Abstract
2-Hydroxy fatty acids (hFA) are important components of a subset of mammalian sphingolipids. The presence of hFA in sphingolipids is best described in the nervous system, epidermis, and kidney. However, the literature also indicates that various hFA-sphingolipids are present in additional tissues and cell types, as well as in tumors. Biosynthesis of hFA-sphingolipids requires fatty acid 2-hydroyxlase, and degradation of hFA-sphingolipids depends, at least in part, on lysosomal acid ceramidase and the peroxisomal fatty acid α-oxidation pathway. Mutations in the fatty acid 2-hydroxylase gene, FA2H, have been associated with leukodystrophy and spastic paraparesis in humans, underscoring the importance of hFA-sphingolipids in the nervous system. In the epidermis, hFA-ceramides are essential for the permeability barrier function. Physiological function of hFA-sphingolipids in other organs remains largely unknown. Recent evidence indicates that hFA-sphingolipids have specific roles in cell signaling.
Keywords: Fatty acid 2-hydroxylase, fatty acid alpha-hydroxylase, FA2H, hydroxy fatty acid, hydroxy sphingolipids
1. INTRODUCTION
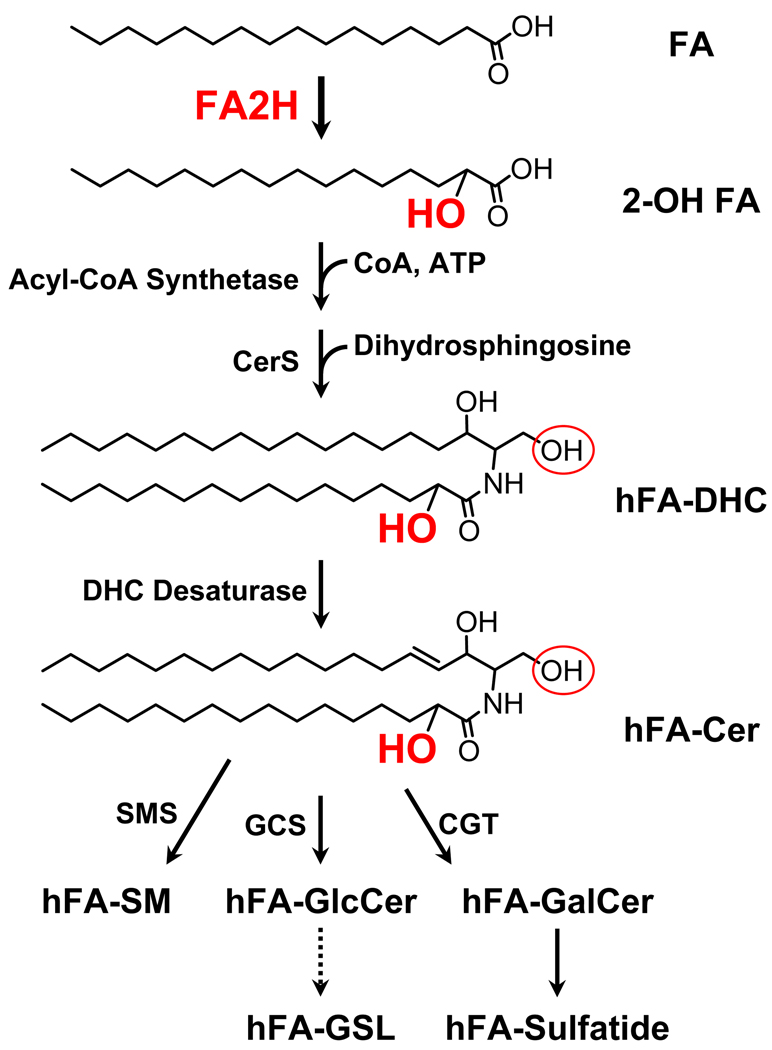
Sphingolipids are ubiquitous eukaryotic membrane lipids with highly diverse structures with hundreds of distinct headgroups attached to various ceramides. In mammalian sphingolipids, both the sphingoid base and the N-acyl chain of ceramide can vary with regard to alkyl chain length, hydroxylation, and desaturation [1]. The topic of this review is a subset of mammalian sphingolipids, hFA-sphingolipids, with 2-hydroxy fatty acid as the N-acyl chain of ceramide. Fatty acid 2-hydroxylase converts fatty acid to hFA, which is then incorporated into hFA-ceramide and complex hFA-sphingolipids (Fig. 1). However there is no evidence in the literature supporting the incorporation of hFA into glycerolipids in mammals.
Fig. 1. Biosynthesis of hFA-sphingolipids.
The pathway is identical to biosynthesis of non-hydroxy sphingolipids, except for the fatty acid 2-hydroxylation step. Note that various fatty acids (mostly C16-C24) can be 2-hydroxylated and incorporated into hFA-ceramide. The 2-hydroxyl group is shown in red, and the hydroxyl group at the C-1 position of ceramide to which a head group is transferred is indicated by a red circle. DHC, dihydroceramide; CerS, dihydroceramide synthase; Cer, ceramide; SMS, sphingomyelin synthase; CGT, UDP-galactose: ceramide galactosyltransferase; GCS, UDP-glucose:ceramide glucosyltransferase; GSL, glycosphingolipids.
The presence of hFA in mammalian sphingolipids has been known since the early 1900’s (for review of early works, see reference [2]). Early investigations described high concentrations of hFA in brain galactosylceramide (GalCer), a major lipid component of the myelin sheath. Decades later, Kishimoto and Radin reported finding hFA-sphingolipids outside of the nervous system [3]. Subsequently, in a number of studies, researchers have shown that hFA-sphingolipids are present in various mammalian tissues and tumors. Despite their prevalence, physiological functions of hFA-sphingolipids remain largely unknown.
In parallel with tissue sphingolipid analyses, in vitro biochemical studies of rat brain fatty acid 2-hydroxylase were initiated by Kishimoto and colleagues [4], and their subsequent studies established key characteristics of this enzyme [5–8]. Molecular genetic approaches led to the identification of the FA2H gene, which encodes a fatty acid 2-hydroxylase with the characteristics of the rat brain enzyme [9, 10].
Recent work by Edvardson et al. describes a leukodystrophy and spastic paraparesis in humans caused by mutations in the FA2H gene [11]. This discovery underscores the importance of hFA-sphingolipids in the nervous system. Also revealed in the study was additional enzyme(s) that can produce hFA outside of the nervous system in the absence of FA2H [11]. Thus, the story of hFA and hFA-sphingolipids is more complex than previously predicted.
Summarized below are the current knowledge about the biosynthesis and turnover of hFA-sphingolipids, tissue distribution of hFA-sphingolipids and their postulated functions, and brief description of recent developments in cell signaling, mediated specifically by hFA-sphingolipids.
2. BIOSYNTHESIS AND TURNOVER OF HFA-SPHINGOLIPIDS
2.1 Fatty acid 2-hydroxylation by FA2H
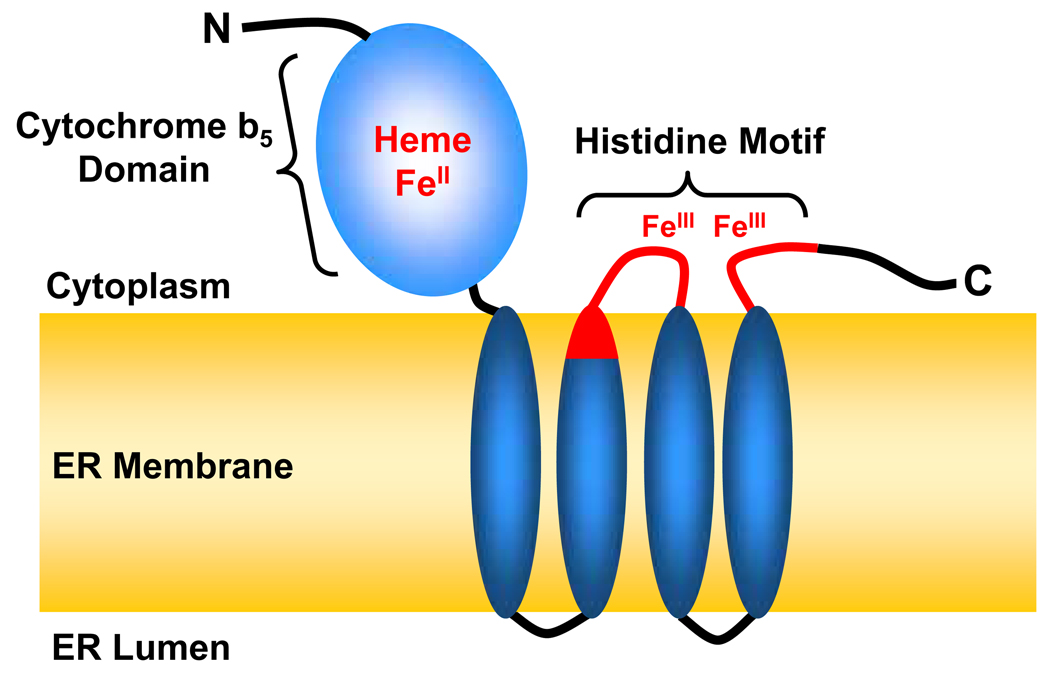
There are at least two types of fatty acid 2-hydroxylase in mammals; the NAD(P)H-dependent monooxygenase encoded by the FA2H gene, and the α-ketoglutarate-dependent monooxygenase encoded by the PHYH gene. FA2H is a 43-kDa (372-aa) integral membrane protein of the ER [9, 10]. Mutations in the FA2H gene are associated with leukodystrophy and spastic paraparesis in humans [11], indicating that its primary function is to synthesize myelin hFA-GalCer (see §3.1). FA2H has two functional domains; an N-terminal cytochrome b5 domain and the C-terminal catalytic domain consisting of four potential transmembrane domains (Fig. 2). The putative catalytic site contains the histidine motif conserved among membrane-bound monooxygenases [consensus: HX(3–4)H X(7–41) HX(2–3)HH X(61–189) (H/Q)X(2–3)HH] [12]. The 8 histidine residues are thought to coordinate the non-heme di-iron cluster at the active site of the enzyme [12]. Studies by Kishimoto and coworkers in the 70’s and 80’s demonstrated that rat brain fatty acid 2-hydroxylase was a microsomal enzyme [5] that required molecular oxygen, Mg2+, pyridine nucleotides (NADPH is preferred to NADH), heat-stable cofactors [4], and microsomal electron transfer proteins [7]. These characteristics indicate that the rat brain enzyme is a mixed-function monooxygenase, which is consistent with the features of FA2H predicted from the primary structure. Indeed, the in vitro fatty acid 2-hydroxylase activity of FA2H is dependent on cytochrome P450 reductase, NADPH, and an NADPH regeneration system [9]. The N-terminal cytochrome b5 domain is also necessary for maximal activity, presumably providing electrons to the catalytic di-iron [9]. Thus, all available evidence suggests that FA2H is the same enzyme previously characterized in rat brain. FA2H converts free fatty acid to free 2-hydroxy fatty acid in vitro [9, 13], which is also likely the case in vivo [14]. The possibility of direct hydroxylation of N-acyl chains of ceramide or other sphingolipids by FA2H has not been examined.
