Abstract
We used event-related potentials to investigate how aging affects local contextual processing. Local context was defined as the occurrence of a short predictive series of visual stimuli before delivery of a target event. Stimuli were presented to either the left or right visual field and consisted of 15% targets (downward facing triangle) and 85% of equal numbers of three types of standards (triangles facing left, upwards and right). Recording blocks consisted of targets preceded by either randomized sequences of standards or by sequences including a three-standard predictive sequence signaling the occurrence of a subsequent target event. Subjects pressed a button in response to targets. Predictive local context affected target detection by reducing the duration of stimulus evaluation compared to detection of non-predictive random targets comparably for both young and older adults, as shown by a P3b latency shift. The peak of an earlier latency context positivity, which was seen only in the predicted compared to the random target condition, was prolonged in the older population compared to young adults. Finally, older subjects elicited a late sustained positivity in the predictive condition, not seen in the younger subjects. Taken together, theses findings suggest that local contextual effects on target detection processes are altered with age.
Keywords: aging, context, P3b, EEG, context positivity
1. Introduction
Contextual processing is essential for the performance of cognitive functions (Braver at al., 2002; 2005) and enables extraction of relevant environmental information to guide our behavior in facilitating the selection of an appropriate task-specific response. For example, we process local contextual information every time we drive our car and see a traffic light turn from green to yellow to red. Utilizing this sequence of events allows us to choose the appropriate response to hit the brakes and to stop our vehicle.
Evidence from neuropsychological, event-related potential (ERP) and neuroimaging studies supports a key role of the prefrontal cortex (PFC) in contextual processing (Barcelo and Knight, 2007; Barch et al., 2001; Huettel et al., 2005; MacDonald et al., 2000). The central proposition is that information such as task instructions, a cue or the processing of preceding sequential stimuli, are maintained in the PFC to facilitate appropriate response to salient target stimuli (Cohen and Servan-Schreiber, 1992; Huettel et al., 2005; MacDonald et al., 2000).
Normal aging is associated with changes in higher cognitive functions (Braver et al., 2002, 2005; Cerella, 1985; Sliwinski and Buschke, 1999; West and Schwarb, 2006) and these changes may be due in part to a decline in efficient processing of contextual information (Bayen et al., 2000; Braver at al., 2002, 2005; Smith et al., 1998; West and Schwarb, 2006). It is known that older adults have specific deficits in processing certain types of contextual information, that are manifested as impairments in activation and updating of context (Braver at al., 2005) and deficits in the integration of context and item (Bayen et al., 2000), specifically in self-initiated effortful integration of contextual information (Smith et al., 1998). These selective deficits in context processing are proposed to be associated with age-related changes in prefrontal cortex function (Braver and Barch, 2002; de Keyser et al., 1990; Raz et al., 1997).
Contextual processing has been linked to the P300 component of the ERP (Barcelo and Knight, 2007; Donchin and Coles, 1988; Polich and Criado, 2006; Poulsen et al., 2005; Squires et al., 1976). The extant literature supports the notion that hypotheses about the environment are continuously generated as a function of incoming information (Donchin and Coles, 1988), and that the target P300 ERP component (P3b) provides a measure of the evaluation of environmental signals as a function of context (Squires et al., 1976). There is evidence to suggest that local contextual processing affects the P3b. A recent study demonstrated that predictive local context reduces the duration of stimulus evaluation of predictive targets compared to random targets, as seen by a P3b latency shift (Fogelson et al., 2009). In this study it was also reported that local predictability was associated with a context positivity, which occurred earlier than the conventional P3b but had a similar scalp distribution. The authors proposed that this context positivity may reflect an early template match process related to early stages of target detection processes or of preparatory attention (Squires et al., 1973; Chao et al., 1995; Donchin and Coles, 1988; Polich 2003; Polich and Criado, 2006). There is evidence that maintaining target templates in working memory and preparatory processes are impaired in elderly subjects (Dujardin et al., 1993; Fabiani et al., 1998; Fjell and Walhovd, 2001; West and Scwarb, 2006; West and Travers, 2008). Thus, it is likely that electrophysiological measures of early latency target detection processes, such as the context positivity, are affected by age.
The P3b component has been shown to be affected by age, such that decreases in amplitude and delays in latency are often observed with increasing age (Ander et al., 1996; Dujardin et al., 1993; Fjell and Walhovd, 2001; Ford et al., 1982; Polich, 1996). Another consistent finding is a shift in P3b activation from posterior to anterior cortical areas in the elderly population compared with young adults who show a traditional posterior scalp P3b (Anderer et al., 1996; Daffner et al., 2005; Dujardin et al., 1993; Fabiani et al., 1998; Fjell and Walhovd, 2001; West and Travers, 2008). This age-related change in P3b topography has been linked to frontal cortex dysfunction (Fabiani et al., 1998). The hypothesis is that elderly subjects have more difficulties in automating the processing of infrequent target stimuli, requiring that PFC dependent working-memory regions are constantly engaged (Dujardin et al., 1993; Fabiani et al., 1998; Fjell and Walhovd, 2001; Daffner et al., 2005). It is important to note that aging has been associated primarily with changes in late ERP components (such as the P300) that are related to late controlled stages of information processing, while early ERP components, related to early perceptual stages of information processing, are not as sensitive to the effects of aging (Dujardin et al., 1993; Looren de Jong et al., 1988; Polich, 1996). Post-target detection processes have also been shown to be affected by age. For example, there is evidence that older subjects have difficulties in reaching confident decisions compared to younger adults (Ford et al., 1979; Looren de Jong et al., 1988).
The aim of the present study was to use electrophysiological measures to explore the effects of aging on processing of local contextual information. Local context was defined as the occurrence of a short predictive series of visual stimuli before the delivery of a target event. We used the P3b, and the difference wave between predicted and random targets to examine the effects of a predictive sequence on local contextual processing in aging, employing a variant of a previously reported paradigm (Fogelson et al., 2009). This study identified three main neural correlates of contextual processing. First, we observed a P3b latency shift between predicted and random targets, associated with faster reaction times. Second, we reported a newly described context positivity (CP) derived from the difference wave between predictive target detection compared to random target detection. Third, local contextual processing was associated with the generation of a robust P3b to the final most-informative stimulus of the predicting sequence. The objective of the current study was to examine the effect of aging on these neural correlates of local contextual processing. We also used the N1 ERP to measure early perceptual processing, predicting that any changes observed in local contextual processing between the age groups will be cognitive rather than perceptual (Dujardin et al., 1993; Fogelson et al., 2009; Polich, 1996). Finally, we examined the effect of age on late latency (400–800 msec post-stimulus onset) processes of post-target detection, and whether these were specific to conditions of local context-dependent target detection.
