Abstract
Introduction
Chest pain and shortness of breath are among the most common symptoms requiring immediate evaluation. Testing for pulmonary embolism (PE) has become easier and widespread due to D-dimer blood tests. Safe use of these tests is only possible if sensitivity is high and they are used in non-high probability patients. We evaluated diagnostic performance of the HemosIL HS D-dimer, which despite FDA approval in 2005, has been minimally reported in prospective standard clinical care.
Materials and Methods
We used a prospective observational study design to follow patients in a single center with the HemosIL HS ordered for symptoms of possible PE with positive test result if >243ng/ml. The outcome was PE or deep venous thrombosis (DVT) at the time of presentation or subsequent 45 days determined by structured evaluation of imaging tests, phone, or medical record follow-up in all patients.
Results
529 patients received a D-dimer and 4.7% were ultimately diagnosed with PE or DVT. The sensitivity of the HemosIL HS was 96.0% (95% CI; 79.6 to 99.9%) specificity was 65.7% (95% CI; 61.4 to 69.8%) and likelihood ratio negative was 0.06 (95% CI; 0.01 to 0.42). The probability of PE in patients with a negative D-dimer was 1/332 or 0.3% (95% CI; 0.01% to 1.67%). The receiver operator curve had an area under the curve of 0.87 and supported the current cut-point as optimal.
Conclusions
The HemosIL HS D-dimer had high sensitivity, very low negative post-test probability and is useful in excluding PE in the acute care setting.
Keywords: Accuracy, D-dimer, pulmonary embolism, sensitivity, specificity
Every year an estimated 10 million emergency department visits in the United States are precipitated by chest pain or shortness of breath. [1]. The exclusion of venous thromboembolism (VTE) – which includes pulmonary embolism (PE) and deep vein thrombosis (DVT) – is a common step in the evaluation of many of these patients. Physicians in US emergency departments (ED) are estimated to order testing to evaluate for PE in 0.5% - 2.6% of all visiting patients. [2,3]. The diagnostic strategy for suspected PE typically includes some estimate of pretest probability of disease, D-dimer testing for low-probability patients and imaging studies in cases of high pretest probability or positive D-dimer assay result. [4,5] The American College of Emergency Physicians (ACEP) endorses the safe exclusion of PE when a negative D-dimer assay with adequate sensitivity is combined with a low structured pretest probability. [6].
The HemosIL HS D-dimer (Instrumentation Laboratories, Lexington, MA) has been in use in the US as an aid in the diagnosis of VTE since FDA approval in 2005. The HemosIL HS was developed to improve on the specificity of the older HemosIL D-dimer assay through reduction in optical interference. The assay employs a suspension of polystyrene latex particles coated with the F(ab′)2 fragment of a monoclonal antibody specific for the D-dimer. When exposed to plasma containing D-dimer molecules, the coated particles agglutinate to a degree proportional to the quantity of D-dimer in the sample. The level of agglutination is determined by measuring the decrease in transmitted light at 671nm using the ACL TOP CTS automated immunoturbidimetric analyzer (Beckman Coulter, Inc., Fullerton, CA). The HemosIL HS functions exclusively on the ACL TOP CTS analyzer which is in use in an estimated 800 institutions worldwide.
While a meta-analysis in 2003 demonstrated that in general, turbidimetric D-dimers perform comparably to rapid ELISA tests and have a pooled sensitivity of 93% and specificity of 51%, [7] the HemosIL HS was not in use at the time of that analysis. To our knowledge, published studies of the clinical performance of the HemosIL HS assay are limited to two letters [8-9] and three abstracts. [10-12]
The purpose of this prospective independent study is to evaluate the real-time clinical performance of the HemosIL HS assay in the acute setting, to describe its sensitivity and specificity and to ascertain whether a negative result indicates a posttest prevalence of disease of less than 1% with an upper limit of the 95% confidence interval of less than 2.0%.
