Abstract
Cytogenetic aberrations identified by metaphase cytogenetics (MC) have important diagnostic, prognostic and therapeutic roles in myelodysplastic syndromes (MDS). Fluorescence in situ hybridization (FISH) complements MC by the ability to evaluate large numbers of both interphase and metaphase nuclei. However, clinically practical FISH strategies are limited to detection of known lesions. Single nucleotide polymorphism array (SNP-A)-based karyotyping can reveal unbalanced defects with superior resolution over MC and FISH and identify segmental uniparental disomy (UPD) undetectable by either method. Using a standardized approach, we focused our investigation on detection of -5/del(5q), -7/del(7q), trisomy 8 and del(20q) in patients with MDS (N=52), MDS/myeloproliferative overlap syndromes (N=7) and acute myeloid leukemia (N=15) using MC, FISH and SNP-A karyotyping. The detection rate for del(5q) was 30%, 32% and 32% by MC, FISH, and SNP-A, respectively. No single method detected all defects, and detection rates improved when all methods were used. The rate for detection of del(5q) increased incrementally to 35% (MC+FISH), 38% (MC+SNP-A), 38% (FISH+SNP-A) and 39% (all 3 methods). Similar findings were observed for -7/del(7q), trisomy 8 and -20/del(20q). We conclude that MC, FISH and SNP-A are complementary techniques that, when applied and interpreted together, can improve the diagnostic yield for identifying genetic lesions in MDS and contribute to the better description of abnormal karyotypes.
Keywords: MDS, FISH, metaphase cytogenetics, SNP arrays
INTRODUCTION
In myelodysplastic syndromes (MDS), cytogenetic aberrations have an important diagnostic and prognostic role and can affect the choice of therapies1,2. Consequently, the impact of cytogenetics is well reflected in its role in the International Prognostic Scoring System (IPSS)3. Three newer technologies that may complement metaphase cytogenetics (MC) include fluorescence in situ hybridization (FISH),4,5 comparative genomic hybridization (CGH) arrays6,7 and single nucleotide polymorphism arrays (SNP-A)8. Several FISH strategies, including combined control and target probes for identifying specific genomic deletions or amplifications, dual fusion probes for identifying specific translocations and break-apart probes for identifying specific gene rearrangements involving multiple potential partner genes are now in widespread use. The most commonly applied FISH panels in MDS include probes for detection of -5/del(5q), -7/del(7q), del(20q) and trisomy 8. The major advantage of FISH is its relatively high sensitivity with regard to the number of scorable cells as compared with the routine analysis of only 20 cells by MC. The clinical relevance of low percentages of abnormal cells that are near the cut-off value in FISH assays remains unclear, apart from residual disease detection in patients with a previously characterized abnormality. In particular, the precision for detecting deletions varies with the probe used, and small populations that represent less than 6–8% of the total cells may fall beneath the threshold of detection for the assay9.
SNP-A-based karyotyping can be applied for cytogenetic analysis of unbalanced rearrangements in interphase cells. Due to its superb resolution and unique ability to detect copy number-neutral loss of heterozygosity (CN-LOH) analogous to uniparental disomy (UPD), this new technology has been shown to effectively complement MC in detection of chromosomal lesions in clonal myeloid disorders including MDS. We and others have previously shown that SNP-A-based karyotyping improves the detection rate of certain lesions8,10,11, often undetectable by MC.
The development of novel therapeutics and prognostic schemes in MDS now requires identification of specific lesions to determine eligibility for clinical protocols and prognosis. Examples include the need to identify del5q- to guide therapy with lenalidomide and the adverse prognostic significance of monosomy 7.
We sought to determine whether the overall diagnostic yield for detecting commonly-recurring genetic defects associated with MDS could be improved using a strategy incorporating MC, FISH and SNP-A karyotyping. Using a standardized approach, we focused our investigation on the detection of -5/del(5q), -7/del(7q), trisomy 8 and del(20q), and analyzed individual and combined results to assess the diagnostic utility of the individual methods.
PATIENTS AND METHODS
Patients
Bone marrow aspirates and/or peripheral blood were collected from 74 patients with myeloid malignancies (mean age 64 years; range 17–87) seen at Cleveland Clinic between 2002 and 2008. Informed consent for sample collection was obtained according to protocols approved by the Cleveland Clinic IRB. Patients were grouped according to WHO classification and IPSS (Table 1).