Fig. 2. FA2H is a membrane-bound monooxygenase.
FA2H (372 aa) consists of two functional domains; the N-terminal cytochrome b5 domain (1–92 aa) and the C-terminal catalytic domain. The topology of FA2H is postulated based on the topology of microsomal cytochrome b5 [111] and the yeast FA2H homologue Scs7p [112, 113]. The 8 conserved histidines (H234, H239, H257, H260, H261, H336, H339, H340) are clustered in the two short segments facing the cytoplasm (shown in red). These histidines are thought to coordinate the non-heme di-iron. The 7 patients with an intronic mutation in the FA2H gene produce aberrant mRNA encoding a protein without the last three transmembrane domains that encompass the putative catalytic site.
2.2 Acyl-CoA 2-hydroxylation by PHYH
The PHYH gene product, phytanoyl-CoA 2-hydroxylase, is an α-ketoglutarate-dependent acyl-CoA 2-hydroxylase in the peroxisomal α-oxidation pathway (for review, see [15]). Its primary function is degradation of branched-chain fatty acids. Deficiencies of this enzyme cause Refsum’s disease characterized by accumulation of phytanic acid in various organs. In addition to branched-chain acyl-CoA, PHYH can catalyze 2-hydroxylation of straight-chain acyl-CoA in vitro [16, 17]. Whether PHYH is responsible for the FA2H-independent fatty acid 2-hydroxylation in dermal fibroblasts reported by Edvardson et al. [11] is an open question. If the FA2H-independent hFA formation occurs in peroxisomes, hFA would need to be transported from peroxisomes to the ER where hFA-ceramide is synthesized. Another possibility might be “alternative trafficking” of PHYH to the ER so that PHYH could contribute to the de novo hFA-ceramide synthesis within the ER.
2.3 Synthesis of hFA-ceramide and complex hFA-sphingolipids
Current evidence supports that hFA-sphingolipids are synthesized by the same set of enzymes as non-hydroxy sphingolipids, except for fatty acid 2-hydroxylase. After 2 hydroxylation, hFA is presumably activated to CoA ester by acyl-CoA synthetases (Fig. 1). In the next step, 2-hydroxy acyl group is transferred to the primary amine of dihydrosphingosine by one of the 6 isoforms of dihydroceramide synthase (CerS), which are all capable of synthesizing hFA-dihydroceramide [18]. Subsequent oxidation by one of the two dihydroceramide desaturases converts hFA-dihydroceramide to hFA-ceramide, which is the common precursor of all complex hFA-sphingolipids. The levels of hFA-ceramide range from 1–3% of total cellular ceramide in many established cell lines (our unpublished data) to over 50% in skin keratinocytes [19] and intestinal epithelial cells [20–22].
Ceramide is converted to sphingomyelin (SM), glucosylceramide (GlcCer), and GalCer, by the addition of phosphocholine, glucose, or galactose, respectively, at the C-1 hydroxyl group of ceramide. Various monosaccharides can be sequentially added to GlcCer to form complex glycosphingolipids, whereas SM is an end product of biosynthesis. GalCer can be converted to two known glycosphingolipids, one of which is another major myelin component sulfatide. Most of the enzymes involved in the synthesis of these sphingolipids appear to utilize hFA-ceramide because various hFA-sphingolipids are present in many different tissues and cell types (see §3). The best-characterized example is UDP-galactose:ceramide galactosyltransferase (CGT), encoded by the UGT8 gene. CGT has strong preference for hFA-ceramide over non-hydroxy ceramide [23]. Both FA2H and CGT are highly upregulated in myelinating oligodendrocytes and Schwann cells, which enables these cells to produces vast quantities of hFA-GalCer in myelin.
2.4 Hydrolysis of hFA-ceramide by ceramidase and saposin D
Complex sphingolipids mostly reside in the extracellular leaflet of the plasma membrane. Once they are internalized by endocytosis and have reached the lysosomes, the cell surface sphingolipids are accessible to lysosomal sphingolipid hydrolases. Several lysosomal enzymes can remove specific sugar headgroups of complex sphingolipids, as well as acid sphingomyelinase and acid ceramidase. Degradation of sphingolipids has been extensively studied in association with the lysosomal storage disorders, which are caused by deficiencies in one of the lysosomal degradation enzymes (for review, see [24]).
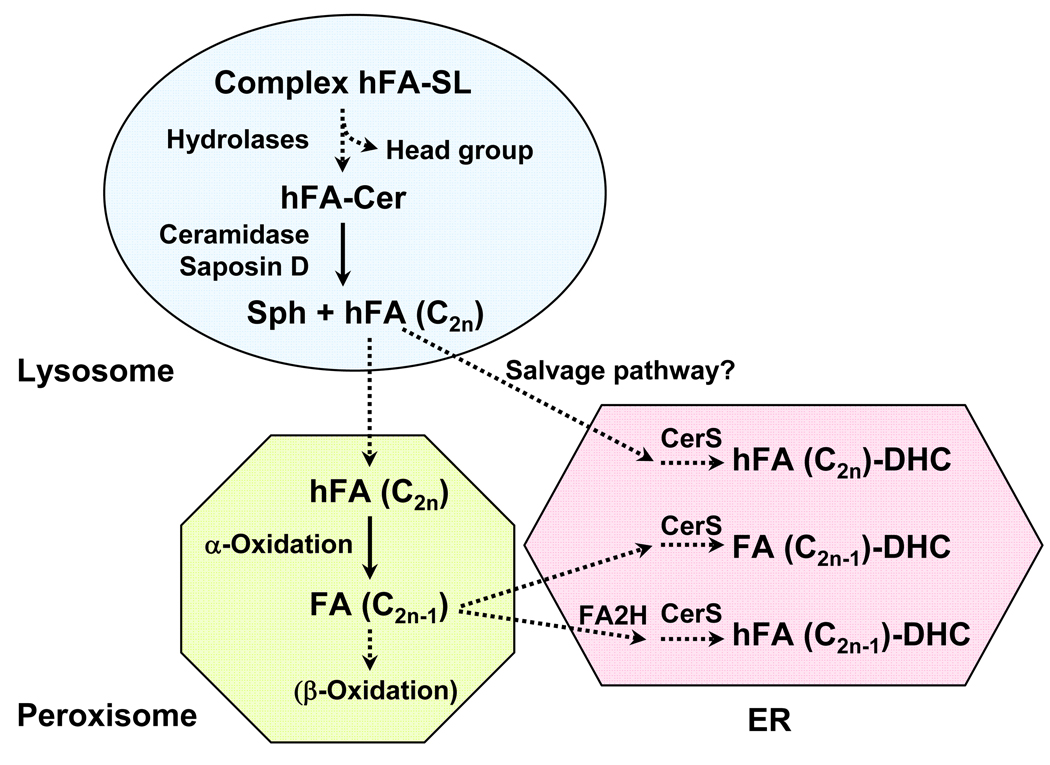
Removal of the headgroup from hFA-sphingolipids generates hFA-ceramide in the lysosome, which can be hydrolyzed by lysosomal acid ceramidase into sphingosine and hFA (Fig. 3). Deficiencies in this enzyme cause the lysosomal storage disorder Farber’s disease. Both ceramide and hFA-ceramide accumulate in the kidney, cerebellum, lung, and stomach of Farber’s disease patients [25, 26], suggesting that hFA-ceramide/sphingolipids are continuously synthesized and turned over in normal cells in these organs. Interestingly, mouse mutants with defective saposin D accumulate hFA-ceramide in kidney and cerebellum, resulting in renal tubular degeneration and a selective loss of cerebellar Purkinje cells [27]. Saposin D is one of five known sphingolipid activator proteins that bind distinct sphingolipids and “present” the sphingolipid substrates to lysosomal hydrolases [28]. Based on the findings in human acid ceramidase deficiency and saposin D knockout mice, it appears that saposin D and acid ceramidase function together to keep hFA-ceramide from accumulating. The reason for the selective susceptibility of certain cell types to accumulated hFA-ceramide could be a “traffic jam” effect that interferes with lipid metabolism, or more specific cytotoxicity of hFA-ceramide. Ceramide can induce apoptosis in many cell types, and there is some evidence for enhanced apoptosis-inducing activity of hFA-ceramide compared to non-hydroxy ceramide [29]. This effect is likely cell type-specific, as hFA-ceramide had significantly lower activity than non-hydroxy ceramide in inducing DNA fragmentation in human promonocytic U937 cells [30].
Fig. 3. Degradation of hFA-sphingolipids.
Endocytosed sphingolipids are first hydrolyzed by lysosomal hydrolases to ceramide. hFA-ceramide is hydrolyzed by acid ceramidase with the assistance of saposin D. Currently, there is no direct evidence for the recycling of hFA via a salvage pathway. The only known degradation pathway of hFA is the peroxisomal α-oxidation pathway, in which one-carbon cleavage of even-chain fatty acids (C2n) generates odd-chain fatty acids (C2n-1). How hFA is transported from the lysosome to peroxisome is not known. SL, sphingolipids; Sph, sphingosine; DHC, dihydroceramide; CerS, dihydroceramide synthase.
2.5 Peroxisomal α-oxidation pathway and odd-chain fatty acids
Hydrolysis of hFA-ceramide by ceramidases yields hFA and sphingosine (Fig. 3). Metabolism of sphingosine has been extensively studied and will not be discussed here. The fate of hFA is not fully understood, and evidence for a salvage pathway to recycle hFA for the biosynthesis of hFA-ceramide is indirect. The only known pathway for degradation of hFA is the peroxisomal α-oxidation [31]. As mentioned in §2.2, this pathway has been studied in association with degradation of branched-chain fatty acids. The study by Foulon et al. demonstrates that the enzyme that catalyzes the one-carbon cleavage, 2-hydroxyacyl-CoA lyase, can utilize straight-chain fatty acyl CoA. When an even-chain hFA is oxidized in this process, an odd-chain fatty acid is produced, which could be further degraded by β-oxidation.
Odd-chain fatty acids can be synthesized de novo by priming of fatty acid synthesis with propionyl-CoA instead of acetyl-CoA, which occurs at a negligible rate in most cells. Interestingly, early studies showed a substantial amount of odd-chain fatty acids in rat brain GalCer (both hydroxy and non-hydroxy) [32, 33], and their levels increased with age [32, 33]. The presence of these odd-chain fatty acids cannot be fully explained by priming with propionyl-CoA. In fact, there is evidence for two pathways for generating odd-chain fatty acids in the brain; one is by the fatty acid synthase reaction primed with propionyl-CoA [34], and the other is shortening of even-chain fatty acid by one carbon via α-oxidation [35]. The study by Foulon et al. provides additional evidence that the peroxisomal α-oxidation pathway is an integral part of the metabolism of hFA in the brain (Fig. 3). Once activated to CoA esters, the hFA entering the peroxisomes would be ready for the cleavage by 2-hydroxyacyl-CoA lyase. A portion of the odd-chain fatty acids produced by α-oxidation may be degraded by β-oxidation, and the remaining may enter the synthesis pathway in the ER, resulting in GalCer with odd-chain fatty acids. Some of the odd-chain fatty acids can be 2-hydroxylated by FA2H in the ER, giving rise to odd-chain hFA-GalCer. This seems an inefficient way of recycling fatty acids, compared to direct recycling of hFA in a salvage pathway. This hypothetical pathway may serve the cells to maintain an optimal hydroxy/non-hydroxy fatty acid ratio, which can be achieved by modulating the balance between fatty acid 2-hydroxylation and α-oxidation of hFA.
