Abstract
Obesity is associated with increased risk for cardiovascular health problems including diabetes, hypertension, and stroke. These cardiovascular afflictions increase risk for cognitive decline and dementia, but it is unknown whether these factors, specifically obesity and Type II diabetes, are associated with specific patterns of brain atrophy. We used tensor‐based morphometry (TBM) to examine gray matter (GM) and white matter (WM) volume differences in 94 elderly subjects who remained cognitively normal for at least 5 years after their scan. Bivariate analyses with corrections for multiple comparisons strongly linked body mass index (BMI), fasting plasma insulin (FPI) levels, and Type II Diabetes Mellitus (DM2) with atrophy in frontal, temporal, and subcortical brain regions. A multiple regression model, also correcting for multiple comparisons, revealed that BMI was still negatively correlated with brain atrophy (FDR <5%), while DM2 and FPI were no longer associated with any volume differences. In an Analysis of Covariance (ANCOVA) model controlling for age, gender, and race, obese subjects with a high BMI (BMI > 30) showed atrophy in the frontal lobes, anterior cingulate gyrus, hippocampus, and thalamus compared with individuals with a normal BMI (18.5–25). Overweight subjects (BMI: 25–30) had atrophy in the basal ganglia and corona radiata of the WM. Overall brain volume did not differ between overweight and obese persons. Higher BMI was associated with lower brain volumes in overweight and obese elderly subjects. Obesity is therefore associated with detectable brain volume deficits in cognitively normal elderly subjects. Hum Brain Mapp, 2010. © 2009 Wiley‐Liss, Inc.
Keywords: brain atrophy, obesity, tensor‐based morphometry
INTRODUCTION
Obesity and Type II, or noninsulin dependent, diabetes mellitus (DM2) are two interlinked conditions that have reached epidemic proportions. There are currently over one billion overweight and 300 million obese persons worldwide [World Health Organization, 2009]. The elderly population has not been spared—40% of men and 45% of women over age 70 are burdened either with obesity or DM2 [Ceska, 2007], increasing their risk for cardiovascular disease and stroke [Mankovsky and Ziegler, 2004]. Obesity is also a risk factor for cognitive decline and dementia, including Alzheimer's disease (AD) [Elias et al., 2005; Wolf et al., 2007]. This added risk may be mediated by DM2, which is associated with a higher probability of having AD [Irie et al., 2008; Leibson et al., 1997].
Previous studies analyzing data from the Cardiovascular Health Study‐Cognition Study (CHS‐CS) indicate that cerebrovascular disease, in addition to age, race, and education level, are associated with cognition and with the development of an intermediate risk state for AD, known as mild cognitive impairment (MCI) [Lopez et al., 2003a]. Cardiovascular risk factors for MCI included white matter lesions, infarcts, hypertension, diabetes mellitus, and heart disease [Lopez et al., 2003a]. Furthermore, patients with AD have abnormally high rates of brain atrophy [Apostolova et al., 2006; Callen et al., 2001; Leow et al., 2009]. Additionally, brain atrophy may be detectable on MRI even before cognitive impairment is clinically evident, as demonstrated in a study showing greater atrophy in asymptomatic APOE4 carriers compared to noncarriers [Morra et al., 2009].
Obesity and DM2 may amplify the risk for dementia by worsening cerebral atrophy even in cognitively intact individuals, raising their vulnerability to future AD neuropathology. Earlier studies, mostly in subjects younger than 65, suggest that increased body tissue fat content (adiposity) is correlated with atrophy in the temporal cortex, frontal lobes, putamen, caudate, precuneus, thalamus, and white matter (WM) [Gustafson et al., 2004; Pannacciulli et al., 2006; Taki et al., 2008]. It is unknown, but of great interest, whether high tissue fat content, as measured by BMI, is associated with differences in brain structure in cognitively normal elderly.
In elderly subjects, DM2 is also associated with brain atrophy in the temporal lobes, hippocampus, and with greater expansion of the lateral ventricles [Korf et al., 2007]. The most common explanation for these effects is WM lesions [Claus et al., 1996] and clinical strokes [Mankovsky and Ziegler, 2004]. DM2 associated brain atrophy may be secondary to increased insulin levels seen in the disease; higher fasting plasma insulin has been associated with cognitive deficits in elderly subjects [Yaffe et al., 2004], and promotes amyloid deposition, thus increasing risk for Alzheimer's disease [Watson et al., 2003]. So far, no other studies have correlated fasting plasma insulin and brain structure, even when DM2 has been examined. A serious potential confound in such studies is the possibility of presymptomatic neurodegenerative changes in the elderly cohorts studied. Since brain atrophy and AD pathology can exist years before onset of clinical symptoms [Braskie et al., 2008; DeKosky et al., 2006], investigations of BMI, DM2 and brain atrophy should be done in individuals for whom incipient AD can be ruled out as far as possible.
Obesity and DM2 may amplify risk for AD by promoting brain atrophy and thus may represent potentially critical risk factors for cognitive decline and dementia. Because these conditions are to some extent preventable and treatable, it is important to identify specifically affected brain structures in the nondemented elderly, both to understand the systems affected, and ultimately to gauge the success of interventions to protect these areas.
We applied tensor‐based morphometry (TBM), a relatively novel method [Hua et al., 2008; Thompson et al., 2000], to generate 3D maps of brain atrophy in a group of nondemented elderly subjects recruited from the Cardiovascular Health Study‐Cognition Study (CHS‐CS), a community‐based cohort of individuals for whom extensive clinical, cognitive, and imaging data exists [Lopez et al., 2004]. On the basis of longitudinal cognitive data, we selected 94 subjects who remained cognitively normal for at least 5 years after their baseline MRI scan; thus minimizing confounding effects of early preclinical neurodegeneration. We regressed BMI (n = 94), FPI (n = 64), and DM2 diagnosis (n = 94) against image‐derived measures of GM and WM volume differences across subjects, to determine whether these variables were associated with brain atrophy. We used bivariate correlation models for an initial exploratory analysis, and then multiple regression models were used to account for potential confounders such as gender and race. We also compared brain structure between normal weight (BMI: 18.5–25), overweight (BMI: 25–30), and obese (BMI: 30+) subjects to assess if these clinical cutoffs for defining higher adiposity are themselves associated with brain atrophy.
MATERIALS AND METHODS
Subjects
The Cardiovascular Health Study Cognition Study (CHS‐CS) is a continuation of the CHS Dementia Study, which began in 2002–2003 to determine the incidence of dementia and mild cognitive impairment (MCI) in a population of normal and MCI subjects identified in 1998–1999 in Pittsburgh [Lopez et al., 2003b]. Of the 927 participants examined in 1998–1999, 532 normal and MCI subjects were available for study in 2002–2003. All subjects had complete neurological and neuropsychological examinations in 1998–1999 and 2002–2003, and an MRI of the brain in 1992–1994, and 295 were scanned with 3D volumetric brain MRI in 1998–1999. From the latter sample, we selected 94 subjects who were cognitively normal in 1997–1998 and 2002–2003. BMI (n = 94) and fasting plasma insulin levels (n = 64) were obtained using standard CHS methods [Fried et al., 1991; McNeill et al., 2006]. All nonimaging statistical analyses were analyzed using the Statistical Package for Social Science (SPSS, version 16.0, SPSS Inc., Chicago, IL).