2. Method
2.1 Subjects
Twelve young adults (mean age = 24 years, 6 females) and eleven older adults (mean age = 65, 6 females) participated in the study. All the subjects were right-handed, had normal vision and had no history of psychiatric or neurological problems. Subjects were consented prior to being tested and were paid for their participation. The Committee for the Protection of Human Subjects for University of California, Berkeley approved the study.
2.2 Task
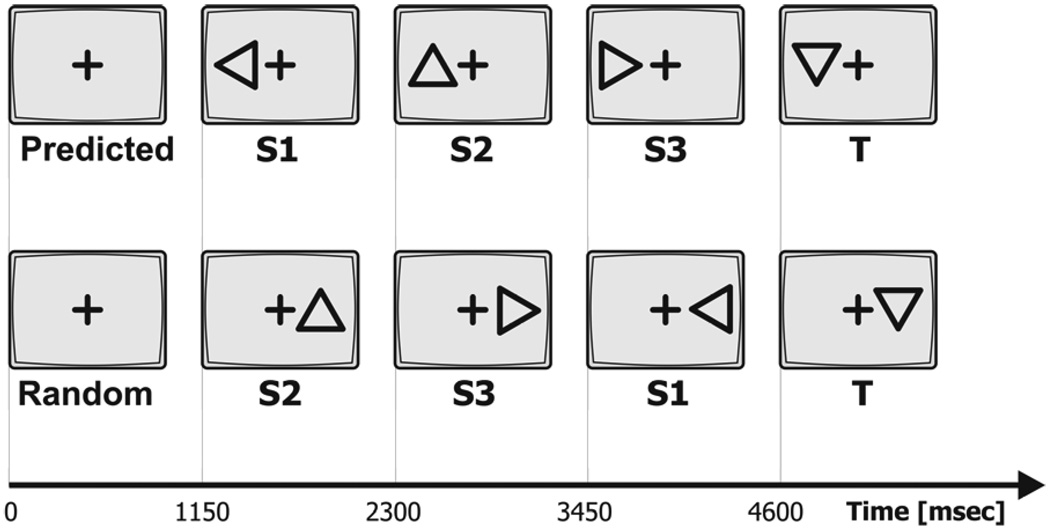
Subjects sat in a sound attenuated booth 110 cm in-front of a 21-inch PC-computer screen. Stimuli were presented to either the left or right visual field 6 degrees from fixation. The subject was asked to centrally fixate throughout the recording. Stimuli consisted of approximately 15% targets (downward facing triangle) and 85% of equal numbers of three types of standards (triangles facing left, upwards and right). In each block a total of 78 stimuli (12 targets, 22 of each standard type) were presented each for 150 msec and ISI of 1 sec. Recording blocks consisted of targets preceded by either randomized sequences of standards or by sequences including a three-standard predictive sequence. The predictive sequence always consisted of the three standards of triangles facing left, up and right, always in that order. Figure 1 illustrates an example of randomized and predicted sequences. Each block consisted of 6 different randomized sequences of standards (3–8 standards long) preceding the target; and 6 sequences of standards (3–8 standards long) with a predictive sequence preceding the target in each. Each recording session consisted of 14 different blocks, displayed in randomized order, each approximately 1.6 minutes long. Blocks were counterbalanced such that there were equal amount of stimuli presented to the right and left visual hemi-field across the blocks.
Figure 1.
Task timeline. Sequences of standards S1, S2 and S3 with a predicted sequence (top) and in randomized order (bottom) preceding the target (T). Stimuli presented to the left or right visual field. Inter-trial intervals, including duration of stimulus presentation (150 msec) are displayed.
Subjects performed a brief training session to ensure they were able to detect the target accurately. Subjects were then introduced to the predictive sequence before the recordings began and were aware that it would be a 100% predictive of a target, but that targets would also appear randomly throughout the block. Subjects were asked to press a button with their right index finger each time a target was presented and to pay attention and look for the predictive sequence. Subjects then performed another brief training session to ensure that they were confident in the detection of the predictive sequence as well as the targets. Stimulus presentation and response recordings were controlled using E-prime (Psychology Software Tools, Inc., Pittsburgh, USA).
2.3 Recording
EEG was recorded from a 64 electrode array using the Active Two system (Biosemi, The Netherlands). External electrodes above and below the right eye monitored vertical eye movements and electrodes placed laterally to the left and right eyes monitored horizontal eye movements. Signals were amplified and digitized at 512 Hz and filtered at 0.16–100 Hz. Post processing and ERP analysis of the data was performed using Brain Vision Analyzer (Brain Products GmbH, Germany). All channels were re-referenced to averaged linked earlobes.
2.4 ERP Analysis
Prior to ERP analysis ocular movements were defined using ICA and were removed by a linear derivation using Brain Vision Analyzer. Epochs containing misses (no button press 150–1150 msec post stimulus onset) were excluded from further analysis. EEG signals were filtered at 0.1–30 Hz for subsequent analysis. EEG signals were sorted and averaged relative to the stimulus onset, with epochs set from −200 to 1000 msec relative to stimulus onset. EEG epochs with amplitude of more than 75 µV at any electrode were excluded.