Materials and Methods
This was a prospective observational study conducted in an urban academic emergency department in the US. Patients were enrolled between September 22, 2006 and August 30, 2007. This study was approved by the Institutional Review Board for the conduct of human subject research. Patients were included if they had signs or symptoms that the treating physician interpreted as sufficient to warrant testing for PE and they indicated willingness to participate by process of written informed consent. Patients were excluded if they were already under treatment for PE or DVT with therapeutic levels of anticoagulation, had received CTPA, VQ or duplex Doppler testing indicating known VTE within prior 7 days, co-morbid conditions that indicated likely death within 45 days or circumstances that would prevent follow-up such as homelessness or imprisonment.
Trained research personnel sequentially monitored ED physician orders for PE testing during weekdays from 9:00 am until 9:00 pm. The HemosIL HS was the D-dimer used in clinical practice during this period. Laboratory clinical and research staff assigned the definition of a positive assay at greater than 243 ng/ml. Standard care included D-dimer use in low pre-test probability patients with the recommendation to treating physicians that negative tests result in no anticoagulation and no further imaging, while positive tests are followed by multidetector CT angiography or VQ scan. (Figure 1).
Figure 1. Course of care.
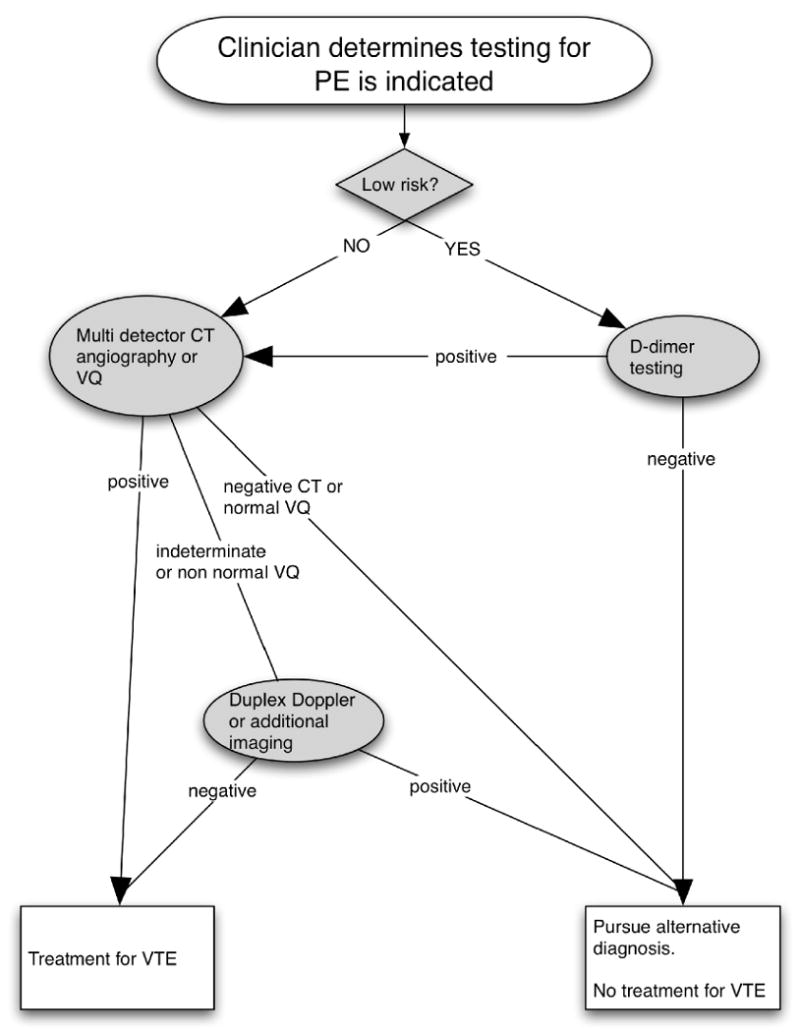
Standard algorithm guiding routine care and decision making for patients requiring testing for pulmonary embolism.