Type II Diabetes Mellitus (DM2)
Classification of DM2 was determined from annually acquired medical data and is described in greater detail in previously published work [Brach et al., 2008]. To summarize, CHS participants were classified as having DM2 if they met any one of the following criteria: (i) use of any DM2 medications; (ii) fasting (≥8 h) glucose ≥126 mg/dL; (iii) nonfasting (8 h) glucose ≥200 mg/dL, or (iv) oral glucose tolerance test ≥200 mg/dL.
MRI Acquisition and Image Correction
All MRI data were acquired at the University of Pittsburgh Medical Center MR Research Center using a 1.5 T GE Signa scanner (GE Medical Systems, Milwaukee, WI, LX Version). A 3D volumetric spoiled gradient recalled acquisition (SPGR) sequence was obtained for the whole brain (TE/TR = 5/25 msec, flip angle = 40°, NEX = 1, slice thickness = 1.5 mm/0 mm interslice gap) set parallel to the AC‐PC line with an in‐plane acquisition matrix of 256 × 256 image elements, 250 × 250 mm field of view and an in‐plane voxel size of 0.98 × 0.98 mm.
Image Preprocessing
Individual scans were linearly registered to the International Consortium for Brain Mapping standard brain image template (ICBM‐53) using a 9‐parameter registration to account for global position and scale differences across individuals, including head size. Globally aligned images were re‐sampled in an isotropic space of 220 voxels along each axis (x, y, and z) with a final voxel size of 1 mm3.
TBM and Three‐Dimensional Jacobian Maps
TBM detects local volumetric differences by averaging rates of volumetric changes (i.e., Jacobian maps), after nonlinearly aligning individual maps of change to a minimal deformation template (MDT). An MDT for this specific study was created from the MRI scans of 40 normal CHS subjects to enable automated image registration, reduce statistical bias, and potentially improve the detection of statistically significant effects [Hua et al., 2008; Kochunov et al., 2002; Lepore et al., 2007]. All scans were nonlinearly aligned to the study‐specific template so that they would all share a common coordinate system, and the local expansion factor of the 3D elastic warping transform, the Jacobian determinant, was plotted for each subject. These 3D Jacobian maps show relative volume differences between each individual and the common template, and may be used to illustrate areas of structural volume reduction such as atrophy of GM and WM. The CHS‐MDT template was manually parcellated using the Brainsuite software program (http://brainsuite.usc.edu/) by a trained anatomist to generate binary masks covering the cerebrum. Correlations between BMI and Jacobian maps were evaluated at each voxel using the general linear model on a whole brain level.
Overview of Statistical Analyses
We performed bivariate statistical tests as an exploratory analysis to identify if obesity and one of its well known complications, DM2, were associated with GM and WM atrophy. We also did this with FPI as increased insulin levels are an early component of DM2 pathology [Ceska, 2007]. We next applied multiple regression analyses to identify which one of these variables accounted for the most variance in our sample. We then used common clinical classifications of normal BMI, overweight, and obesity to conduct ANCOVA analyses. The purpose of this was to express our BMI findings in terms that can be understood in a clinical context.
Bivariate Statistical Analyses
In an initial exploratory analysis, we used a bivariate model to correlate Jacobian maps, which provide information on both tissue atrophy and CSF expansion relative to a standard template, with the possible predictor variables BMI, FPI, and DM2. We conducted separate tests of negative, positive, and two‐tailed correlations. The statistical significance of these associations is reported using omnibus P‐values. Since our hypothesis focused on GM and WM atrophy, we report only P‐values for negative associations (i.e., based on one‐tailed testing). Permutation tests (with N = 10,000 randomizations) [Edgington, 1995] were performed to correct for multiple comparisons. We derived corrected P‐values for the overall pattern of effects, by computing the probability of observing the suprathreshold volume of statistics under the null hypothesis, i.e., by chance, when covariates and groups were randomly assigned (setting a voxel‐level threshold of P = 0.01). Statistically significant associations were projected as maps of P‐values and correlation coefficient r‐values onto the CHS‐MDT using the Shiva viewer (http://www.loni.ucla.edu/Software/Software_Detail.-jsp?software_id=12) and displayed with standard scales.
Multiple Regression Statistical Analyses
After applying a bivariate approach in our exploratory analyses, we fitted a multiple regression statistical model to better understand which of these variables (BMI, FPI, DM2) best accounted for the variance in brain volumes in the cohort. We analyzed the Jacobian maps using Statistical Parametric Mapping software (SPM2, http://www.fil.ion.ucl.ac.uk/spm/), inputting BMI, DM2, age, gender, and race in the same general linear model. The influence of FPI was separately tested in interaction models with BMI.
To better understand how the inverse associations between BMI and brain structure were distributed in groups who were obese and to put such associations in a clinical context, we ran a series of between‐group ANCOVA analyses in SPM2. These included: (i) Obese versus normal BMI groups; (ii) Overweight versus normal BMI groups; (iii) Obese versus overweight groups. All between‐group comparisons controlled for age, gender, race, and DM2. Correction for multiple comparisons was achieved using the False Discovery Rate (FDR) method [Genovese et al., 2002] in which findings were only declared as significant if the expected rate of false positives in the map was less than 5%. Voxel‐level t‐values were converted to point biserial correlations (r) as a measure of effect size, using the cg_spmT2x.m script in SPM2. This was done for all analyses so that the effect sizes of all results could be compared using the same measure. The r‐values were projected onto orthogonal sections of the standard single subject MNI template [Holmes et al., 1998] in MRIcron (http://www.sph.sc.edu/comd/rorden/MRIcron/) for display purposes.
RESULTS
Subject demographics are shown in Table I, divided into three BMI categories for normal, overweight and obese (BMI Range: 18.5–36.2). Only BMI and FPI levels differed between the groups—as expected, or by definition, overweight and obese persons had higher BMI and FPI levels than the normal BMI group (P ≤ 0.001). There was no correlation between DM2 and FPI levels (r(64) = 0.01, P = 0.92). Additionally, DM2 subjects did not have higher FPI levels than non‐FPI subjects (t(62) = −0.09, P = 0.92).
Table I.
Subject characteristics
| Variable | Normal BMI (18.5–25) | Overweight (25–29) | Obese (30+) | T‐test(t, P)/χ2 |
|---|---|---|---|---|
| Sample size (n) | 29 | 51 | 14 | — |
| BMI | 22.5 ± 1.9 | 27.5 ± 1.4 | 32.9 ± 2.5 | −15.5, <0.001* |
| −13.2, <0.001** | ||||
| −10.5, <0.001*** | ||||
| Age | 77.5 ± 4.0 | 77.2 ± 2.6 | 76.9 ± 2.8 | 0.49, 0.63* |
| 0.35, 0.73** | ||||
| −0.40, 0.69*** | ||||
| Female Gender | 48% (14) | 53% (27) | 64% (9) | 0.98, 0.61 |
| Education (Grade 12 +) | 69% (20) | 67% (34) | 50% (7) | 1.65, 0.44 |
| Non‐White | 17% (5) | 10% (5) | 14% (2) | 0.95, 0.62 |
| MRI Infarcts (47) | 24% (7) | 23% (12) | 7% (1) | 1.97, 0.37 |
| Type II diabetes mellitus | 3% (1) | 16% (8) | 14% (2) | 2.79. 0.25 |
| WMH Grade >3 (48) | 28% (8) | 28% (14) | 29% (4) | 0.001, 0.98 |
| Hypertension | 28% (8) | 31% (16) | 43% (6) | 1.03, 0.59 |
| APOE 4 genotype | 29% (7) | 24% (11) | 7% (1) | 2.55, 0.28 |
| Fasting Plasma Insulin (n = 64) | 10.1 ± 2.8 | 14.6 ± 6.3 | 15.4 ± 4.4 | −4.1, <0.001* |
| −3.1, 0.001** | ||||
| 0.37, 0.71*** |
Normal BMI compared with obese on t‐test.
