.g., Mather et al., 2004; St. Jacques et al., 2009). By contrast, the size judgment used in the present study did not require participants to focus on the emotional salience of the information. It is possible that age-related changes in the processing of negative information are more notable when the affective content of the stimuli must be consciously processed and verbalized.
In the present study, older adults’ affective modulation of the hippocampus existed not only for negative information but also for positive information, whereas young adults’ modulation was limited to the encoding of negative items. These results suggest that older (but not younger) adults may exhibit mnemonic benefits for positive items because only they benefit from positive connections between affect-processing regions and the hippocampus. However, older adults’ benefits for positive information may also stem from changes in connectivity beyond the hippocampus. There were interesting distinctions in the connectivity among prefrontal regions when young and older adults were encoding positive information. Older adults showed strong positive connectivity among the vmPFC, dmPFC and the OFC, whereas young adults showed an inverse relation between the dmPFC and OFC. These results may suggest that older adults have greater synergy among different types of affective processing, perhaps including self-referential processing (often reliant on medial PFC processes; e.g., Burgess et al., 2007; Northoff and Bermpohl, 2004) and affective-evaluative processing (often associated with OFC activity; e.g., Rolls, 2004).
Though future research will be needed to examine the reasons for this positive connectivity in older adults, one possibility is that older adults may be more likely than young adults to process positive information in a self-relevant manner or to consider how that information makes them feel (discussed by Kensinger and Leclerc, in press), perhaps allowing them to capitalize on links between medial PFC processing and more general mnemonic processing (see Gutchess et al., 2008 for discussion of self-referential processing in older adults). These changes in information processing could be tied to motivational changes that occur with aging; as noted in the introduction, older adults often have an increased desire to find emotional gratification in daily activities, and for this reason they may be more likely to process positive information in a manner that connects that information to themselves. The present study was not designed to isolate the thought processes that may be tied to these changes in older adults’ prefrontal connectivity, but this would appear to be a fruitful avenue for future research.
When considering the pattern of connectivity revealed here, it is worthwhile to note that in the present study (as well as in some prior research, Charles et al., 2003; Kensinger et al., 2007), older adults showed preserved memory for positive information but poorer memory for negative and neutral information as compared to young adults. Reductions in episodic memory, and in episodic encoding processes more specifically, are very consistent findings within the cognitive aging literature (e.g., Logan et al., 2002; Nielsen-Bohlman and Knight, 1995). The present data suggest that older adults may be able to overcome these encoding deficits when they receive a unique boost from affective modulation of hippocampal processes, as occurs with positive information. By contrast, for negative items (which benefit from such modulation in both young and older adults), there is no unique benefit conferred upon older adults, and thus their memory levels cannot reach those displayed by young adults.
It also is worthwhile to consider that these age-related differences in effective connectivity arose even though the present analyses were restricted to activity during the successful encoding of positive items. Therefore, these differences in connectivity cannot be explained by failures to encode the stimuli. Rather, what these results suggest is that there are basic differences in the types of processes that underlie young and older adults’ successful encoding of positive items. When older adults remember positive images, strong affective modulation of hippocampal processes seems to be an essential contributor. By contrast, when young adults remember positive images, other processes, including thalamic modulation of hippocampal and fusiform processes, appear to contribute disproportionately.
As described in the introduction, much of the research into the “positivity effect” has situated the findings within the motivation framework of Socioemotional Selectivity Theory (Carstensen et al., 1999; Mather and Carstensen, 2005). Many of these discussions have focused on how older adults respond to, regulate, and reappraise negative experiences so as to make them less aversive (e.g., Gross et al., 1997; Labouvie-Vief and Medler, 2002; Lawton, et al,, 1992). While older adults can engage in these types of processes to alter their experiences with negative information, in the present study, age-related changes in effective connectivity were apparent only during the successful encoding of positive items and not during the successful encoding of negative items. This pattern of results could be consistent with motivational theories of the “positivity effect”: changes in how older adults encode positive information could be tied to their increased desire to maximize positive affect. However, the results suggest that the motivational effects – at least as they relate to subsequent memory performance - can be tied to age-related changes in the processing of positive information rather than to changes in older adults’ regulation of negative affect. Future research will be required to clarify the basis for the alterations in older adults’ processing of positive information, but the present research provides an important first step by revealing that there are situations in which older adults’ “positivity effect” arises not from changes in how negative information is processed, but rather from changes in how positive information is encoded. Of course, while the present results suggest that there are not fundamental age-related changes in the set of processes that lead young and older adults to remember negative information, future research will be needed to more carefully investigate whether there are instances in which older adults’ “positivity effect” is best characterized as a disruption in the circuitry recruited to encoding negative information (see St. Jacques et al., 2009 for discussion).