After providing informed consent, all subjects had a structured interview with data recorded in a web based data collection instrument with preformed fields and drop down menus to prevent miss-keyed or missing data. Uploading of the form was not possible until all data were complete. All clinical data including signs, symptoms, Wells Score components [13] and medical history were collected prior to results of final PE testing while in the ED. Research staff obtained pretest probability from the treating physician according to unstructured clinical judgment (categorized as low <15%, moderate 15-40%, or high >40%) prior to physician knowledge of PE testing results. For the purposes of this analysis patients were classified as low risk if they had either a Wells score <=4 OR they were deemed to be <15% probability of PE by the treating physicians at the time of test ordering.
Follow-up was performed 45 days after the index visit for all enrolled subjects via telephone interview, medical record review or search of the Social-Security Death Index. Patients and medical records were queried for subsequent acute care visits, hospitalization, cardiopulmonary imaging, tests for VTE, new or changed anticoagulation, or death. The criterion standard for the diagnosis of VTE was based on image results of CTPA, VQ or vascular duplex Doppler examination at the index visit or over the subsequent 45 days. Patients were considered positive for the primary outcome of VTE (PE or DVT) based on any of the following: CTPA documented by an attending radiologist as positive for acute filling defect of a pulmonary artery, VQ scan documented as high probability for PE, a duplex Doppler exam with acute proximal or distal DVT, or any death with autopsy determined PE. VTE determination for this study also required documentation of the intent or initiation of anticoagulation or inferior vena cava filter placement. As routine clinical care, radiologists were not blinded to D-dimer results, but results were not part of the standard required dataform required to make a radiology requisition.
Results
During the study period, 680 patients were enrolled for PE testing. (Figure 2) Of these, 529 were tested with the HemosIL HS assay while the remainder had exclusively image-based evaluation. The results and analyses below pertain only to the 529 patients who underwent HemosIL HS testing. The mean age was 46.3 years (95% CI 45.0 - 47.7) and 70.5% were female. (Table 1). The primary symptoms were dyspnea (49.5%) and chest pain (47.0%). At least one of the following traditional risk factors for PE were reported in 25.7% of patients (some reported multiple factors); active cancer n=19, trauma within four weeks n=5, surgery within 4 weeks n=13, personal history of VTE n=27, or concurrent estrogen therapy n= 81. Ninety-six percent (n=507) of patients tested with the HemosIL HS had either a Wells Score ≤4 or were deemed by the clinician to be low risk by unstructured physician assessment. Ninety five percent of all subjects had follow-up status verified by phone interview or medical record review.
Figure 2. Patient outcomes.