Normal BMI compared with overweight on t‐test.
Overweight compared with obese on t‐test.
Bivariate Statistical Analyses
Potential confounders
In our TBM maps correlating potential confounding variables with brain structure, increasing age showed a trend‐level association with lower brain volumes in this sample but this was not statistically significant (P = 0.07, corrected; permutation test). Age and BMI were not significantly correlated in our sample (r (92) = −0.04, P = 0.90) nor was age correlated with insulin levels (r (64) = 0.06, P = 0.66) or with DM2 diagnosis (r (92) = −0.05, P = 0.61). Additionally, APOE4 genotype, which increases the risk for sporadic AD, was not related to detectable alterations in brain structure as assessed with TBM in this sample (P = 0.39, permutation test). Education, defined categorically as progression beyond high school, was also not statistically significant in its correlation with TBM measures of GM and WM atrophy either negatively (P = 0.92, permutation test) or positively (P = 0.12, permutation test). A clinical designation of hypertension (systolic/diastolic >140/90 mm hg or use of antihypertensive medications) also had no statistically significant negative correlation with brain structure in our sample (P = 0.33, permutation test).
Body mass index
Higher BMI was significantly correlated with lower GM and WM volumes throughout the brain (P < 0.001, permutation test). Figure 1a shows the correlation coefficients for the inverse association of BMI with brain structure projected onto the CHS‐specific minimum deformation template (the CHS‐MDT). Blue colors represent areas of higher negative correlation; values typically range from −0.30 to 0.30. The areas of positive correlation in red and yellow were not statistically significant. Areas of strongest negative correlation (r ≤ −0.30) were found in the orbital frontal cortex (red arrow at x = −9, y = 57, z = 29, r = −0.31), the hippocampus (gold arrows: left at x = −31, y = −2, z = 25, r = −0.32; right at x = 32, y = 9, z = 18, r = −0.31) and subcortical areas (white asterisks: left at x = −28, y = −14, z = 1, r = −0.30; right at x = 29, y = −15, z = 1, r = −0.34) including the putamen, globus pallidus, and thalamus. These results suggest atrophy in people with higher body tissue fat. Figure 1b shows the corresponding significance (P‐value) map. Darker colors indicate areas with lower P‐values.
Figure 1.
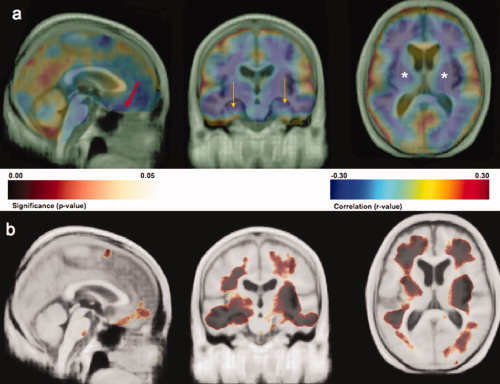
Part a shows an r‐value (Pearson correlation coefficient) map highlighting the negative and positive correlations between BMI and brain structure projected onto cardinal sections of the Cardiovascular Health Study Minimal Deformation Template (CHS‐MDT). Blue colors show stronger negative correlations while red and yellow colors show positive correlations; only negative correlations were statistically significant (p < 0.001; permutations test). An inverse association between BMI and brain volume is observed in orbital frontal cortex (red arrow at x = −9, y = 57, z = 29, r = −0.31), the hippocampus (gold arrows: left at x = −31, y = −2, z = 25, r = −0.32; right at x = 32, x = 9, z = 18, r = −0.31) and subcortical areas (white asterisks: left at x = −28, y = −14, z = 1, r = −0.30; right at x = 29, y = −15, z = 1, r = −0.34) including the putamen, globus pallidus, and thalamus. Part b shows a P‐value image of BMI main effects on brain structure projected onto the CHS‐MDT. All images are in neurological convention (left on left). Dark colors indicate atrophy in both GM and WM; darker colors denote lower P‐values. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Fasting plasma insulin
Higher FPI was associated with lower regional brain volumes (P = 0.01, permutation test) in both GM and WM. Higher FPI were associated with brain atrophy in the frontal lobes, hippocampus, and the splenium of the corpus callosum. These results are shown in Figures 2a,b. Figure 2a shows the correlation coefficient map in which higher FPI is correlated with lower volumes in the splenium of the corpus callosum (red arrow: x = −3, y = 12, z = −12, r = −0.27), orbital frontal cortex (orange arrow: x = −3, y = −39, z = 31, r = −0.33) and hippocampus (gold arrows: left at x = −24, y = −1, z = 24, r = −0.31; right at x = 31, y = 3, z = 21, r = −0.33). Figure 2b shows the corresponding P‐value map. These results suggest that high insulin levels, an early component of DM2 pathology, may be linked to atrophy in brain regions with cognitive function such as the splenium of corpus callosum (interhemispheric transfer of visual and other cognitive information), the orbital frontal cortex (executive function), and hippocampus (learning and memory).
Figure 2.
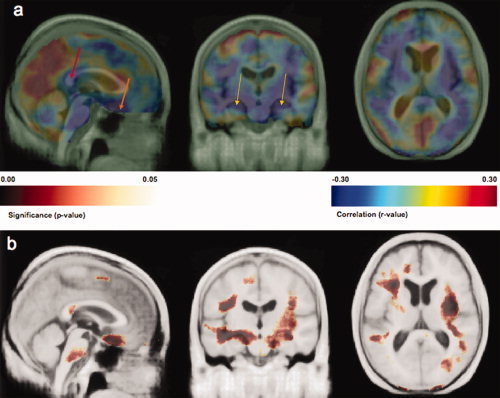
Part a shows a correlation map projected onto the CHS‐MDT template indicating where brain atrophy (volume reduction) is related to higher fasting plasma insulin levels. Higher FPI is correlated to lower volumes of the splenium of the corpus callosum (red arrow: x = −3, y = 12, z = −12, r = −0.27), orbital frontal cortex (orange arrow: x = −3, y = −39, z = 31, r = −0.33) and hippocampus (gold arrows: left at x = −24, y = −1, z = 24, r = −0.31; right at x = 31, x = 3, z = 21, r = −0.33). Part b shows the corresponding significance maps. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Type II diabetes mellitus
DM2 was also associated with brain atrophy (P < 0.001, permutation test) in the bivariate analysis. Those participants diagnosed with DM2 had lower volumes in multiple brain regions including the frontal lobes, prefrontal cortex, genu and splenium of the corpus callosum, middle cingulate gyrus, superior parietal lobule, the occipital lobes, and the cerebellum. The basal ganglia, including the caudate, putamen, and globus pallidus were also atrophied in those with a diagnosis of DM2. None of our DM2 subjects had either a history of clinical stroke or MRI‐identified infarcts. The DM2‐related areas of atrophy are displayed in Figures 3a (correlation coefficient map) and 3b (P‐value map). This figure shows that DM2 is associated with lower volumes in splenium of the corpus callosum (Fig. 3b, significance map, black arrow, corresponding r‐value = −0.21 at x = −4, y = 14, z = −17), genu of the corpus callosum (Fig. 3b, green arrow, corresponding r‐value = −0.17 at x = 4, y = −49, z = 1) and the frontal lobes (Fig. 3b, red arrow, corresponding r‐value = −0.24 at x = −7, y = −77, z = 7).