Table 1.
General characteristics of patient cohort.
| WHO Classification | N | % | Sex (M/F) | Age, y (SE) | Mean IPSS |
|---|---|---|---|---|---|
| MDS | |||||
| Low grade | |||||
| RA/RCMD | 13 | 17.6 | 6/7 | 67 ± 21 | 0.65§ |
| RARS/RCMD-RS | 9 | 12.2 | 5/4 | 69 ± 8 | 0.72 |
| 5q- syndrome | 4 | 5.4 | 1/3 | 54 ± 22 | 0.25 |
| MDS-U | 4 | 5.4 | 3/1 | 70 ± 11 | 0.5§ |
| Advanced grade | |||||
| RAEB1/2 | 22 | 29.7 | 17/5 | 64 ± 13 | 1.89§ |
| MDS/MPN | |||||
| MDS/MPN-U | 4 | 5.4 | 3/1 | 79 ± 5 | 1.25§ |
| CMML1/2 | 3 | 4.1 | 3/0 | 76 ± 2 | 0.5 |
| AML | |||||
| Primary AML | 4 | 5.4 | 2/2 | 57 ± 22 | NA |
| Secondary AML* | 11 | 14.8 | 7/4 | 72 ± 7 | 2.5ψ |
NA, not applicable.
RAEB-T accounts for 3 of 8 secondary AML (WHO).
includes patients that cannot be analyzed for IPSS due to non-informative cytogenetics.
Only patients with secondary AML who fulfilled FAB criteria for RAEB-T were included in IPSS.
Cytogenetic analysis
Cytogenetic analysis was performed on marrow aspirates and/or peripheral blood according to standard methods. 20 metaphase spreads were examined per patient, if available. Chromosome preparations were G-banded using trypsin and Giemsa (GTG) and karyotypes were described according to ISCN (2005).
Fluorescence in situ hybridization
FISH analysis was performed on cell pellets from unstimulated cytogenetic cultures. Thresholds for interpretation as a positive result were established for each probe at 3 standard deviations above the mean of 20 normal bone marrow samples. In 27 cases, FISH analysis was performed at an outside reference laboratory using the following dual color probe sets: 5p15.2 (normal range; 0–4%)/EGR1 (5q31) (0–6%), CEP7 (0–5%)/7q31 (0–7%), CEP8 (0–2%)/MYC (8q24) (0–2%) and 20q12 (0–5%)/20qter (0–5%). In 47 cases, FISH was performed at Cleveland Clinic using three dual color probe sets (Abbott Molecular, Abbott Park, IL). The first probe set consisted of D5S23, D5S721 (5p15.2) labeled in Spectrum Green (0–6%) and EGR1 (5q31) labeled in Spectrum Orange (0-6%). The second probe set consisted of the chromosome 7 centromere labeled in Spectrum Green (0–5%) and D7S486 (7q31) labeled in Spectrum Orange (0–7%). The third probe set consisted of the chromosome 8 centromere labeled in Spectrum Green (0–8%) and D20S108 (20q12) labeled in Spectrum Orange (0–4%).
DNA extraction
DNA was extracted from whole bone marrow with the ArchivePure Kit (5Prime, Gaithersburg, MD) per manufacturer’s instructions. The concentration of the DNA was obtained using a ND-1000 spectrophotometer (NanoDrop, Wilmington, DE, USA). To study the germ line, T lymphocytes (CD3+) were isolated using RoboSep according to manufacturer’s protocol (StemCell Technologies, Vancouver, BC, Canada).
SNP array analysis
Affymetrix Gene Chip Mapping 250K Assay Kit or Genome-Wide Human SNP Assay Kit 6.0 (Affymetrix, Santa Clara, CA) was used for analysis of 52 and 22 samples, respectively. Following Nsp I digestion (New England Biolabs, Ipswich, MA), fragmented DNA was ligated to adaptor using T4 ligase (New England Biolabs) followed by PCR amplification. The PCR product was hybridized to the GeneChip Mapping 250K Array or Genome-Wide Human SNP Array 6.0, processed with the Fluidic Station 450 and scanned using the Gene Chip Scanner 3000 (Affymetrix).
