Abstract
In the present study, we manipulated the cognitive effort in an associative encoding task using fMRI. Older and younger adults were presented with two objects that were either semantically related or unrelated, and were required to form a relationship between the items. Both groups self-reported greater difficulty in completing the unrelated associative encoding task providing independent evidence of the associative difficulty manipulation. On both the low and high difficulty tasks, older adults showed a typical pattern of increased right inferior frontal recruitment relative to younger adults. Of particular interest was the finding that both groups showed increased activation as task difficulty increased in the left inferior frontal and left hippocampus. Overall, the results suggest that the aging brain is characterized by greater prefrontal processing, but that as cognitive demand increases, the networks used by older and younger adults are the largely the same.
Keywords: Aging, Relational Memory, Prefrontal Cortex, Hippocampus, Encoding
1. INTRODUCTION
The present study focuses on the neural substrates of relational encoding in younger and older adults. Prior investigations involving younger adults have shown that regions of the medial temporal lobe (Davachi, Mitchell, & Wagner, 2003; Davachi, Maril & Wagner, 2001; Prince, Daselaar, & Cabeza, 2005; Sperling, Bates, Cocchiarella, Schacter, Rosen, & Albert, 2001) and the prefrontal cortex (Achim & Lepage, 2005; Addis & McAndrews, 2007; Dolan & Fletcher, 1997) are important for memory formation, with the hippocampus especially crucial for relational encoding (Cohen, Ryan, Hunt, Romine, Wszalek, Nash, 1999; Davachi et al., 2003; Henke, Weber, Kneifel, Weiser & Buck, 1999). There is considerable evidence that when older adults encode information they show both heightened functional activity in frontal regions (Cabeza, et al. 1997; Reuter-Lorenz, et al, 2000; Cabeza, 2002) and decreased medial temporal activation on a broad range of memory tasks in comparison to the young (Daselaar, Veltman, Rombouts, Raaijmakers, & Jonker, 2003; Grady et al., 1995; Gutchess et al., 2005; Park & Gutchess, 2004).
While it is clear that the hippocampus and frontal regions are engaged in associative encoding, little is known about whether manipulation of associative difficulty modulates activity in these critical memory regions, and further, whether these changes differ as a function of age. The general view of functional activity with age is that as task demands increase, older adults bring on additional frontal sites, typically in contralateral, homologous regions, to provide additional neural resources for task performance (Park & Reuter-Lorenz, 2009; Cabeza, 1997). A few imaging studies have examined age-related changes on tasks that differ as a function of difficulty (Grady, 2002), but these studies—often employing a levels-of-processing manipulation—confound the type of encoding task on which subjects engage, with encoding difficulty (e.g. perceptual encoding of stimulus as compared with semantic encoding), so that the effect of task difficulty cannot be assessed with precision. To accurately assess the effect of increased task difficulty, it is vital to maintain encoding strategy while implementing the difficulty manipulation. One of the few studies that successfully manipulated difficulty involved a verb generation task (Persson, Sylvester, Nelson, Walsh, Jonides, Reuter-Lorenz, 2004). In that study, participants generated verbs to noun targets that had either high- or low-selection demands. Using an ROI approach, Persson et al. (2007) showed that young adults increased suppression of default activity compared to the older adults as difficulty increased. Put simply, young adults, when faced with cognitive challenge, were more likely than older adults to switch out of a resting state and actively suppress this mode of neural activity. While providing important insights into effects of task difficulty on default activity, the Persson et al. (2007) study yielded little information about associative encoding processes since the task was not a memory task.
To get more insight into how frontal and hippocampal sites’ activation levels change with age when confronted with associative challenge, we developed a paradigm that a) involved a manipulation of task difficulty that was not confounded by encoding task differences, and, b) utilized a relational memory paradigm to maximize the functional contributions of the prefrontal cortex and the hippocampus. We adopted a relational memory task that manipulated task difficulty by varying the semantic relationship between to-be-encoded object pairs. In the present task, subjects viewed pairs of pictures and were instructed to form a meaningful sentence integrating the two objects. Behavioral evidence for this task suggests that relationally encoding unrelated objects is a more challenging task than encoding related pairs (Park, et al., 1990; Smith, Park, Earles, Shaw & Whiting, 1998). In addition, subjects rated the difficulty of each integration in this task, providing an independent measure of task difficulty.
We expected that for younger adults, as difficulty increased, both frontal and hippocampal activation would increase. In older adults, we considered two possible patterns of functional activity as difficulty level increased; based on previous work the first was a pattern of increased frontal activity and diminished hippocampal activity in the older adults relative to the young. Such a pattern in frontal and medial temporal regions was observed by Gutchess et al. (2005) and occurs broadly in the literature, leading to the frontal-hippocampal hypothesis, presented by Park and Gutchess (2004). This view suggests that enhanced frontal activity in older adults above the level of younger adults is compensatory for deficient hippocampal activations.
A second outcome we thought possible was a pattern of similar activity increases across both age groups, with the older adults showing a larger overall response to compensate for the many structural declines that occur in the brain with age (Park & Reuter-Lorenz, in press.) Often, neuroimaging studies reporting different patterns of neural activation with age have compared encoding conditions where no strategy is contrasted with a guided encoding condition where a strategy is prescribed. For example, Logan and colleagues (2002) showed that age differences were larger in prefrontal regions when subjects performed an intentional encoding task with no prescribed strategy compared to a guided incidental semantic encoding task. Similarly, Grady (1999) reported that requiring participants to process target items deeply at encoding through elaboration helped reduce age-related functional differences in the left prefrontal cortex and bilaterally in the medial temporal lobes during the processing of pictures relative to an intentional encoding condition.
Of the two potential outcomes, the first explanation (more frontal recruitment in the older adults) suggests that the hippocampus shows compromise with age—unable to show concomitant functional increases that correspond to task difficulty—which the regions of the frontal lobes must compensate for. The second explanation (similar activity increases for both age groups) argues that once a subject is focused on a task, modulation of the hippocampus and frontal cortex are roughly equivalent across age groups, and that age-related differences are linked more to selection of strategy than limitations in neural responsiveness of some brain regions. This latter explanation predicts that older adults will show a more young-like pattern of activation with increases in both frontal and hippocampal activation as task difficulty increases (Grady et al., 1999).
To determine the pattern of activation associated with increased encoding difficulty unconfounded by encoding strategy differences, we presented subjects with an associative memory task at two levels of difficulty. We hypothesized that if older adults are fundamentally limited in their ability to engage the hippocampus, increasing cognitive challenge would result in a pattern of lower medial temporal activation along with increased frontal activity for the old adults compared to younger adults. If, in contrast, aging differences observed in frontal/hippocampal recruitment patterns are due to strategy selection differences with age, we expected to see similar increases in frontal and hippocampal activations in older and younger adults as task demand increased. Particularly notable in the present study was the inclusion of difficulty ratings by subjects for each association formed, so our expectation that task difficulty actually varied between the two conditions could be independently verified.
2. METHODS
2.1. Subjects
A total of 18 older (10 females) and 19 younger adults (9 females) participated in the study. Mean age was 65.7 years (5.0 SD; range 60 – 80) for the older group and 20.9 years (2.1 SD; range 18–26) for the young. Both groups were restricted to right-handed, healthy individuals free of neurological disorders and items contraindicated by MRI (i.e., implanted ferrous metals). Informed consent was obtained from all subjects in accordance with the Institutional Review Board at the University of Illinois. Both groups had equivalent years of education, but the older group had a lower MMSE score (Folstein, Folstein & McHugh, 1975) than the younger adults (See Table 1). On measures of fluid intelligence (digit symbol and verbal memory), older adults perform more poorly than did the younger adults, while on crystallized measures (Shipley Vocabulary), the older adults performed somewhat better than the young, but not significantly so. This is a pattern that has been widely reported (Park et al., 1996, 2002), suggesting that the older adult in this study are typical of those sampled in other aging studies.
Table 1.
Comparison of Younger and Older adults on Neuropsychological measures (Means and Standard Deviation)
| Young | Older | p-value | |||
|---|---|---|---|---|---|
| Education | 14.63 | (1.67) | 15.63 | (2.61) | 0.14 |
| Health Rating1 | 3.89 | (0.66) | 4.17 | (0.71) | 0.23 |
| Health Satisfaction1 | 4.11 | (.81) | 4.28 | (.83) | 0.53 |
| Digit Symbol | 69.21 | (10.98) | 56.89 | (5.28) | 0.00 |
| Verbal Paired Associates2 | 26.39 | (5.42) | 22.22 | (7.82) | 0.07 |
| Dot Comparison2 | 50.39 | (10.62) | 37.83 | (5.76) | 0.00 |
| Controlled Oral Word Association (FAS) | 15.50 | (3.1) | 13.78 | (5.6) | 0.26 |
| Letter Number2 | 13.56 | (3.07) | 11.44 | (2.48) | 0.03 |
| Shipley's Vocabulary2 | 32.00 | (3.53) | 33.56 | (5.2) | 0.30 |
| MMSE | 29.42 | (0.77) | 28.44 | (1.38) | 0.01 |
Health Rating and Health Satisfaction were measured on a 1 to 5 scale. A rating of 4 equals better than average.
Young N=18
2.2. Stimuli
Stimuli were pairs of line-drawing images taken from the Snodgrass and Vanderwart (1980) pictorial set, the International Picture Naming Project (Szekely et al., 2004), and Clipart.com. The pictures were black and white line-drawings of common objects, such as animals, household items, and fruit.