Figure 3.
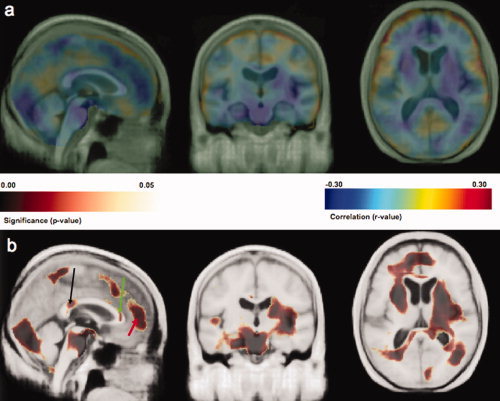
The r‐value image in part a shows the negative correlation between a categorical diagnosis of DM2 and atrophy in GM and WM. DM2 is associated with lower volumes in splenium of the corpus callosum (Fig. 3 b, significance map, black arrow, corresponding r‐value = −0.21 at x = −4, y = 14, z = −17), genu of the corpus callosum (Fig. 3 b, green arrow, corresponding r‐value = −0.17 at x = 4, y = −49, z = 1) and the frontal lobes (Fig. 3b, red arrow, corresponding r‐value = −0.24 at x = −7, y = −77, z = 7). All results in this image were projected onto the CHS‐MDT. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Multiple Regression Analyses
In the multiple regression models, BMI was the only variable that was significantly linked with brain atrophy in GM and WM (expected FDR< 5%); there were no independent associations between FPI, DM2, age, gender, or race with the degree of brain atrophy once BMI was accounted for. The inverse association of BMI and brain structure is shown in Figure 4. Higher BMI was associated with lower GM and WM volumes in orbital frontal cortex (part a, blue box), anterior cingulate gyrus (part a, blue box), medial temporal lobe (part b, black arrows), and subcortical WM (part c, black asterisks). The effect sizes for this association were large (r ≥ 0.30). In Supporting information Figure 1, we show a beta image of the main effects of BMI. This image represents the slope of the regression line, showing the percentage brain volume (in cc) lost for every one standard deviation gain in BMI after adjusting for the other variables in the model. In a restricted region of the orbital frontal cortex/anterior cingulate, for instance, purple colors show that more than 4% of brain volume is lost for every one standard deviation gain in BMI. Consequently, a person in the top 5% of BMI (i.e., two standard deviations from the mean), would exhibit a maximum 8% focal deficit in an area such as the orbital frontal cortex. The arrows and asterisks identify matching anatomical areas between the correlation map and the beta image.
Figure 4.
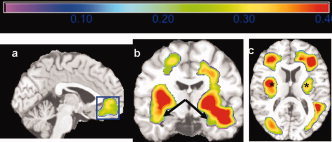
This figure shows a map of correlation values (r‐value map) projected onto the Standard Single Subject MNI brain template for display purposes. The correlation shown is between higher BMI and GM/WM atrophy controlling for age, gender, race, and DM2. Hotter colors denote stronger correlation effect sizes, which range from 0 to 0.4. Higher BMI was associated with lower GM and WM volumes in orbital frontal cortex (a, blue box), anterior cingulate gyrus (a, blue box), medial temporal lobe (b, black arrows), and subcortical WM (c, black asterisks). [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
We detected no independent associations of age, DM2, gender, or race on GM or WM volumes in our sample once BMI was accounted for. Interaction analyses also showed that BMI‐related atrophy did not vary as a function of any one of these variables. A separate BMI by FPI interaction analysis (n = 64) showed that BMI effects on brain structure did not vary as a function of FPI. To better understand how the BMI associated atrophy was distributed, we also compared GM and WM volumes of persons with three discrete diagnostic classifications, i.e., normal BMI, overweight, and obese.
Between‐Group ANCOVA Analyses
Obesity versus normal BMI
In comparing obese subjects (BMI: 30+) to those with a normal BMI (BMI: 18.5–25), we found lower GM and WM volumes (FDR < 5%) in the obese group despite controlling for age, gender, race, and DM2. This atrophy is shown in Figure 5 as an r‐image projected onto the Standard Single Subject MNI template, with red colors corresponding to a higher correlation effect size (r > 0.50). Obese persons had lower GM and WM volumes in the frontal lobes, anterior cingulate gyrus (part a, blue arrow), hippocampus (part b, black arrow), and basal ganglia (part c, green box). These maps suggest that being obese is associated with atrophy in brain regions important for cognitive function such as the anterior cingulate, which participates in attention and executive function.
Figure 5.
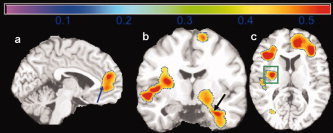
Correlation map (r‐value image) effect sizes for a comparison of 14 obese persons (BMI > 30) to 29 normal weight persons (18.5–25). Obese persons had lower GM and WM volumes in the frontal lobes, anterior cingulate gyrus (a, blue arrow), hippocampus (b, black arrow), and basal ganglia (c, green box). Correlation coefficients range from 0 to 0.5. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Overweight versus normal BMI
Figure 6 shows that overweight (BMI: 25–30) subjects have lower brain volumes than those with Normal BMI in the basal ganglia (part a, black arrow; part b, red arrow; part c, blue arrow), corona radiata (part b, black box), and parietal lobe (part c, purple arrow). These associations were generally lower in magnitude (|r| = 0.3–0.4) compared to the obese‐normal BMI results. Unlike obese persons, the overweight group did not show atrophy in such paralimbic areas, such as the anterior cingulate gyrus and hippocampus. There were no statistically significant differences in GM and WM between the obese and overweight groups. All analyses were controlled for age, gender, race, and DM2.
Figure 6.