Biostatistical evaluation of SNP-A data
For GeneChip Mapping 250K Array data, signal intensity and SNP calls were determined using Gene Chip Genotyping Analysis Software Version 4.0 (GTYPE). Copy number and LOH were investigated using Copy Number Analyzer for Affymetrix GeneChip Mapping (CNAG v. 3.0). For Genome-Wide Human SNP Array 6.0, the genotype calls for each individual were determined by the Birdseed version 1 genotype-calling algorithm, embedded in the software included with the Affymetrix Genotyping Console 2.0 (Affymetrix).
For detection of lesions we used the following diagnostic algorithm: lesions identified by SNP-A were compared with the Database of Genomic Variants (http://projects.tcag.ca/variation/) and our own internal control series to exclude known copy number variants (CNVs). In our internal control cohort, the largest area of CN-LOH we observed was 52.5 Mb and the average size of CN-LOH was 7.2 Mb. In addition, we observed that areas of LOH in controls were exclusively interstitial. Consequently, areas of LOH <24.8 Mb (mean size ± 2 SD) were excluded from analysis in the patient set. Deletions and gains of chromosomal material seen on metaphase karyograms and SNP-A samples that showed a concordantly normal karyotype by both metaphase cytogenetics and SNP-A testing were not further confirmed. When possible, all other remaining new defects were confirmed using paired analysis of CD3+ cells.
Statistical analysis
The detection rates of karyotypic abnormalities were evaluated by the Cochran Q test. The positivities of cytogenetic aberrations were compared by the Mann-Whitney U test or Spearman’s Rank Correlation Test.
RESULTS
Characteristics of patients and methodology
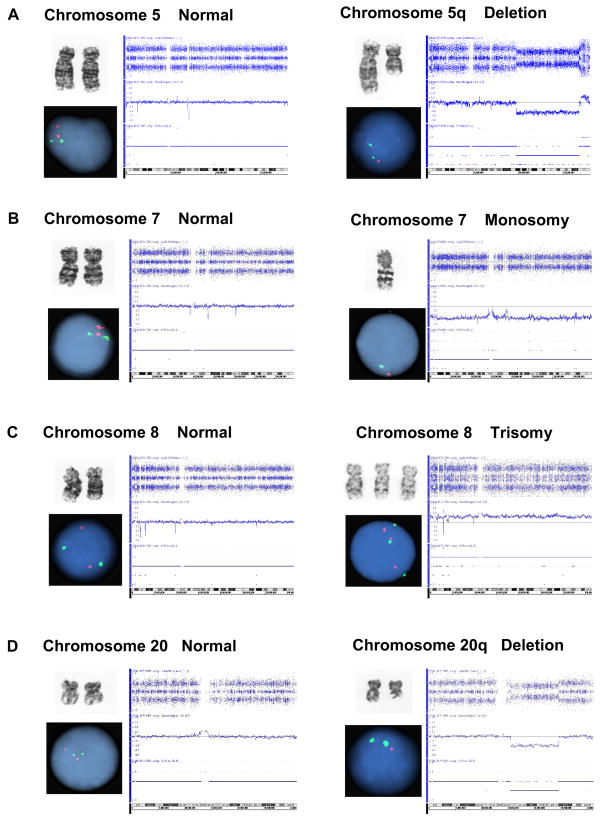
Our study included 74 patients with various forms of MDS evaluated at our institution for whom metaphase cytogenetics, FISH MDS panel and SNP-A-based karyotyping were available (Table 1). Only patients in whom all 3 methods were performed were compared, irrespective of the results. This is a logical approach to compare the diagnostic yield of various technologies. Examples of normal and abnormal results are presented in Figure 1. In the patient cohort studied, 40.6% showed low-risk MDS (RA, RCMD, RARS, RCMD-RS, or 5q-), 44.5% had high-risk disease (RAEB-1/2, secondary acute myeloid leukemia (sAML)), 9.5% had chronic myelomonocytic leukemia (CMML) 1/2 or other forms of myelodysplastic/myeloproliferative overlap syndromes (MDS/MPD) and 5.4% were diagnosed with de novo AML (Table 1). Routine metaphase cytogenetics allowed for detection of chromosomal abnormalities in 39 (52.7%) patients.
Figure 1. Results of metaphase cytogenetic, FISH and SNP-A analysis.
Examples of normal (left panels) and abnormal (right panels) results are presented. A. Deletion of chromosome 5q. B. Monosomy of chromosome 7. C. Trisomy of chromosome 8. D. Deletion of chromosome 20q.