In sum, the results of the present study indicate that older adults’ “positivity effect” can be rooted in changes in the processes engaged during the initial encoding of emotional information. While there are no age-related differences in the connectivity of the network supporting encoding of negative items, age-related differences are evident during the successful encoding of positive information, when only older adults show affective modulation of hippocampal mnemonic processes.
Acknowledgments
This research was supported by grants from the National Institutes of Health (MH080833) and the Searle Scholars program (to E.A.K.). The authors thank Lisa Feldman Barrett, Angela Gutchess, Heathery Urry, Robert Waldinger, and Chris Wright for helpful discussion.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Addis DR, Moscovitch M, McAndrews MP. Consequences of hippocampal damage across the autobiographical memory network. Brain. 2007;130:2327–2342. doi: 10.1093/brain/awm166. [DOI] [PubMed] [Google Scholar]
- Brett M, Anton J-L, Valabregue R, Poline J-B. Region of interest analysis using an SPM toolbox. NeuroImage. 2002;16:497. [Google Scholar]
- Burgess PW, Gilbert SJ, Dumontheil I. Function and localization within rostral prefrontal cortex (area 10) Philos Trans R Soc Lond B: Biol Sci. 2007;29:887–899. doi: 10.1098/rstb.2007.2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carstensen LL, Isaacowitz D, Charles ST. Taking time seriously: A theory of socioemotional selectivity. The American Psychologist. 1999;54:165–181. doi: 10.1037//0003-066x.54.3.165. [DOI] [PubMed] [Google Scholar]
- Carstensen LL, Mikels JA. At the intersection of emotion and cognition: Aging and the positivity effect. Current Directions in Psychological Science. 2005;14:117–121. [Google Scholar]
- Charles ST, Mather M, Carstensen LL. Aging and emotional memory: The forgettable nature of negative images for older adults. Journal of Experimental Psychology: General. 2003;132:310–324. doi: 10.1037/0096-3445.132.2.310. [DOI] [PubMed] [Google Scholar]
- Comblain C, D’Argembeau A, Van der Linden M. Phenomenal characteristics of autobiographical memories for emotional and neutral events in older and younger adults. Experimental Aging Research. 2005;31:173–189. doi: 10.1080/03610730590915010. [DOI] [PubMed] [Google Scholar]
- Dale AM, Buckner RL. Selective averaging of rapidly presented individual trials using fMRI. Human Brain Mapping. 1997;5:329–340. doi: 10.1002/(SICI)1097-0193(1997)5:5<329::AID-HBM1>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Dennis NA, Hayes SM, Prince SE, Madden DJ, Huettel SA, Cabeza R. Effects of aging on the neural correlates of successful item and source memory encoding. J Exp Psychol Learn Mem Cogn. 2008;34:791–808. doi: 10.1037/0278-7393.34.4.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duverne S, Motamedinia S, Rugg MD. The relationship between aging, performance, and the neural correlates of successful memory encoding. Cereb Cortex. doi: 10.1093/cercor/bhn122. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer H, Sandblom J, Gavazzeni J, Fransson P, Wright CI, Backman L. Age differential patterns of brain activation during perception of angry faces. Neuroscience Letters. 2005;386:99–104. doi: 10.1016/j.neulet.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-mental state: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatry Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Gross JJ, Carstensen LL, Pasupathi M, Tsai J, Skorpen CG, Hsu AYC. Emotion and aging: Experience, expression, and control. Psychology and Aging. 1997;12:590–599. doi: 10.1037//0882-7974.12.4.590. [DOI] [PubMed] [Google Scholar]
- Gunning-Dixon FM, Gur RC, Perkins AC, Schroder L, Turner T, Turetsky BI, et al. Age-related differences in brain activation during emotional face processing. Neurobiology of Aging. 2003;24:285–295. doi: 10.1016/s0197-4580(02)00099-4. [DOI] [PubMed] [Google Scholar]