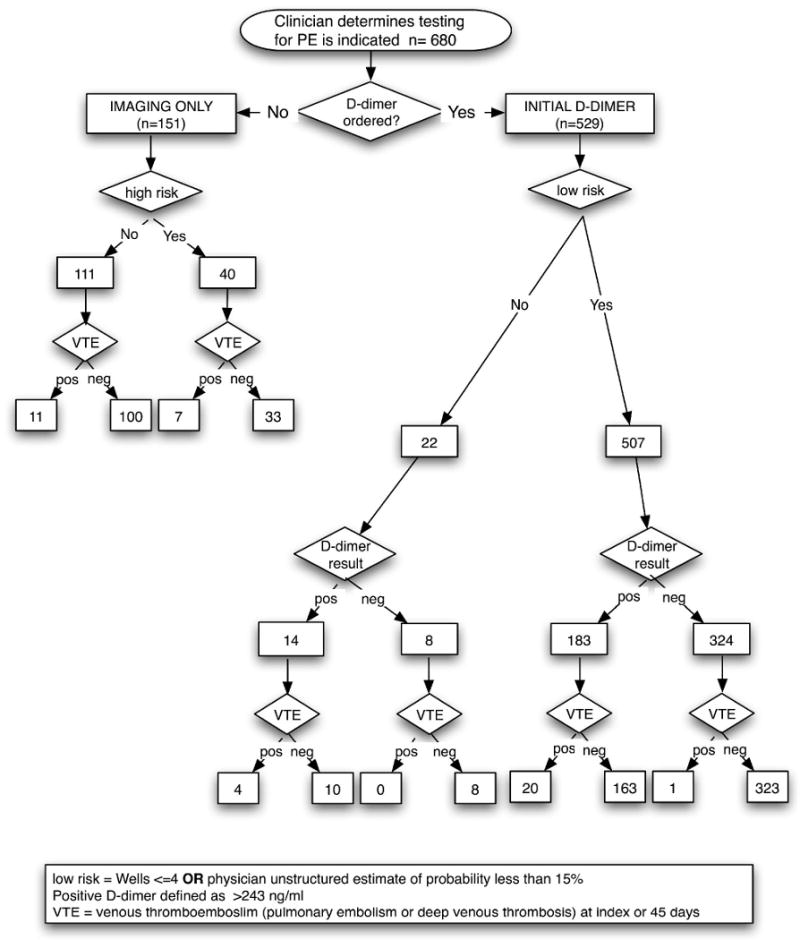
Table 1. Demographic and clinical characteristics of the sample.
| n (%) | 95% CI | |
|---|---|---|
| % Female | 373 (70.5%) | 66.4 to 74.4% |
| Age | 46.3 (mean) | 45.0 to 47.7 |
| Demographics | ||
| White | 300 (56.7%) | 52.4 to 61.0% |
| African American | 170 (32.1%) | 28.2 to 36.3% |
| Hispanic | 35 (6.6%) | 4.6 to 9.1% |
| Other | 24 (4.5%) | 2.9 to 6.7% |
| Symptoms | ||
| Chest pain | 250 (47.2%) | 42.9 to 51.6% |
| Dyspnea | 262 (49.5%) | 45.2 to 53.9% |
| Disposition | ||
| Discharged | 229 (43.3%) | 39.0 to 47.6% |
| Admitted | 172 (32.5%) | 28.5 to 36.7% |
| ED Observation | 121 (22.9%) | 19.3 to 26.7% |
| ICU | 7 (1.3%) | 5.3 to 2.7% |
| Pretest probability | ||
| Wells ≤4 | 490 (92.6%) | 90.0 to 94.7% |
| MD estimate <15% | 441 (83.4%) | 79.9 to 86.4% |
| Outcome of VTE | ||
| Overall | 25 (4.7%) | 3.1 to 6.9% |
| PE only | 15 (2.8%) | 1.6 to 4.6% |
| PE and DVT | 7 (1.3%) | 0.5% to 2.7% |
| DVT only | 3 (0.6%) | 0.1 to 1.6% |
The overall prevalence of VTE in this sample of 529 patients tested with the HemosIL HS was 4.7% (95% CI 2.9 - 6.5%); with 15 being PE only, 3 being DVT only, and 7 with both PE and DVT. All positive diagnoses were established during the index visit. No subject with D-dimer testing was determined to have newly diagnosed VTE or sudden death during the 45-day follow-up period. Twenty-two patients (4%) were non-low risk by Wells Score or physician's estimate, but received D-dimer testing at the discretion of the treating physician. (Figure 2) Of these, four were ultimately found to be VTE positive. All four had a HemosIL test that was positive, progressed to CTPA scan and had PE diagnosed in the ED at index visit. Not all patients tested for PE received a D-dimer. 151 patients were interpreted as being too high risk for D-dimer testing or had other indications for CT chest imaging and progressed directly to imaging tests. Eighteen or 11.9% (95% CI 7.7 - 18.1%) of these patients were VTE positive.