Detection of del(5q), -7/del(7q), del(20q), and trisomy 8 with metaphase cytogenetics, FISH and SNP-array
Based on the hypothesis that a combination of cytogenetic technologies will improve the overall diagnostic yield for recurrent defects seen in MDS, we focused our investigations on the most frequently encountered lesions targeted by commercially-available MDS FISH probe panels including del(5q), -7/del(7q), trisomy 8 and del(20q) lesions (Figure 1). For del(5q), comparison of the methodologies showed a detection rate of 30%, 32%, and 32% by MC, FISH, and SNP-A, respectively. There was no significant difference in detection rates among these 3 methods. However, no single method detected all defects. Thus, combining these tests appears to increase the overall diagnostic yield. Similar results were obtained for -7/del(7q) with a detection rate of 10%, 12% and 13% for MC, FISH and SNP-A, respectively, for trisomy 8 with a detection rate of 8%, 8% and 12% for MC, FISH and SNP-A, respectively, and for del(20q) with a detection rate of 10%, 12% and 12% for MC, FISH and SNP-A, respectively (Table 2).
Table 2.
Abnormalities detected using metaphase cytogenetics, FISH and SNP-A alone or in combination.
| Anomaly | Number Examined | MC | FISH | SNP | MC+FISH | MC+SNP | FISH+SNP | MC+FISH+SNP |
|---|---|---|---|---|---|---|---|---|
| del(5q) | 74 | 22 | 24 | 24 | 26 | 28 | 28 | 29 |
| 30% | 32% | 32% | 35% | 38% | 38% | 39% | ||
| -7/del(7q) | 60 | 6 | 7 | 8 | 8 | 9 | 10 | 10 |
| 10% | 12% | 13% | 13% | 15% | 17% | 17% | ||
| Trisomy 8 | 60 | 5 | 5 | 7 | 6 | 8 | 9 | 9 |
| 8% | 8% | 12% | 10% | 13% | 15% | 15% | ||
| del(20q) | 60 | 6 | 7 | 7 | 7 | 8 | 8 | 8 |
| 10% | 12% | 12% | 12% | 13% | 13% | 13% | ||
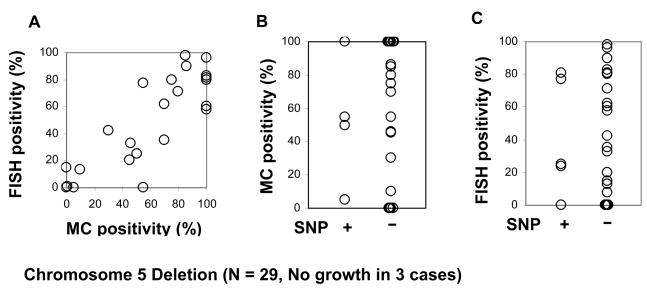
Next, we compared the size of the clones detected by FISH and MC. As expected, a strong correlation was found as demonstrated, for example, by del(5q) (r2= 0.82, P<0.0001; Fig 2A). However, the size of the clone as expressed by the number of positive metaphases (Fig. 2B) or by the percentage of positive cells by FISH (Fig. 2C) did not correlate with results of SNP-A karyotyping. For example, in some patients in whom defects were found by SNP-A, metaphase cytogenetics was negative, likely due to the low fraction of clonal cells undergoing division. Similar observations were also made for -7/del(7q), trisomy 8 and del(20q) (data not shown).
Figure 2. Comparison of detection rates of MC, FISH and SNP-A.
A. FISH results vs. MC results. A strong correlation was found when the proportion of del5q positive cells detected by FISH and the percentage of metaphase cells with del5q by MC were compared (r2 = 0.82, P<0.0001). B. MC results vs. SNP results. No correlation was found, likely due to the low fraction of clonal cells undergoing division in some patients. A similar observation was also made for del7/7q, trisomy8 and del20/20q (data not shown). C. FISH results vs. SNP results. No correlation was found between FISH and SNP-A findings.