2.4.1 P3b
P3b was measured as the most positive point in the latency range of 250–700 msec in the young adults and 250–800 msec in the older adults. In order to restrict the number of comparisons and to determine if any significant lateralization effects of hemisphere or visual field existed, an omnibus ANOVA was first performed. In this ANOVA we used the P3b peak amplitude at electrode sites (F3, F4, Fz, C3, C4, Cz, P3, P4, Pz) and from both visual fields of stimuli presentation (right and left) for six different conditions (predictive and random targets, standards and the three standards comprising the predicting sequence) across both groups (young and older adults). No differences were observed between the two visual fields of presentation, or between left and right hemisphere electrode sites. Thus, all P3b ERP data were collapsed across visual fields for subsequent analysis. In addition we concentrated on midline electrode sites (AFz, Fz, FCz, Cz, CPz, and Pz) to explore anterior versus posterior topographical differences of the P3b for the different target conditions. Furthermore, since robust P3b components were induced only for the two target conditions (random and predictive) and for the last most-informative standard of the predicting sequence, we used these for the further statistical analysis of P3b amplitude effects (see Fogelson et al., 2009, for detailed discussion of all 6 conditions). Thus, peak P3b amplitude (measured in µV) at AFz, Fz, FCz, Cz, CPz, and Pz were evaluated for 4 conditions: targets after predictive sequences (predicted), targets after non predictive random sequences (random), random preceding standards (standards excluding those comprising of the predicting sequence) and the last most-informative standard of the predicting sequence (n−1) for both groups. Peak P3b latency (measured in msecs) was compared between the two target conditions. Since there were no significant differences in peak P3b latency between the six electrodes, we used the electrode with maximal P3b amplitude for statistical analysis.
There were comparable number of trials for predicted (57 ± 4, young and 56 ± 7, older adults), random (56 ± 4, young and 55 ± 6, older adults) and n−1 (60 ± 3, young and 57 ± 5, older adults) conditions after removal of misses and artifacts.
2.4.2 Context positivity and late sustained activity
A difference wave subtracting random targets from predicted targets was derived, to extract the context positivity (CP) that was observed in the predicted target compared to the random target condition. Peak amplitude and latency were evaluated for CP by determining the most positive point in the latency range of 150–400 msec. Like P3b no field effects were observed and data were collapsed across visual fields for subsequent analysis. The CP was evaluated at AFz, Fz, FCz, Cz, CPz, and Pz. In addition, the mean difference wave (DW) amplitude for the range 400–800 msec post stimulus onset (late DW) was evaluated for each of the six midline electrode sites, to extract the late sustained activity observed in the predicted target compared to random target condition in older compared to younger adults.
2.4.3 N1
To assess whether any age-related changes in local contextual processing are affected by early perceptual processes we analyzed N1 for the two target conditions. Peak N1 amplitudes (measured in µV) were determined at PO7 and PO8 for both predicted and random targets presented to the right or left visual field. N1 was determined as the most negative peak in the latency range of 50–200 msec.
Analysis of variance (ANOVA) was performed with the Greenhouse-Geisser correction, followed by post-hoc parametric paired t-tests, Sidak corrected for multiple comparisons unless otherwise stated. All t-tests reported are two-sided. Mean values with standard error of the mean (SEM) are used throughout the text. Partial eta squared (ηp 2) values are reported where applicable.
3. Results
3.1 Behavioral results
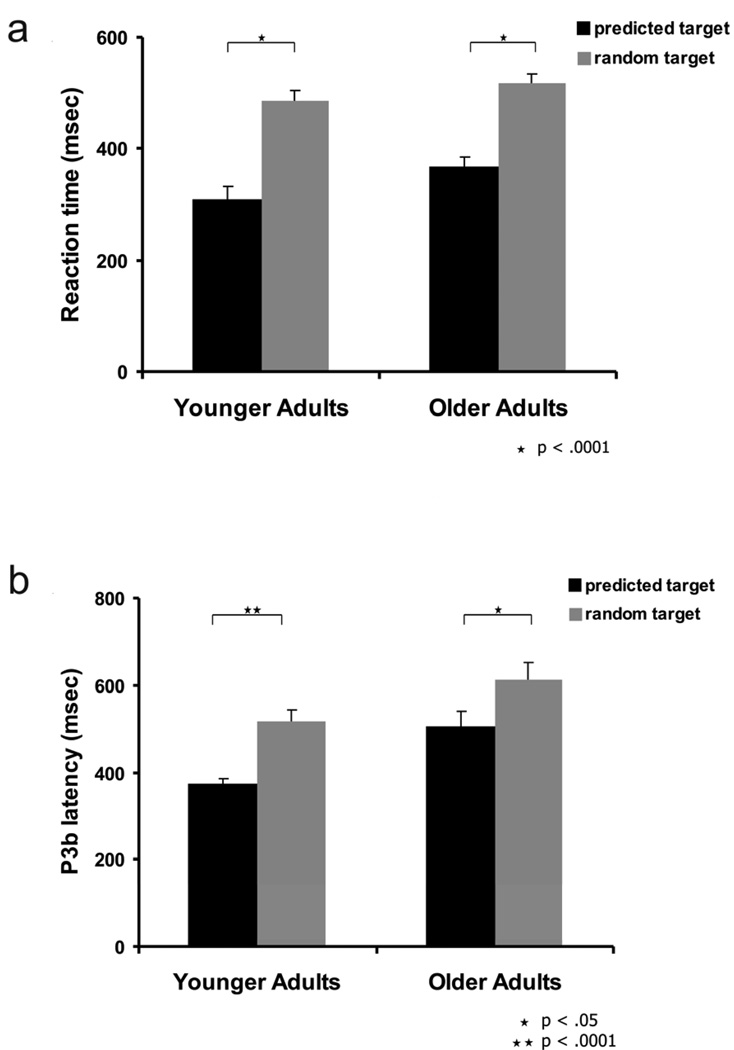
Mean accuracy was 97 ± .6 % and 99 ± .3 % for the young and older adults, respectively. To compare the reaction times (RT) for the targets between the groups we performed an ANOVA with group (young vs. older) as the between-subject factor and condition (predicted vs. random targets) as the repeated measures factor. There was a main effect for condition (F(1,21) = 105.47, p< .0001, ε = 1, ηp 2 = .83). However, there was no significant overall effect of age (F(1,21) = 3.81, p = .07, ηp 2 = .15), neither a condition x group interaction (F(1,21) = .67, p=0.4, ηp 2 = .03). Post hoc t-tests showed that the reaction times for the predicted targets (mean RT = 309 ± 23 msec and 366 ± 18 msec for young and older adults, respectively) were shorter than those for random targets (mean RT = 486 ± 19 msec and 517 ± 17 msec for young and older adults, respectively) in both groups (t(11) = 7.39, p< .0001 and t(10) = 7.29, p< .0001 for young and older adults, respectively). RT values are illustrated in Figure 2a.