D-dimer assay results were positive in 197 patients (37.2%). In these cases, definitive diagnosis was obtained uniquely or in combination by: computerized tomography in 162 (82.2%), ventilation-perfusion scan in 12 (6.1%) and by venous duplex doppler in 66 (33.5%) patients. The sensitivity of the D-dimer was 96.0% (95% CI; 79.6 to 99.9%) and the specificity was 65.7% (95% CI; 61.4 to 69.8%). The likelihood ratio negative was 0.06 (95% CI; 0.01 to 0.42) with a likelihood ratio positive of 2.8 (95% CI; 2.4 to 3.2). The primary outcome - prevalence of VTE on index visit or follow-up in subjects with negative D-dimer result - was 1/332 or 0.3% (95% CI; 0.01% to 1.67%). (Tables 2, 3)
Table 2. 2 × 2 Summary of assay results and outcomes.
| VTE diagnosis (PE or DVT at index visit of subsequent 45 days) |
||||
|---|---|---|---|---|
| Positive | Negative | Total | ||
| HemosIL HS positive if >243 ng/mL | Positive | 24 | 173 | 197 |
| Negative | 1 | 331 | 332 | |
| Total | 25 | 504 | 529 | |
Table 3. Assay performance statistics.
| >243 ng/mL cutoff (95% CI) |
|
|---|---|
| Sensitivity | 96.0% (79.6 to 99.9%) |
| Specificity | 65.7% (61.3 to 69.8%) |
| Positive predictive value | 12.2% (8.0 to 17.6%) |
| Negative predictive value | 99.7% (98.3 to 100.0%) |
| Likelihood ratio negative | 0.06 (0.01 to 0.42) |
| Likelihood ratio positive | 2.80 (2.42 to 3.23) |
| Negative post-test probability | 0.3% (0.01 to 1.67) |
| True positives | 24 |
| False positives | 173 |
| True negatives | 331 |
| False negatives | 1 |
The cutoff for a positive assay in our analysis was >243 ng/ml. This level is suggested in the package insert and is used for reporting purposes in our clinical lab as well as for medical decision making by treating clinicians. We constructed a Receiver-Operator Curve to describe the overall test performance with area under the curve analysis and also to explore the optimal cutoff threshold for positive results. Figure 3 depicts the ROC analysis with an area under the curve (AUC) of 0.87 (95% CI; 0.84 - 0.90). Using a lowered cutpoint of 200 ng/ml would likely result in no added sensitivity in this sample as the one patient with a false negative D-dimer had a result of 150 ng/ml. The cutoff used in clinical care closely approximates the inflexion point of the ROC analysis and optimizes both sensitivity and specificity According our analysis from this cohort, only trivially increased specificity (65.7 to 65.9%) would result if the cutoff were raised to the maximum level consistent with maintained sensitivity (positive if ≥247 ng/ml).
Figure 3. D-dimer receiver operator curve.
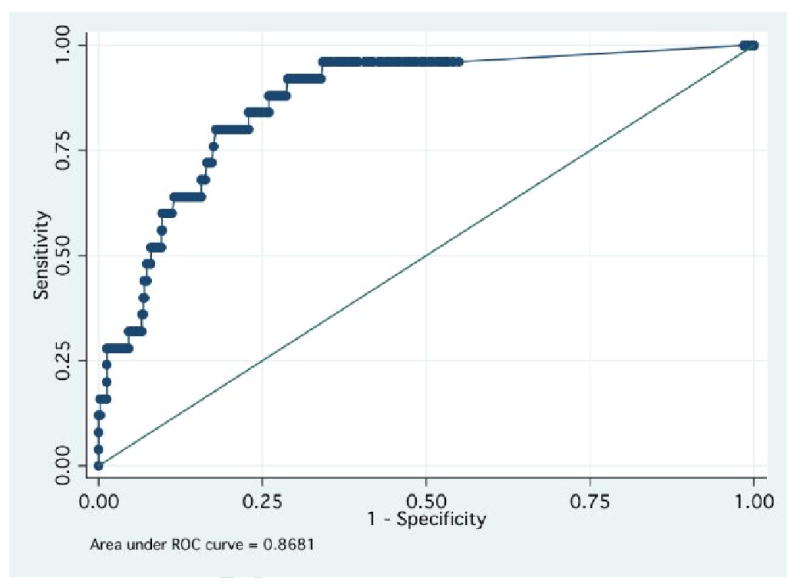
receiver operator characteristic curve demonstrating overall diagnostic performance of the HemosIL HS D-dimer. The Y axis plots sensitivity (true positive rate); the X axis plots 1-specificity (the false positive rate). The area under the curve is 0.87 with 95% confidence interval of 0.84 to 0.90.