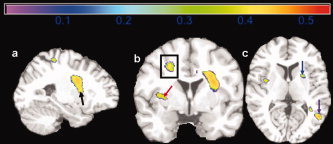
Maps of correlation coefficients are shown for a group comparison of 51 overweight persons (BMI: 25–30) versus 29 normal weight persons (18.5–25). Atrophy in the overweight group is seen in the basal ganglia (a, black arrow; b, red arrow; c, blue arrow), corona radiata (b, black box), and parietal lobe (c, purple arrow). Correlations range from 0 to 0.5. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
DISCUSSION
Here we report several key findings relating brain structural deficits to obesity, higher BMI, FPI, and DM2 in cognitively normal elderly individuals drawn from a community cohort. First, higher body tissue fat was strongly associated with brain volume deficits in cognitively normal elderly subjects, even when controlling for potential confounds such as age, sex, and race. Second, FPI and DM2 showed inverse associations with brain structure in a bivariate analysis, but these correlations were not statistically significant when controlling for BMI. Third, negative correlations between body tissue fat and brain structure were strongest in obese persons but were also seen in overweight individuals. Although we acknowledge that the effects of obesity may be secondary to generally poor health, this is less likely in our sample because (i) those with very poor health are less likely to survive to the advanced age (mean: 77.3 years) in our study; (ii) no correlation was detected between BMI and death rates in our cohort 10 years after their scan (r(94) = 0.07, P = 0.47); and (iii) the three BMI groups did not differ in their rates of vascular diseases that increase morbidity and mortality (Table I). Therefore, even in persons with normal cognition who survive to old age, higher body tissue adiposity may have deleterious consequences on brain structure.
Our finding of BMI‐associated brain atrophy in cognitively normal elderly is supported by studies from younger samples. A study of Japanese males (mean age: 46.1) showed reduced GM volumes in association with increasing BMI in medial temporal lobes, hippocampus, and precuneus [Taki et al., 2008]. Another study (mean age: 32) showed greater GM volume loss in obese individuals in the frontal operculum, postcentral gyrus, and putamen [Pannacciulli et al., 2006]. A recent MR spectroscopy study revealed metabolic abnormalities in frontal lobe GM and WM in a group of younger obese persons (mean age: 41.7) [Gazdzinski et al., 2008].
The correlation between BMI and brain volumes is unlikely to be direct in the sense of one causing the other; therefore, it is of interest to identify factors or mechanisms that might tend to cause brain volume reduction and obesity in the same subjects. The most commonly proposed mediators for the relationship between higher body tissue adiposity and brain structure include hypercortisolemia [Lupien et al., 1998], reduced exercise [Colcombe et al., 2003], impaired respiratory function [Guo et al., 2006], inflammation [van Dijk et al., 2005], cardiovascular/hypertension/hyperlipidemia [Breteler et al., 1994; Swan et al., 1998], and Type II diabetes mellitus [den Heijer et al., 2003; Ferguson et al., 2003]. The manifestations of brain structural deficits in these studies were hippocampal atrophy, cortical volume loss, and WM hyperintensities. We found no interaction between BMI and DM2, so the effects of BMI are unlikely to be mediated by that mechanism in our sample. Additionally, our BMI results did not change when controlling for hypertension and WM hyperintensities as assessed by standardized CHS criteria [Dai et al., 2008; Yue et al., 1997]. These results may reflect a survivor effect, as persons with both high BMI and clinically severe cerebrovascular disease are less likely to live to the age range of our study population (70–89 years). Additionally, we cannot rule out the possibility that BMI relationships with brain atrophy in our elderly cohort are more directly mediated through any one or any combination of the other mechanisms listed above.
Having established that BMI is associated with brain atrophy in the elderly, we also acknowledge that controversy exists in the literature about how this association is influenced by sex differences. A group of elderly (70–84 years) Swedish women showed substantial temporal lobe atrophy that was associated with BMI, on computed tomography [Gustafson et al., 2004] while another study found BMI associated cerebral volume loss in Japanese men but not in women [Taki et al., 2008]. To determine whether or not correlations between BMI and brain structure are influenced by gender in our study, we modeled a BMI by gender interaction in our multiple regression analyses and did not detect a sex difference in BMI‐related brain atrophy. Our study therefore suggests that the deleterious effects of higher tissue adiposity on brain structure may be gender independent; however, this finding merits further investigation in future studies.
Even though the unadjusted correlations of FPI, DM2, and brain atrophy were not statistically significant in the adjusted models, they may merit discussion due to a growing literature on the effects of hyperinsulinemia and DM2 on the brain. In the early stages of DM2, insulin resistance is associated with a compensatory hyperinsulinemia [Yaffe et al., 2004], and high insulin levels are associated with cognitive impairment, even in subjects who will not develop DM2 [van Oijen et al., 2008], suggesting that hyperinsulinemia can alter brain structure. Multiple mechanisms are involved in the impact of hyperinsulinemia on brain function and structure, including vasoactive effects on cerebral arteries, neurotoxicity due to impaired clearance of amyloid from the brain and stimulation of the formation of neurofibrillary tangles through advanced glycation end‐product metabolism [Bian et al., 2004; Watson et al., 2003]. The insulin effect is observed here in multiple areas relevant to cognitive function such as the orbital frontal cortex and the hippocampus. This is consistent with the notion that hyperinsulinemia affects brain structures involved in cognition; it may also lead to subtle cognitive decline before clear clinical symptoms of dementia are detectable [Kalmijn et al., 1995].
DM2 was associated with lower GM and WM volumes areas of cognitive relevance such as the frontal lobes and large WM tracts (splenium of the corpus callosum), suggesting that DM2 has a widespread association with brain atrophy. DM2 can reduce brain volume through a progressive cerebrovascular process that leads to stroke and infarcts [Ikram et al., 2008; Knopman et al., 2005]. DM2 can exert damage through advanced glycation of key structural proteins, imbalance between production and elimination of reactive oxygen species, and through perturbations of hexosamine and polyol pathways, causing the basement membranes of cerebral capillaries to thicken [Arvanitakis et al., 2006]. Such microvascular changes, which frequently occur with other obesity consequences such as hypertension, can lead to chronic subclinical ischemia, impaired neuronal energy consumption, and atrophy in brain areas with delicately vulnerable vasculature such as the lenticulostriate arteries of the basal ganglia [Breteler et al., 1994]. Basal ganglial findings in TBM analyses can also be noticeable due to a comparative lack of sensitivity TBM has to volume changes in the cortical surface due to smoothness of the deformation fields and resulting partial volume effects [Hua et al., 2009; Leow et al., 2009]. Our bivariate DM2 results are consistent with prior findings that GM and WM are affected in DM2 [Korf et al., 2007; Tiehuis et al., 2008] and with FDG‐PET studies that showed hypometabolism in frontal, temporal, and parietal association regions, and posterior cingulate gyrus in cognitively normal subjects with mild hyperglycemia [Kawasaki et al., 2008].
The DM2 association did not survive the adjusted multiple regression models, which may be due to the small number of DM2 subjects in the study (n = 11), that itself may be a consequence of a survivor effect. That is, many persons with DM2 may not have lived long enough to undergo scanning as part of the CHS. This bias may have led to lack of power in the multiple regression models and lack of a statistically significant interaction between BMI and DM2. This issue could be overcome in future studies by analyzing larger numbers of cognitively normal elderly DM2 persons. Such work could elucidate a possible mediation role for DM2 with respect to obesity and brain atrophy. While it is tempting to speculate that obese and overweight persons harbor early subclinical DM2 pathology (as reflected by obese and overweight persons having higher FPI) and that this drives the relationship between BMI and brain atrophy, future work would have to verify this as we found no statistically significant interactions between BMI and DM2 or FPI.