Figure 2.
Reaction times (a) and P3b peak latency at CPz (b) for predicted and random targets in young and older adults. Bars = standard errors of mean.
3.2 P3b
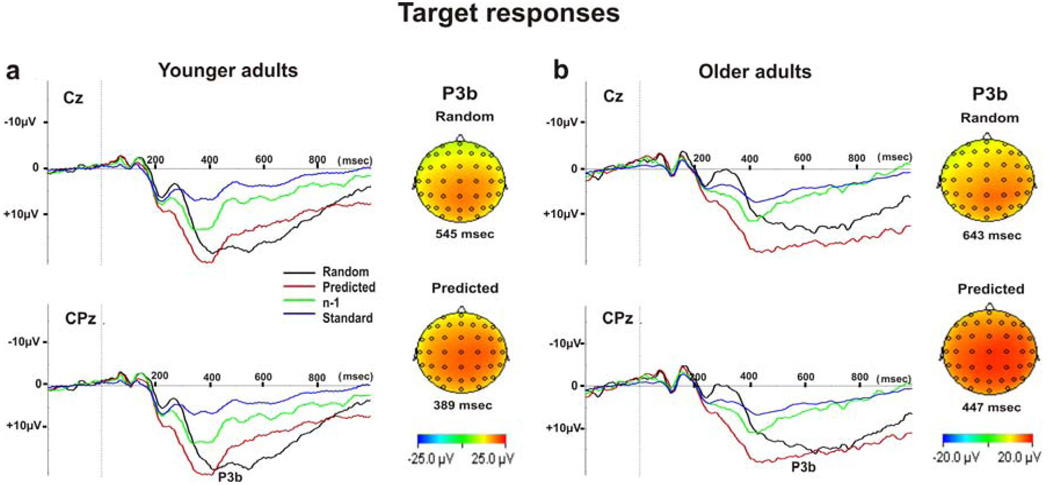
Scalp distributions of the grand-averaged ERPs across the 12 subjects in the young group and across the 11 subjects in the older group at Cz and CPz elicited by predicted and random targets, standards and n−1, the last most-informative stimulus of the predicting sequence, are shown for the young and older adults in Figure 3. Both groups show a robust posterior P3 component for targets with a shift in latency between predicted and random targets.
Figure 3.
Grand average at Cz and CPz for the 4 conditions: targets after random non-predictive (Random) and predictive sequences (Predicted), the last most informative standard comprising the predicting sequence (n−1) and random preceding standards (Standard) for young (a) and older (b) adults. Vertical dotted line indicates time of stimulus presentation onset. Topographical maps for the maximal peak P3b at CPz are shown for random and predicted targets.
To compare peak P3b amplitudes we performed an ANOVA with group (young vs. older) as the between-subject factor and with condition (predicted, random targets, n-1, and standards) and electrode (AFz, Fz, FCz, Cz, CPz, and Pz) as the repeated measures factors. There was no significant overall effect of age in the comparison of peak P3b amplitude (F(1,21) = .70, p = .41, ηp 2 = .03). However, there was a main effect for condition (F(3,63) = 35.6, p< .0001, ε = .67, ηp 2 = .63) and electrode (F(5,105) = 68.56, p< .0001, ε = .33, ηp 2 = .77), with significant electrode x group (F(5,105) = 4.81, p= .019, ηp 2 = .19) and electrode x condition (F(15,315)=17.11, p< .0001, ε = .31, ηp 2 = .45) interactions.
Both groups showed maximal and minimal P3b amplitudes at CPz and AFz, respectively. Post-hoc tests, corrected for multiple comparisons, showed that peak P3b amplitude at CPz was larger for predicted targets (mean P3b amplitude = 23.66 ± 2.37 µV, p< .0001 in young and 20.81 ± 2.75 µV, p< .0001 in older adults), random targets (mean P3b amplitude = 22.65 ± 2.24 µV, p< .0001 in young and 18.31 ± 2.11 µV, p< .0001 in older adults), and n−1 condition (mean P3b amplitude = 16.36 ± 1.71 µV, p = .013 in young and 13.11 ± 2.0 µV, p< .0001 in older adults) compared to standards (mean P3b amplitude = 9.30 ± .86 µV in young and 7.76 ± 1.60 µV in older adults). P3b amplitude in the n-1 condition was smaller compared to predicted and random targets (p = .037) in the older adults. However, there was no significant difference in P3b amplitude between random and predicted targets in both groups. Topographical maps of P3b amplitudes in the two age groups, displayed in Figure 3, indicate a more frontal distribution of P3b in the older adults compared to the younger adults. For instance, post-hoc tests corrected for multiple comparisons, showed that in younger adults P3b amplitude for random targets was smaller at AFz (12.51 ± 1.33 µV) compared to all the other 5 midline electrodes (21.28 ± 2.40 µV at Pz, 22.65 ± 2.24 µV at CPz, 21.55 ± 2.12 µV at Cz, 18.09 ± 1.79 µV at FCz, 14.98 ± 1.53 µV at Fz, p≤ .003). In older adults P3b amplitude for random targets was smaller at AFz (12.41 ± 2.60 µV) compared to Pz (16.65 ± 2.17 µV, p = .02), CPz (18.31 ± 2.11 µV, p = .002), Cz (17.05 ± 2.53 µV, p = .008) and FCz (15.44 ± 2.61 µV, p = .007), but not compared to Fz (13.71 ± 2.68 µV, p = .09).