3. TISSUE DISTRIBUTION OF HFA-SPHINGOLIPIDS AND THEIR BIOLOGICAL FUNCTIONS
3.1 hFA-sphingolipids in the nervous system
In the mammalian nervous system, nerve conduction is greatly facilitated by myelin, a lipid-rich membrane that wraps around the axon. The myelin sheath is a specialized structure with distinct lipid and protein constituents. Galactosylceramide (GalCer) and sulfatide make up approximately 30% of total myelin lipids, and more than half of these galactolipids contains hFA as their N-acyl chains [36]. No other mammalian tissues contain such high concentrations of hFA. The specific function of hFA-galactolipids in myelin is not fully understood, but there has been significant advance in our knowledge in the past few years.
The necessity of hFA-sphingolipids for the normal function of the nervous system became evident when mutations in the FA2H gene were discovered in patients with hereditary leukodystrophy with spastic paraparesis [11]. This study was conducted with 9 patients in three families who had normal early development but presented with gait disturbance due to spasticity in the lower limbs at 4–6 years old. In 2 patients from one family the disease was restricted to the lower limbs with no cognitive or speech impairment. In contrast, the disease rapidly progressed in 7 patients from the other two families. These 7 patients required walking aids at 7 years old and presented with spasticity extended to the upper limbs; dystonia in the trunk, limbs, and face; upper-motor neuron deficits; decline in cognitive abilities; and cerebellar dysfunction. Brain MRI indicated progressive white matter degeneration.
Homozygosity mapping and subsequent sequencing of candidate genes led to identification of two mutations in the FA2H gene. The 7 patients with the severe form of the disease carried a homozygous intronic point mutation that resulted in aberrant FA2H mRNA lacking exon 5 and exon 6. These exons encode 3 of the 4 putative transmembrane domains of FA2H that encompass the 8 conserved histidine residues (see Fig. 2). Targeted deletion of these exons in mice resulted in loss of hFA-galactolipids in both the central and peripheral nervous systems, providing additional evidence that the loss of hFA-galactolipids is the cause of this disorder (our unpublished data).
The two patients with the milder form of the disease carried a homozygous point mutation resulting in replacement of Asp 35 to Tyr in the N-terminal cytochrome b5 domain of FA2H. The Asp residue is conserved in all known cytochrome b5, including cytochrome b5 domains of various enzymes, suggesting an essential function of this Asp in electron transport. The signs and symptoms of the milder form of the disease fall under the category of hereditary spastic paraplegia. Interestingly, one of the loci associated with hereditary spastic paraplegia, SPG35, has been mapped to chromosome region 16q21-q23 [37], the same region as the FA2H locus. It is possible that SPG35 is an allelic form of FA2H.
Interestingly, fibroblasts isolated from patients with FA2H mutations showed fatty acid 2-hydroxylase activity in vitro, indicating that there is a second enzyme that is not present in the nervous system [11]. The identity of the hypothetical second enzyme is currently unknown.
The clinical findings of human FA2H deficiency indicate that hFA-sphingolipids are dispensable for the onset of myelination, but myelin is highly unstable in the absence of hFA-galactolipids and begins to disintegrate even before myelination is complete. Interestingly, targeted inactivation of the mouse Fa2h gene presented a different picture [38]. Despite the complete loss of hFA-sphingolipids, cellular, histological, and clinical phenotypes of Fa2h null mice are reportedly indistinguishable from the wild type mice for the most of their life. However, aged Fa2h-null mice had scattered axonal degeneration and loss of myelin in both the central and peripheral nervous systems. These observations indicate that hFA-sphingolipids are entirely dispensable for developmental myelination in mice, and are needed only for long-term stability of axons and myelin. Possibly, mice may have a mechanism that compensates for the deficits caused by the loss of hFA-sphingolipids. Such a mechanism could provide insight into the development of potential therapeutics to prevent and treat human FA2H deficiency.
3.2 hFA-Sphingolipids in the skin
The outermost layer of mammalian epidermis is the stratum corneum, which is made of flattened, enucleated keratinocytes and a unique extracellular lipid matrix produced by differentiating keratinocytes. The stratum corneum provides the permeability barrier against water and various environmental agents, such as chemicals and microorganisms. About half of the lipids in the stratum corneum are mixtures of ceramides, 40% of which contain hFA [39]. These ceramides, together with cholesterol and free fatty acids (25% and 10% of the stratum corneum lipids, respectively) are essential for the permeability barrier function of the skin [40, 41].
A study with cultured human skin keratinocytes demonstrated that FA2H provides the precursor hFA for the synthesis of the unique epidermal hFA-ceramides [19]. Interestingly, FA2H-silencing in cultured human keratinocytes greatly disturbed formation of the unique extracellular lipid matrix. During normal differentiation, these cells produce characteristic “lamellar bodies” filled with pieces of lipid lamellae, which are secreted outside the cell and assembled into extracellular lipid layers. With FA2H-silencing, formation and secretion of lamellar bodies were markedly diminished, and extracellular lipid layers were not formed. Thus, hFA-sphingolipids are not only the cargo in these lamellar bodies, but also play an essential role in controlling formation and transport of lamellar bodies. It should be noted that neither human FA2H deficiency, nor targeted inactivation of mouse Fa2h gene, causes obvious skin permeability barrier dysfunction. This is likely due to a second fatty acid 2-hydroxylase that allows the cells to proceed with differentiation and to form functional stratum corneum in the absence of FA2H. The second enzyme may be the same as the one observed in fibroblasts from patients with FA2H deficiency.
3.3 hFA-sphingolipids in other tissues
Kishimoto and Radin first reported the presence of sphingolipid-associated hFA in several extraneural tissues (spleen, kidney, lung, skin, plasma) in 1963 [3]. Subsequently, a number of reports described various hFA-sphingolipids in many tissues and organs. The most extensively documented example outside of the nervous system and epidermis is hFA-GalCer in kidney and other organs. First described by Makita in 1964, GalCer in human kidney is primarily hFA-GalCer [42]. The presence of hFA-GalCer in several organs was also shown in the twitcher mouse, a mutant mouse line that possesses a mutation in the gene encoding galactosylceramidase (Galc) and cannot hydrolyze the head group of GalCer. Consequently, GalCer (predominantly hFA form) accumulates in the kidney, liver, and lung of the twitcher mice [43]. It became evident that in many instances, the N-acyl chain of GalCer is predominantly hFA, which gave rise to the misconception that hFA is only associated with GalCer and not with other sphingolipids. Apparently, hFA is readily incorporated into GalCer because of the subcellular localization and substrate preference of CGT. Among the biosynthetic enzymes that transfer a head group to ceramide, CGT is the only one localized in the ER [44]. Consequently, CGT comes in contact with ceramide/hFA-ceramide before other enzymes do in other organelles (mostly Golgi). With CGT’s strong preference for hFA-ceramide over non-hydroxy ceramide [23], most hFA-ceramide would be converted to GalCer in the ER if sufficient CGT was present, leaving little or no hFA-ceramide for SM or GlcCer synthesis. However, this does not mean that SM synthases and GlcCer synthase (GCS) are incapable of utilizing hFA-ceramide. In fact, a number of reports describe various hFA-sphingolipids other than hFA-GalCer in various organs. Representative studies are listed in Table 1. Based on the literature, it is safe to say that hFA can be incorporated into most, if not all, sphingolipids.
TABLE 1.