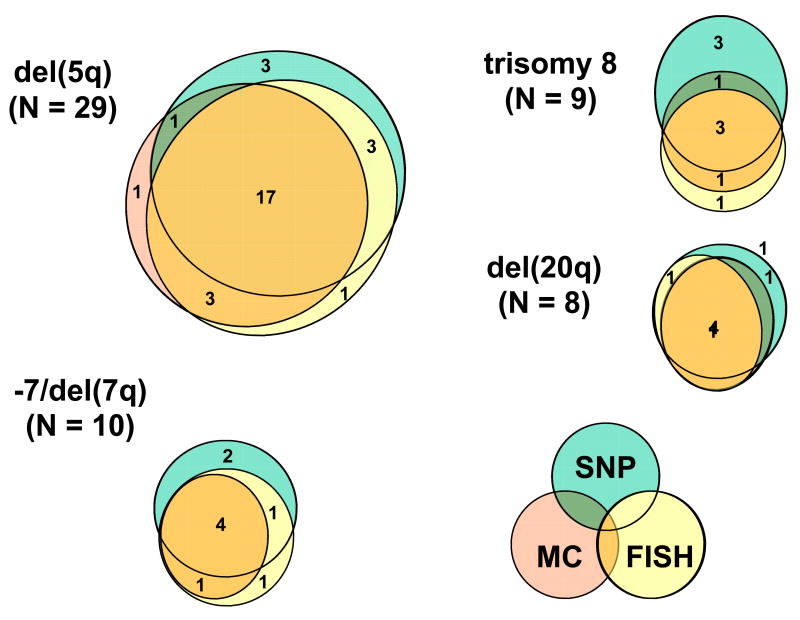
Overall, a combination of the 3 cytogenetic methods improved the detection rates for the abnormalities tested (Figure 3); e.g., for del(5q) the detection rate was increased to 35% (MC+FISH), 38% (MC+SNP-A), 38% (FISH+SNP-A) and 39% when all 3 methods were applied. Similar observations were made for -7/del(7q), trisomy 8, and del(20q): after combining all methods the detection rates improved from 10% to 17%, from 8% to 15%, and from 10% to 13%, respectively, as compared to MC alone (Table 2).
Figure 3. Combining cytogenetic techniques improves detection rate of chromosomal abnormalities in MDS.
Overall, a combination of the 3 cytogenetic methods improved the detection rates for the abnormalities tested. Shown are the results for del(5q), -7/del(7q), trisomy 8 and del(20q).
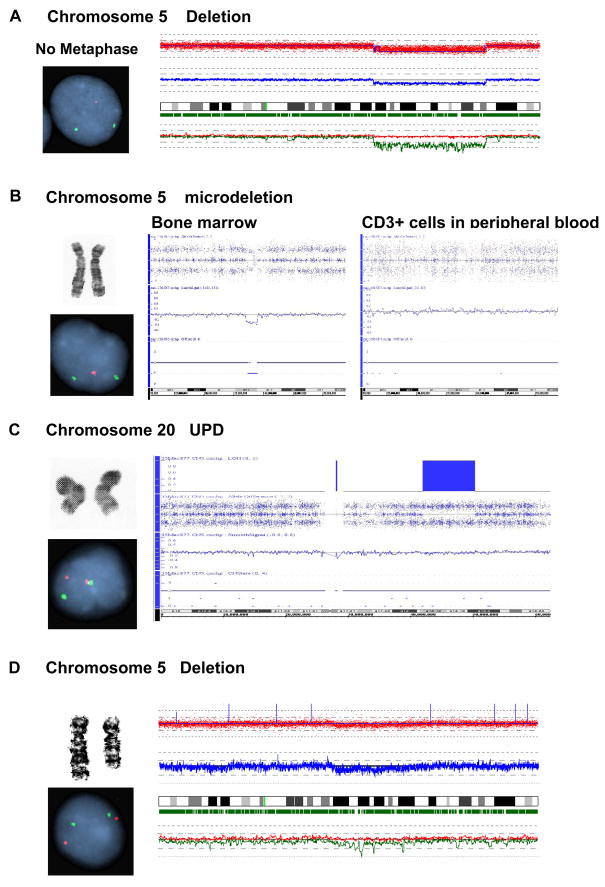
In some instances discrepant results were obtained for the specific techniques (Table 3). For MC, these were attributed to poor growth (N=3) (Figure 4A) and a low percentage of positive metaphases (small clonal size; N=2). In addition, small somatic deletions (N=6) and UPD (N=2) (Figure 4B, C) detected by SNP-A were not detected, as expected, by the other methods. Additionally, larger defects detected by SNP-A (e.g., from 5q14.2 to q23.1) were not detectable by FISH because the deletion did not involve either the control or target FISH probe sites located at 5p15.2 (D5S630) or 5q31 (EGR1), respectively (Figure 4D).