Since peak P3b amplitudes were largest at CPz we used this electrode, for both target conditions in all the subjects, in the comparison of peak P3b latencies and performed an ANOVA with group (young vs. older) as the between-subject factor and with condition (predicted vs. random targets) as the repeated measures factor. There was an overall significant effect of age in the comparison of peak P3b latency (F(1,21) = 10.62, p= .004, ηp 2 = .34) with slower P3b latencies in the older adults compared to the younger adults. There was a main effect for condition (F(1,21) = 35.82, p< .0001, ε = 1, ηp 2 = .63). However, there was no significant condition x group interaction (F(1,21) = .71, p = .41, ηp 2 = .03). In both groups peak P3b latency was shorter for predicted targets (mean P3b latency = 373 ± 11 msec and 504 ± 34 msec for young and older adults, respectively) compared to the peak P3b latency for random targets (mean P3b latency = 517 ± 26 msec and 613 ± 39 msec for young and older adults, respectively; t(11) = 6.7, p< .0001 for young and t(10) = 2.9, p= .016 for older adults). These comparisons are displayed in Figure 2b.
3.3 Context Positivity
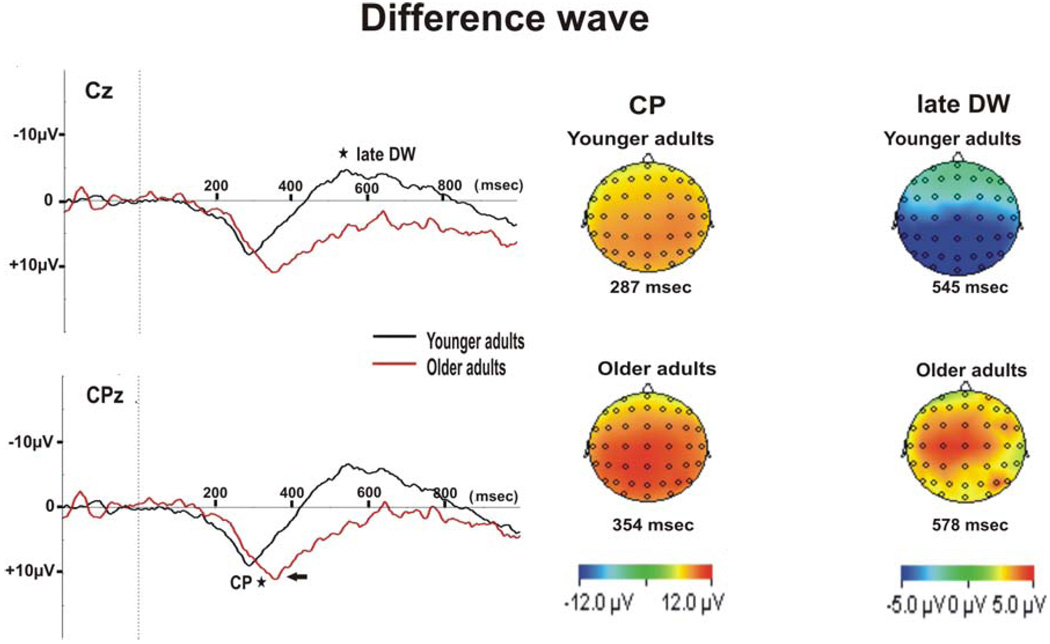
We compared the peak context positivity (CP) amplitude (derived from the difference wave between predicted and random targets), demonstrating the early latency context dependent positive shift observed in the predicted target compared to the random target condition. We performed an ANOVA with group (young vs. older) as the between-subject factor and electrode (AFz, Fz, FCz, Cz, CPz, and Pz) as the repeated measures factor. There was no significant overall effect of age in the comparison of peak CP amplitude (F(1,21) = .36, p = .55, ηp 2 = .02). There was a main effect for electrode (F(5,105) = 24.54, p< .0001, ε = .46, ηp 2 = .54). However, there was no significant electrode x group interaction (F(5,105) = 1.46, p = .24, ηp 2 = .07). Peak CP amplitudes were maximal at CPz and minimal at AFz across age groups. Thus, the CP has a posterior – parietal scalp distribution similar to that of P3b as is illustrated in Figure 4. Since CPz had maximal CP amplitudes we used this electrode to compare CP peak latencies between the two age groups. An independent t-test showed significantly shorter peak CP latencies at CPz in the young adults (mean CP latency = 306 ± 11 msec) compared to the older adults (mean CP latency = 346 ± 11 msec, t(21) = 2.55, p= .019), as is illustrated in Figure 4.
Figure 4.
Grand average for the difference wave (DW) between predicted and random targets at Cz and CPz in young and older adults. The peak of the context positivity (CP) is indicated by an arrow. Topographical maps the CP peak at CPz and for late DW at Cz, for young and older adults are displayed. Age-related differences (p < 0.05) in peak CP latency at CPz and in late DW mean amplitude (400–800 msec post stimulus onset) at Cz are indicated by a star.
T-tests comparing CP peak latency versus P3b peak latency for targets at CPz showed CP peak latency to be shorter (mean CP latency = 306 ± 11 msec and 346 ± 11 msec for young and older adults, respectively) than P3b peak latency for both predicted (mean P3b latency = 373 ± 11 msec, t(11) = 5.02, p< .0001 for young and 504 ± 34 msec, t(10) = 4.41, p= .001 for older adults) and random targets (mean P3b latency = 517 ± 26 msec, t(11) = 7.87, p< .0001 for young and 613 ± 39 msec, t(10)=6.85, p< .0001 for older adults).
3.4 Late sustained activity
We compared the mean amplitude (400–800 msec post stimulus onset) of the difference wave between predicted and random targets (late DW), using an ANOVA with group (young vs. older) as the between-subject factor and electrode (AFz, Fz, FCz, Cz, CPz, and Pz) as the repeated measures factor. There was a significant overall effect of age in the comparison of late DW mean amplitude (F(1,21) = 6.06, p= .023, ηp 2 = .22). There was a main effect for electrode (F(5,105) = 8.68, p= .001, ε = .4, ηp 2 = .29) and a significant electrode x group interaction (F(5,105) = 7.36, p= .002, ηp 2 = .26). Late DW mean amplitude showed the maximal negative shift at CPz in younger adults and the maximal positive shift at Cz in older adults. However, independent t-tests revealed group differences between late DW amplitudes to be maximal at Cz, with a sustained positive shift in older adults (mean DW amplitude = 4.61 ± 1.83 µV) compared to young adults (mean DW amplitude = −2.52 ± 1.49 µV, t(21) =3.04, p= .0006; Fig. 4). Note that the difference in late DW between the two age groups is due to a late sustained positivity in the predicted target condition in older adults that is not observed in the younger adults.