Our findings, taken in the context of earlier studies, suggest that elderly persons with higher adiposity are at increased risk for brain atrophy and consequently dementia. Even our elderly subjects, who were very healthy and confirmed to be cognitively stable for at least five years after baseline scanning, were afflicted with brain atrophy associated with obesity. Our results suggest that individuals may have a greater extent of brain atrophy due to obesity or factors that promote obesity and that this atrophy may, in turn, predispose them to future cognitive impairment and dementia. The implications of this cycle include: (i) amplified morbidity/mortality in the elderly; (ii) higher health care costs due to obesity‐related dementia; and (iii) emotional and other nonfinancial burdens on caretakers and healthcare providers. Obesity associations with brain atrophy and dementia risk therefore present a potential public health challenge.
This study used neuroimaging methods to explore the effects of higher BMI, insulin, and DM2 in an elderly community cohort who remained cognitively normal for five years after their scan. Such results are therefore more likely to reflect brain changes in the general elderly population as they avoid the referral biases of studies that draw subjects from specialty clinics. TBM offers high resolution mapping of anatomical differences, offering excellent sensitivity to systematic structural differences in the brain, and lacks the selection bias of ROI tracings that examine only part of the brain. We used TBM because of its effectiveness in analyzing volumetric group differences in the entire brain. In other types of voxel‐based studies, such as voxel‐based morphometry [Ashburner and Friston, 2000], a question sometimes arises as to whether the findings may be attributable to imperfect registration. This question arises because in VBM, smoothed maps of classified gray matter are automatically aligned across subjects and smoothed, and then statistical inferences are made regarding group differences, by voxel‐by‐voxel subtraction of the group‐averaged images. As such it is possible that a difference detected at any one location is due to imperfect registration [Thacker et al., 2004].
In TBM, however, the signals analyzed are based only on the registrations of the images and not the aligned gray matter classifications, so it is not required that the gray matter be perfectly registered across subjects as the gray matter density is not analyzed at each stereotactic location. As such, false positive findings due to systematic group differences in registration errors are less likely. Even so, there may be false negative findings, because the power to detect morphometric differences depends on the scale at which anatomic data can be matched by the warping algorithm. Finer‐scale morphometric differences (e.g., in the hippocampus or cortical thickness) may be better detected using other methods that model those structures explicitly. However, we preferred use of TBM over cortical pattern matching as TBM is able to process larger numbers of subjects more efficiently [Xue et al., 2008]. TBM is therefore less vulnerable to registration bias than VBM and more efficient for analyzing larger numbers of subjects than cortical surface modeling and cortical pattern matching.
Our findings are limited by the cross‐sectional design, though longitudinal follow‐up was used to inform subject selection to minimize confounding from those experiencing early neurodegeneration from Alzheimer's or other dementias. Our multiple regression approach accounted for the potentially confounding effects of age, gender, and race and DM2. We did not include APOE4 genotype in this model, as the variable showed no statistically significant relationships in the bivariate analysis (P = 0.39, permutation test).
With an increasing number of persons becoming both obese and elderly, a detailed understanding of brain structural abnormalities in this group is vital. Studies such as this suggest why these individuals may have an increased risk for dementia. Even elderly individuals who remained cognitively normal long after their MRI had BMI associated atrophy in brain areas targeted by neurodegeneration: hippocampus, frontal lobes, and thalamus. Such individuals may benefit from interventions to reduce body tissue fat and experience better brain health in aging.
Supporting information
Additional Supporting Information may be found in the online version of this article.
Supporting Information Figure 1. This figure shows two columns of sagittal and axial images. The first column shows the main effects of BMI from the r image of Figure 4. The second column shows the corresponding beta image or the slope of the multiple regression of BMI on brain structure. This picture shows an estimate, based on that multiple regression, of the percentage brain volume (in cc) lost for every one standard deviation gain in BMI. In the orbital frontal cortex/anterior cingulate areas, for instance, purple colors show that greater than 4% of brain volume is lost for every one standard deviation gain in BMI. The arrows and asterisks identify matching anatomical areas between the main effects r images and the beta image.
Acknowledgements
Algorithm development for this study was funded by the NIA, NIBIB, and the NCRR (AG016570, EB01651, RR019771 to PT). This study was also supported by funds from the National Institute of Aging to O.L.L. (AG 20098, AG05133) and L.H.K. (AG15928) and an American Heart Association Pre‐doctoral Grant to C.A.R. (0815465D). A full list of participating CHS investigators and institutions is available at http://www.chs-nhlbi.org. C.A.R. would like to acknowledge Dr. William E. Klunk for his mentorship and support.
REFERENCES
- Apostolova LG, Dutton RA, Dinov ID, Hayashi KM, Toga AW, Cummings JL, Thompson PM ( 2006): Conversion of mild cognitive impairment to Alzheimer disease predicted by hippocampal atrophy maps. Arch Neurol 63: 693–699. [DOI] [PubMed] [Google Scholar]
- Arvanitakis Z, Schneider JA, Wilson RS, Li Y, Arnold SE, Wang Z, Bennett DA ( 2006): Diabetes is related to cerebral infarction but not to AD pathology in older persons. Neurology 67: 1960–1965. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ ( 2000): Voxel‐based morphometry—The methods. Neuroimage 11: 805–821. [DOI] [PubMed] [Google Scholar]
- Bian L, Yang JD, Guo TW, Sun Y, Duan SW, Chen WY, Pan YX, Yeng GY, He L ( 2004): Insulin‐degrading enzyme and Alzheimer disease. Neurology 63: 241–245. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E ( 1991): Neuropathological stageing of Alzheimer‐related changes. Acta Neuropathol 82: 239–259. [DOI] [PubMed] [Google Scholar]