Table 3.
Interpretation of discrepancy among karyotypic lesions using MC, FISH and SNP-A.
| Anomaly | Patient | Metaphase cytogenetics | FISH | SNP array | Interpretation |
|---|---|---|---|---|---|
| 5q deletion | 1 | + | − | − | Small population was detected by MC [1/20]. |
| 21 | − | + | + | No metaphase cells were seen. | |
| 26 | − | − | + | UPD was detected by SNP array. | |
| 34 | − | − | + | A small lesion was detected by SNP array. | |
| 37 | − | − | + | UPD was detected by SNP array. | |
| 46 | − | + | + | A submicroscopic lesion in 5q31 was detected by FISH and SNP array. | |
| 51 | − | + | − | Small population (8%) was detected by FISH. | |
| 52 | − | + | + | No metaphase cells were seen. | |
| 55 | − | + | − | No metaphase cells were seen. Abnormality was seen in FISH using BM but not in SNP array using PB. | |
| 63 | + | + | − | Abnormality was seen in MC [11/20] and FISH using BM but not in SNP array using PB. | |
| 68 | + | + | − | Abnormality was seen in MC [8/8] and FISH using BM but not in SNP array using PB. | |
| 69 | + | + | − | Abnormality was seen in MC and FISH using [20/20] BM but not in SNP array using PB. | |
| 73 | + | − | + | Deletion was detected between 5q14.2 and 5q23.1 by SNP array. FISH probes were on 5p15.2 (D5S630) and 5q31. del(5)(q12q33) was seen in MC [11/20] | |
| -7/7q deletion | 8 | − | − | + | A small lesion was detected by SNP array. |
| 15 | − | − | + | A small lesion was detected by SNP array. | |
| 21 | − | + | − | No metaphase cells were seen in specimen used for FISH. SNP array specimen was from a different day. | |
| 27 | + | − | + | Metaphase cells had monosomy 7 [6/20], which was also detected by SNP array. | |
| 44 | − | + | + | Complex karyotype contained chromosome 7 and many marker chromosomes. | |
| 56 | + | + | − | Small population was detected by MC [8/20] and FISH. | |
| Trisomy 8 | 16 | − | + | − | Small population (4%) was detected by FISH. |
| 27 | + | − | + | Metaphase cells [6/20] showed increased number of chromosomes 8, which was also detected by SNP array. | |
| 42 | − | − | + | A small lesion was detected by SNP array. | |
| 45 | + | + | − | SNP array was not done on the same sample used for MC [20/20] and FISH. | |
| 47 | − | − | + | A small lesion was detected by SNP array. | |
| 58 | − | − | + | SNP array was not done on the same sample used for MC and FISH. | |
| 20q deletion | 25 | + | + | − | MC [20/20] and FISH using BM but SNP array using PB. |
| 33 | − | − | + | UPD was detected by SNP array. | |
| 41 | + | − | + | del(20q) 31,405,908-39,898,208 bp in SNP array and seen by MC [14/20]. FISH probe (D20S108) located at 20q 40,263,862-40,264,096 bp. | |
| 52 | − | + | + | No metaphase cells were seen. | |
Figure 4. Causes of discrepancies between the results of MC, FISH and SNP-A.
In some instances discrepant results were obtained for the specific techniques, due to specific limitations of the individual techniques. A. Failure of MC analysis due to lack of growth. B. Failure of MC due to small size of lesion. C. Failure of MC and FISH due to UPD. D. Failure of FISH due to limitations of probes used.
DISCUSSION
The purpose of this manuscript was to evaluate the effectiveness of combining these three different cytogenetic methods for the detection of common recurrent abnormalities in MDS. We chose to focus on chromosomes 5, 7, 8 and 20 for which also FISH probes are applied in many institutions as a routine diagnostic test. Our results indicate that lesions identified by SNP-A (we focused on exemplary well described and most recurrent abnormalities of chromosomes 5, 7, 8 and 20) may change the risk classification.
Detection of chromosomal defects is of critical importance for the diagnosis and prognostic classification of myeloid malignancies, and is particularly pertinent in MDS where prognosis is dependent on the presence or absence of certain chromosome gains or losses. While metaphase cytogenetics has been the predominant method for identifying genetic abnormalities in MDS, newer techniques for genetic diagnosis are gaining more widespread clinical application. The need for sensitive and precise diagnosis of clonal chromosomal defects in MDS is clear, and has only gained in importance now that certain recurrent lesions may indicate responsiveness to novel therapeutics, as in the case of 5q- syndrome and lenalidomide.