3.5 N1
We utilized an ANOVA with group (young vs. older) as the between-subject factor and electrode (PO7 and PO8), visual field (left vs. right) and condition (predicted vs. random targets) as repeated measures factors, to compare the peak N1 amplitude between predicted and random targets. There was no significant overall effect of age in the comparison of peak N1 amplitude (F(1,21) = .42, p = .52, ηp 2 = .02) and no significant group interactions with any of the factors. However, there was a significant interaction between electrode locations and visual field of stimuli presentation for N1 amplitude (F(1,21) = 26.76, p< .0001, ηp 2 = .56) across both groups. N1 ERPs were enhanced to contralaterally presented stimuli.
4. Discussion
We observed that predictive local context affects target detection by reducing the duration of stimulus evaluation in both young and older adults. This effect was associated with both faster reaction times and shortened P3b latencies. An earlier latency context positivity was observed only in the predicted compared to the random target condition. CP peak latency was slower in the older population compared to young adults. Furthermore, older subjects elicited a late latency sustained positivity in the predictive target condition, that was not observed in younger adults. Taken together these findings reveal that certain aspects of local contextual processing are altered with age.
4.1 P3b and contextual processing
P3b amplitude was not affected by age in the current study. However, a scalp topographical posterior-anterior shift of P3b amplitude was observed in the older adults. This is consistent with studies showing little effect of age on P3b amplitude in some conditions (Daffner et al., 2005; Kray et al., 2005; West and Travers, 2008) but a more consistent finding of P3b topographical changes in the elderly (Anderer et la., 1996; Daffner et al., 2005; Dujardin et al., 1993; Fabiani et al., 1998; Fjell and Walhovd, 2001; West and Travers, 2008).
P3b amplitude increased with task-informative stimuli, with a large P3b induced by the last most-informative standard of the predicting sequence, with P3b amplitude reaching its maximum in the two target conditions for both the young and older adults. This is in line with studies showing P3b amplitude increases as a function of task relevance (Fogelson et al., 2009; Sawaki and Katayama, 2006). However, there were no significant differences in P3b amplitude between predicted and random targets in both age groups, suggesting that in this easy discrimination task, what determined the magnitude of the P3b amplitude was the task-relevance of the stimulus (Fogelson et al., 2009). The last most-informative stimuli of the predicting sequence induced a significant P3b compared to standards in both young and older adults. This suggests that it became a secondary target for the subjects and thus another electrophysiological indicator of successful local contextual processing.
We replicated previous findings of slower P3b latency in older adults compared to younger adults (Ander et al., 1996; Bashore et al., 1997; Dujardin et al., 1993; Fjell and Walhovd, 2001; Ford et al., 1982; Polich, 1996). P3b latency was shorter for sequence predicted targets than for targets after non-predictive sequences, in both age groups. These findings suggest that predictive local context affects target detection by reducing the duration of stimulus evaluation (Duncan-Johnson, 1981; Duncan-Johnson and Donchin, 1982; Hillyard and Kutas, 1983; Fogelson et al., 2009; Ford et al., 1982; Kutas et al., 1977; McCarthy and Donchin, 1981) in both young and older adults. Latency differences in our study were associated with parallel behavioral results showing shorter reaction times for predicted targets compared to random targets, in both age groups. However, there were no differences in reaction times across the age groups, a finding that has been shown by several other studies (Daffner et al., 2005; Dujardin et al., 1993; Fabiani et al., 1998; Ford et al., 1982). Furthermore, our findings suggest that this facilitation in stimulus evaluation is cognitive rather than perceptual, since targets were identical in their physical features and there were no significant N1 amplitude differences between predicted and random targets (Hillyard and Kutas, 1983), in both age groups nor was there an effect of age on N1 amplitude (Dujardin et al., 1993).
Overall, effects of local contextual processing on P3b were comparable across age groups. Both groups showed faster P3b latencies and faster reaction times for predicted compared to random targets, increases in P3b amplitude with increasing task relevance and a robust P3b amplitude to the last most-informative stimuli of the predictive sequence.
4.2 Context positivity and contextual processing
An earlier latency context dependent positive shift was observed only in the predicted target as compared to the random target condition. The CP has a similar distribution to the P3b. However, it is has a shorter peak latency than P3b, being generated approximately 200 msec earlier than the P3b. Peak CP amplitude was slower in the older population compared to young adults, suggesting that contextual effects on target detection processes are slowed with age, and that this slowing is specific to earlier latency processes of target detection (Bashore et al., 1997). This finding supports other evidence of contextual processing deficits in older adults, specifically in self-initiated effortful integration of contextual information (Smith et al., 1998), such as that utilized in the present study, where subjects had to consciously look for the predictive sequence. We propose that the CP may reflect an early template match process (Squires et al., 1973; Chao et al., 1995), and that this early context dependent process is slowed with age. This is supported by hypothesis implicating that elderly subjects have impairment in the ability to maintain templates of the target stimuli in working-memory (Dujardin et al., 1993; Fabiani et al., 1998; Fjell and Walhovd, 2001) and with evidence suggesting that preparatory processes may be disrupted in the elderly (West and Schwarb, 2006; West and Travers, 2008).
4.3 Sustained positivity and contextual processing
Age-related differences were also observed in a late time-window (400–800 msec post-stimulus onset). This effect was due to a late sustained positivity after the detection of predicted targets, similar to that seen after the detection of random targets, observed only in older adults. We propose that this continued processing may indicate an abnormality in disengagement after the detection of predicted targets. Similar findings of a late sustained positivity in the elderly, reported after ERPs to attended non-targets (Looren de Jong et al., 1988), have been interpreted as an indication of reprocessing after uncertain decisions, and suggest that older subjects have difficulties in reaching confident decisions (Ford et al., 1979; Looren de Jong et al., 1988). This interpretation fits well with our data, suggesting that older adults have deficits in resolving uncertainty in conditions where local contextual information had to be utilized in order to predict targets. The lateral prefrontal cortex has been reported to have a critical role in the resolution of short term uncertainty (Huettel et al., 2005) and this area has been shown to be susceptible to the effects of aging (Braver and Barch, 2002; de Keyser et al., 1990; Raz et al., 1997), suggesting that this may contribute to our aging effects. Another possible interpretation for the age-related changes in post-decision processing, observed as a delay in return to baseline in the predicted target condition, is that it reflects compensatory mechanisms (Grady et al., 1995; Grady, 2008). However, considering the comparable behavioral performance of young and older adults in the present study, the possibility of non-selective brain activation (as opposed to over-recruitment in mechanisms of compensation) in the older adults cannot be ruled out (Grady, 2008).