- Brach JS, Talkowski JB, Strotmeyer ES, Newman AB ( 2008): Diabetes mellitus and gait dysfunction: Possible explanatory factors. Phys Ther 88: 1365–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braskie MN, Klunder AD, Hayashi KM, Protas H, Kepe V, Miller KJ, Huang SC, Barrio JR, Ercoli LM, Siddarth P, Satyamurthy N, Liu J, Toga AW, Bookheimer SY, Small GW, Thompson PM ( 2008): Plaque and tangle imaging and cognition in normal aging and Alzheimer's disease. Neurobiol Aging. In press. doi:10.1016/j.neurobiolaging.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breteler MM, van Swieten JC, Bots ML, Grobbee DE, Claus JJ, van den Hout JH, van Harskamp F, Tanghe HL, de Jong PT, van Gijn J ( 1994): Cerebral white matter lesions, vascular risk factors, and cognitive function in a population‐based study: The Rotterdam Study. Neurology 44: 1246–1252. [DOI] [PubMed] [Google Scholar]
- Callen DJA, Black SE, Gao F, Caldwell CB, Szalai JP ( 2001): Beyond the hippocampus. MRI volumetry confirms widespread limbic atrophy in AD. Neurology 57: 1669–1674. [DOI] [PubMed] [Google Scholar]
- Ceska R ( 2007): Clinical implications of the metabolic syndrome. Diab Vasc Dis Res 4 ( Suppl 3): S2–S4. [DOI] [PubMed] [Google Scholar]
- Claus JJ, Breteler MM, Hasan D, Krenning EP, Bots ML, Grobbee DE, van Swieten JC, van Harskamp F, Hofman A ( 1996): Vascular risk factors, atherosclerosis, cerebral white matter lesions and cerebral perfusion in a population‐based study. Eur J Nucl Med 23: 675–682. [DOI] [PubMed] [Google Scholar]
- Colcombe SJ, Erickson KI, Raz N, Webb AG, Cohen NJ, McAuley E, Kramer AF ( 2003): Aerobic fitness reduces brain tissue loss in aging humans. J Gerontol A Biol Sci Med Sci 58: 176–180. [DOI] [PubMed] [Google Scholar]
- Dai W, Lopez OL, Carmichael OT, Becker JT, Kuller LH, Gach HM ( 2008): Abnormal regional cerebral blood flow in cognitively normal elderly subjects with hypertension. Stroke 39: 349–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeKosky ST, Mathis CA, Price JC, Lopresti BJ, Meltzer CC, Ziolko SK, Hoge JA, Tsopelas N, Klunk WE ( 2006): Human amyloid‐imaging studies with Pittsburgh Compound‐B in Mild Cognitive Impairment (MCI): Is MCI the critical period of amyloid plaque deposition? Alzheimer's and Dementia 1: S84. [Google Scholar]
- den Heijer T, Vermeer SE, van Dijk EJ, Prins ND, Koudstaal PJ, Hofman A, Breteler MM ( 2003): Type 2 diabetes and atrophy of medial temporal lobe structures on brain MRI. Diabetologia 46: 1604–1610. [DOI] [PubMed] [Google Scholar]
- Edgington ES ( 1995): Randomization Tests, 3rd ed New York: Marcel Dekker. [Google Scholar]
- Elias MF, Elias PK, Sullivan LM, Wolf PA, D'Agostino RB ( 2005): Obesity, diabetes and cognitive deficit: The Framingham Heart Study. Neurobiol Aging 26 ( Suppl 1): 11–16. [DOI] [PubMed] [Google Scholar]
- Ferguson SC, Blane A, Perros P, McCrimmon RJ, Best JJ, Wardlaw J, Deary IJ, Frier BM ( 2003): Cognitive ability and brain structure in type 1 diabetes: Relation to microangiopathy and preceding severe hypoglycemia. Diabetes 52: 149–156. [DOI] [PubMed] [Google Scholar]
- Fried LP, Borhani NO, Enright P, Furberg CD, Gardin JM, Kronmal RA, Kuller LH, Manolio TA, Mittelmark MB, Newman A ( 1991): The Cardiovascular Health Study: Design and Rationale. Ann Epidemiol 1: 263–276. [DOI] [PubMed] [Google Scholar]
- Gazdzinski S, Kornak J, Weiner MW, Meyerhoff DJ ( 2008): Body mass index and magnetic resonance markers of brain integrity in adults. Ann Neurol 63: 652–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, Nichols TE ( 2002): Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage 15: 870–878. [DOI] [PubMed] [Google Scholar]
- Guo X, Pantoni L, Simoni M, Gustafson D, Bengtsson C, Palmertz B, Skoog I ( 2006): Midlife respiratory function related to white matter lesions and lacunar infarcts in late life: The Prospective Population Study of Women in Gothenburg, Sweden Stroke 37: 1658–1662. [DOI] [PubMed] [Google Scholar]
- Gustafson D, Lissner L, Bengtsson C, Bjorkelund C, Skoog I ( 2004): A 24‐year follow‐up of body mass index and cerebral atrophy. Neurology 63: 1876–1881. [DOI] [PubMed] [Google Scholar]
- Holmes CJ, Hoge R, Collins L, Woods R, Toga AW, Evans AC ( 1998): Enhancement of MR images using registration for signal averaging. J Comput Assist Tomogr 22: 324–333. [DOI] [PubMed] [Google Scholar]
- Hua X, Leow AD, Parikshak N, Lee S, Chiang MC, Toga AW, Jack CR Jr, Weiner MW, Thompson PM ( 2008): Tensor‐based morphometry as a neuroimaging biomarker for Alzheimer's disease: An MRI study of 676 AD, MCI, and normal subjects. Neuroimage 43: 458–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikram MA, Vrooman HA, Vernooij MW, van der Lijn F, Hofman A, van der Lugt A, Niessen WJ, Breteler MM ( 2008): Brain tissue volumes in the general elderly population. The Rotterdam Scan Study. Neurobiol Aging 29: 882–890. [DOI] [PubMed] [Google Scholar]
- Irie F, Fitzpatrick AL, Lopez OL, Kuller LH, Peila R, Newman AB, Launer LJ ( 2008): Enhanced risk for Alzheimer disease in persons with type 2 diabetes and APOE epsilon4: The Cardiovascular Health Study Cognition Study. Arch Neurol 65: 89–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalmijn S, Fesken EM, Launer LJ, Stignen T, Kromhout D ( 1995): Glucose intolerance, hyperinsulinemia, and cognitive function in a general population of elderly men. Diabetologica 38: 1096–1102. [DOI] [PubMed] [Google Scholar]
- Kawasaki K, Ishii K, Saito Y, Oda K, Kimura Y, Ishiwata K ( 2008): Influence of mild hyperglycemia on cerebral FDG distribution patterns calculated by statistical parametric mapping. Ann Nucl Med 22: 191–200. [DOI] [PubMed] [Google Scholar]
- Knopman DS, Mosley TH, Catellier DJ, Sharrett AR ( 2005): Cardiovascular risk factors and cerebral atrophy in a middle‐aged cohort. Neurology 65: 876–881. [DOI] [PubMed] [Google Scholar]
- Kochunov P, Lancaster J, Thompson P, Toga AW, Brewer P, Hardies J, Fox P ( 2002): An optimized individual target brain in the Talairach coordinate system. Neuroimage 17: 922–927. [PubMed] [Google Scholar]
- Korf ES, van Straaten EC, de Leeuw FE, van der Flier WM, Barkhof F, Pantoni L, Basile AM, Inzitari D, Erkinjuntti T, Wahlund LO, Rostrup E, Schmidt R, Fazekas F, Scheltens P; LADIS Study Group ( 2007): Diabetes mellitus, hypertension and medial temporal lobe atrophy: The LADIS study. Diabet Med 24: 166–171. [DOI] [PubMed] [Google Scholar]
- Leibson CL, Rocca WA, Hanson VA ( 1997): Risk of dementia among persons with diabetes mellitus: a population‐based cohort study. Am J Epidemiol 145: 301–308. [DOI] [PubMed] [Google Scholar]