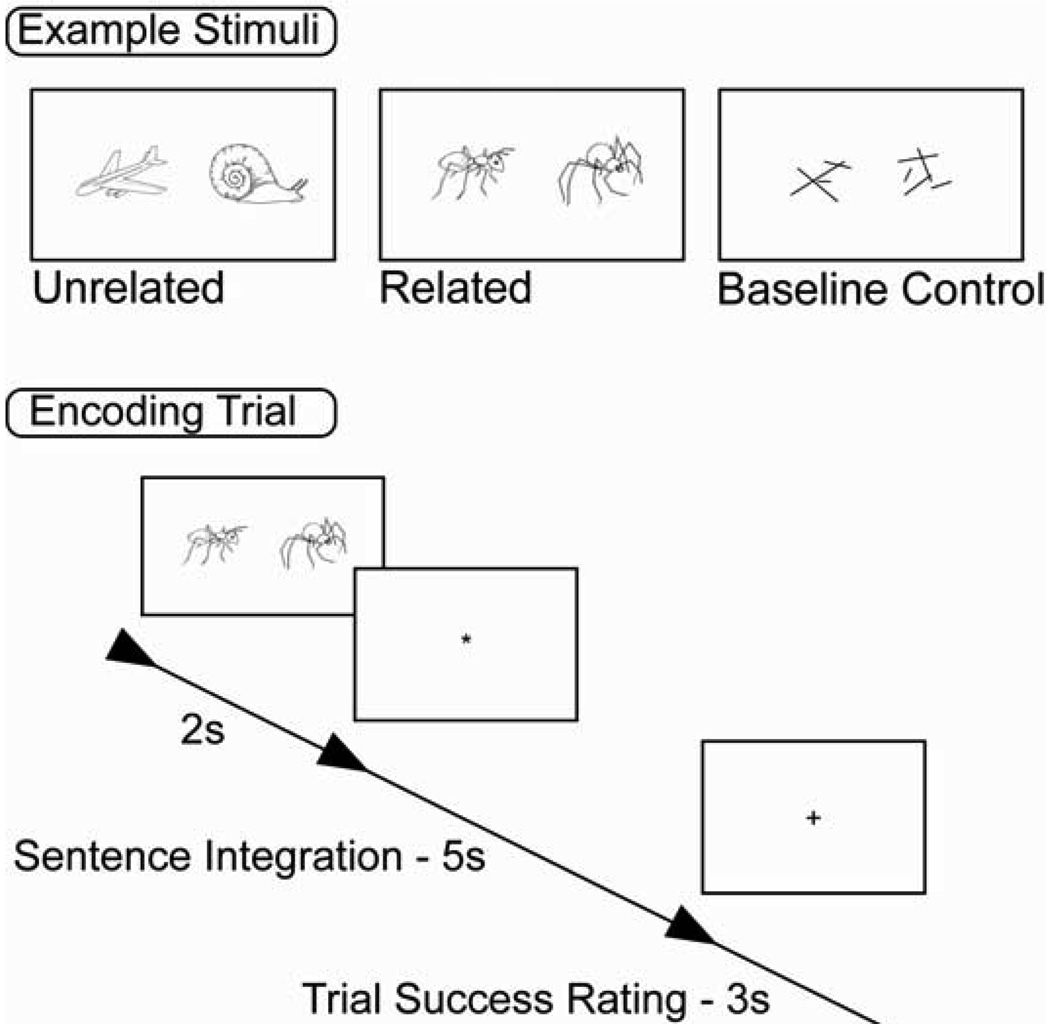
From the pool of line-drawings, related and unrelated pairs were created. Related pairs were sets of objects that were functionally (e.g. cart-horse), or categorically (e.g., apple-pear) related. Unrelated pairs were created by randomly pairing items together and discarding items judged by the experimenters to be obviously or even remotely related. An independent sample of 20 pilot subjects judged the relatedness of each pair on a scale of 1 to 7, with 7 being highly related. Related pairs had a mean rating of 6.52 (SD = .22) and the unrelated pairs a mean rating of 1.41 (SD = .33). A third set of stimuli was created to serve as the baseline control task. These stimuli consisted of pairs of randomly distributed lines which served as a control for the visual stimulation provided by the related and unrelated pairs (See Figure 1).
Figure 1.
Example stimuli and encoding trial schematic.
2.3. Design
A mixed design was employed with age (young or old) as a between-subjects variable and task difficulty (related, unrelated or control) as a within-subjects variable. There were 60 stimuli of each type presented across four runs. Within a run, there were 45 trials (15 of each stimulus type) presented in pseudorandom order. The control task served as the baseline condition against which both the related and unrelated trials were compared. The pseudorandomized presentation of the three trial types allowed for sufficient temporal separation between to allow for the deconvolution of the signal associated with each trial.
2.4. Procedure
Subjects were tested over two sessions. During the first session, they completed demographic and neuropsychological testing. During the second session, they received pre-training on the encoding task using color photographs rather than line-drawing images to prevent interference with the fMRI task. After successful completion of the training, they were placed in a Siemens Allegra 3T head-only scanner (Siemens, Erlanger, Germany). Subject’s vision was corrected to at least 20/30 with corrective lenses. Subjects were further provided noise-dampening ear plugs, a right handed five button response pad, and a headset to communicate with the MRI technician. Images were back-projected onto a screen and viewed through a mirror mounted on the head coil. First, T1-weighted structural scan (MPRAGE) were run. Next, during the four encoding runs, 32 slice T2*-weighted echo planar images (EPI) were acquired (TR = 2000 ms; TE = 25 ms, FoV = 22 cm, FA = 80°) with a slice thickness of 4 mm (3.4 mm in-plane resolution) and a ten percent interslice gap (0.4 mm). Run length was 470 seconds resulting in the collection of 235 volumes per run, or 940 volumes over the four EPI sessions.
A trial lasted 10 seconds and was composed of three epochs (for trial schematic, see Figure 1). For the first two seconds of the trial, the pair of pictures was displayed. After the initial two seconds, the stimulus pair was replaced by an asterisk that remained on screen for five seconds. During this 5 second interval, subjects performed the association task, and were instructed to construct a sentence associating the two objects together (e. g., “The spider bit the ant”). During the control condition, subjects were instructed to look at the abstract line drawings, but not to compose an integrating sentence. At the end of the five seconds, the asterisk was replaced by a fixation cross for the final three seconds of the trial. When the fixation cross appeared, subjects were instructed to rate their success at developing an integrative sentence for that trial. Following Davachi & Wagner (2003), subjects rated their success on a four choice scale (1: Succeeded with ease; 2: Succeeded with effort; 3: Partially Successful; 4: Unsuccessful), while in the control condition, subjects were instructed to always press “1”. Each trial was separated by a two second ITI.
Following the encoding trials, subjects were given a surprise recognition test that occurred approximately ten minutes after the last encoding run. During the recognition test subject were shown: Intact pairs that were identical to those shown during encoding, novel pairs that consisted of two items not seen before, and rearranged pairs which were novel pairings of old items seen during encoding. Of the original 60 related and 60 unrelated pairs presented during encoding, 40 pairs from each of the experimental conditions were shown intact and 20 pairs were shown rearranged. The semantic relationship between the items in the rearranged pairs was the same as in encoding (e.g., related or unrelated). Subjects were instructed to report a pair as old only if the pair was presented exactly as it was in the encoding session, and that any new pairing—pairing of novel items, or novel pairings of old items—was to be considered a new pair. Related and unrelated pairs were presented in a pseudo-randomized order. For each pair, subjects had a maximum of five seconds to make an old/don’t know/new judgment. All recognition trials were separated by a 1500 ms fixation interval. The recognition task was used only to calculate behavioral memory performance for the encoded items and fMRI data from recognition will not be reported here.
2.5. fMRI preprocessing and random effects analysis
All preprocessing and General Linear Model (GLM) estimation was carried out using SPM2 (Wellcome Department of Cognitive Neurology, London, UK). The initial five volumes of each run were discarded in order to allow for equilibrium; the remaining volumes were then corrected for slice acquisition time and for participant motion. The resultant images were warped to a standardized space (Montreal Neurological Institute; MNI), resampled into 2-mm cubic voxels, and then spatially smoothed using a 5-mm full-width-at-half-maximum (FWHM) gaussian kernel. Contrast images that were entered into the group-level random effects analysis were additionally smoothed with a 9-mm gaussian kernel.
For each 10 second trial, two separate regressors were modeled—one for the first 7 seconds and one for the final 3 seconds of each trial. The first 7 seconds of each trial contained the encoding and association task, whereas the last 3 seconds of each trial involved rating the success of encoding for that trial. Additionally, discrete regressors were modeled for successful and unsuccessful trials. Successful trials (a term that will be used throughout this text) were defined as trials where a subject reported success during the encoding task (rating of 1 or 2) and correctly recognized the intact pair at recognition. Data reported here are based only on the successful trials. Unless otherwise noted, all contrast maps were thresholded at p < 0.001, uncorrected, with a minimum cluster size of 10 voxels.
The fMRI analysis proceeded in a series of steps. In the first step, we examined the effect of difficulty separately for the younger adults and the older adults, by contrasting the unrelated with the related items in a single sample t-test for each group. Next, we examined the effect of difficulty across all subject using a single sample t-test. Based on this contrast, two regions of interest (ROIs) were selected for further age comparisons from the regions that showed a main effect of difficulty: a region in the left hippocampus (peak voxel = −36 −34 −8) and a region in the left inferior frontal gyrus (peak voxel= −52 30 10). The left hippocampal ROI consisted of an 8-mm spheres drawn around the peak voxel. Because the left inferior frontal peak voxel was close to the cortical surface, we drew a smaller ROI (6-mm) to ensure that no regions outside the cortical surface were included in the ROI analysis. In the second step of the analysis, we looked at the age effect collapsed across the difficulty conditions by directly contrasting the younger adults with the older adults using a 2-sample t-test, and then at the age effect for the unrelated and related conditions separately. Based on these contrasts, we examined age and encoding condition effects associated with two sets of ROIs—frontal regions in the right inferior frontal gyrus (peak voxel, MNI space = 40 28 18) and the left Middle frontal gyrus (peak voxel = −46 46 20) and a set of default regions that included the right superior parietal lobe (peak voxel = 32 −40 54), the right inferior parietal lobe (peak voxel = 60 −38 54), and the right Precuneus (peak voxel = 6 −60 58). The ROIs were constructed by including all functionally active voxels within an 8-mm sphere drawn around the peak voxel for each cluster. Mean parameter estimates were extracted for both groups using MarsBaR (Brett, Anton, Valabregue, & Poline, 2002).