The diagnostic workup for MDS now frequently includes FISH panels using multiple probes for the most common unbalanced chromosomal defects. Since FISH can be performed on interphase nuclei, these panels allow for targeted detection of specific defects even when metaphase cytogenetics is not possible or is unsuccessful due to a lack of growth. Drawbacks of FISH include the unclear clinical significance of small clones and a detection cutoff value that varies from probe to probe in the range of 0–8%, an inherent consequence of the technical aspects of this method when unbalanced defects are investigated.
The clinical implications of previously cryptic defects detected by newer whole genome scanning technologies such as CGH or SNP-A-based karyotyping are less well established, but several recent publications suggest that SNP-A detected lesions have a similar impact on clinical outcomes as do lesions detected by metaphase cytogenetics (for example, UPD7)10. Moreover, unlike targeted FISH, SNP-A karyotyping can be used as a screening method and is similarly useful when no viable cells can be obtained or when cellular proliferation cannot be enhanced in culture. Due to its high level of resolution, SNP-A allows for detection of smaller defects and CN-LOH not identifiable by MC or FISH. The relevance of smaller lesions has to be tested and clearly it depends upon their location. For example, we have shown that TET2, mutated in myeloid malignancies, maps to a microdeletion of 4q2412.
While resolution is the major advantage of SNP-A, its sensitivity is low. However, smaller clones may not be clinically relevant. This issue has not been resolved even after many decades of experience with MC and FISH. Its relative insensitivity to detecting small clones may actually be an advantage when interpreting results, in that defects within large clinically-relevant clones are more likely to be identified, while smaller, possibly transient clones will be diluted by the dominant clonal cell population.
We conceived this study fully aware that new technologies are not designed to replace or compete with MC. In an earlier study, the number of positive metaphases and percentage of positive cells by FISH did not correlate when these parameters were study in a cohort of 13 patients7. In contrast, our larger cohort showed correlation between metaphase cytogenetics and FISH results fitting with the intuitive expectation that higher numbers of dividing cells with a clonal marker will be also associated with higher numbers of interphase FISH positive cells. However, these findings discrepant from the previous report may be due to very sample size and differences in the technique.
Next, we hypothesized that they could complement this time-tested technique and improve the diagnostic yield. Our results indicate that, while most of the cytogenetic gains and losses were seen using either of the techniques, combining the findings of the individual technologies resulted in, on average, a 5% increase in the diagnostic yield. Discrepant results between the methods are particularly instructive for understanding the biology of the clonal defects. These can be explained by the variable proportions of non-clonal and clonal cells within the interphase cells used for FISH and SNP-A, the differential growth of clonal versus non-clonal precursors, and variable sensitivity of the individual methods with regard to the size of the dysplastic (clonal) cell population. Consequently, the diagnostic yield of this global screening method may be higher, in particular, when affected regions do not match with the probe used for FISH. This was the case in 2 patients in whom del 7q not involving the centromere and D7S486 locus was detected by SNP-A. In 2 cases discrepant for FISH and SNP-A in evaluation of trisomy 8, small lesions were detected by SNP-A, but they didn’t contain the region targeted by FISH probe. In some cases, MC and FISH (not SNP-A) were performed on bone marrow while SNP-A was done on DNA from whole blood likely explaining the discrepancy.
Complex karyotypes change the risk factor for MDS. While for recurrent lesions such as those screened by FISH the impact on prognosis is clearer, for a majority of less recurrent defects the associated prognostic impact is not clear. However, in some situations identification of additional defect may not be relevant for example; according to current schemes one cannot upstage complex karyotype or monosomy 7.
The clinical impact of alterations found by SNP-A or array CGH is a subject of intense research and it is likely due to its objectivity, precision, ability to detect CN-LOH, automation and the steadily decreasing cost, this technology will be included into the tool box of clinical cytogeneticists. Our study indicates that SNP-A may improve detection of chromosomal lesions of prognostic significance in MDS. While the additional diagnostic gains may be small in comparison to current FISH panels that test for the most common abnormalities in MDS, SNP-A produces equivalent results in a single test and can identify CN-LOH not recognizable by other methods.