HFA-SPHINGOLIPIDS IN MAMMALSa
| hFA-SL species and source | hFA content | Major hFAb | Note | Ref |
|---|---|---|---|---|
| hFA-Ceramide | ||||
| Human erythrocyte | 0.9% | 24:0 (58.7%) 16:0 (18%) 22:0 (10.5%) | Cer content was 5.6 µmol/100 ml packed cells. |
[87] |
| Monkey intestinal mucosa | 63.1% in small intestine |
Cer was 22.4% of neutral SL. | [22] | |
| 72.9% in colon | Cer was 35.1% of neutral SL. | |||
| Horse kidney | 11.6% | 24:0 (29%) 23:0 (26%) 22:0 (22%) 20:0 (10%) |
[88] | |
| Sheep small intestinal mucosa |
31.6% in fetal tissue | 28:0 (34.8%) 26:0 (13.0%) 18:1 (9.8%) 30:0 (8.9%) 18:0 (6.6%) |
Cer was 40.2% of neutral SL. | [21] |
| 70.2% in adult tissue | 28:0 (35.5%) 30:0 (8.5%) 20:2 (6.8%) 18:1 (6.1%) |
Cer was 65.0% of neutral SL. | ||
| Sheep colonic mucosa | 29.7% in fetal tissue | Cer was 62.7% of neutral SL. | [20] | |
| 67.1% in adult tissue | Cer was 62.5% of neutral SL. | |||
| Rat small intestine | 11% | C20–C24 | Cer content was 0.16 µmol/ml packed cells. |
[89] |
| Rat liver | 0.5% at P10 1.4% in adult |
Cer content was 320 µg/g dry wt at P6, 700 µg/g dry wt at P14. |
[90] | |
| Rat kidney | 0.6% at P10 3.2% in adult |
Cer content was 180 µg/g dry wt at P6, 500 µg/g dry wt at P14. |
||
| Guinea pig Harderian gland | 14% | 18:0 (19.9%) 23:0 (18.5%) 19:0 (12.3%) 22:0 (11.7%) 20:0 (10.9%) |
Also contained branched-chain hFA (5.8% of hFA). |
[91] |
| hFA-SM | ||||
| Bovine Rennet stomach | 25% | 16:0 (28%) 24:0 (22%) 23:0 (20%) 22:0 (15%) |
[92] | |
| Bovine rennet stomach | 25% | 16:0 (38%) 23:0 (16%) 22:0 (12%) 24:0 (12%) |
[93] | |
| Bovine kidney, intestinal mucosa |
22:0, 23:0, 24:0 | [94] | ||
| Bovine milk | <1% | 23:0 (31.5%) 24:0 (21.8%) 22:0 (17.2%) 16:0 (9.2%) |
[95] | |
| Guinea pig Harderian gland | 30.5% | 18:0 (30.2%) 19:0 (20.0%) 20:0 (16.4%) | Also contained 1.4% branched-chain hFA (16-methyl 19:0). |
[96] |
| Ram, bull, rat, boar testes and spermatozoa; human spermatozoa |
In rat testis, undetectable at P20; 49% at P55 |
In rat testis, 28:4, 30:5, 31:5, 32:5 | [97] | |
| hFA-GlcCer/MHC | ||||
| Human liver GlcCer | 20% | 24:0 (46%) 22:0 (17%) 23:0 (17%) 24:1 (11%) |
GlcCer was 19.1% of neutral GSL. | [98] |
| Human kidney GlcCer | 49% | 24:0 (32.1%) 22:0 (23.2%) 23:0 (19.2%) 24:1 (12.5%) |
MHC (6–7% of neutral GSL) was a mixture of GlcCer and GalCer (1:1). |
[99] |
| Human ureteral epithelial cells GlcCer |
90% | 24:0, 20:0, 21:0, 22:0, 23:0, 25:0 | GlcCer was 75% of GSL | [100] |
| Human uterine endometrium GlcCer |
30.9% in the secretory phase 3.8% in the proliferative phase |
In the secretory phase, 24:0 (54.7%)24:1 (14.9 %) 16:0 (11.7%) 22:0 (11.3%) |
[101] | |
| Monkey intestinal mucosa MHC |
88.4% in small intestine |
MHC (21.8% of neutral SL) was a mixture of GlcCer (90%) and GalCer (10%). |
[22] | |
| 93.4% in colon | MHC (14.8% of neutral SL) was a mixture of GlcCer (82%) and GalCer (18%). |
|||
| Bovine kidney MHC | >95% | 22:0 16:0 | MHC was a mixture of GlcCer (75%) and GalCer (25%). |
[102] |
| Bovine milk GlcCer | <1% | 23:0 (31.0%) 24:0 (19.7%) 22:0 (16.7%) 16:0 (12.6%) |
[95] | |
| Horse kidney MHC | 78.9% | 23:0 (25%) 24:0 (23%) 22:0 (20%) | MHC was a mixture of GlcCer (70.5%) and GalCer (29.5%). |
[88] |
| Sheep small intestinal mucosa MHC |
36.3% in fetal tissue | 18:0 (23.1%) 18:2 (15.2%) 16:1 (12.9%) 22:0 (12.7%) 16:0 (12.1%) 20:0 (5.8%) |
MHC (25.5% of neutral SL) was a mixture of GlcCer (80%) and GalCer (20%). |
[21] |
| 69.1% in adult tissue | 18:0 (41.5%) 16:0 (21.4%) 18:1 (4.1%) | MHC (13.6% of neutral SL) was a mixture of GlcCer (90%) and GalCer (10%). |
||
| Sheep colonic mucosa MHC |
32.6% in fetal tissue | 18:1 (38.7%) 18.0 (36.8%) 18:2 (4.3%) | MHC (10.8% of neutral SL) was a mixture of GlcCer (80%) and GalCer (20%). |
[20] |
| 64.2% in adult tissue | 26:0 (45.2%) 16:0 (14.2%) 30:0 (12.2%) 28:0 (8.5%) 20:2 (6.8%) |
MHC (12.6% of neutral SL) was a mixture of GlcCer (80%) and GalCer (20%). |
||
| Rat stomach MHC | 55.5% in 20-day embryo 82.4% at P60 |
In 20-day embryo, 24:0 (57.8%) 22:0 (14.2%) 20:0 (7.0%) In P60, 24:0 (54%) 23:0 (21.7%) 22:0 (17.6%) |
MHC was a mixture of GlcCer (95%) and GalCer (5%). |
[103] |
| Rat intestinal villus and crypt cells GlcCer |
63–68% | C24 (28–29%) C22 (20–24%) C20 (10–16%) C24:1 (10–12%) |
[104] | |
| Rat small intestinal epithelial cells |
66% in villus tip | 24:0 (34.7%) 16:0 (19.7%) 22:0 (15.9%) 24:1 (14.0%) 20:0 (9.2%) 23:0 (5.4%) |
[105] | |
| 32% in crypt cells | 24:0 22:0 16:0 20:0 24:1 | |||
| Rat colon MHC | 40% at birth 75% in adult |
MHC (45–50% of neutral GSL) was mostly GlcCer (>90%) at birth and in adult |
[46] | |
| Mouse intestinal mucosa GlcCer |
90.5% | 24:1 (27.8%) 22:0 (26.0%) 24:0 (13.5%) 23:0 (12.0%) 16:0 (11.5%) 20:0 (6.4%) |
Asialo GM1 and GlcCer were the two major neutral GSL. |
[106] |
| hFA-LacCer | ||||
| Human liver | 10% | C24 (33%) C24:1 (18%) C23 (15%) C22 (13%) |
LacCer was 30.3% of neutral GSL. | [98] |
| Human Uterine endometrium |
26.2% in the secretory phase 4.7% in the proliferative phase |
In the secretory phase, C24:0 (50.4%) C24:1 (21.8 %) 16:0 (8.0%) |
[101] | |
| Monkey intestinal mucosa | 91.2% in small intestine |
28:0 (42.5%) 26:0 (11.4%) 18:0 (10.2%) | LacCer was 27.6% of neutral SL. | [22] |
| 93.6% in colon | 28:0 (43.9%) 18:2 (14.2%) 18:1 (13.6%) 26:0 (7.4%) |
LacCer was 26.6% of neutral SL. | ||
| Bovine milk | <1% | 24:0 (29.5%) 23:0 (26.9%) 22:0 (15.4%) 16:0 (10.5%) |
[95] | |
| hFA-GM3 | ||||
| Human liver | 43.1% | 22:0 (28.8%) 18:0 (17.7%) 16:0 (17.4%) | (84 yr old female) | [107] |
| Human liver | 30% | C24 (37%) C22 (20%) C23 (17%) C24:1 (15%) |
GM3 was 91.6% of liver gangliosides (11 yr old male). |
[98] |
| Human liver | 10% at 20 yr old 50% at 80 yr old |
22:0 (32.3%) 24:0 (25.0%) 23:0 (16.1%) 24:1 (9.8%) |
hFA composition was determined with a pool of 31 samples, 19–85 yr old. |
[48] |
| Monkey intestinal mucosa | 70.3% in small intestine |
28:0 (42.2%) 30:0 (31.2%) 16:1 (7.2%) | GM3 was 60.7% of gangliosides. | [22] |
| 76.4% in colon | 30:0 (50.9%) 28:0 (20.8%) 16:1 (7.9%) 26:0 (7.3%) |
GM3 was 61.6% of gangliosides. | ||
| Sheep small intestinal mucosa |
41.1% in fetal tissue | GM3 was 41.5% of gangliosides. | [21] | |
| 72.3% in adult tissue | GM3 was 25.2% of gangliosides. | |||
| Sheep colonic mucosa | 36.3% in fetal tissue | 26:0 (81.8%) 28:0 (5.5%) 24:0 (3.4%) 18:0 (2.4%) |
GM3 was 41.2% of gangliosides. | [20] |
| 72.1% in adult tissue | 30:0 (44.7%) 24:0 (16.1%) 28:0 (15.6%) 18:0 (8.8%) 26:0 (9.9%) 18.1 (2.7%) |
GM3 was 22.3% of gangliosides. | ||
| Rat intestinal villus and crypt cells |
71–72% | C24 (23–25%) C22 (21–22%) C16 (14–15%) C24:1 (10–15%) C20 (9–14%) |
[104] | |
| Rat small intestine | 69% | 16:0 20:0 22:0 24:0 24:1 | hFA-GM3 was concentrated in the epithelial tissue. GM3 in non-epithelial tissue contained no hFA. |
[108] |
| Rat small intestine | no hFA at P0; 26.0% at P21; 41.5% at P38 |
Majority of hFA-GM3 was in epithelium. |
[45] | |
| Adult rat small intestinal epithelium |
49.4–82% among different strains |
[109] | ||
| Other hFA-SLs | ||||
| Gangliosides in human kidney |
11.7–28.9% | 24:0 23:0 24:1 22:0 | Values are for 2 main gangliosides and 2 minor gangliosides. |
[110] |
| Globotriaosyl ceramide in human uterine endometrium |
22.2% in the secretory phase 4.8% in the proliferative phase |
In the secretory phase, C24:0 (51.8%) C24:1 (29.7 %) 16:0 (7.7%) |
[101] | |
| THC in sheep small intestinal mucosa |
42.2% in fetal tissue | 28:0 (29.4%) 26:0 (16.1%) 24:0 (11.8%) 16:0 (5.7) |
THC was 17.3% of neutral SL. | [21] |
| 68.3% in adult tissue | 18:0 (64.1%) 16:0 (20.8%) 18:1 (10.4%) 18:2 (4.2%) |
THC was 18.9% of neutral SL. | ||
| GD1a in sheep small intestinal mucosa |
34.3% in fetal tissue | 24:0 (28.0%) 26:0 (25.1%) 18:0 (24.2%) 16:0 (7.3%) 20:0 (6.4%) 20:1 (4.4%) |
GD1a was 47.2% of gangliosides. | |
| 68.3% in adult tissue | 26:0 (55.9%) 18:0 (16.7%) 24:0 (14.6%) 22:1 (4.5%) |
GD1a was 48.3% of gangliosides. | ||
| THC in sheep colonic mucosa |
40.1% in fetal tissue | THC was 25.5% of neutral SL. | [20] | |
| 69.4% in adult tissue | THC was 14.7% of neutral SL. | |||
| GD1a in sheep colonic mucosa |
31.2% in fetal tissue | GD1a was 47.5% of gangliosides. | ||
| 67.4% in adult tissue | GD1a was 50.7% of gangliosides. | |||
| Asialo GM1 in mouse intestinal mucosa |
79.8% | 22:0 (27.2%) 24:1 (23.6%) 16:0 (21.4%) 23:0 (10.3%) 20:0 (10.2%) 24:0 (7.0%) |
Asialo GM1 and GlcCer were the two major neutral GSL. |
[106] |
This table does not include hFA-sphingolipids that have been extensively documented (GalCer and sulfatide in the nervous system, kidney, and other organs; epidermal sphingolipids).
Percent composition was calculated among hFA and does not include non-hydroxy FA.
P, postnatal day; MHC, monohexosylceramide; DHC, dihexosylcermaide; THC, tetrahexosylceramide. Neutral SL includes ceramide.
In most cases shown in Table 1, physiological functions of hFA-sphingolipids are unknown, but a couple of interesting facts are noteworthy. When developing animals were studied, a dramatic increase in hFA content was found in different tissues. For instance, myelin GalCer is mostly the non-hydroxy form, and the hFA content increased during developmental myelination [6]. A similar developmental change occurs in intestinal GlcCer. In neonatal rodents, intestinal GlcCer contains mostly non-hydroxy FA, and shifts to predominantly hFA during development [45–47]. A number of other examples are shown in Table 1. Another interesting transition is the ganglioside GM3 in human liver. Riboni et al. found that hFA-GM3 was relatively minor in the liver of young adults, but that it continued to increase with age [48]. Although the physiological significance of such a transition is not fully understood, it may relate to the developmental and/or environmental requirement for higher stability/rigidity of the membrane in which these lipids reside.