Finally, we conducted a beta series connectivity analysis that allowed us to assess differences in functional networks associated with age and task difficulty, using seed voxels within the hippocampus (after Rissman & Gazzeley, 2004). The hippocampus seed regions were isolated for each subject from the left hippocampus, using the hippocampal ROI isolated in the first step of the fMRI analysis (Table 3C). For both the related and unrelated conditions, the 7 most active contiguous voxels, as determined by the related minus control and unrelated minus control were selected. General linear models for each subject were constructed that included separate regressors for the encoding portion (7 s) of each trial. Condition-specific time courses for the seed regions were then averaged and correlated with the condition-specific time courses of every other voxel in the brain, generating two whole-brain maps for each individual: related trial voxel-to-seed correlation and unrelated trial voxel-to-seed correlation. These maps were then z-transformed, and entered into a second-level group analysis. Group level whole brain 2-sample t-tests were then carried out. As in the univariate analysis, all correlational analyses were thresholded at p < 0 .001, uncorrected, with a minimum cluster size of 10 voxels.
Table 3.
Regions of activity in MNI coordinate space showing effects of the difficulty manipulation when the unrelated condition was contrasted with the related condition. Effects are shown for A) the older adults, B) the Younger adults, and C) both the younger and older adults (collapsed across age; BA = Brodmann’s Area).
| Region | Hemisphere | Coordinate (mm) | BA | Cluster Size |
t-value | ||
|---|---|---|---|---|---|---|---|
| x | y | Z | |||||
| A. Unrelated - Related contrast, Older | |||||||
| Inferior Frontal | L | −48 | 30 | 10 | 46 | 231 | 5.94 |
| Hippocampus | L | −36 | −32 | −10 | 36 | 166 | 5.82 |
| Cerebellum | L | −30 | −78 | −38 | 55 | 5.42 | |
| Middle Occipital | R | 52 | −68 | 26 | 39 | 356 | 5.38 |
| Angular | L | −48 | −76 | 24 | 39 | 376 | 5.37 |
| Middle Occipital | L | −42 | −76 | 24 | 39 | 4.99 | |
| Fusiform | R | 32 | −54 | −16 | 37 | 329 | 4.87 |
| Parahippocampus | R | 30 | −34 | −14 | 36 | 4.52 | |
| Middle Temporal | L | −60 | −56 | −4 | 37 | 209 | 4.56 |
| Inferior Temporal | L | −52 | −60 | −6 | 19 | 4.33 | |
| Cerebellum | R | 16 | −78 | −40 | 49 | 4.56 | |
| Middle Occipital | R | 38 | −84 | 8 | 19 | 174 | 4.49 |
| Middle Occipital | R | 34 | −88 | 16 | 19 | 4.45 | |
| Angular | L | −40 | −54 | 24 | 39 | 39 | 4.43 |
| Inferior Frontal | L | −32 | 26 | −14 | 47 | 33 | 4.34 |
| Cerebellum | R | 42 | −64 | −32 | 65 | 4.31 | |
| Precuneus | R | −6 | −58 | 64 | 7 | 94 | 4.28 |
| B. Unrelated - Related contrast, Younger | |||||||
| Middle Temporal | L | −48 | −48 | 20 | 40 | 1677 | 5.68 |
| Parahippocampus | L | −34 | −40 | −6 | 37 | 5.49 | |
| Middle Occipital | L | −34 | −90 | 10 | 19 | 5.34 | |
| Postcentral | L | −60 | −24 | 50 | 2 | 102 | 4.17 |
| Superior Occipital | R | 24 | −98 | 10 | 18 | 122 | 4.17 |
| Putamen | L | −18 | 16 | 4 | 23 | 4.15 | |
| Angular | L | −46 | −74 | 28 | 39 | 169 | 4.12 |
| Angular | L | −42 | −66 | 26 | 39 | 4.08 | |
| Inferior Temporal | R | 46 | −60 | −6 | 19 | 28 | 4.04 |
| Inferior Temporal | L | −56 | 32 | 10 | 46 | 43 | 4.03 |
| C. Unrelated - Related contrast, Collapsed Across Age | |||||||
| Angular | L | −46 | −74 | 26 | 39 | 6677 | 6.19 |
| Hippocampus | L | −36 | −34 | −8 | 6.13 | ||
| Middle Temporal | L | −54 | −54 | 4 | 21 | 5.66 | |
| Inferior Frontal | L | −52 | 30 | 10 | 46 | 1759 | 6.03 |
| Middle Occipital | R | 34 | −84 | 6 | 19 | 828 | 5.13 |
| Fusiform | R | 38 | −52 | −16 | 37 | 2244 | 5.11 |
| Inferior Temporal | R | 50 | −62 | −8 | 37 | 4.85 | |
| Angular | R | 52 | −62 | 26 | 39 | 714 | 5.09 |
| Superior Medial | |||||||
| Frontal | L | −4 | 58 | 42 | 9 | 146 | 4.44 |
| Precuneus | L | −8 | −54 | 60 | 7 | 229 | 3.93 |
| Middle Frontal | L | −40 | 8 | 58 | 6 | 101 | 3.83 |
3. RESULTS
3.1. Behavioral performance
3.1.1 Sentence Formation Ratings
The sentence formation ratings were used to establish the success of the encoding difficulty manipulation. Ratings from the sentence formation task were categorized into two groups: Complete (ratings of 1, “succeeded with ease”, and 2, “succeeded with effort”) or Incomplete (ratings of 3, “partially successful”, or 4, “unsuccessful”) encoding trials. An analysis indicated that age did not affect sentence ratings (F (1, 35) = .1, p = .75), but that condition did (F (1, 35) = 44.83, p < .01), with fewer completed encoding trials occurring for the unrelated compared to the related condition. This difference is important, because it provides independent verification of the experimental manipulation of encoding difficulty, indicating that both older and younger adults engaged in more processing to perform the associational task for unrelated compared to related items. Importantly, the high proportion of complete encoding trials (See Table 2A) suggests that both age groups were readily able to perform the sentence association task.
Table 2.
Encoding task success as represented by the percentage of complete and incomplete encoding trials is shown in (A). Complete encoding trials received a sentence formation rating of either 1 or 2, whereas incomplete trials received a rating of 3 or 4. Recognition accuracy represented by hits, false alarms and A’ are shown in (B). A’ scores were computed with the false alarms for the novel items (A’-New) and for the rearranged items (A’-Rearranged). The mean number of successful trials (trials receiving a sentence formation rating of 1 or 2 that were subsequently recognized) for each condition by age group is shown in (C).
| A. Percentage of Complete and Incomplete trials at Encoding | ||||||
|---|---|---|---|---|---|---|
| Related | Unrelated | |||||
| Complete (1 & 2) | Incomplete (3 & 4) | Complete (1 & 2) | Incomplete (3 & 4) | |||
| Young | 95.9% | 4.1% | 84.9% | 15.1% | ||
| Older | 97.7% | 2.3% | 81.6% | 18.4% | ||
| B. Recognition Accuracy: Hits, False Alarms (FA), and A' Prime scores | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Related | Unrelated | |||||||||
| Hit | FA-New | FA- Rearranged |
A'-New | A'- Rearranged |
Hit | FA-New | FA- Rearranged |
A'-New | A'- Rearranged |
|
| Young | 0.95 | 0.01 | 0.2 | 0.98 | 0.93 | 0.84 | 0.01 | 0.13 | 0.96 | 0.91 |
| Older | 0.96 | 0.02 | 0.41 | 0.98 | 0.87 | 0.82 | 0.01 | 0.27 | 0.95 | 0.85 |
| C. Mean Number of Successful and Unsuccessful Trials (out of 40) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Related | Unrelated | |||||||||
| Successful | Unsuccessful | Successful | Unsuccessful | |||||||
| Young | 35.8 | 4.2 | 28.4 | 11.6 | ||||||
| Older | 36.1 | 3.9 | 26.4 | 13.6 | ||||||
3.1.2. Recognition performance
A-prime (A’) scores were calculated using the hits for the intact pairs and the false alarms to the new pairs (A’-new; for A’ prime formula, see Stanislaw & Todorov, 1999), and the means associated with this analysis are displayed in Table 2B. An analysis using this A’ measure showed an effect of condition (F (1, 35) = 72.70, p < .01) but no effect of age (F (1, 35) = .17, p = .68), or age by condition interaction (F (1, 35) = .22, p = .65). A second A’ (A’-rearranged) was calculated using the hit rate for the intact pairs and the false alarm rate for the rearranged pairs. This measure of A’ resulted in an age main effect (F (1, 35) = 8.63, p < .01), but no effect of difficulty (F (1, 35) = 1.78, p = .19) or interaction (F (1, 35) = 0, p = .99). Taken together, the A’ analysis indicated that if a traditional memory measure was used (hits versus false alarms to new items), both groups performed similarly. However, when a more stringent test of memory was used by presenting rearranged items that the subject should reject, substantial effects of age emerged, reflecting the increased difficulty of the memory task.