Supplementary Material
Acknowledgments
This work was supported by NIH R01 HL082983 (JPM), U54 RR019391 (JPM), K24 HL077522 (JPM) and a charitable donation from the Robert Duggan Cancer Research Fund
Footnotes
Author’s contributions: H.M. performed experiments, analyzed the data and wrote the manuscript, M.R. performed experiments and analyzed the data, L.P.G. analyzed the data, J.H. analyzed the data, J.R.C. reviewed cytogenetic data and edited the manuscript, K.S.T. reviewed cytogenetic data and edited the manuscript, M.A.S. enrolled patients and helped write the manuscript, E.K. edited the manuscript, C.O. helped analyze the data and write the manuscript, J.P.M. conceived the idea, designed the experiments, provided financial and administrative support and wrote the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mufti GJ. Chromosomal deletions in the myelodysplastic syndrome. Leuk Res. 1992;16:35–41. doi: 10.1016/0145-2126(92)90097-q. [DOI] [PubMed] [Google Scholar]
- 2.Morel P, Hebbar M, Lai JL, Duhamel A, Preudhomme C, Wattel E, et al. Cytogenetic analysis has strong independent prognostic value in de novo myelodysplastic syndromes and can be incorporated in a new scoring system: a report on 408 cases. Leukemia. 1993;7:1315–23. [PubMed] [Google Scholar]
- 3.Greenberg P, Cox C, LeBeau MM, Fenaux P, Morel P, Sanz G, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. 1997;89:2079–88. [PubMed] [Google Scholar]
- 4.Jenkins RB, Le Beau MM, Kraker WJ, Borell TJ, Stalboerger PG, Davis EM, et al. Fluorescence in situ hybridization: a sensitive method for trisomy 8 detection in bone marrow specimens. Blood. 1992;79:3307–15. [PubMed] [Google Scholar]
- 5.Flactif M, Lai JL, Preudhomme C, Fenaux P. Fluorescence in situ hybridization improves the detection of monosomy 7 in myelodysplastic syndromes. Leukemia. 1994;8:1012–18. [PubMed] [Google Scholar]
- 6.Paulsson K, Heidenblad M, Strombeck B, Staaf J, Jonsson G, Borg A, et al. High-resolution genome-wide array-based comparative genome hybridization reveals cryptic chromosome changes in AML and MDS cases with trisomy 8 as the sole cytogenetic aberration. Leukemia. 2006;20:840–6. doi: 10.1038/sj.leu.2404145. [DOI] [PubMed] [Google Scholar]
- 7.Evers C, Beier M, Poelitz A, Hildebrandt B, Servan K, Drechsler M, et al. Molecular definition of chromosome arm 5q deletion end points and detection of hidden aberrations in patients with myelodysplastic syndromes and isolated del(5q) using oligonucleotide array CGH. Genes Chromosomes Cancer. 2007;46:1119–28. doi: 10.1002/gcc.20498. [DOI] [PubMed] [Google Scholar]
- 8.Maciejewski JP, Mufti GJ. Whole genome scanning as a cytogenetic tool in hematologic malignancies. Blood. 2008;112:965–74. doi: 10.1182/blood-2008-02-130435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cuneo A, Bigoni R, Roberti MG, Bardi A, Rigolin GM, Piva N, et al. Detection and monitoring of trisomy 8 by fluorescence in situ hybridization in acute myeloid leukemia: a multicentric study. Haematologica. 1998;83:21–6. [PubMed] [Google Scholar]
- 10.Gondek LP, Tiu R, O’Keefe CL, Sekeres MA, Theil KS, Maciejewski JP. Chromosomal lesions and uniparental disomy detected by SNP arrays in MDS, MDS/MPD, and MDS-derived AML. Blood. 2008;111:1534–42. doi: 10.1182/blood-2007-05-092304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tiu R, Gondek L, O’keefe C, Maciejewski JP. Clonality of the stem cell compartment during evolution of myelodysplastic syndromes and other bone marrow failure syndromes. Leukemia. 2007;21:1648–57. doi: 10.1038/sj.leu.2404757. [DOI] [PubMed] [Google Scholar]
- 12.Jankowska AM, Szpurka H, Tiu RV, Makishima H, Afable M, Huh J, et al. Loss of heterozygosity 4q42 and TET2 mutations associated with myelodysplatic/myeloproliferative neoplasms. Blood. 2009;113:6303–10. doi: 10.1182/blood-2009-02-205690. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.