In conclusion, the present study provides evidence of age-related changes in neural correlates associated with processing of local contextual information. These include age-related slowing of an early latency context positive shift, reflecting contextual effects on target detection processing, and generation of a late sustained positivity after the detection of predicted targets, which is absent in younger adults. This sustained positivity may indicate continued processing due to deficits in resolution of uncertainty in conditions where contextual information was utilized. These findings provide neurophysiological evidence that aging is associated with task-specific slowing of higher order processes, and that local contextual effects on target detection processes are altered with age.
Acknowledgments
This research was supported by grants from the National Institute of Health (NINDS Grant NS21135 and PO40813). We thank Kilian Koepsell for his contributions to the design of the paradigm and to Jeffrey Lewis for assistance with programming of the task.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderer P, Semlitsch HV, Saletu B. Multichannel auditory event-related brain potentials: effects of normal aging on the scalp distribution of N1, P2, N2 and P300 latencies and amplitudes. Electroencephalography and clinical Neurophysiology. 1996;99:458–472. doi: 10.1016/s0013-4694(96)96518-9. [DOI] [PubMed] [Google Scholar]
- Barcelo F, Knight RT. An information-theoretical approach to contextual processing in the human brain: evidence from prefrontal lesions. Cerebral Cortex. 2007;17 Suppl 1:i51–i60. doi: 10.1093/cercor/bhm111. [DOI] [PubMed] [Google Scholar]
- Barch DM, Carter CS, Braver TS, Sabb FW, MacDonald A, Noll DC, Cohen JD. Selective deficits in prefrontal cortex function in medication-naive patients with schizophrenia. Archives of General Psychiatry. 2001;58:280–288. doi: 10.1001/archpsyc.58.3.280. [DOI] [PubMed] [Google Scholar]
- Bashore TR, van der Molen MW, Ridderinkhof KR, Wylie SA. Is the age-complexity effect mediated by reductions in a general processing resource? Biological Psychology. 1997;45:263–282. doi: 10.1016/s0301-0511(96)05231-3. [DOI] [PubMed] [Google Scholar]
- Bayen UJ, Phelps MP, Spaniol J. Age-related differences in the use of contextual information in recognition memory: a global matching approach. Journal of Gerontology. 2000;55B:131–141. doi: 10.1093/geronb/55.3.p131. [DOI] [PubMed] [Google Scholar]
- Braver TS, Barch DM. A theory of cognitive control, aging cognition, and neuromodulation. Neuroscience and Biobehavioral Reviews. 2002;26:809–817. doi: 10.1016/s0149-7634(02)00067-2. [DOI] [PubMed] [Google Scholar]
- Braver TS, Satpute AB, Rush BK, Racine CA, Barch DM. Context processing and context maintenance in healthy aging and early stage dementia of the Alzheimer’s type. Psychology and Aging. 2005;20:33–46. doi: 10.1037/0882-7974.20.1.33. [DOI] [PubMed] [Google Scholar]
- Cerella J. Information processing rates in the elderly. Psychological Bulletin. 1985;98:67–83. [PubMed] [Google Scholar]
- Chao LL, Nielsen-Bohlman L, Knight RT. Auditory event-related potentials dissociate early and late memory processes. Electroencephalography and clinical Neurophysiology. 1995;96:157–168. doi: 10.1016/0168-5597(94)00256-e. [DOI] [PubMed] [Google Scholar]
- Cohen JD, Servan-Schreiber D. Context, cortex, and dopamine: a connectionist approach to behavior and biology in schizophrenia. Psychological Review. 1992;99:45–77. doi: 10.1037/0033-295x.99.1.45. [DOI] [PubMed] [Google Scholar]
- Daffner KR, Ryan KK, Williams DM, Budson AE, Rentz DM, Scinto LFM, Holcomb PJ. Age-related differences in novelty and target processing among cognitively high performing adults. Neurobiology of Aging. 2005;26:1283–1295. doi: 10.1016/j.neurobiolaging.2004.11.007. [DOI] [PubMed] [Google Scholar]
- de Keyser J, De Backer J-P, Vauquelin G, Ebinger G. The effect of aging on the D1 dopamine receptors in human frontal cortex. Brain Research. 1990;528:308–310. doi: 10.1016/0006-8993(90)91672-4. [DOI] [PubMed] [Google Scholar]
- Donchin E, Coles MGH. Is the P300 component a manifestation of context updating? Behavioral and Brain Sciences. 1988;11:357–374. [Google Scholar]
- Dujardin K, Derambure P, Bourriez JL, Jacquesson JM, Guieu JD. P300 component of the event-related potentials (ERP) during an attention task: effects of age, stimulus modality and event probability. International Journal of Psychophysiology. 1993;14:255–267. doi: 10.1016/0167-8760(93)90040-v. [DOI] [PubMed] [Google Scholar]
- Duncan-Johnson CC. P300 latency: a new metric of information processing. Psychophysiology. 1981;18:207–215. doi: 10.1111/j.1469-8986.1981.tb03020.x. [DOI] [PubMed] [Google Scholar]
- Duncan-Johnson CC, Donchin E. The P300 component of the event-related brain potential as an index of information processing. Biological Psychology. 1982;14:1–52. doi: 10.1016/0301-0511(82)90016-3. [DOI] [PubMed] [Google Scholar]
- Fabiani M, Friedman D, Cheng J. Individual differences in P3 scalp distribution in older adults, and their relationship to frontal lobe function. Psychophysiology. 1998;35:698–708. [PubMed] [Google Scholar]
- Fjell AM, Walhovd KB. P300 and neuropsychological tests as measures of aging: scalp topography and cognitive changes. Brain Topography. 2001;14:25–40. doi: 10.1023/a:1012563605837. [DOI] [PubMed] [Google Scholar]