3.1.3. Successful Trials
Trials entered into the random effects fMRI analysis were “Complete” encoding trials (sentence formation rating of 1 or 2), that were subsequently remembered. The mean number of successful trials (out of a maximum of 40) is displayed in Table 2C. The number of successful trials was entered into a 2 (age) by 2 (task difficulty) ANOVA, which resulted in a main effect of encoding condition (F (1, 35) = 89.90, p < .01), but no main effect of age (F (1, 35) = .361, p<.55) or age by condition interaction (F (1, 35) = 1.64, p = .21). Like the data from the encoding ratings, both groups had equivalent numbers of successful trials within each of the two conditions. 1
3.2. fMRI Results
3.2.1. Differences in neural activation as a function of relatedness
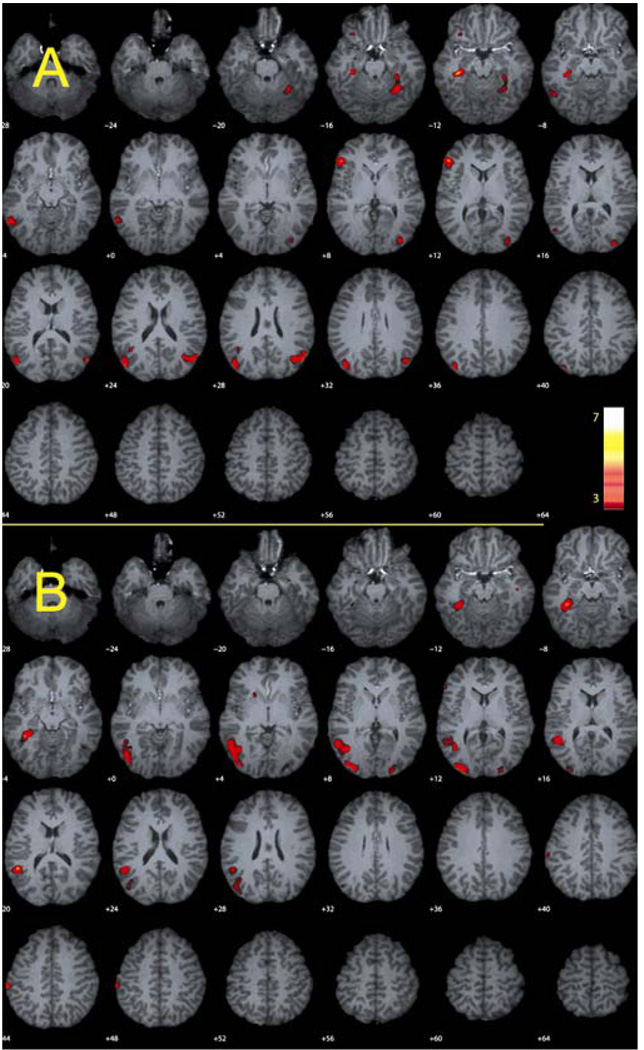
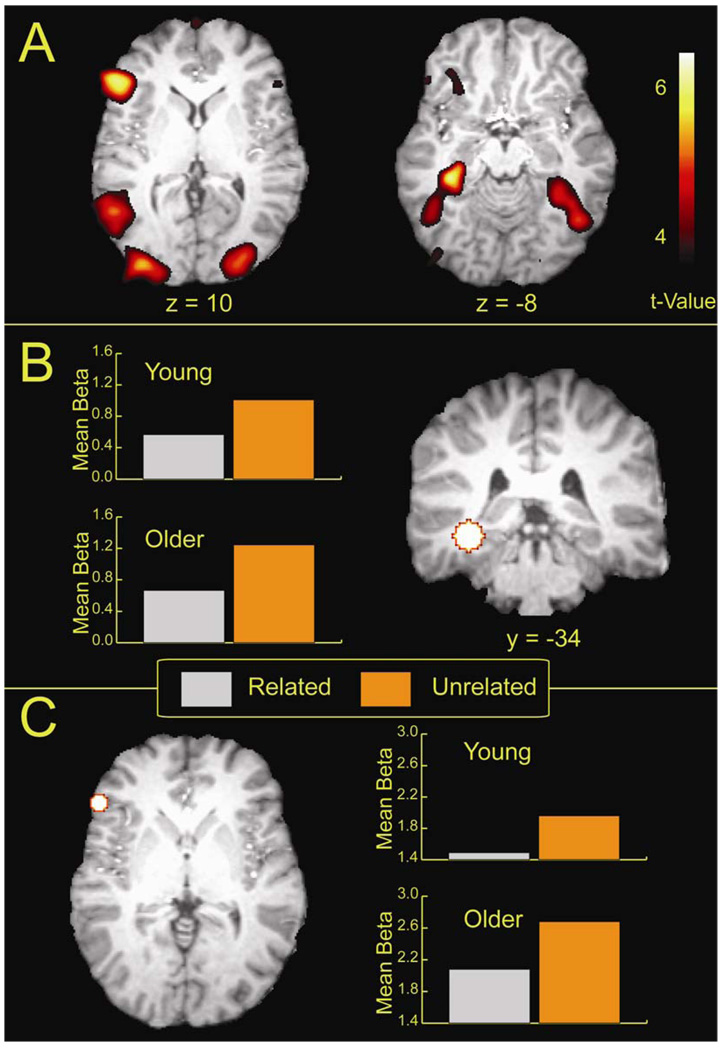
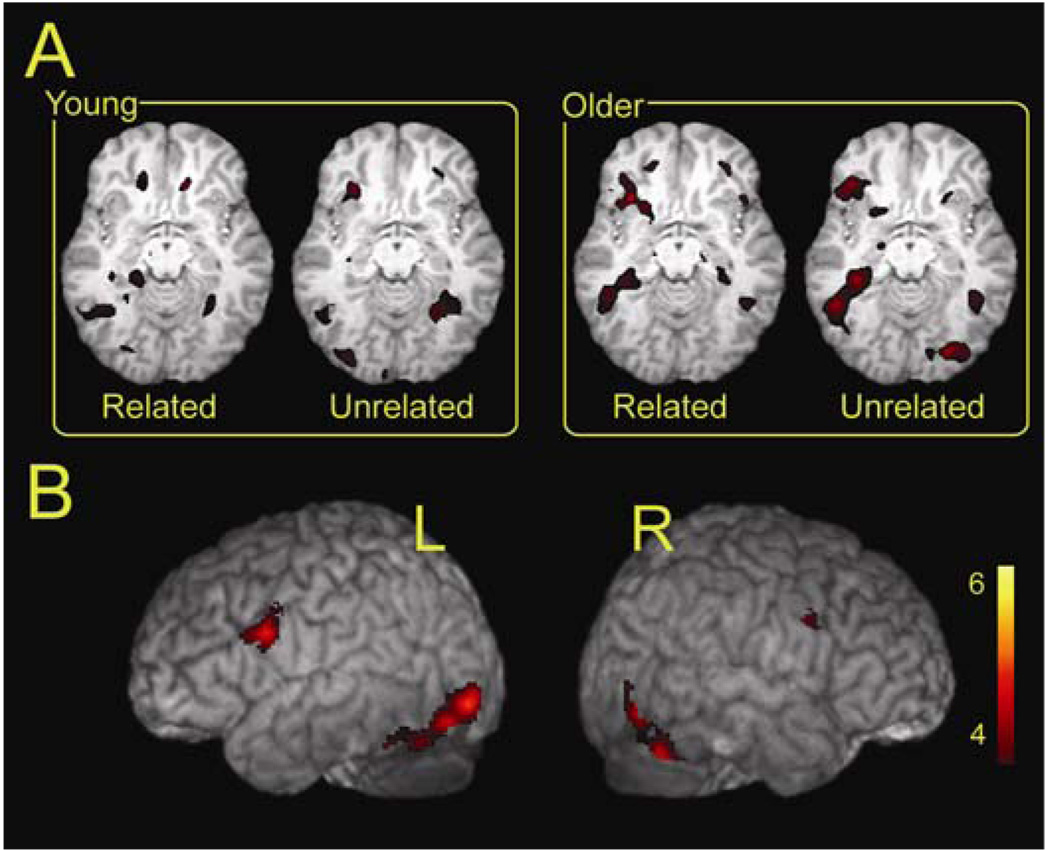
To assess the main effect of the encoding difficulty manipulation, we first contrasted the unrelated with the related condition separately for the younger and older adults. The older adults responded to relational difficulty by engaging inferior frontal, hippocampal and occipital cortex regions (See Table 3A and Figure 2A). Younger adults engaged middle temporal regions which extended into the parahippocampus, occipital areas and inferior frontal regions (See Table 3B and Figure 2B). The frontal regions engaged by the younger adults were somewhat spatially reduced in comparison to the older adults, but were still robust regions of activation. The analysis showed that both younger and older engaged a similar set of regions in response to the difficulty manipulation, which included medial temporal, inferior frontal, and middle occipital regions. While the regions recruited were similar across age, the older adults did show bilateral activity in medial temporal and in middle occipital regions. We next examined the effects of difficulty collapsed across age. Results of the analysis yielded several regions of activity distributed across the brain that included the angular gyrus, and both the left hippocampus and the left inferior frontal gyrus (see Table 3C). This analysis showed that both younger and older adults exhibited more activation for the difficult unrelated condition relative to the related condition, with little evidence of a greater effect in the older adults as a function of difficulty. The largest peaks of activation in the left hippocampus and the left inferior frontal gyrus (See Figure 3A) were then selected for further analysis.
Figure 2.
Regions showing the effect of difficulty (Unrelated – Related contrast) are depicted for the (A) older adults and the (B) younger adults. Both groups showed activity in medial temporal, occipital and inferior frontal regions. Threshold, p < .001, uncorrected, 10 voxel minimum cluster size.
Figure 3.
Activity from the supergroup analysis is shown in two slices that correspond to the left prefrontal and medial temporal activity (A). The ROI mask and the mean parameter estimates (betas) for the related and the unrelated conditions are shown for the left hippocampal ROI (B) and the left inferior frontal ROI (C).