An important physical characteristic of sphingolipids is the hydrogen-bonding capacity of ceramide, which allows lateral intermolecular interactions and influences the stability and permeability of the membrane [49]. Pascher speculated that the renal tubular cells and intestinal epithelial cells stand the physical stress in part due to the high hFA-sphingolipid contents [49]. Several biophysical studies support this hypothesis. The crystal structure of synthetic GalCer with 2-hydroxyoctadecanoic acid indicated that the 2-hydroxyl group participated in an extensive lateral network of hydrogen bonds between the galactose head group and the polar part of the ceramide backbone [50]. The monolayer behavior of synthetic ceramides revealed that the 2-hydroxyl group promoted the condensation to a close-packed arrangement [51]. Boggs and coworkers demonstrated that the 2-hydroxyl group in sphingolipids increased their phase transition temperature, suggesting that the 2-hydroxyl group contributes to the hydrogen-bonding network and stabilizes the gel phase of the lipids [52]. To confirm these effects in vivo, genetically modified cells and animals with or without hFA-sphingolipids would be needed. One such study using Fa2h knockout mice showed that detergent resistance of myelin lipid microdomains was indistinguishable between control and mutant mice, whereas long-term stability of myelin was clearly compromised in the mutant mice [38]. Human patients with FA2H deficiency underwent normal early development, followed by progressive white matter degeneration [11]. These studies foretell that efforts to determine physiological roles of hFA-sphingolipids, especially the long-term effect, will likely face significant challenges.
3.4 hFA-sphingolipids in cancer
Cancer cells often express aberrant cell surface antigens. Glycosphingolipids (GSL) are the first demonstrated tumor-associated cell surface antigens [53], many of which facilitate uncontrolled cell growth and metastasis [54, 55]. Interestingly, some human tumors express aberrant GSL containing relatively high levels of hFA. These tumors include ovarian tumors [56], neuroblastomas [57], small cell lung carcinomas [58], and colon and liver adenocarcinomas [59, 60]. Based on these observations, Ladisch proposed that elevated fatty acid 2-hydroxylation is a characteristic metabolic change in some human tumors [57]. In a study of transformed human urothelial cell lines, hFA-GSL were found in highly tumorigenic and invasive cell lines, but not in non-tumorigenic, non-invasive cell lines [61]. Whether hFA-GSL has specific roles in facilitating tumorigenesis and metastasis is a fascinating question, and could be addressed by targeted silencing/deletion of FA2H or another fatty acid 2-hydroxylase, whichever present in the cancer cells of interest.
Recently, Iwamori and colleagues reported a striking increase in hFA-GSL in drug-resistant human ovarian carcinoma cell lines [62, 63]. The drug-resistance in these cells correlates with the amount of drug efflux pumps, suggesting an intriguing possibility that hFA-GSL may modulate the expression, stability, and/or activities of these pumps. It is also conceivable that elevated hFA-GSL is a byproduct of metabolic activities associated with drug resistance, such as upregulation of cytochrome P450 enzymes, many of which are hydroxylases. Some of them may catalyze fatty acid 2-hydroxylation, resulting in elevated hFA.
In a recent study of the antitumor drug PM02734, Herrero et al. reported that overexpression of FA2H increased the sensitivity of human colorectal and cervical carcinoma cells to this drug, while silencing FA2H rendered the cells resistant to the drug [64]. It is unlikely that the observed effect was non-specific permeabilization of the plasma membrane because the sensitivity to methyl methanesulfonate was not affected. It appears that hFA-sphingolipids interact with this drug and facilitate its uptake by the cell.
4. HFA-SPHINGOLIPIDS IN CELL SIGNALING
The remarkable expansion of the research on sphingolipid-mediated cell signaling was triggered by the discovery of ceramide as potential lipid second messenger [65]. A key discovery was the activation of hydrolysis of SM by vitamin D, leading to a transient increase of ceramide, which in turn stimulated cell differentiation [66]. In subsequent studies, enhanced de novo ceramide synthesis and reduced conversion to GlcCer were also found to be part of the stress response that increase cellular ceramide concentrations (for review, see [67]). In most ceramide-mediated signaling studies, no attention was paid to hFA-ceramide. As mentioned in §2.4, one study reported inactivity of hFA-ceramide in inducing DNA fragmentation in human promonocytic U937 cells [30].
In a recent study, we provided evidence that hFA-sphingolipids regulate cAMP-signaling in rat D6P2T Schwannoma cells [68]. Similar to Schwann cells, D6P2T cells respond to cAMP and show differentiation phenotypes, including withdrawal from the cell cycle and upregulation of myelin basic protein [69, 70]. The cAMP-induced exit from the cell cycle is, in part, mediated by upregulation of the cyclin-dependent kinase inhibitors p21 and p27 [70, 71]. We found that cAMP-induced upregulation of p21 and p27 is greatly diminished by silencing Fa2h. This finding suggests that hFA or hFA-sphingolipids contribute to cAMP-dependent signaling pathways that regulate the cell cycle, thereby facilitating Schwann cell differentiation. Though the exact hFA-lipid species involved in this regulation has not been defined, altered Fa2h expression appears to influence phosphorylation/dephosphorylation status of a subset of proteins in response to cAMP (our unpublished observation). Identification of these proteins will provide clues to the pathways regulated by hFA-lipids.
For hFA-sphingolipids to participate in specific signaling pathways, there must be specific targets that recognize hFA-sphingolipids. A question arises as to whether proteins and other molecules can distinguish hFA-sphingolipids from non-hydroxy counterparts, and compelling evidence suggests that this is true for hFA-GSL. Some anti-GSL monoclonal antibodies and bacterial toxins have higher affinity for hFA-GSL than for non-hydroxy equivalents [72–74]. Cell surface GSLs often serve as specific binding sites for bacteria, some of which bind only to hFA-GSL and not to the non-hydroxy form, and vice versa [75–79]. The effects of hFA in these examples could be attributed to the hydrogen bonding capability mentioned in §3.3. The 2-hydroxyl group can influence the conformation of the carbohydrate head groups via hydrogen bonds [50], which could affect affinity for specific binding proteins. The evidence for molecular recognition between hFA-ceramide and non-hydroxy ceramide is scarcer. As mentioned in §2.4, there are indications for higher apoptosis-inducing activity of hFA-ceramide in some cell types [29].
In a non-sphingolipid area of lipid research, studies have uncovered potent biological activities of 2-hydroxyoleic acid (HOA). HOA was initially developed as a synthetic oleic acid analog and found to have potent anti-cancer activities in vitro and in animal models [80–82]. The anti-cancer activity of HOA is mediated, at least in part, by downregulation of dihydrofolate reductase [83]. HOA also has anti-hypertensive activity in rats [84] and modulates contractility of cardiomyocytes [85]. The mechanism of HOA action is not fully understood, and it has been attributed to its structural effects on cell membranes, rather than specific interactions with target proteins. However, specific fatty acid-protein interactions are well-documented, and there is an example of a G protein-coupled receptor GPR109B that binds with hFA (both 2- and 3-hydroxyoctanoic acid) [86]. Hence, bioactivities of HOA could well be mediated by specific interactions with target proteins.
What is not mentioned in the literature of HOA is that endogenous HOA is present as a component of hFA-sphingolipids in rat kidney, lung, plasma, and skin [3], and in intestinal epithelial cells of monkey [22] and sheep [20, 21]. Thus, endogenous and exogenous HOA appear to show distinct biological activities. One possible explanation for this might be that endogenous free hFA is very rapidly converted to hFA-ceramide in the ER or degraded in the peroxisomes, which would keep cellular free hFA levels extremely low. If this is the case, modulating the rate of hFA-ceramide synthesis or hFA degradation could be an effective mechanism to regulate cell signaling mediated by HOA and possibly by other hFA. This is highly speculative, and further investigation is warranted to determine the mechanism of action of HOA and to determine whether specific binding proteins for hFA and hFA-sphingolipids exist.
5. FUTURE PERSPECTIVES
This review illustrates a few issues that were often overlooked in mammalian sphingolipid studies. First, hFA can be incorporated into many, if not all, complex sphingolipids. Second, hFA-sphingolipids are present in many mammalian tissues and cell types. It may even be true that all cells contain hFA-sphingolipids, ranging from barely detectable levels to relatively high levels. As evident from this review, the current knowledge about the metabolism and physiological function of hFA-sphingolipids is very limited. At the cellular level, many questions remain to be addressed, such as trafficking and subcellular localization, potential targets, and selective stability/instability of hFA-sphingolipids.
A recent and emerging theme in sphingolipid biology is that the bioactivity of sphingolipids can be greatly influenced by the structure of the sphingoid base and the N-acyl chain of ceramide [67]. The physiological significance and the underlying mechanism for such diversity is only beginning to be understood. The precise composition of various ceramide species is determined by the relative activities of enzymes responsible for synthesis and degradation of various sphingoid bases, fatty acids, and ceramides. In the case of hFA-sphingolipids, the expression of FA2H is highly variable among cell types, and is inducible during cellular differentiation and by certain stimuli. In addition, there is a second enzyme that can produce hFA. With combinatorial up- and down-regulation of enzymes, the cell can adjust the portfolio of various ceramides in response to the ever-changing cellular environment, thereby fine-tuning the overall metabolic outcomes regulated by bioactive sphingolipids. In order to draw a complete picture of biology of not only hFA-sphingolipids but of all sphingolipids, the “ceramide code” needs to be deciphered. Fortunately, the field of sphingolipid biology is advancing rapidly with ever-evolving analytical technologies, molecular genetic tools and animal models. The study of hFA-sphingolipids will no doubt benefit from these advances, and many unanswered questions will be addressed in the near future. The complete translation of the ceramide code may become available soon.