- Fogelson N, Wang X, Lewis JB, Kishiyama MM, Ding M, Knight RT. Multimodal effects of local context on target detection: evidence from P3b. Journal of Cognitive Neuroscience. 2009 doi: 10.1162/jocn.2009.21071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford JM, Duncan-Johnson CC, Pfefferbaum A, Kopell BS. Expectancy for events in old age: stimulus sequence effects on P300 and reaction time. Journal of Gerontology. 1982;37:696–704. doi: 10.1093/geronj/37.6.696. [DOI] [PubMed] [Google Scholar]
- Ford JM, Roth WT, Mohs RC, Hopkins WF, III, Bert S. Event-related potentials recorded from young and old adults during a memory retrieval task. Electroencephalography and clinical Neurophysiology. 1979;47:450–459. doi: 10.1016/0013-4694(79)90161-5. [DOI] [PubMed] [Google Scholar]
- Grady CL. Cognitive neuroscience of aging. Annals of the New York Academy of Sciences. 2008;1124:127–144. doi: 10.1196/annals.1440.009. [DOI] [PubMed] [Google Scholar]
- Grady CL, McIntosh AR, Horwitz B, Maisog JM, Ungerleider LG, Mentis MJ, Pietrini P, Schapiro MB, Haxby JV. Science. 1995;269:218–221. doi: 10.1126/science.7618082. [DOI] [PubMed] [Google Scholar]
- Hillyard SA, Kutas M. Electrophysiology of cognitive processing. Annual Review of Psychology. 1983;34:33–61. doi: 10.1146/annurev.ps.34.020183.000341. [DOI] [PubMed] [Google Scholar]
- Huettel SA, Song AW, McCarthy G. Decisions under uncertainty: probabilistic context influences activation of prefrontal and parietal cortices. The Journal of Neuroscience. 2005;25:3304–3311. doi: 10.1523/JNEUROSCI.5070-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kray J, Eppinger B, Mecklinger A. Age differences in attentional control: an even-related potential approach. Psychophysiology. 2005;42:407–416. doi: 10.1111/j.1469-8986.2005.00298.x. [DOI] [PubMed] [Google Scholar]
- Kutas M, McCarthy G, Donchin E. Augmenting mental chronometry: the P300 as a measure of stimulus evaluation time. Science. 1977;197:792–795. doi: 10.1126/science.887923. [DOI] [PubMed] [Google Scholar]
- Looren de Jong H, Kok A, van Rooy JCGM. Early and late selection in young and old adults: an event-related potential study. Psychophysiology. 1988;25:657–671. doi: 10.1111/j.1469-8986.1988.tb01904.x. [DOI] [PubMed] [Google Scholar]
- MacDonald AW, Cohen JD, Stenger VA, Carter CS. Dissociating the role of the dorsolateral prefrontal and anterior cingulated cortex in cognitive control. Science. 2000;288:1835–1838. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- McCarthy G, Donchin E. A metric for thought: a comparison of P300 latency and reaction time. Science. 1981;211:77–80. doi: 10.1126/science.7444452. [DOI] [PubMed] [Google Scholar]
- Polich J. Meta-analysis of P300 normative aging studies. Psychophysiology. 1996;33:334–353. doi: 10.1111/j.1469-8986.1996.tb01058.x. [DOI] [PubMed] [Google Scholar]
- Polich J. Overview of P3a and P3b. In: Polich J, editor. Detection of change: event-related potential and fMRI findings. Boston: Kluwer Academic Press; 2003. pp. 83–98. [Google Scholar]
- Polich J, Criado JR. Neuropsychological and neuropharmacology of P3a and P3b. International Journal of Psychophysiology. 2006;60:172–185. doi: 10.1016/j.ijpsycho.2005.12.012. [DOI] [PubMed] [Google Scholar]
- Poulsen C, Luu P, Davey C, Tucker DM. Dynamics of task sets: evidence from dense-array event-related potentials. Cognitive Brain Research. 2005;24:133–154. doi: 10.1016/j.cogbrainres.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Raz N, Gunning FM, Head D, Dupuis JH, McQuain J, Briggs SD, Loken WJ, Thornton AE, Acker JD. Selective aging of the human cerebral cortex observed in vivo: differential vulnerability of the prefrontal gray matter. Cerebral Cortex. 1997;7:268–282. doi: 10.1093/cercor/7.3.268. [DOI] [PubMed] [Google Scholar]
- Sawaki R, Katayama J. Stimulus context determines whether non-target stimuli are processed as task-relevant or distractor information. Clinical Neurophysiology. 2006;117:2532–2539. doi: 10.1016/j.clinph.2006.06.755. [DOI] [PubMed] [Google Scholar]
- Sliwinski M, Buschke H. Cross-sectional and longitudinal relationships among age, cognition, and processing speed. Psychology and Aging. 1999;14:18–33. doi: 10.1037//0882-7974.14.1.18. [DOI] [PubMed] [Google Scholar]
- Smith AD, Park DC, Earles JLK, Shaw RJ, Whiting WL., IV Age differences in context integration in memory. Psychology and Aging. 1998;13:21–28. doi: 10.1037//0882-7974.13.1.21. [DOI] [PubMed] [Google Scholar]
- Squires KC, Hillyard SA, Lindsay PH. Vertex potentials evoked during auditory signal detection: relation to decision criteria. Perception & Psychophysics. 1973;14:265–272. [Google Scholar]
- Squires KC, Wickens C, Squires NK, Donchin E. The effect of stimulus sequence on the waveform of the cortical event-related potential. Science. 1976;193:1142–1146. doi: 10.1126/science.959831. [DOI] [PubMed] [Google Scholar]
- West R, Schwarb H. The influence of aging and frontal function on the neural correlates of regulative and evaluative aspects of cognitive control. Neuropsychology. 2006;20:468–481. doi: 10.1037/0894-4105.20.4.468. [DOI] [PubMed] [Google Scholar]
- West R, Travers S. Differential effects of aging on processes underlying task switching. Brain and Cognition. 2008 doi: 10.1016/j.bandc.2008.03.001. [DOI] [PubMed] [Google Scholar]