An analysis of the parameter estimates extracted from the left hippocampal ROI (Figure 3B) resulted in a main effect of condition (F (1, 35) = 29.18, p < .01) due to the greater activation in the unrelated compared to related condition, but no age Effect or interaction (F < .01) . The hippocampal activity exhibited here is consistent with prior literature showing hippocampal involvement in associative memory (Davachi et al., 2003; Henke et al., 1997; Henke et al., 1999). An age by difficulty ANOVA conducted on the parameter estimates extracted from the left inferior frontal ROI (See Figure 3C), yielded a main effect of difficulty (F (1, 35) = 35.19, p < .01), and a marginally significant effect of age (F (1, 35) = 3.20, p = .08), but no interaction (F (1, 35) = .74, p = .49). Activity in this region has been associated with semantic processing (Kelley, Miezin, McDermott, Buckner, Raichle, Cohen et al. 1998) and is commonly seen during encoding tasks. For both age groups the unrelated condition elicited greater functional recruitment than the related condition. Additionally, the trend towards an age effect in the left inferior frontal gyrus suggests that the older adults were showing an increased response compared to the younger adults.
3.2.2 Age Effects
A series of contrasts of the age differences yielded consistent evidence for greater activation in older compared to younger adults across the Task difficulty manipulation. In an initial contrast—all trials (collapsed across difficulty) minus baseline—the younger adults showed greater activation in the left supramarginal gyrus compared to the older adults (Table 4A). Previous studies have implicated this region in the maintenance of verbal information in working memory in studies with younger adults (Gold & Buckner, 2002; Paulesu, Frith, & Frackowiak, 1993). Such an account is consistent with the nature of the encoding task in the present study given that subjects maintained the object names while constructing the sentences. The reverse contrast, the older minus younger adults, revealed a number of posterior parietal and frontal regions that were significantly more active for the older adults, as shown in Table 4B consistent with many previous reports of increased activation in elderly samples on encoding tasks (see Park and Reuter-Lorenz, 2009, for a review).
Table 4.
Regions showing an aging main effect in MNI coordinate space for areas (A) more active for the young than the older adults collapsed across the difficulty manipulation, (B) more active for the older adults than the young collapsed across difficulty, (C) more active for the young than the older adults in the unrelated minus control contrast, (D) more active for the older than young adults in the unrelated minus control contrast, and (E) more active for the older than the young adults in the related minus control contrast. (BA = Brodmann’s Area; Note: There were no significant regions showing age effects when all pictures were contrasted against the control or for the related minus control contrast.)
| Region | Hemisphere | Coordinate (mm) | Cluster | BA | t -value |
||
|---|---|---|---|---|---|---|---|
| x | y | Z | |||||
| A. All Pictures - Baseline Control, Young>Old | |||||||
| Supramarginal | L | −54 | −44 | 24 | 40 | 40 | 3.85 |
| B. All Pictures - Baseline Control, Old>Young | |||||||
| Inferior Parietal | R | 60 | −38 | 54 | 894 | 40 | 4.93 |
| Superior Parietal | R | 32 | −40 | 54 | 40 | 4.52 | |
| Postcentral | R | 36 | −40 | 62 | 5 | 4.23 | |
| Superior Frontal | R | 36 | −2 | 68 | 44 | 6 | 4.39 |
| Precentral | R | −66 | −8 | 28 | 134 | 6 | 4.34 |
| Postcentral | L | −64 | −2 | 20 | 6 | 3.85 | |
| Precuneus | R | 6 | −60 | 58 | 331 | 7 | 4.14 |
| Cingulate | R | 2 | −40 | 48 | 5 | 3.41 | |
| Precentral | L | −32 | −26 | 72 | 25 | 4 | 4.02 |
| Inferior Frontal | R | 40 | 28 | 18 | 128 | 46 | 4.01 |
| Postcentral | R | 50 | −20 | 56 | 140 | 3 | 4 |
| Postcentral | R | 52 | −4 | 32 | 265 | 6 | 3.95 |
| Precentral | R | 40 | 2 | 30 | 6 | 3.61 | |
| Anterior Cingulate | L | 0 | 48 | −4 | 24 | 32 | 3.82 |
| Middle Frontal | L | −46 | 46 | 20 | 10 | 10 | 3.78 |
| Insula | L | −36 | −4 | −10 | 26 | 21 | 3.76 |
| Cingulate | L | −14 | 32 | −2 | 26 | 10 | 3.75 |
| Angular Gyrus | R | 40 | −72 | 38 | 106 | 19 | 3.73 |
| Superior Parietal | R | 34 | −76 | 50 | 7 | 3.59 | |
| Angular Gyrus | R | 36 | −66 | 50 | 7 | 3.4 | |
| C. Unrelated - Baseline Control, Young>Old | |||||||
| Supramarginal | L | −54 | −44 | 24 | 29 | 40 | 3.85 |
| D. Unrelated − Baseline Control, Old>Young | |||||||
| Precentral | R | −66 | −8 | 26 | 127 | 4 | 4.51 |
| Postcentral | R | 36 | −40 | 64 | 507 | 5 | 4.44 |
| Superior Parietal | R | 34 | −38 | 56 | 40 | 4.36 | |
| Inferior Parietal | R | 60 | −38 | 52 | 127 | 40 | 4.26 |
| Inferior Frontal | R | 38 | 26 | 20 | 82 | 9 | 4.03 |
| Lingual | R | 8 | −58 | −2 | 65 | 19 | 3.93 |
| Precentral | R | 50 | −4 | 34 | 219 | 6 | 3.82 |
| Superior Frontal | R | 36 | −2 | 68 | 17 | 6 | 3.77 |
| Postcentral | R | 50 | −20 | 58 | 61 | 3 | 3.69 |
| Insula | R | −34 | −2 | −14 | 20 | 3.66 | |
| Postcentral | L | −42 | −10 | 32 | 31 | 6 | 3.65 |
| Lingual | L | −12 | −44 | 0 | 18 | 30 | 3.6 |
| Operculum | R | 60 | 0 | 10 | 66 | 6 | 3.58 |
| Precuneus | R | 2 | −62 | 60 | 44 | 7 | 3.57 |
| Cingulate | R | −12 | 34 | −4 | 12 | 10 | 3.56 |
| E. Related - Baseline Control, Old>Young | |||||||
| Precuneus | R | 8 | −60 | 58 | 403 | 7 | 4.69 |
| Inferior Parietal | R | 58 | −40 | 54 | 513 | 40 | 4.63 |
| Superior Parietal | R | 32 | −40 | 52 | 40 | 4.01 | |
| Postcentral | R | 36 | −26 | 46 | 3 | 3.82 | |
| Superior Frontal | R | 34 | −2 | 68 | 30 | 6 | 4.18 |
| Superior Parietal | R | 36 | −74 | 50 | 186 | 7 | 4.15 |
| Postcentral | R | 54 | −2 | 32 | 171 | 6 | 3.95 |
| Postcentral | L | −32 | −28 | 72 | 22 | 4 | 3.86 |
| Superior Frontal | R | 20 | 30 | 40 | 48 | 8 | 3.85 |
| Anterior Cingulate | L | 0 | 48 | −4 | 25 | 32 | 3.85 |
| SupraMarginal | L | −64 | −24 | 32 | 55 | 40 | 3.82 |
| Inferior Parietal | L | −60 | −30 | 42 | 2 | 3.46 | |
| Inferior Frontal | R | 42 | 16 | 24 | 60 | 46 | 3.8 |
| Postcentral | R | 50 | −22 | 56 | 43 | 1 | 3.79 |
| Lingual | R | 10 | −74 | −4 | 32 | 18 | 3.78 |
| Angular | R | 42 | −50 | 28 | 52 | 39 | 3.73 |
| Precentral | R | −66 | −4 | 26 | 53 | 6 | 3.65 |
| Cingulate | L | −20 | 18 | 34 | 14 | 8 | 3.65 |
| Middle Frontal | R | 26 | 18 | 52 | 17 | 6 | 3.53 |
| Precentral | R | 36 | −2 | 50 | 14 | 6 | 3.47 |
We next examined the age effects separately for the unrelated and related conditions. In the related condition, the younger adults showed no regions of significantly greater activity relative to the older adults, and in the unrelated condition the young again showed a single region of greater activation in the left supramarginal gyrus (see Table 4C). The reverse age contrast, however, yielded evidence for large differences across both frontal and parietal sites in the related and the unrelated conditions. In the unrelated condition (shown in Table 4D), the older adults showed greater activation in several parietal regions including the right superior parietal lobule, the right inferior parietal sulcus, as well as in the inferior frontal gyrus compared to the young. In the related condition (shown in Table 4E), older adults again showed greater activity in multiple areas including the right precuneus and the inferior and superior right parietal lobe.