Discussion
This large prospective study assessed the diagnostic accuracy of a new, rapid, turbidimetric D-dimer assay in clinical use in a busy emergency department. Using a sample of 529 prospectively enrolled patients, we report sensitivity of 96.0% (CI; 79.6% - 99.9%) and specificity of 65.7% (CI; 61.4% -69.8%) at the cutoff value of 243 ng/ml. It is also of note that in this real-time observational study of physician test utilization, the posttest prevalence of disease remained small (0.3%). Thus, our study supports the use of a negative HemosIL HS assay for the safe exclusion of disease in ED patients with low suspicion for VTE.
The performance measures compare favorably to assessments of the diagnostic accuracy of other latex-based turbidimetric D-dimer tests. A 2003 meta-analysis of turbidimetric D-dimer tests reported an overall sensitivity of 93% (CI; 89% - 96%) and specificity of 51% (CI; 42% - 59%). [8] More recently,studies of another widely used latex-based turbidimetric assay – the Liatest D-Di (Diagnostica Stago, Parsippany, NJ) – have reported safe use with sensitivity of 97-100% and specificity of 36-57%. [14, 15]
To our knowledge, this is the largest independently funded prospective study of the performance of the HemosIL HS in real time clinical use to evaluate for PE. In 2005, an abstract using blood bank samples reported a normal range, precision, processing speed of 130 samples/hour, and minimal optical interference. [10]. Also in 2005, a letter reported retrospective analysis of 300 stored samples from a 1999 management study of European and Canadian outpatients suspected of PE or DVT and compared performance of the HemosIL HS to the older HemosIL assay and the VIDAS immunoassay (bioMérieux Worldwide, Marcy l'Étoile, France). [8]. Authors reported an optimal cutpoint as determined by ROC analysis of 267 ng/ml resulting in a sensitivity of 100% (95% CI 95.4 to 100%) and a specificity of 51.8% (95% CI 45.0 to 58.5%). Subsequently in 2006, an abstract compared performance of the HemosIL HS with its predecessor and the VIDAS assay using samples from 166 patients. They report sensitivity of 100% for all three assays, but methods of testing for PE, follow-up, and confidence intervals were not reported. [12]. More recently a study done in several centers evaluated the HemosIL HS from either fresh and frozen samples for use in the exclusion of both DVT and PE among clinic and emergency department patients. [9] They included 361 patients with suspected PE and in that subset reported a sensitivity of 100% (93.8 to 100.0%) and specificity of 35.6% (30.2 to 41.3%) among all samples. This lower specificity is perhaps a result of the inclusion of older patients and the use of a lower cut-point (230 ng/ml). The sensitivity they report is consistent with our findings.