- Gutchess AH, Kensinger EA, Schacter DL. Aging, self-referencing, and medial prefrontal cortex. Social Neuroscience. 2007;2:117–133. doi: 10.1080/17470910701399029. [DOI] [PubMed] [Google Scholar]
- Hamann S. Cognitive and neural mechanisms of emotional memory. Trends Cogn Sci. 2001;5:394–400. doi: 10.1016/s1364-6613(00)01707-1. [DOI] [PubMed] [Google Scholar]
- Joreskog KG, Sorbom D. LISREL 8: Users’ reference guide. Chicago: Scientific Software International; 1993. [Google Scholar]
- Kennedy Q, Mather M, Carstensen LL. The role of motivation in the age- related positivity effect in autobiographical memory. Psychological Science. 2004;15:208–214. doi: 10.1111/j.0956-7976.2004.01503011.x. [DOI] [PubMed] [Google Scholar]
- Kensinger EA. Remembering the details: Effects of emotion. Emotion Review. doi: 10.1177/1754073908100432. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kensinger EA, Corkin S. Two routes to emotional memory: Distinct neural processes for valence and arousal. Proceedings of the National Academy of Sciences, USA. 2004;101:3310–3315. doi: 10.1073/pnas.0306408101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kensinger EA, Garoff-Eaton RJ, Schacter DL. Effects of emotion on memory specificity in young and older adults. J Gerontol B Psychol Sci Soc Sci. 2007;62:208–15. doi: 10.1093/geronb/62.4.p208. [DOI] [PubMed] [Google Scholar]
- Kensinger EA, Leclerc CM. Age-related changes in the neural mechanisms supporting emotion processing and emotional memory. European Journal of Cognitive Psychology. in press. [Google Scholar]
- Kensinger EA, Schacter DL. Neural processes supporting young and older adults’ emotional memories. J Cogn Neurosci. 2008;20:1161–73. doi: 10.1162/jocn.2008.20080. [DOI] [PubMed] [Google Scholar]
- Kilpatrick L, Cahill L. Amygdala modulation of parahippocampal and frontal regions during emotionally influenced memory storage. Neuroimage. 2003;20:2091–2099. doi: 10.1016/j.neuroimage.2003.08.006. [DOI] [PubMed] [Google Scholar]
- LaBar KS, Cabeza R. Cognitive neuroscience of emotional memory. Nat Rev Neurosci. 2006;7:54–64. doi: 10.1038/nrn1825. [DOI] [PubMed] [Google Scholar]
- Labouvie-Vief G, Medler M. Affect optimization and affect complexity: Modes and styles of regulation in adulthood. Psychology and Aging. 2002;17:571–588. doi: 10.1037//0882-7974.17.4.571. [DOI] [PubMed] [Google Scholar]
- Lawton MP, Kleban MH, Rajagopal D, Dean J. Dimensions of affective experience in three age groups. Psychology and Aging. 1992;7:171–184. doi: 10.1037//0882-7974.7.2.171. [DOI] [PubMed] [Google Scholar]
- Leclerc CM, Kensinger EA. Age-related differences in medial prefrontal activation in response to emotional images. Cogn Affect Behav Neurosci. 2008;8:153–64. doi: 10.3758/cabn.8.2.153. [DOI] [PubMed] [Google Scholar]
- Leclerc CM, Kensinger EA. Neural processing of emotional pictures and words: A comparison of young and older adults. Developmental Neuropsychology. doi: 10.1080/87565641.2010.549864. in press. [DOI] [PubMed] [Google Scholar]
- Logan JM, Sanders AL, Snyder AZ, Morris JC, Buckner RL. Under- recruitment and nonselective recruitment: dissociable neural mechanisms associated with aging. Neuron. 2002;33:827–840. doi: 10.1016/s0896-6273(02)00612-8. [DOI] [PubMed] [Google Scholar]
- Mather M. Why memories may become more positive with age. In: Uttl B, Ohta N, Siegenthaler AL, editors. Memory and emotion: Interdisciplinary perspectives. Blackwell Publishing; 2006. pp. 135–158. [Google Scholar]
- Mather M, Canli T, English T, Whitfield S, Wais P, Ochsner K, et al. Amygdala responses to emotionally valenced stimuli in older and younger adults. Psychological Science. 2004;15:259–263. doi: 10.1111/j.0956-7976.2004.00662.x. [DOI] [PubMed] [Google Scholar]