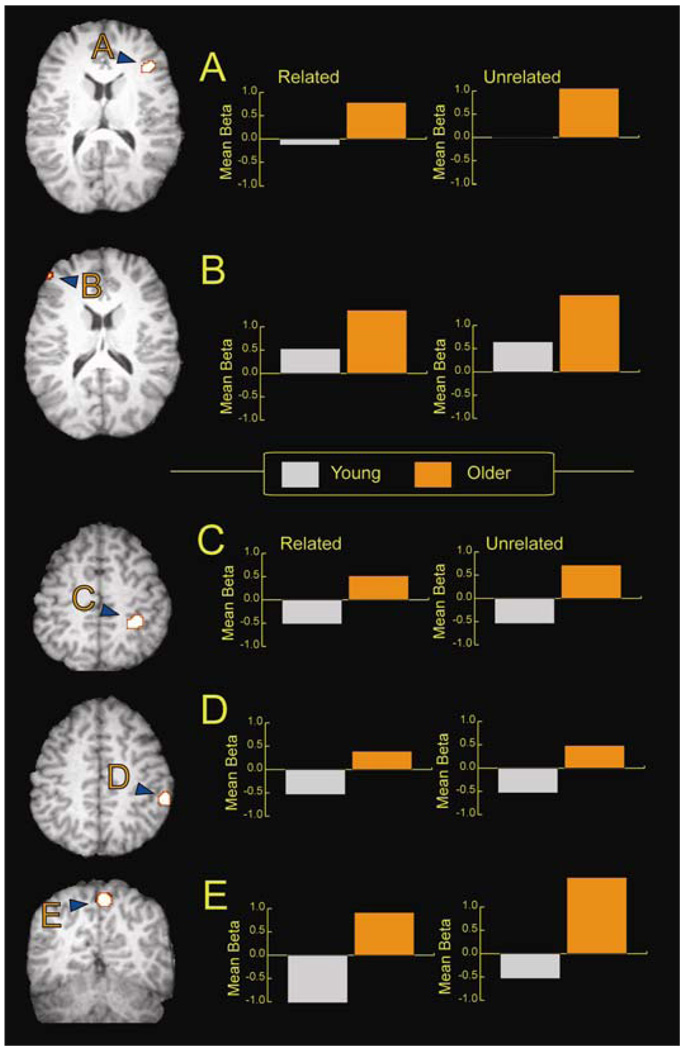
Based on the regions showing age effects, a further analysis was carried out on the two frontal ROI and the three default regions of interest to determine the nature of the age differences in these regions. In the right inferior frontal gyrus, an age effect emerged (F (1, 35) = 14.74, p < .01), but there was neither a main effect of condition (F (1, 35) = 3.49, p = .07), or interaction (F (1, 35) = .51, p = .48), showing that the older adults engaged in more right frontal activation than did the younger adults (Figure 4A). In the left middle frontal gyrus, there was again an age effect (F (1, 35) = 13.71, p < .01) and also a main effect of condition (F (1, 35) = 4.46, p < .05), but no interaction (F (1, 35) = .90, p = .35), again showing greater recruitment of the older adults relative to the younger adults (Figure 4B). Turning to the default ROIs, in the right superior parietal region, the significant effect of age (F (1, 35) = 18.39, p < .01) was due to persistent deactivations on the part of the young in contrast to activations in the older adults, as shown in Figure 4C. Similarly, age effects surface in both the right inferior parietal (F(1, 35) = 19.44, p < .01), and the right precuneus (F(1,35) = 15.98, p < .01), as can be seen in Figure 4D and 4E. Note that the younger adults show persistent deactivation relative to the older adults who showed above-baseline activity. These pattern of results are in line with previous reports of suppression deficits in older adults in default regions (Lustig, Snyder, Bhakta, O’Brien, McAvoy, Raichle et al., 2003; Persson et al., 2007). Overall, the analyses reported thus far suggest a typical pattern of age effects with old showing increased frontal engagement and disrupted function of the default network.
Figure 4.
Shown are the regions of interest and the averaged parameter estimates for two frontal ROIs, the right inferior frontal gyrus (A), and the left middle frontal gyrus (B), and for a set of regions in the default network including the right superior lobule (C), the right inferior parietal lobule (D), and the right precuneus (E). These ROIs were regions showing age effects for the encoding of all pictures (collapsed across difficulty).
3.3. Connectivity Analysis Results
In order to determine differences that might occur in the networks during the associative encoding tasks we next performed a connectivity analysis. The first connectivity analysis assessed whether encoding network differences occurred across age groups (collapsed across stimulus condition). Results of this analysis indicated that the younger adults evidenced more bilateral connectivity between the inferior occipital cortex and the hippocampus compared to the older adults (See Table 5A). In the reverse contrast, older adults showed no additional connectivity relative to younger adults. These results suggests that the younger adults utilized a network that showed a greater reliance on posterior region compared to old, a finding congruent with results reported by Davis et al. (2008).
Table 5.
Results from the connectivity analysis showing the age main effect in regions that expressed correlated activity with a seed region in the hippocampus (A) greater for the encoding of all the pictures (collapsing across stimulus condition) in younger compared to older adults, and (B) greater for the unrelated condition in the younger compared to the older adults. There were no regions showing greater connectivity for the related condition in the younger adults. In addition, the older adults showed no regions greater than that of the younger adults across any of the conditions (All Pictures, Unrelated trials, Related trials; BA = Brodmann’s Area.)
| Region | Hemisphere | Coordinate (mm) | Cluster | BA | t-value | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| A. Pictures - Baseline Control, Young > Old | |||||||
| Inferior Occipital | R | 24 | −84 | 0 | 21 | 18 | 3.78 |
| Inferior Occipital | L | −30 | −82 | −6 | 32 | 18 | 3.7 |
| B. Unrelated - Baseline Control, Young > Old | |||||||
| Inferior Occipital | L | −36 | −88 | −6 | 582 | 18 | 4.85 |
| Fusiform | L | −40 | −78 | −16 | 19 | 4.41 | |
| Cerebellum | L | −48 | −66 | −24 | 3.92 | ||
| Inferior Frontal | L | −42 | 6 | 28 | 371 | 9 | 4.68 |
| Inferior Frontal | L | −56 | 14 | 28 | 9 | 4.08 | |
| Precentral | L | −44 | 4 | 38 | 6 | 3.73 | |
| Cerebellum | R | 50 | −62 | −32 | 293 | 4.48 | |
| Inferior Temporal | R | 46 | −74 | −10 | 19 | 4.23 | |
| Middle Occipital | R | 44 | −76 | 0 | 19 | 3.66 | |
| Precentral | R | 50 | 8 | 34 | 58 | 9 | 3.86 |
| Superior Medial | |||||||
| Frontal | L | −6 | 18 | 40 | 6 | 32 | 3.71 |
| Middle Occipital | L | −28 | 70 | 24 | 12 | 31 | 3.58 |
A direct contrast of connectivity between the unrelated and the related networks however, yielded no significant differences. Next, connectivity networks for both the younger and older adults were examined separately for the related and unrelated conditions (Figure 5A). Finally, connectivity differences as a function of age were examined separately for the related and unrelated conditions. There were no differences between the age groups in the less-demanding related condition. In the unrelated condition, however, younger adults showed a more extensive network of prefrontal and occipital regions in comparison to the older group (See Figure 5B, Table 5B), mirroring the age difference in connectivity reported across difficulty.
Figure 5.
Results from the connectivity analysis show (A) networks correlated with the hippocampus for the encoding of related and unrelated items for the younger and older adults, respectively, and (B) a network of greater connectivity in the younger adults compared to the older adults during the encoding of unrelated pairs.
4. DISCUSSION
The main findings from this study are as follows: First, both younger and older adults rated the unrelated items more difficult to relationally encode than the related, providing independent evidence for a successful manipulation of associative difficulty. Second, younger and older adults showed equivalent increases in hippocampal and inferior frontal areas in the more demanding condition. Third, in a contrast between the unrelated and control conditions, older adults failed to disengage from default regions in line with previous reports of age-related default network changes, but showed greater activation of the right inferior and left middle frontal regions. Fourth, younger adults showed more connectivity between the hippocampus and posterior occipital sites than older adults for the demanding, unrelated task.
A comparison across the difficulty manipulation showed that both younger and older adults engaged a similar set of cortical regions to respond to the increasing relational load. Specifically, in a contrast between the unrelated and related trials both groups engaged medial temporal, middle occipital, inferior temporal, and frontal regions. Previous work in younger adults has shown that these regions are important for meeting increasing relational demands for verbal stimuli (Davachi et al., 2002, Lepage, et al., 2000). While the younger adults showed only a left lateralized parahippocampal activity, the older adults showed a more bilateral pattern engaging the left hippocampal and right parahippocampal activity to meet the task demands. More bilateral recruitment in older adults, such as that seen in this study, has been frequently reported across various task paradigms (for Review see Dennis & Cabeza, 2008). In particular, a pattern of more bilateral recruitment has been shown in older adults during successful memory formation (Morcom, et al., 2003). As we note at the outset of this report, we were interested in exploring the possibility that, when given a highly focused encoding strategy, older adults would engage cortical regions, most notably medial temporal areas, to a similar extent as younger adults. The evidence from this report found that the older and younger adults recruited similar regions to perform the task. The finding of such similar networks between the older and younger adults suggests that the encoding task effectively constrained both younger and older adults in their processing strategies. While it is possible that strategy differences across age were present, we see this as unlikely. First, the fact that both groups showed a similar set of regions, including frontal and medial temporal areas, suggests that both groups were similarly engaged. Second, a prior behavioral study, which engaged participants in an identical sentence generation task for related and unrelated items, showed that both groups produced qualitatively identical sentences, adding to the case that encoding strategy in the present study was well-matched across age (Smith, et al., 1998).
Of particular interest in these data was the finding that older adults responded similarly to the young as cognitive demands increased in the left inferior frontal gyrus and left hippocampus. It is widely reported that the left inferior frontal gyrus and the hippocampus regions are crucial for the formation of long-term memory (for review, see Simons & Spiers, 2003), and are commonly activated during encoding in studies involving younger adults (Addis & McAndrews, 2006; Bunge, Burrows, & Wagner, 2004; Dolan & Fletcher, 1997); the present findings extend these results to older adults. Addis and McAndrews (2006) reported activity in both the left prefrontal cortex and the left medial temporal lobe in associating word triads, a memory task that is strongly relational in nature. Evidence from a path analysis in that study suggested that the left prefrontal cortex was important for generating the associations between the word triads, and that the hippocampus then bound these associations into memory. Given the nature of the present task, i.e. the dependence on creating associations between objects pairs, it is likely that this process was the basis for the joint activation of the left prefrontal cortex and the left hippocampus in both age groups.