We acknowledge limitations to our work. Foremost, as this study was observational, physicians were not required to follow mandated imaging algorithms. There is, therefore, the possibility that patients may have had delayed non-recognized VTE. The design of this study included structured medical record review, phone follow-up to query for new anticoagulation, diagnosis or imaging to minimize this possibility. We also recognize that physicians were not required to calculate a Wells Score prior to ordering a D-dimer. However, physicians were required to prospectively report their interpretation of pretest probability as <15%, 15 to 40%, or >40% and all Wells Score variables were recorded before PE test results were returned. We observed that 96% of patients with D-dimer testing, were low risk by Wells score <=4 or physician unstructured estimate of <15% probability for PE. Although 4% of patients having testing with this D-dimer were non-low risk, and 4 of these 22 patients had VTE, all 4 were detected with an abnormal HemosIL D-dimer and confirmed with subsequent CTPA demonstrating acute PE.
Also it is important to point out that the low post-test probability that we report in patients with a negative test is a function of the low pre-test probability or prevalence of disease in this sample. However, we find that the prevalence of PE in our sample of 4.7% is not dissimilar to other ED based studies of PE, particularly in the US, and may reflect the current climate of test ordering driven by low risk patients with undifferentiated symptoms seeking acute care, medico-legal factors that promote over-testing, as well as the ease of current blood based testing for PE.
The present study contributes toward the understanding of the performance of the HemosIL HS when used under actual clinical conditions: where physicians may or may not calculate a Wells score and where D-dimer results guide the need for subsequent image-based testing. While consistent with the limited existing data available as to overall safety and accuracy of the HemosIL HS, our work reports sensitivity of 96%, specificity of 66% and prevalence of disease in patients with a negative test of 0.3% with an upper limit of the 95% CI of 1.7%. Our study is unique in employing a large racially diverse sample with prospective data collection and outcome determination and all decision making being done by clinicians at the time of test results.
We conclude that the HemosIL HS D-dimer is a safe, effective assay for use in exclusion of pulmonary embolism in Emergency Department patients with low pretest probability of disease.
Acknowledgments
Funding Source: 5K23HL77404-4 (DMC) from National Heart Lung and Blood Institute.
The authors thank the Northwestern Emergency Medicine research assistants and clinical laboratory personnel for their logistic assistance.
Abbreviations
- PE
pulmonary embolism
- HS
high sensitivity
- FDA
United States Food and Drug Administration
- DVT
deep venous thrombosis
- CI
confidence interval
- VTE
venous thromboembolism
- ED
emergency department
- ACEP
American College of Emergency Physicians
- ELISA
Enzyme Linked Immunosorbent Assay
- VQ
ventilation/perfusion scan
- LR
likelihood ratio
- ROC
receiver-operator curve
Footnotes
Previous Presentations: Society for Academic Emergency Medicine, Washington, DC, June 1 2008
Conflicts of Interest: None of the authors have any conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
D. Mark Courtney, Northwestern University, Chicago, IL.
Justin M. Steinberg, Northwestern University, Chicago, IL.
Jennifer C. McCormick, Northwestern University, Chicago, IL.