Acknowledgement
The author thanks Dr. Chiara Luberto for critically reading the manuscript. The work from the author’s laboratory was supported by National Institutes of Health Grants NS056075 and NS060807.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Pruett ST, Bushnev A, Hagedorn K, Adiga M, Haynes CA, Sullards MC, Liotta DC, Merrill AH., Jr Biodiversity of sphingoid bases ("sphingosines") and related amino alcohols. J Lipid Res. 2008;49:1621–1639. doi: 10.1194/jlr.R800012-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Deuel HJ. Chemistry of the Phosphatides and Cerebrosides. In: Deuel HJ, editor. The Lipids, Their Chemistry and Biochemistry. vol. 1. New York: Chemistry, Interscience Publishers; 1951. pp. 405–496. [Google Scholar]
- 3.Kishimoto Y, Radin NS. Occurrence of 2-Hydroxy Fatty Acids in Animal Tissues. J Lipid Res. 1963;4:139–143. [PubMed] [Google Scholar]
- 4.Hoshi M, Kishimoto Y. Synthesis of cerebronic acid from lignoceric acid by rat brain preparation. Some properties and distribution of the -hydroxylation system. J Biol Chem. 1973;248:4123–4130. [PubMed] [Google Scholar]
- 5.Akanuma H, Kishimoto Y. Synthesis of ceramides and cerebrosides containing both alpha-hydroxy and nonhydroxy fatty acids from lignoceroyl-CoA by rat brain microsomes. J Biol Chem. 1979;254:1050–1060. [PubMed] [Google Scholar]
- 6.Kishimoto Y, Akanuma H, Singh I. Fatty acid alpha-hydroxylation and its relation to myelination. Mol Cell Biochem. 1979;28:93–105. doi: 10.1007/BF00223361. [DOI] [PubMed] [Google Scholar]
- 7.Shigematsu H, Hisanari Y, Kishimoto Y. Alpha-hydroxylation of lignoceroyl-CoA in rat brain microsomes: involvement of NADPH-cytochrome c reductase and topical distribution. Int J Biochem. 1990;22:1427–1432. doi: 10.1016/0020-711x(90)90233-s. [DOI] [PubMed] [Google Scholar]
- 8.Shigematsu H, Kishimoto Y. Alpha-hydroxylation of lignoceroyl-CoA by a cyanide-sensitive oxygenase in rat brain microsomes. Int J Biochem. 1987;19:41–46. doi: 10.1016/0020-711x(87)90121-2. [DOI] [PubMed] [Google Scholar]
- 9.Alderson NL, Rembiesa BM, Walla MD, Bielawska A, Bielawski J, Hama H. The human FA2H gene encodes a fatty acid 2-hydroxylase. J Biol Chem. 2004;279:48562–48568. doi: 10.1074/jbc.M406649200. [DOI] [PubMed] [Google Scholar]
- 10.Eckhardt M, Yaghootfam A, Fewou SN, Zoller I, Gieselmann V. A mammalian fatty acid hydroxylase responsible for the formation of alpha-hydroxylated galactosylceramide in myelin. Biochem J. 2005;388:245–254. doi: 10.1042/BJ20041451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Edvardson S, Hama H, Shaag A, Gomori JM, Berger I, Soffer D, Korman SH, Taustein I, Saada A, Elpeleg O. Mutations in the fatty acid 2-hydroxylase gene are associated with leukodystrophy with spastic paraparesis and dystonia. Am J Hum Genet. 2008;83:643–648. doi: 10.1016/j.ajhg.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shanklin J, Cahoon EB. Desaturation and related modifications of fatty acids. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1998;49:611–641. doi: 10.1146/annurev.arplant.49.1.611. [DOI] [PubMed] [Google Scholar]
- 13.Alderson NL, Walla MD, Hama H. A novel method for the measurement of in vitro fatty acid 2-hydroxylase activity by gas chromatography-mass spectrometry. J Lipid Res. 2005;46:1569–1575. doi: 10.1194/jlr.D500013-JLR200. [DOI] [PubMed] [Google Scholar]
- 14.Alderson NL, Maldonado EN, Kern MJ, Bhat NR, Hama H. FA2H-dependent fatty acid 2-hydroxylation in postnatal mouse brain. J Lipid Res. 2006;47:2772–2780. doi: 10.1194/jlr.M600362-JLR200. [DOI] [PubMed] [Google Scholar]
- 15.Wanders RJ, Jansen GA, Lloyd MD. Phytanic acid alpha-oxidation, new insights into an old problem: a review. Biochim Biophys Acta. 2003;1631:119–135. doi: 10.1016/s1388-1981(03)00003-9. [DOI] [PubMed] [Google Scholar]
- 16.Mukherji M, Kershaw NJ, Schofield CJ, Wierzbicki AS, Lloyd MD. Utilization of sterol carrier protein-2 by phytanoyl-CoA 2-hydroxylase in the peroxisomal alpha oxidation of phytanic acid. Chem Biol. 2002;9:597–605. doi: 10.1016/s1074-5521(02)00139-4. [DOI] [PubMed] [Google Scholar]
- 17.Searls T, Butler D, Chien W, Mukherji M, Lloyd MD, Schofield CJ. Studies on the specificity of unprocessed and mature forms of phytanoyl-CoA 2-hydroxylase and mutation of the iron binding ligands. J Lipid Res. 2005;46:1660–1667. doi: 10.1194/jlr.M500034-JLR200. [DOI] [PubMed] [Google Scholar]
- 18.Mizutani Y, Kihara A, Chiba H, Tojo H, Igarashi Y. 2-Hydroxy-ceramide synthesis by ceramide synthase family: enzymatic basis for the preference of FA chain length. J Lipid Res. 2008;49:2356–2364. doi: 10.1194/jlr.M800158-JLR200. [DOI] [PubMed] [Google Scholar]
- 19.Uchida Y, Hama H, Alderson NL, Douangpanya S, Wang Y, Crumrine DA, Elias PM, Holleran WM. Fatty acid 2-hydroxylase, encoded by FA2H, accounts for differentiation-associated increase in 2-OH ceramides during keratinocyte differentiation. J Biol Chem. 2007;282:13211–13219. doi: 10.1074/jbc.M611562200. [DOI] [PubMed] [Google Scholar]
- 20.Dahiya R, Brasitus TA. Glycosphingolipids of fetal and adult sheep colonic mucosa. Lipids. 1985;20:331–336. doi: 10.1007/BF02534198. [DOI] [PubMed] [Google Scholar]
- 21.Dahiya R, Brasitus TA. Glycosphingolipid patterns of fetal and adult small intestinal mucosa in the sheep. Biochim Biophys Acta. 1986;875:220–226. doi: 10.1016/0005-2760(86)90171-2. [DOI] [PubMed] [Google Scholar]
- 22.Dahiya R, Brown MD, Brasitus TA. Distribution of glycosphingolipids of monkey small and large intestinal mucosa. Lipids. 1986;21:107–111. doi: 10.1007/BF02534429. [DOI] [PubMed] [Google Scholar]
- 23.Schaeren-Wiemers N, van der Bijl P, Schwab ME. The UDP-galactose:ceramide galactosyltransferase: expression pattern in oligodendrocytes and Schwann cells during myelination and substrate preference for hydroxyceramide. J Neurochem. 1995;65:2267–2278. doi: 10.1046/j.1471-4159.1995.65052267.x. [DOI] [PubMed] [Google Scholar]
- 24.Futerman AH, van Meer G. The cell biology of lysosomal storage disorders. Nat Rev Mol Cell Biol. 2004;5:554–565. doi: 10.1038/nrm1423. [DOI] [PubMed] [Google Scholar]
- 25.Sugita M, Connolly P, Dulaney JT, Moser HW. Fatty acid composition of free ceramides of kidney and cerebellum from a patient with Farber's disease. Lipids. 1973;8:401–406. doi: 10.1007/BF02531715. [DOI] [PubMed] [Google Scholar]
- 26.Sugita M, Iwamori M, Evans J, McCluer RH, Dulaney JT, Moser HW. High performance liquid chromatography of ceramides: application to analysis in human tissues and demonstration of ceramide excess in Farber's disease. J Lipid Res. 1974;15:223–226. [PubMed] [Google Scholar]
- 27.Matsuda J, Kido M, Tadano-Aritomi K, Ishizuka I, Tominaga K, Toida K, Takeda E, Suzuki K, Kuroda Y. Mutation in saposin D domain of sphingolipid activator protein gene causes urinary system defects and cerebellar Purkinje cell degeneration with accumulation of hydroxy fatty acid-containing ceramide in mouse. Hum Mol Genet. 2004;13:2709–2723. doi: 10.1093/hmg/ddh281. [DOI] [PubMed] [Google Scholar]
- 28.Kolter T, Sandhoff K. Principles of lysosomal membrane digestion: stimulation of sphingolipid degradation by sphingolipid activator proteins and anionic lysosomal lipids. Annu Rev Cell Dev Biol. 2005;21:81–103. doi: 10.1146/annurev.cellbio.21.122303.120013. [DOI] [PubMed] [Google Scholar]
- 29.Kyogashima M, Tadano-Aritomi K, Aoyama T, Yusa A, Goto Y, Tamiya-Koizumi K, Ito H, Murate T, Kannagi R, Hara A. Chemical and apoptotic properties of hydroxy-ceramides containing long-chain bases with unusual alkyl chain lengths. J Biochem. 2008;144:95–106. doi: 10.1093/jb/mvn050. [DOI] [PubMed] [Google Scholar]
- 30.Ji L, Zhang G, Uematsu S, Akahori Y, Hirabayashi Y. Induction of apoptotic DNA fragmentation and cell death by natural ceramide. FEBS Lett. 1995;358:211–214. doi: 10.1016/0014-5793(94)01428-4. [DOI] [PubMed] [Google Scholar]
- 31.Foulon V, Sniekers M, Huysmans E, Asselberghs S, Mahieu V, Mannaerts GP, Van Veldhoven PP, Casteels M. Breakdown of 2-hydroxylated straight chain fatty acids via peroxisomal 2-hydroxyphytanoyl-CoA lyase: a revised pathway for the alpha-oxidation of straight chain fatty acids. J Biol Chem. 2005;280:9802–9812. doi: 10.1074/jbc.M413362200. [DOI] [PubMed] [Google Scholar]
- 32.Kishimoto Y, Radin NS. Isolation and determination methods for brain cerebrosides, hydroxy fatty acids, and unsaturated and saturated fatty acids. J. Lipid Res. 1959;1:72–78. [Google Scholar]
- 33.Kishimoto Y, Radin NS. Composition of cerebroside acids as a function of age. J. Lipid Res. 1959;1:79–82. [Google Scholar]
- 34.Hajra AK, Norman SR. Biosynthesis of the cerebroside odd-numbered fatty acids. J. Lipid Res. 1962;3:327–332. [Google Scholar]
- 35.Mead JF, Levis GM. A 1 carbon degradation of the long chain fatty acids of brain sphingolipids. J Biol Chem. 1963;238:1634–1636. [PubMed] [Google Scholar]
- 36.Norton WT, Cammer W. In: Isolation and characterization of myelin. Morell P, editor. New York: Myelin, Plenum; 1984. [Google Scholar]
- 37.Dick KJ, Al-Mjeni R, Baskir W, Koul R, Simpson MA, Patton MA, Raeburn S, Crosby AH. A novel locus for an autosomal recessive hereditary spastic paraplegia (SPG35) maps to 16q21-q23. Neurology. 2008;71:248–252. doi: 10.1212/01.wnl.0000319610.29522.8a. [DOI] [PubMed] [Google Scholar]