- Leow AD, Yanovsky I, Parikshak N, Hua X, Lee S, Toga AW, Jack CR Jr, Bernstein MA, Britson PJ, Gunter JL, Ward CP, Borowski B, Shaw LM, Trojanowski JQ, Fleisher AS, Harvey D, Kornak J, Schuff N, Alexander GE, Weiner MW, Thompson PM; Alzheimer's Disease Neuroimaging Initiative ( 2009): Alzheimer's disease neuroimaging initiative: A one‐year follow up study using tensor‐based morphometry correlating degenerative rates, biomarkers and cognition. Neuroimage 45: 645–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leporé N, Brun C, Pennec X, Chou YY, Lopez OL, Aizenstein HJ, Becker JT, Toga AW, Thompson PM ( 2007): Mean template for tensor‐based morphometry using deformation tensors In: Ayache N, Ourselin S, Maeder A, editors. MICCAI2007, Part II, LNCS 4792. Berlin Heidelberg: Springer‐Verlag; p 826–833. [DOI] [PubMed] [Google Scholar]
- Lopez OL, Jagust WJ, Dulberg C, Becker JT, DeKosky ST, Fitzpatrick A, Breitner J, Lyketsos C, Jones B, Kawas C, Carlson M, Kuller LH ( 2003a): Risk factors for mild cognitive impairment in the Cardiovascular Health Study Cognition Study: Part 2. Arch Neurol 60: 1394–1399. [DOI] [PubMed] [Google Scholar]
- Lopez OL, Kuller LH, Fitzpatrick A, Ives D, Becker JT, Beauchamp N ( 2003b): Evaluations of dementia in the cardiovascular health cognition study. Neuroepidemiology 22: 1–12. [DOI] [PubMed] [Google Scholar]
- Lopez OL, Kuller LH, Becker JT, Jagust JW, Fitzpatrick A, Carlson M, Breimer J, Lyketsos C ( 2004): Classification of vascular dementia in the Cardiovascular Health Study cognition study. Neurobiol Aging 25( Suppl 1): S483. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, de Leon M, de Santi S, Convit A, Tarshish C, Nair NP, Thakur M, McEwen BS, Hauger RL, Meaney MJ ( 1998): Cortisol levels during human aging predict hippocampal atrophy and memory deficits. [see comment][erratum appears in Nat Neurosci 1998 Aug;1(4):329]. Nat Neurosci 1: 69–73. [DOI] [PubMed] [Google Scholar]
- Mankovsky BN, Ziegler D ( 2004): Stroke in patients with diabetes mellitus. Diabetes Metab Res Rev 20: 268–287. [DOI] [PubMed] [Google Scholar]
- Morra JH, Tu Z, Apostolova LG, Green AE, Avedissian C, Madsen SK, Parikshak N, Toga AW, Jack CR Jr, Schuff N, Weiner MW, Thompson PM, ADNI ( 2009): Automated mapping of hippocampal atrophy in 1‐year repeat MRI data from 490 subjects with Alzheimer's disease, mild cognitive impairment, and elderly controls. Neuroimage 45( 1 Suppl): S3–S15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeill AM, Katz R, Girman CJ, Rosamond WD, Wagenknecht LE, Barzilay JI, Tracy RP, Savage PJ, Jackson SA ( 2006): Metabolic syndrome and cardiovascular disease in older people: The cardiovascular health study. J Am Geriatr Soc 54: 1317–1324. [DOI] [PubMed] [Google Scholar]
- Pannacciulli N, Del Parigi A, Chen K, Le DS, Reiman EM, Tataranni PA ( 2006): Brain abnormalities in human obesity: A voxel‐based morphometric study. Neuroimage 31: 1419–1425. [DOI] [PubMed] [Google Scholar]
- Swan GE, DeCarli C, Miller BL, Reed T, Wolf PA, Jack LM, Carmelli D ( 1998): Association of midlife blood pressure to late‐life cognitive decline and brain morphology. Neurology 51: 986–993. [DOI] [PubMed] [Google Scholar]
- Taki Y, Kinomura S, Sato K, Inoue K, Goto R, Okada K, Uchida S, Kawashima R, Fukuda H ( 2008): Relationship between body mass index and gray matter volume in 1,428 healthy individuals. Obesity (Silver Spring) 16: 119–124. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Giedd JN, Woods RP, MacDonald D, Evans AC, Toga AW ( 2000): Growth patterns in the developing brain detected by using continuum‐mechanizal tensor maps. Nature 404: 190–193. [DOI] [PubMed] [Google Scholar]
- Thacker NA, Williamson DC, Pokric M ( 2004): Voxel based analysis of tissue volume from MRI data. Br J Radiol 77: S114–S125. [DOI] [PubMed] [Google Scholar]
- Tiehuis AM, van der Graaf Y, Visseren FL, Vincken KL, Biessels GJ, Appelman AP, Kappelle LJ, Mali WP ( 2008): Diabetes increases atrophy and vascular lesions on brain MRI in patients with symptomatic arterial disease. Stroke 39: 1600–1603. [DOI] [PubMed] [Google Scholar]
- van Dijk EJ, Prins ND, Vermeer SE, Vrooman HA, Hofman A, Koudstaal PJ, Breteler MM ( 2005): C‐reactive protein and cerebral small‐vessel disease: The Rotterdam Scan Study. Circulation 112: 900–905. [DOI] [PubMed] [Google Scholar]
- van Oijen M, Okereke OI, Kang JH, Pollak MN, Hu FB, Hankinson SE, Grodstein F ( 2008): Fasting insulin levels and cognitive decline in older women without diabetes. Neuroepidemiology 30: 174–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson GS, Peskind ER, Asthana S, Purganan K, Wait C, Chapman D, Schwartz MW, Plymate S, Craft S ( 2003): Insulin increases CSF A‐Beta‐42 levels in normal older adults. Neurology 60: 1899–1903. [DOI] [PubMed] [Google Scholar]
- WHO ( 2009): Obesity and overweight. World Health Organization. Available at: http://www.who.int/dietphysicalactivity/publications/facts/obesity/en/. Accessed on April 19, 2009.
- Wolf PA, Beiser A, Elias MF, Au R, Vasan RS, Seshadri S ( 2007): Relation of obesity to cognitive function: Importance of central obesity and synergistic influence of concomitant hypertension. The Framingham Heart Study. Curr Alzheimer Res 4: 111–116. [DOI] [PubMed] [Google Scholar]
- Yaffe K, Blackwell T, Kanaya AM, Davidowitz N, Barrtett‐Connor E, Krueger K ( 2004): Diabetes, impaired fasting glucose, and development of cognitive impairment in older women. Neurology 63: 658–663. [DOI] [PubMed] [Google Scholar]
- Yue NC, Arnold AM, Longstreth WT, Elster AD, Jungreis CA, O'Leary DH, Poirier VC, Bryan RN ( 1997): Sulcal, ventricular, and white matter changes at MR imaging in the aging brain: Data from the Cardiovascular Health Study. Radiology 202: 33–39. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found in the online version of this article.
Supporting Information Figure 1. This figure shows two columns of sagittal and axial images. The first column shows the main effects of BMI from the r image of Figure 4. The second column shows the corresponding beta image or the slope of the multiple regression of BMI on brain structure. This picture shows an estimate, based on that multiple regression, of the percentage brain volume (in cc) lost for every one standard deviation gain in BMI. In the orbital frontal cortex/anterior cingulate areas, for instance, purple colors show that greater than 4% of brain volume is lost for every one standard deviation gain in BMI. The arrows and asterisks identify matching anatomical areas between the main effects r images and the beta image.