References
- 1.McCaig LF, Nawar EW. National hospital ambulatory medical care survey: 2004 emergency department summary. Adv data. 2006;372:1–29. [PubMed] [Google Scholar]
- 2.Kline JA, Webb WB, Jones AE, Hernandez-Nino J. Impact of a rapid rule-out protocol for pulmonary embolism on the rate of screening, missed cases, and pulmonary vascular imaging in an urban US emergency department. Ann Emerg Med. 2004;44:490–502. doi: 10.1016/j.annemergmed.2004.03.018. [DOI] [PubMed] [Google Scholar]
- 3.Kabrhel C, Matts C, McNamara M, Katz J, Ptak T. A highly sensitive ELISA D-dimer increases testing but not diagnosis of pulmonary embolism. Acad Emerg Med. 2006;13:519–524. doi: 10.1197/j.aem.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 4.Stein PD, Hull RD, Patel KC, Olson RE, Ghali WA, Brant R, Biel RK, Bharadia V, Kalra NK. D-dimer for the exclusion of acute venous thrombosis and pulmonary embolism: a systematic review. Ann Intern Med. 2004;140:589–602. doi: 10.7326/0003-4819-140-8-200404200-00005. [DOI] [PubMed] [Google Scholar]
- 5.Wells PS. Integrated strategies for the diagnosis of venous thromboembolism. J Thromb Hemost. 2007;5(Supplement 1):41–50. doi: 10.1111/j.1538-7836.2007.02493.x. [DOI] [PubMed] [Google Scholar]
- 6.Clinical policy: critical issues in the evaluation and management of adult patients presenting with suspected pulmonary embolism. Ann Emerg Med. 2003;41:257–270. doi: 10.1067/mem.2003.40. [DOI] [PubMed] [Google Scholar]
- 7.Brown MD, Lau J, Nelson RD, Kline JA. Turbidimetric D-dimer test in the diagnosis of pulmonary embolism: a metaanalysis. Clin Chem. 2003;49:1846–1853. doi: 10.1373/clinchem.2003.022277. [DOI] [PubMed] [Google Scholar]
- 8.De Moerloose P, Vanrusselt M, Reber G, Arnout J. Performances of the HemosIL D-dimer HS assay for the exclusion of venous thromboembolism. J Thromb Hemost. 2005;3:2361–2363. doi: 10.1111/j.1538-7836.2005.01582.x. [DOI] [PubMed] [Google Scholar]
- 9.Scarvelis D, Palareti G, Toulon P, Wells PS, Wu JR. HemosIL D-dimer HS assay in the diagnosis of deep vein thrombosis and pulmonary embolism. Results of a multicenter management study. J Thromb Hemost. 2008;6:1973–1975. doi: 10.1111/j.1538-7836.2008.03155.x. [DOI] [PubMed] [Google Scholar]
- 10.Arza B, Sanchez T, Sales M, d'Amunt L. Analytical performance of the new HemosIL TM D-DimerHS Assay that overcomes the Rheumatoid Factor (RF) Interference. J Thromb Hemost. 2005;3(Supplement 1) [Google Scholar]
- 11.Morelli B, Sales M, Arza B, Spagnotto M. Clinical evaluation of thromboembolic samples with the new HemosIL TM D-Dimer HS and the HemosIL TM D-Dimer Assays on the ACL TOP. J Thromb Hemost. 2005;3(Supplement 1 abstract P0285) [Google Scholar]
- 12.Toulon P, Levraut J, Appert A, Oualid H, Fisher F, Jambou D. Performance of a new rapid quantitative D-dimer assay (HemosIL D-dimer Plus) in the diagnosis of pulmonary embolism. Comparison with other quantitative assays. Preliminary results. J Thromb Hemost. 2006;4(Supplement 1):93. [Google Scholar]
- 13.Wells PS, Anderson DR, Rodger M, Stiell I, Dreyer JF, Barnes D, Forgie M, Kovacs G, Ward J, Kovacs MJ. Excluding pulmonary embolism at the bedside without diagnostic imaging: management of patients with suspected pulmonary embolism presenting to the emergency department by using a simple clinical model and d-dimer. Ann Intern Med. 2001;135:98–107. doi: 10.7326/0003-4819-135-2-200107170-00010. [DOI] [PubMed] [Google Scholar]
- 14.Ghanima W, Abnelnoor M, Mowinckel M-C, Sandset PM. The performance of STA-Liatest D-dimer assay in out-patients with suspected pulmonary embolism. Br J Haematol. 2006;132:210–215. doi: 10.1111/j.1365-2141.2005.05859.x. [DOI] [PubMed] [Google Scholar]
- 15.Bosson JL, Barro C, Satger B, Carpentier PH, Polack B, Pernod G. Quantitative high D-dimer value is predictive of pulmonary embolism occurrence independently of clinical score in a well-defined low risk factor population. J Thromb Hemost. 2005;3:93–99. doi: 10.1111/j.1538-7836.2004.01045.x. [DOI] [PubMed] [Google Scholar]