- Mather M, Carstensen LL. Aging and motivated cognition: the positivity effect in attention and memory. Trends Cogn Sci. 2005;9:496–502. doi: 10.1016/j.tics.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Mather M, Knight M. Goal-directed memory: The role of cognitive control in older adults’ emotional memory. Psychology and Aging. 2005;20:554–570. doi: 10.1037/0882-7974.20.4.554. [DOI] [PubMed] [Google Scholar]
- McIntosh AR, Gonzalez-Lima F. Network interactions among limbic cortices, basal forebrain, and cerebellum differentiate a tone conditioned as a Pavlovian excitor or inhibitor: fluorodeoxyglucose mapping and covariance structural modeling. J Neurophysiol. 1994;72:1717–1733. doi: 10.1152/jn.1994.72.4.1717. [DOI] [PubMed] [Google Scholar]
- McIntosh AR. Mapping cognition to the brain through neural interactions. Memory. 1999;7:523–548. doi: 10.1080/096582199387733. [DOI] [PubMed] [Google Scholar]
- Murphy NA, Isaacowitz DM. Preferences for emotional information in older and younger adults: a meta-analysis of memory and attention tasks. Psychol Aging. 2008;23:263–86. doi: 10.1037/0882-7974.23.2.263. [DOI] [PubMed] [Google Scholar]
- Nielsen-Bohlman L, Knight RT. Prefrontal alterations during memory processing in aging. Cereb Cortex. 1995;5:541–549. doi: 10.1093/cercor/5.6.541. [DOI] [PubMed] [Google Scholar]
- Northoff G, Bermpohl F. Cortical midline structures and the self. Trends Cogn Sci. 2004;8:102–107. doi: 10.1016/j.tics.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Paller KA, Wagner AD. Observing the transformation of experience into memory. Trends Cogn Sci. 2002;6:93–102. doi: 10.1016/s1364-6613(00)01845-3. [DOI] [PubMed] [Google Scholar]
- Patterson DW, Schmidt LA. Neuroanatomy of the human affective system. Brain Cogn. 2003;52:24–26. doi: 10.1016/s0278-2626(03)00005-8. [DOI] [PubMed] [Google Scholar]
- Phelps EA. Human emotion and memory: interactions of the amygdala and hippocampal complex. Curr Opin Neurobiol. 2004;14:198–202. doi: 10.1016/j.conb.2004.03.015. [DOI] [PubMed] [Google Scholar]
- Phelps EA, LeDoux JE. Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron. 2005;48 :175–187. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Postle B. The Hippocampus, Memory, and Consciousness. In: Laureys S, Tononi G, editors. The Neurology of Consciousness. Elsevier Press; 2009. pp. 326–338. [Google Scholar]
- Rolls ET. The functions of the orbitofrontal cortex. Brain Cogn. 2004;55:11–29. doi: 10.1016/S0278-2626(03)00277-X. [DOI] [PubMed] [Google Scholar]
- Satpute AB, Lieberman MD. Integrating automatic and controlled processes into neurocognitive models of social cognition. Brain Research. 2006;1079:86–97. doi: 10.1016/j.brainres.2006.01.005. [DOI] [PubMed] [Google Scholar]
- St Jacques PL, Dolcos F, Cabeza R. Effects of aging on functional connectivity of the amygdala for subsequent memory of negative pictures: a network analysis of functional magnetic resonance imaging data. Psychol Sci. 2009;20 :74–84. doi: 10.1111/j.1467-9280.2008.02258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Jacques PL, Dolcos F, Cabeza R. Effects of aging on functional connectivity of the amygdala during negative evaluation: A network analysis of fMRI data. Neurobiology of Aging. doi: 10.1016/j.neurobiolaging.2008.03.012. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessitore A, Hariri AR, Fera F, Smith WG, Das S, Weinberger DR, Mattay VS. Functional changes in the activity of brain regions underlying emotion processing in the elderly. Psychiatry Research. 2005;139:9–18. doi: 10.1016/j.pscychresns.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Petrovich GD. What is the amygdala? Trends Neurosci. 1998;21:323–31. doi: 10.1016/s0166-2236(98)01265-x. [DOI] [PubMed] [Google Scholar]
- Williams LM, Brown KJ, Palmer D, Liddell BJ, Kemp AH, Olivieri G, Peduto A, Gordon E. The mellow years? Neural basis of improving emotional stability over age. Journal of Neuroscience. 2006;26:6422–6430. doi: 10.1523/JNEUROSCI.0022-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]