On the surface, the finding of equivalent hippocampal activation in the older adults compared to the young seems somewhat surprising and at odds with prior neuroimaging studies that have suggested that older adults under-recruit the hippocampal region (Chee et al., 2006; Daselaar et al., 2003; Grady et al., 1995; Gutchess et al., 2005; though see Rand-Giovanetti, 2006). The present study, however, differs from studies that have found decreased hippocampal recruitment in older relative to younger adults in important ways. First, this is perhaps the only aging study where the encoding process is held constant across conditions and only difficulty varies between the two conditions. A review of existing studies suggests that age differences observed in these studies have contrasted an easy control task with a more demanding task, but one that involved qualitatively different processing (e.g., Grady, et al., 2002). We conclude that using a highly constrained encoding task that did not vary as a function of difficulty resulted in the engagement of relatively similar functional activity in the younger and older groups. While it is also the case that this task varied from other memory paradigms in that there was a working memory burden at encoding with subjects performing the encoding task while the images were not on screen, it should be noted that this difference cannot account for the relative equivalence of hippocampal engagement across age groups observed in this study; previous investigations of working memory function have also reported deficits in hippocampal involvement in older adults (Park et al., 2003).
Although the older adults generally showed similar activations when difficulty was assessed, secondary analyses yielded evidence for some differences across age. A direct age comparison resulted in a large set of regions more active for older adults than younger adults. In contrasts of all items, the related and the unrelated trials, with baseline, age-related differences were uncovered in a wide set of regions. The largest of these regions that differed with age were in parietal regions associated with the default network. A further analysis of these regions showed that the young showed suppression of default regions when performing the encoding task, whereas the older adults did not. The default network is a complex of sites that are engaged primarily during self-referential or self-focused cognition (For review see, Buckner, et al., 2008), and is a set of regions which must be deactivated when performing other cognitive tasks such as memory encoding. Older adults, however, show substantial reductions in the suppression of these areas during the performance of a variety of cognitive tasks (Lustig et al., 2003; Miller, et al, 2008). The activity in the right superior parietal, right inferior parietal, and right precuneus all showed the pattern of deactivations in the younger adults relative to the older adults suggestive of an inability of the older adults to inhibit default activity. It has been theorized that default activation reflects “mind-wandering” and may thus be a sign of poor attentional gating during cognitive tasks (Mason, et al, 2007). One possibility then, is that the pattern of failed suppression of the default network in older adults might represent an inhibition failure in line with Hasher and Zack’s, inhibition theory (Hasher & Zacks 1998), reflecting the older adults reduced ability to stay task-focused. It is noteworthy to mention that other areas besides the default regions showed age differences in this study, such as in frontal sites. Older adults showed greater activity in right inferior and left middle frontal ROIs compared to the younger adults. Previous work has effectively tied enhanced bilateral frontal recruitment to better memory performance in older adults (Cabeza, 2002, Gutchess, et al, 2005, Morcom, et al, 2003), a pattern evident here. It may be then, that older adults engage enhanced prefrontal recruitment to compensate for the cognition failures denoted by reduced suppression of the default network. This possibility, however, is speculative given that this study did not directly provide such evidence, and thus should be the focus of future research.
Further age differences emerged from the connectivity analyses. That analysis presents evidence suggestive of age-related differences in the networks engaged to perform this task. In a direct comparison across age, the older adults showed a sparser network than did the younger adults in the demanding, unrelated condition. The younger adults invoked posterior occipital regions in concert with the hippocampus, whereas the older subjects did not show the additional involvement of this occipital/hippocampal circuit (Figure 5B). Results from the connectivity analysis demonstrated, first, that the younger adults engaged a network more reliant upon earlier (occipital) processing regions, a finding consistent with the emerging view that older adults experience a posterior to anterior shift in functional recruitment (Simons, et al., 2008). Second, this analysis complements the whole brain analysis by demonstrating an important way that younger adults differed in functional recruitment patterns relative to the older adults. Results from the whole brain, direct age comparison showed a pattern of increased recruitment in the older adults, with little evidence of increased activity in the younger adults (Table 4). Inclusion of the connectivity analysis makes an important point suggesting that more efficient networks were engaged by the younger adults in performance of the task.
Overall, the pattern observed in other studies combined with the results from the present study suggests the following. First, when encoding processing is highly constrained, younger and older adults will show equivalent activity in frontal and hippocampal areas. Second, we tentatively suggest that when encoding occurs in undirected conditions, older adults will show the most evidence for age differences denoted by diminished hippocampal recruitment. This hypothesis is consistent with both Craik’s (1983) view that older adults are particularly deficient in “self-initiated processing,” and with the frontal-hippocampal hypothesis of aging proposed by Park & Gutchess, 2004.
In sum, the main contribution of the present study is the finding that the typical pattern of deficient medial temporal lobe/increased inferior frontal activation with age suggested as a signature of encoding with age by Park and Gutchess (2004) may be confined to unconstrained encoding conditions. The present study demonstrated that when encoding was rigorously constrained and there were objective measures of increased difficulty in encoding both age groups showed enhanced recruitment of frontal and medial temporal areas with increased difficulty. Although more studies are needed, this pattern suggests that decreased functional activation in critical neural regions can be “repaired” to some extent by prescribing specific encoding strategies in older adults. It may be the case then that age differences are more likely to occur under intentional encoding because older and younger adults are using different strategies. Such an interpretation concurs with evidence from Kirchhoff and Buckner (2006) demonstrating differences in patterns of activation at encoding in young adults based on self-reported strategy use. The present findings are a cause for optimism when compared with other studies in the literature as the findings tentatively suggest that recruitment patterns are largely similar and intact across age groups when encoding is guided and patterns differ primarily when young and older adults are free to engage in a strategy of choice.
Acknowledgments
This research was supported by National Institute on Aging grant R01 AGO6265, awarded to Denise C. Park. Eric D. Leshikar was supported by the National Institute of Mental Health training grant T32 MH19554. The authors gratefully acknowledge the various contributions of Brian Gonsalves, Robert Welsh, Lucas Jenkins, and Josh Goh in this project.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
It should be noted that the focus of this study was on neural activation patterns at encoding as a function of the two trial types, and consequently, the present study was not designed for a subsequent memory analysis, where remembered items (hits) are contrasted with forgotten items (misses). Such an analysis, to be truly informative, requires a fairly high miss rate (which did not occur with the distinct pictures used) and also many trials of one type, so that the inclusion of the unrelated/related manipulation that occurred in this study resulted in limited power for such an analysis.
Contributor Information
Eric D. Leshikar, Beckman Institute, University of Illinois, at Urbana-Champaign, Urbana, IL
Angela H. Gutchess, Brandeis University and Massachusetts, General Hospital, Cambridge, MA
Andrew C. Hebrank, Center for Brain Health and University, of Texas at Dallas, Dallas, TX
Bradley P. Sutton, Beckman Institute, University of Illinois at Urbana-Champaign, Urbana, IL
Denise C. Park, Center for Brain Health and University of Texas at Dallas, Dallas, TX
References
- Addis DR, McAndrews MP. Prefrontal and hippocampal contributions to the generation and binding of semantic associations during successful encoding. NeuroImage. 2006;33:1194–1206. doi: 10.1016/j.neuroimage.2006.07.039. [DOI] [PubMed] [Google Scholar]
- Achim AM, Lepage M. Neural correlates of memory for items and for associations: An event-related functional magnetic resonance imaging study. Journal of cognitive neuroscience. 2005;17(4):652–667. doi: 10.1162/0898929053467578. [DOI] [PubMed] [Google Scholar]
- Brett M, Anton J-L, Valabregue R, Poline J-B. Region of interest analysis using an SPM toolbox [abstract]; Presented at the 8th International Conference on Functional Mapping of the Human Brain; 2002. Available on CD-ROM in NeuroImage. [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain's default network: Anatomy, function, and relevance to disease. Annals of the New York Academy of Sciences. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Bunge SA, Burrows B, Wagner AD. Prefrontal and hippocampal contributions to visual associative recognition: Interactions between cognitive control and episodic retrieval. Brain and cognition. 2004;56:141–152. doi: 10.1016/j.bandc.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Cabeza R. Hemispheric asymmetry reduction in older adults: The HAROLD model. Psychology and Aging. 2002;17:85–100. doi: 10.1037//0882-7974.17.1.85. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Grady CL, Nyberg L, McIntosh AR, Tulving E, Kapur S, et al. Age-related differences in neural activity during memory encoding and retrieval: A positron emission tomography study. Journal of Neuroscience. 1997;17:391–400. doi: 10.1523/JNEUROSCI.17-01-00391.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castel AD, Craik FIM. The effects of aging and divided attention on memory for item and associative information. Psychology and aging. 2003;18(4):873–885. doi: 10.1037/0882-7974.18.4.873. [DOI] [PubMed] [Google Scholar]