- 38.Zoller I, Meixner M, Hartmann D, Bussow H, Meyer R, Gieselmann V, Eckhardt M. Absence of 2-hydroxylated sphingolipids is compatible with normal neural development but causes late-onset axon and myelin sheath degeneration. J Neurosci. 2008;28:9741–9754. doi: 10.1523/JNEUROSCI.0458-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Downing DT. Lipid and protein structures in the permeability barrier of mammalian epidermis. J. Lipid Res. 1992;33:301–313. [PubMed] [Google Scholar]
- 40.Feingold KR. Thematic review series: skin lipids. The role of epidermal lipids in cutaneous permeability barrier homeostasis. J Lipid Res. 2007;48:2531–2546. doi: 10.1194/jlr.R700013-JLR200. [DOI] [PubMed] [Google Scholar]
- 41.Holleran WM, Takagi Y, Uchida Y. Epidermal sphingolipids: metabolism, function, and roles in skin disorders. FEBS Lett. 2006;580:5456–5466. doi: 10.1016/j.febslet.2006.08.039. [DOI] [PubMed] [Google Scholar]
- 42.Makita A. Biochemistry of Organ Glycolipids. Ii. Isolation of Human Kidney Glycolipids. J Biochem. 1964;55:269–276. doi: 10.1093/oxfordjournals.jbchem.a127880. [DOI] [PubMed] [Google Scholar]
- 43.Igisu H, Suzuki K. Glycolipids of the spinal cord, sciatic nerve, and systemic organs of the twitcher mouse. J Neuropathol Exp Neurol. 1984;43:22–36. doi: 10.1097/00005072-198401000-00002. [DOI] [PubMed] [Google Scholar]
- 44.Sprong H, Kruithof B, Leijendekker R, Slot JW, van Meer G, van der Sluijs P. UDP-galactose:ceramide galactosyltransferase is a class I integral membrane protein of the endoplasmic reticulum. J Biol Chem. 1998;273:25880–25888. doi: 10.1074/jbc.273.40.25880. [DOI] [PubMed] [Google Scholar]
- 45.Bouhours D, Bouhours JF. Developmental changes of hematoside of rat small intestine. Postnatal hydroxylation of fatty acids and sialic acid. J Biol Chem. 1983;258:299–304. [PubMed] [Google Scholar]
- 46.Bouhours D, Bouhours JF. Developmental changes of monohexosylceramide and free ceramide in the large intestine of the rat. J Biochem. 1985;98:1359–1366. doi: 10.1093/oxfordjournals.jbchem.a135403. [DOI] [PubMed] [Google Scholar]
- 47.Bouhours JF, Bouhours D, Hansson GC. Developmental changes of glycosphingolipid composition of epithelia of rat digestive tract. Adv Lipid Res. 1993;26:353–372. [PubMed] [Google Scholar]
- 48.Riboni L, Acquotti D, Casellato R, Ghidoni R, Montagnolo G, Benevento A, Zecca L, Rubino F, Sonnino S. Changes of the human liver GM3 ganglioside molecular species during aging. Eur J Biochem. 1992;203:107–113. doi: 10.1111/j.1432-1033.1992.tb19834.x. [DOI] [PubMed] [Google Scholar]
- 49.Pascher I. Molecular arrangements in sphingolipids. Conformation and hydrogen bonding of ceramide and their implication on membrane stability and permeability. Biochim. Biophys. Acta. 1976;455:433–451. doi: 10.1016/0005-2736(76)90316-3. [DOI] [PubMed] [Google Scholar]
- 50.Pascher I, Sundell S. Molecular arrangements in sphingolipids. The crystal structure of cerebroside. Chem. Phys. Lipids. 1977;20:175–191. [Google Scholar]
- 51.Lofgren H, Pascher I. Molecular arrangements of sphingolipids. The monolayer behavior of ceramides. Chem. Phys. Lipids. 1977;20:273–284. doi: 10.1016/0009-3084(77)90068-8. [DOI] [PubMed] [Google Scholar]
- 52.Boggs JM, Koshy KM, Rangaraj G. Influence of structural modifications on the phase behavior of semi- synthetic cerebroside sulfate. Biochim. Biophys. Acta. 1988;938:361–372. doi: 10.1016/0005-2736(88)90134-4. [DOI] [PubMed] [Google Scholar]
- 53.Rapport MM, Graf L. Immunochemical reactions of lipids. Prog Allergy. 1969;13:273–331. [PubMed] [Google Scholar]
- 54.Birkle S, Zeng G, Gao L, Yu RK, Aubry J. Role of tumor-associated gangliosides in cancer progression. Biochimie. 2003;85:455–463. doi: 10.1016/s0300-9084(03)00006-3. [DOI] [PubMed] [Google Scholar]
- 55.Hakomori S. Tumor malignancy defined by aberrant glycosylation and sphingo(glyco)lipid metabolism. Cancer Res. 1996;56:5309–5318. [PubMed] [Google Scholar]
- 56.Kiguchi K, Takamatsu K, Tanaka J, Nozawa S, Iwamori M, Nagai Y. Glycosphingolipids of various human ovarian tumors: a significantly high expression of I3SO3GalCer and Lewis antigen in mucinous cystadenocarcinoma. Cancer Res. 1992;52:416–421. [PubMed] [Google Scholar]
- 57.Ladisch S, Sweeley CC, Becker H, Gage D. Aberrant fatty acyl alpha-hydroxylation in human neuroblastoma tumor gangliosides. J Biol Chem. 1989;264:12097–12105. [PubMed] [Google Scholar]
- 58.Nilsson O, Brezicka FT, Holmgren J, Sorenson S, Svennerholm L, Yngvason F, Lindholm L. Detection of a ganglioside antigen associated with small cell lung carcinomas using monoclonal antibodies directed against fucosyl-GM1. Cancer Res. 1986;46:1403–1407. [PubMed] [Google Scholar]
- 59.Hakomori S, Nudelman E, Levery SB, Kannagi R. Novel fucolipids accumulating in human adenocarcinoma. I. Glycolipids with di- or trifucosylated type 2 chain. J Biol Chem. 1984;259:4672–4680. [PubMed] [Google Scholar]
- 60.Yang HJ, Hakomori SI. A sphingolipid having a novel type of ceramide and lacto-N-fucopentaose 3. J Biol Chem. 1971;246:1192–1200. [PubMed] [Google Scholar]
- 61.Ugorski M, Pahlsson P, Dus D, Nilsson B, Radzikowski C. Glycosphingolipids of human urothelial cell lines with different grades of transformation. Glycoconj J. 1989;6:303–318. doi: 10.1007/BF01047850. [DOI] [PubMed] [Google Scholar]
- 62.Iwamori M, Iwamori Y, Kubushiro K, Ishiwata I, Kiguchi K. Characteristic expression of Lewis-antigenic glycolipids in human ovarian carcinoma-derived cells with anticancer drug-resistance. J Biochem. 2007;141:309–317. doi: 10.1093/jb/mvm031. [DOI] [PubMed] [Google Scholar]
- 63.Kiguchi K, Iwamori Y, Suzuki N, Kobayashi Y, Ishizuka B, Ishiwata I, Kita T, Kikuchi Y, Iwamori M. Characteristic expression of globotriaosyl ceramide in human ovarian carcinoma-derived cells with anticancer drug resistance. Cancer Sci. 2006;97:1321–1326. doi: 10.1111/j.1349-7006.2006.00326.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Herrero AB, Astudillo AM, Balboa MA, Cuevas C, Balsinde J, Moreno S. Levels of SCS7/FA2H-mediated fatty acid 2-hydroxylation determine the sensitivity of cells to antitumor PM02734. Cancer Res. 2008;68:9779–9787. doi: 10.1158/0008-5472.CAN-08-1981. [DOI] [PubMed] [Google Scholar]
- 65.Hannun YA. Functions of ceramide in coordinating cellular responses to stress. Science. 1996;274:1855–1859. doi: 10.1126/science.274.5294.1855. [DOI] [PubMed] [Google Scholar]
- 66.Okazaki T, Bell RM, Hannun YA. Sphingomyelin turnover induced by vitamin D3 in HL-60 cells. Role in cell differentiation. J Biol Chem. 1989;264:19076–19080. [PubMed] [Google Scholar]
- 67.Hannun YA, Obeid LM. Principles of bioactive lipid signalling: lessons from sphingolipids. Nat Rev Mol Cell Biol. 2008;9:139–150. doi: 10.1038/nrm2329. [DOI] [PubMed] [Google Scholar]
- 68.Alderson NL, Hama H. Fatty acid 2-hydroxylase regulates cAMP-induced cell cycle exit in D6P2T Schwannoma cells. J Lipid Res. 2009;50:1203–1208. doi: 10.1194/jlr.M800666-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Clark R, Stewart M, Miskimins WK, Miskimins R. Involvement of MAP kinase in the cyclic AMP induction of myelin basic protein gene expression. Int J Dev Neurosci. 1998;16:323–331. doi: 10.1016/s0736-5748(98)00045-8. [DOI] [PubMed] [Google Scholar]
- 70.Friessen AJ, Miskimins WK, Miskimins R. Cyclin-dependent kinase inhibitor p27kip1 is expressed at high levels in cells that express a myelinating phenotype. J Neurosci Res. 1997;50:373–382. doi: 10.1002/(SICI)1097-4547(19971101)50:3<373::AID-JNR3>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 71.Atanasoski S, Boller D, De Ventura L, Koegel H, Boentert M, Young P, Werner S, Suter U. Cell cycle inhibitors p21 and p16 are required for the regulation of Schwann cell proliferation. Glia. 2006;53:147–157. doi: 10.1002/glia.20263. [DOI] [PubMed] [Google Scholar]
- 72.Kannagi R, Stroup R, Cochran NA, Urdal DL, Young WW, Jr, Hakomori S. Factors affecting expression of glycolipid tumor antigens: influence of ceramide composition and coexisting glycolipid on the antigenicity of gangliotriaosylceramide in murine lymphoma cells. Cancer Res. 1983;43:4997–5005. [PubMed] [Google Scholar]
- 73.Nakakuma H, Arai M, Kawaguchi T, Horikawa K, Hidaka M, Sakamoto K, Iwamori M, Nagai Y, Takatsuki K. Monoclonal antibody to galactosylceramide: discrimination of structural difference in the ceramide moiety. FEBS Lett. 1989;258:230–232. doi: 10.1016/0014-5793(89)81660-6. [DOI] [PubMed] [Google Scholar]
- 74.Binnington B, Lingwood D, Nutikka A, Lingwood CA. Effect of globotriaosyl ceramide fatty acid alpha-hydroxylation on the binding by verotoxin 1 and verotoxin 2. Neurochem Res. 2002;27:807–813. doi: 10.1023/a:1020261125008. [DOI] [PubMed] [Google Scholar]
- 75.Angstrom J, Teneberg S, Milh MA, Larsson T, Leonardsson I, Olsson BM, Halvarsson MO, Danielsson D, Naslund I, Ljungh A, Wadstrom T, Karlsson KA. The lactosylceramide binding specificity of Helicobacter pylori. Glycobiology. 1998;8:297–309. doi: 10.1093/glycob/8.4.297. [DOI] [PubMed] [Google Scholar]
- 76.Stromberg N, Karlsson KA. Characterization of the binding of propionibacterium granulosum to glycosphingolipids adsorbed on surfaces. An apparent recognition of lactose which is dependent on the ceramide structure. J Biol Chem. 1990;265:11244–11250. [PubMed] [Google Scholar]
- 77.Stromberg N, Karlsson KA. Characterization of the binding of Actinomyces naeslundii (ATCC 12104) and Actinomyces viscosus (ATCC 19246) to glycosphingolipids, using a solid-phase overlay approach. J Biol Chem. 1990;265:11251–11258. [PubMed] [Google Scholar]
- 78.Stromberg N, Ryd M, Lindberg AA, Karlsson KA. Studies on the binding of bacteria to glycolipids. Two species of Propionibacterium apparently recognize separate epitopes on lactose of lactosylceramide. FEBS Lett. 1988;232:193–198. doi: 10.1016/0014-5793(88)80415-0. [DOI] [PubMed] [Google Scholar]
- 79.Tang W, Seino K, Ito M, Konishi T, Senda H, Makuuchi M, Kojima N, Mizuochi T. Requirement of ceramide for adhesion of Helicobacter pylori to glycosphingolipids. FEBS Lett. 2001;504:31–35. doi: 10.1016/s0014-5793(01)02759-4. [DOI] [PubMed] [Google Scholar]
- 80.Llado V, Gutierrez A, Martinez J, Casas J, Teres S, Higuera M, Galmes A, Saus C, Besalduch J, Busquets X, Escriba PV. Minerval Induces Apoptosis in Jurkat and Other Cancer Cells. J Cell Mol Med. 2008 doi: 10.1111/j.1582-4934.2008.00625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Martinez J, Gutierrez A, Casas J, Llado V, Lopez-Bellan A, Besalduch J, Dopazo A, Escriba PV. The repression of E2F-1 is critical for the activity of Minerval against cancer. J Pharmacol Exp Ther. 2005;315:466–474. doi: 10.1124/jpet.105.088716. [DOI] [PubMed] [Google Scholar]
- 82.Martinez J, Vogler O, Casas J, Barcelo F, Alemany R, Prades J, Nagy T, Baamonde C, Kasprzyk PG, Teres S, Saus C, Escriba PV. Membrane structure modulation, protein kinase C alpha activation, and anticancer activity of minerval. Mol Pharmacol. 2005;67:531–540. doi: 10.1124/mol.104.000778. [DOI] [PubMed] [Google Scholar]
- 83.Llado V, Teres S, Higuera M, Alvarez R, Noguera-Salva MA, Halver JE, Escriba PV, Busquets X. Pivotal role of dihydrofolate reductase knockdown in the anticancer activity of 2-hydroxyoleic acid. Proc Natl Acad Sci U S A. 2009;106:13754–13758. doi: 10.1073/pnas.0907300106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Alemany R, Teres S, Baamonde C, Benet M, Vogler O, Escriba PV. 2-hydroxyoleic acid: a new hypotensive molecule. Hypertension. 2004;43:249–254. doi: 10.1161/01.HYP.0000107778.85528.b5. [DOI] [PubMed] [Google Scholar]
- 85.Borchert GH, Giggey M, Kolar F, Wong TM, Backx PH, Escriba PV. 2-hydroxyoleic acid affects cardiomyocyte [Ca2+]i transient and contractility in a region-dependent manner. Am J Physiol Heart Circ Physiol. 2008;294:H1948–H1955. doi: 10.1152/ajpheart.01209.2007. [DOI] [PubMed] [Google Scholar]
- 86.Ahmed K, Tunaru S, Langhans CD, Hanson J, Michalski CW, Kolker S, Jones PM, Okun JG, Offermanns S. Deorphanization of GPR109B as a receptor for the beta-oxidation intermediate 3-OH-octanoic acid and its role in the regulation of lipolysis. J Biol Chem. 2009;284:21928–21933. doi: 10.1074/jbc.M109.019455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bouhours JF, Bouhours D. Identification of free ceramide in human erythrocyte membrane. J Lipid Res. 1984;25:613–619. [PubMed] [Google Scholar]
- 88.Hara A, Taketomi T. Long chain base and fatty acid compositions of equine kidney sphingolipids. J Biochem. 1975;78:527–536. doi: 10.1093/oxfordjournals.jbchem.a130937. [DOI] [PubMed] [Google Scholar]
- 89.Bouhours JF, Guignard H. Free ceramide, sphingomyelin, and glucosylceramide of isolated rat intestinal cells. J Lipid Res. 1979;20:879–907. [PubMed] [Google Scholar]
- 90.Iwamori M, Costello C, Moser HW. Analysis and quantitation of free ceramide containing nonhydroxy and 2-hydroxy fatty acids, and phytosphingosine by high-performance liquid chromatography. J Lipid Res. 1979;20:86–96. [PubMed] [Google Scholar]
- 91.Yasugi E, Kasama T, Seyama Y. Composition of long chain bases in ceramide of the guinea pig Harderian gland. J Biochem. 1991;110:202–206. doi: 10.1093/oxfordjournals.jbchem.a123557. [DOI] [PubMed] [Google Scholar]
- 92.Karlsson K, Nilsson K, Samuelsson BE, Steen GO. The presence of hydroxy fatty acids in sphingomyelins of bovine rennet stomach. Biochim Biophys Acta. 1969;176:660–663. doi: 10.1016/0005-2760(69)90239-2. [DOI] [PubMed] [Google Scholar]
- 93.Breimer ME. Distribution of molecular species of sphingomyelins in different parts of bovine digestive tract. J Lipid Res. 1975;16:189–194. [PubMed] [Google Scholar]
- 94.Breimer ME, Karlsson KA, Samuelsson BE. Presence of phytosphingosine combined with 2-hydroxy fatty acids in sphingomyelins of bovine kidney and intestinal mucosa. Lipids. 1975;10:17–19. doi: 10.1007/BF02532188. [DOI] [PubMed] [Google Scholar]
- 95.Morrison WR, Hay JD. Polar lipids in bovine milk. II. Long-chain bases, normal and 2-hydroxy fatty acids, and isomeric cis and trans monoenoic fatty acids in the sphingolipids. Biochim Biophys Acta. 1970;202:460–467. doi: 10.1016/0005-2760(70)90116-5. [DOI] [PubMed] [Google Scholar]
- 96.Yasugi E, Kasama T, Shibahara M, Seyama Y. Composition of long-chain bases in sphingomyelin of the guinea pig Harderian gland. Biochem Cell Biol. 1990;68:154–160. doi: 10.1139/o90-021. [DOI] [PubMed] [Google Scholar]
- 97.Robinson BS, Johnson DW, Poulos A. Novel molecular species of sphingomyelin containing 2-hydroxylated polyenoic very-long-chain fatty acids in mammalian testes and spermatozoa. J Biol Chem. 1992;267:1746–1751. [PubMed] [Google Scholar]
- 98.Nilsson O, Svennerholm L. Characterization and quantitative determination of gangliosides and neutral glycosphingolipids in human liver. J Lipid Res. 1982;23:327–334. [PubMed] [Google Scholar]
- 99.Martensson E. Neutral glycolipids of human kidney isolation, identification, and fatty acid composition. Biochim Biophys Acta. 1966;116:296–308. doi: 10.1016/0005-2760(66)90012-9. [DOI] [PubMed] [Google Scholar]
- 100.Breimer ME, Hansson GC, Leffler H. The specific glycosphingolipid composition of human ureteral epithelial cells. J Biochem. 1985;98:1169–1180. doi: 10.1093/oxfordjournals.jbchem.a135383. [DOI] [PubMed] [Google Scholar]
- 101.Mikami M, Tukazaki K, Nozawa S, Iwamori M, Nagai Y. Menstrual cycle-associated expression of 2-hydroxy fatty acyl phytosphingosine-containing GlcCer, LacCer and Gb3Cer in human uterine endometrium. Biochim Biophys Acta. 1992;1125:104–109. doi: 10.1016/0005-2760(92)90162-o. [DOI] [PubMed] [Google Scholar]
- 102.Karlsson KA, Samuelsson BE, Steen GO. Separation of monoglycosylceramides (cerebrosides) of bovine kidney into subgroups and characterization by mass spectrometry. Biochim Biophys Acta. 1973;306:317–328. doi: 10.1016/0005-2760(73)90237-3. [DOI] [PubMed] [Google Scholar]
- 103.Bouhours D, Bouhours JF. Developmental changes of the lipidic part of the neutral glycosphingolipids of the rat stomach. J Biol Chem. 1985;260:2172–2177. [PubMed] [Google Scholar]
- 104.Bouhours JF, Glickman RM. Rat intestinal glycolipids. III. Fatty acids and long chain bases of glycolipids from villus and crypt cells. Biochim Biophys Acta. 1977;487:51–60. doi: 10.1016/0005-2760(77)90043-1. [DOI] [PubMed] [Google Scholar]
- 105.Breimer ME, Hansson GC, Karlsson KA, Leffler H. Studies on differentiating epithelial cells of rat small intestine. Alterations in the lipophilic part of glycosphingolipids during cell migration from crypt villus tip. Biochim Biophys Acta. 1982;710:415–427. doi: 10.1016/0005-2760(82)90125-4. [DOI] [PubMed] [Google Scholar]
- 106.Umesaki Y, Suzuki A, Kasama T, Tohyama K, Mutai M, Yamakawa T. Presence of asialo GM1 and glucosylceramide in the intestinal mucosa of mice and induction of fucosyl asialo GM1 by conventionalization of germ-free mice. J Biochem. 1981;90:1731–1738. doi: 10.1093/oxfordjournals.jbchem.a133650. [DOI] [PubMed] [Google Scholar]
- 107.Seyfried TN, Ando S, Yu RK. Isolation and characterization of human liver hematoside. J Lipid Res. 1978;19:538–543. [PubMed] [Google Scholar]
- 108.Angstrom J, Breimer ME, Falk KE, Griph I, Hansson GC, Karlsson KA, Leffler H. Separation and characterization of hematosides with different sialic acids and ceramides from rat small intestine. Different composition of epithelial cells versus non-epithelial tissue and of duodenum versus jejunum-ileum. J Biochem. 1981;90:909–921. doi: 10.1093/oxfordjournals.jbchem.a133579. [DOI] [PubMed] [Google Scholar]
- 109.Bouhours D, Bouhours JF. Tissue-specific expression of GM3(NeuGc) and GD3(NeuGc) in epithelial cells of the small intestine of strains of inbred rats. Absence of NeuGc in intestine and presence in kidney gangliosides of brown Norway and spontaneously hypertensive rats. J Biol Chem. 1988;263:15540–15545. [PubMed] [Google Scholar]
- 110.Rauvala H. Gangliosides of human kidney. J Biol Chem. 1976;251:7517–7520. [PubMed] [Google Scholar]
- 111.Ito A, Sato R. Proteolytic microdissection of smooth-surfaced vesicles of liver microsomes. J Cell Biol. 1969;40:179–189. doi: 10.1083/jcb.40.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Dunn TM, Haak D, Monaghan E, Beeler TJ. Synthesis of monohydroxylated inositolphosphorylceramide (IPC-C) in Saccharomyces cerevisiae requires Scs7p, a protein with both a cytochrome b5-like domain and a hydroxylase/desaturase domain. Yeast. 1998;14:311–321. doi: 10.1002/(SICI)1097-0061(19980315)14:4<311::AID-YEA220>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 113.Mitchell AG, Martin CE. Fah1p, a Saccharomyces cerevisiae cytochrome b5 fusion protein, and its Arabidopsis thaliana homolog that lacks the cytochrome b5 domain both function in the alpha-hydroxylation of sphingolipid-associated very long chain fatty acids. J Biol Chem. 1997;272:28281–28288. doi: 10.1074/jbc.272.45.28281. [DOI] [PubMed] [Google Scholar]