- Chalfonte BL, Johnson MK. Feature memory and binding in young and older adults. Memory & cognition. 1996;24(4):403–416. doi: 10.3758/bf03200930. [DOI] [PubMed] [Google Scholar]
- Chee MWL, Goh JOS, Venkatraman V, Tan JC, Gutchess A, Sutton B, Hebrank A, Leshikar E, Park D. Age-related changes in object processing and contextual binding revealed using fMR-Adaptation. Journal of Cognitive Neuroscience. 2006;18:495–507. doi: 10.1162/jocn.2006.18.4.495. [DOI] [PubMed] [Google Scholar]
- Craik FIM. On the transfer of information from temporary to permanent memory. Philosophical Transactions of the Royal Society of London. 1983;B302:341–359. [Google Scholar]
- Cohen NJ, Ryan E, Hunt C, Romine L, Wszalek T, Nash C. Hippocampal system and declarative (relational) memory: Summarizing the data from functional neuroimaging studies. Hippocampus. 1999;9(1):83–98. doi: 10.1002/(SICI)1098-1063(1999)9:1<83::AID-HIPO9>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Daselaar SM, Veltman DJ, Rombouts SA, Raaijmakers JG, Jonker C. Deep processing activates the medial temporal lobe in young but not in old adults. Neurobiology of aging. 2003;24(7):1005–1011. doi: 10.1016/s0197-4580(03)00032-0. [DOI] [PubMed] [Google Scholar]
- Davis SW, Dennis NA, Daselaar SM, Fleck MS, Cabeza R. Que pasa? The posterior-anterior shift in aging. Cerebral Cortex. 2008;18:1201–1209. doi: 10.1093/cercor/bhm155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davachi L, Maril A, Wagner AD. When keeping in mind supports later bringing to mind: Neural markers of phonological rehearsal predict subsequent remembering. Journal of cognitive neuroscience. 2001;13(8):1059–1070. doi: 10.1162/089892901753294356. [DOI] [PubMed] [Google Scholar]
- Davachi L, Mitchell JP, Wagner AD. Multiple routes to memory: Distinct medial temporal lobe processes build item and source memories. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(4):2157–2162. doi: 10.1073/pnas.0337195100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davachi L, Wagner AD. Hippocampal contributions to episodic encoding: insights from relational and item-based learning. Journal of Neurophysiology. 2002;88:982–990. doi: 10.1152/jn.2002.88.2.982. [DOI] [PubMed] [Google Scholar]
- Dennis NA, Cabeza R. Neuroimaging of healthy cognitive aging. In: Craik FIM, Salthouse TA, editors. Handbook of aging and cognition: Third edition. Mahwah, NJ: Erlbaum; 2008. pp. 1–54. [Google Scholar]
- Dolan RJ, Fletcher PC. Dissociating prefrontal and hippocampal function in episodic memory encoding. Nature. 1997;388(6642):582–585. doi: 10.1038/41561. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-mental state: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Grady CL, McIntosh AR, Horwitz B, Maisog JM, Ungerleider LG, Mentis MJ, et al. Age-related reductions in human recognition memory due to impaired encoding. Science. 1995;269(5221):218–221. doi: 10.1126/science.7618082. [DOI] [PubMed] [Google Scholar]
- Grady CL, McIntosh AR, Rajah N, Beig S, Craik FIM. The effects of age on the neural correlates of episodic encoding. Cerebral Cortex. 1999;9:805–814. doi: 10.1093/cercor/9.8.805. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Srivastava G, Reiss AL, Menon V. Default mode activity distinguishes alzheimer's disease from healthy aging: Evidence from functional MRI. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(13):4637–4642. doi: 10.1073/pnas.0308627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold B, Buckner R. Common prefrontal regions coactivate with dissociable posterior regions during controlled semantic and phonological tasks. Neuron. 2002;35:803–812. doi: 10.1016/s0896-6273(02)00800-0. [DOI] [PubMed] [Google Scholar]
- Gutchess AH, Welsh RC, Hedden T, Bangert A, Minear M, Liu LL, et al. Aging and the neural correlates of successful picture encoding: Frontal activations compensate for decreased medial-temporal activity. Journal of cognitive neuroscience. 2005;17(1):84–96. doi: 10.1162/0898929052880048. [DOI] [PubMed] [Google Scholar]
- Hasher L, Zacks RT. Working memory, comprehension, and aging: a review and a new view. In: Bower GH, editor. The psychology of learning and motivation. Vol. 22. San Diego: Academic Press; 1998. pp. 193–225. [Google Scholar]
- Henke K, Weber B, Kneifel S, Wieser HG, Buck A. Human hippocampus associates information in memory. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(10):5884–5889. doi: 10.1073/pnas.96.10.5884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley W, Miezin FM, McDermott KB, Buckner RL, Raichle ME, Cohen NJ, et al. Hemispheric specialization in human dorsal frontal cortex and medial temporal lobe for verbal and nonverbal memory encoding. Neuron. 1998;20:927–936. doi: 10.1016/s0896-6273(00)80474-2. [DOI] [PubMed] [Google Scholar]
- Kirchhoff BA, Buckner RL. Functional-anatomic correlates of individual differences in memory. Neuron. 2006;51(2):263–274. doi: 10.1016/j.neuron.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Lepage M, Habib R, Cormier H, Houle S, McIntosh AR. Neural correlates of semantic associative encoding in episodic memory. Cognitive Brain Research. 2000;9:271–280. doi: 10.1016/s0926-6410(00)00005-7. [DOI] [PubMed] [Google Scholar]
- Logan JM, Sanders AL, Snyder AZ, Morris JC, Buckner RL. Under-recruitment and nonselective recruitment: Dissociable neural mechanisms associated with aging. Neuron. 2002;33(5):827–840. doi: 10.1016/s0896-6273(02)00612-8. [DOI] [PubMed] [Google Scholar]
- Lustig C, Snyder AZ, Bhakta M, O'Brien KC, McAvoy M, Raichle ME, et al. Functional deactivations: Change with age and dementia of the Alzheimer type. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:14504–14509. doi: 10.1073/pnas.2235925100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason MF, Norton MI, Van Horn JD, Wegner DM, Grafton ST, Macrae CN. Wandering mind: the default network and stimulus-independent though. Science. 2007;315:393–395. doi: 10.1126/science.1131295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morcom AM, Good CD, Frackowiak RS, Rugg MD. Age effects on the neural correlates of successful memory encoding. Brain: a journal of neurology. 2003;126(Pt 1):213–229. doi: 10.1093/brain/awg020. [DOI] [PubMed] [Google Scholar]
- Naveh-Benjamin M, Brav TK, Levy O. The associative memory deficit of older adults: The role of strategy utilization. Psychology and aging. 2007;22(1):202–208. doi: 10.1037/0882-7974.22.1.202. [DOI] [PubMed] [Google Scholar]
- Naveh-Benjamin M, Guez J, Kilb A, Reedy S. The associative memory deficit of older adults: Further support using face-name associations. Psychology and aging. 2004;19(3):541–546. doi: 10.1037/0882-7974.19.3.541. [DOI] [PubMed] [Google Scholar]
- Park DC, Gutchess AH. Long-term memory and aging: A cognitive neuroscience perspective. In: Cabeza R, Nyberg L, Park DC, editors. Cognitive neuroscience of aging: Linking cognitive and cerebral aging. New York: Oxford Press; 2004. [Google Scholar]
- Park DC, Reuter-Lorenz P. The adaptive brain: Aging and neurocognitive scaffolding. Annual Review of Psychology. doi: 10.1146/annurev.psych.59.103006.093656. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park DC, Smith AD, Morrell RW, Puglisi JT, Dudley WN. Effects of contextual integration on recall of pictures by older adults. Journal of gerontology. 1990;45(2):P52–P57. doi: 10.1093/geronj/45.2.p52. [DOI] [PubMed] [Google Scholar]
- Paulesu E, Frith CD, Frackowiak The neural correlates of verbal component of working memory. Nature. 1993;362:342–345. doi: 10.1038/362342a0. [DOI] [PubMed] [Google Scholar]
- Prince SE, Daselaar SM, Cabeza R. Neural correlates of relational memory: Successful encoding and retrieval of semantic and perceptual associations. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25(5):1203–1210. doi: 10.1523/JNEUROSCI.2540-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rand-Giovannetti E, Chua EF, Driscoll AE, Schacter DL, Albert MS, Sperling RA. Hippocampal and neocortical activation during repetitive encoding in older persons. Neurobiology of aging. 2006;27(1):173–182. doi: 10.1016/j.neurobiolaging.2004.12.013. [DOI] [PubMed] [Google Scholar]
- Ranganath C, Heller A, Cohen MX, Brozinsky CJ, Rissman Functional connectivity with the hippocampus during successful memory formation. Hippocampus. 2005;15:997–1005. doi: 10.1002/hipo.20141. [DOI] [PubMed] [Google Scholar]
- Reuter-Lorenz P, Jonides J, Smith EE, Hartley A, Miller A, Marshuetz C, Koeppe R. Age differences in the frontal lateralization of verbal and spatial working memory revealed by PET. Journal of Cognitive Neuroscience. 2000;12(1):174–187. doi: 10.1162/089892900561814. [DOI] [PubMed] [Google Scholar]
- Simons JS, Spiers HJ. Prefrontal and medial temporal lobe interactions in long-term memory. Nature Reviews Neuroscience. 2003;4(8):637–648. doi: 10.1038/nrn1178. [DOI] [PubMed] [Google Scholar]
- Smith AD, Park DC, Earles JL, Shaw RJ, Whiting WL., 4th Age differences in context integration in memory. Psychology and aging. 1998;13(1):21–28. doi: 10.1037//0882-7974.13.1.21. [DOI] [PubMed] [Google Scholar]
- Snodgrass JG, Vanderwart M. A standardized set of 260 pictures: Norms for name agreement, image agreement, familiarity, and visual complexity. Journal of experimental psychology. Human learning and memory. 1980;6(2):174–215. doi: 10.1037//0278-7393.6.2.174. [DOI] [PubMed] [Google Scholar]
- Sperling RA, Bates JF, Cocchiarella AJ, Schacter DL, Rosen BR, Albert MS. Encoding novel face-name associations: A functional MRI study. Human brain mapping. 2001;14(3):129–139. doi: 10.1002/hbm.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanislaw H, Todorov N. Calculation of signal detection theory measures. Behavior research methods, instruments, & computers: a journal of the Psychonomic Society, Inc. 1999;31(1):137–149. doi: 10.3758/bf03207704. [DOI] [PubMed] [Google Scholar]
- Szekely A, Jacobsen T, D'Amico S, Devescovi A, Andonova E, Herron D, Lu C, et al. A new on-line resource for psycholinguistic studies. Journal of Memory and Language. 2004;51(2):247–250. doi: 10.1016/j.jml.2004.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]