Abstract
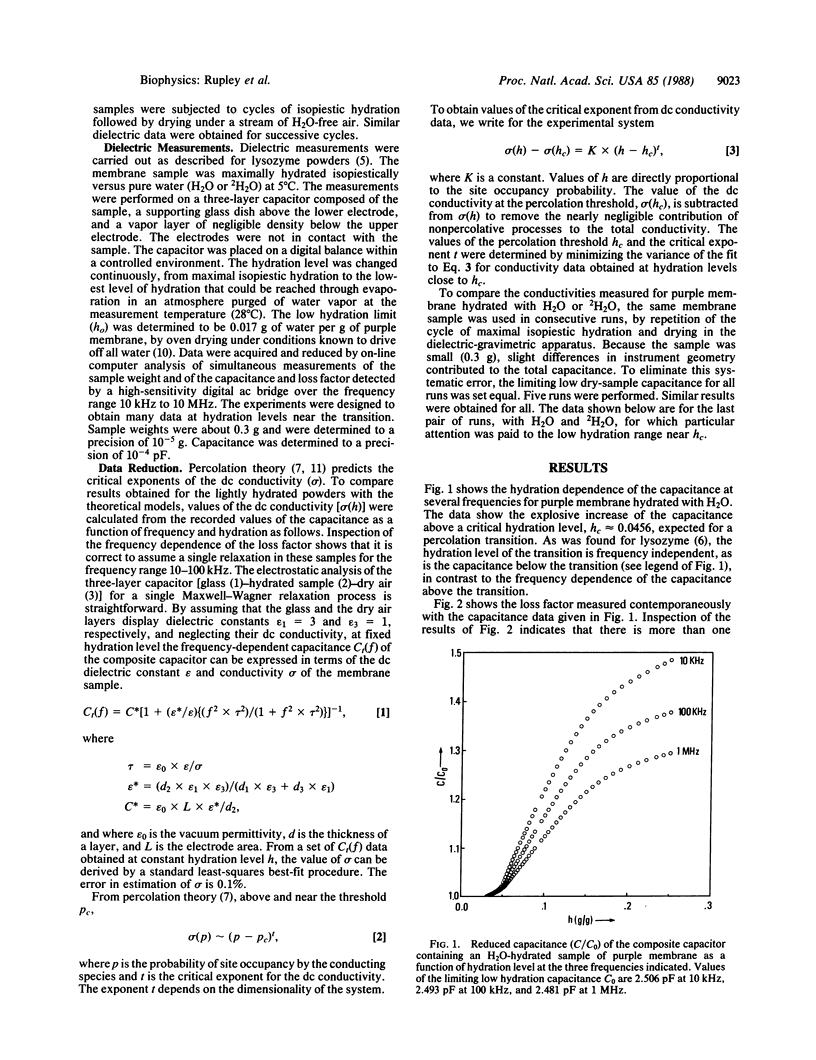
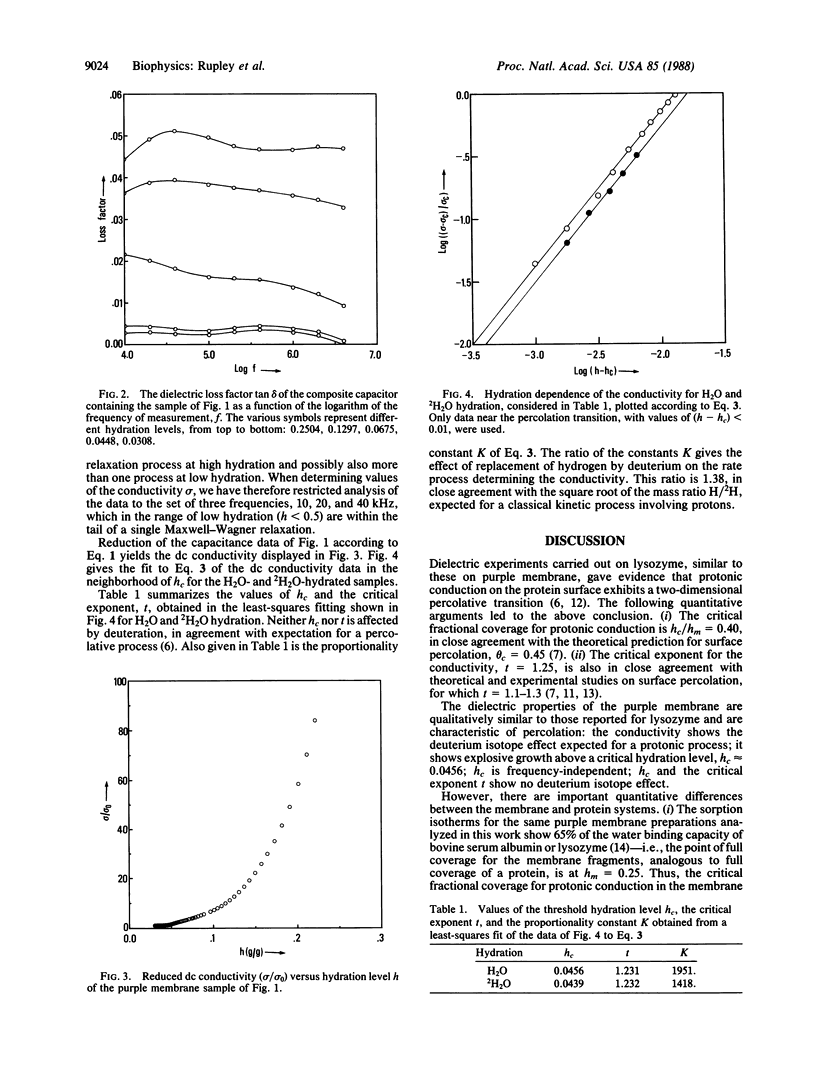
The capacitance and dielectric loss factor were measured for a sample of purple membrane of Halobacterium halobium as a function of hydration level (0.017 to >0.2 g of water/g of membrane) and frequency (10 kHz to 10 MHz). The capacitance and the derived conductivity show explosive growth above a threshold hydration level, hc ≈ 0.0456. The conductivity shows a deuterium isotope effect, H/2H = 1.38, in close agreement with expectation for a protonic process. The level hc is frequency independent and shows no deuterium isotope effect. These properties are analogous to those found for lysozyme in a related study. Protonic conduction for the purple membrane can be considered, as for lysozyme, within the framework of a percolation model. The critical exponent, t, which describes the conductivity of a percolative system near the threshold, has the value 1.23. This number is in close agreement with expectation from theory for a two-dimensional percolative process. The dielectric properties of the purple membrane are more complex than those of lysozyme, seen in the value of hc and in the frequency and hydration dependence of the loss factor. There appear to be preferred regions of proton conduction. The percolation model is based upon stochastic behavior of a system partially populated with conducting elements. This model suggests that ion transport in membranes and its control can be based on pathways formed of randomly connected conducting elements and that a fixed geometry (a proton wire) is not the only possible basis for a mechanism of conduction.
Keywords: dielectric, hydration, proton transfer
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Careri G., Geraci M., Giansanti A., Rupley J. A. Protonic conductivity of hydrated lysozyme powders at megahertz frequencies. Proc Natl Acad Sci U S A. 1985 Aug;82(16):5342–5346. doi: 10.1073/pnas.82.16.5342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Careri G., Giansanti A., Rupley J. A. Proton percolation on hydrated lysozyme powders. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6810–6814. doi: 10.1073/pnas.83.18.6810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oesterhelt D., Stoeckenius W. Isolation of the cell membrane of Halobacterium halobium and its fractionation into red and purple membrane. Methods Enzymol. 1974;31:667–678. doi: 10.1016/0076-6879(74)31072-5. [DOI] [PubMed] [Google Scholar]
- Rogan P. K., Zaccai G. Hydration in purple membrane as a function of relative humidity. J Mol Biol. 1981 Jan 5;145(1):281–284. doi: 10.1016/0022-2836(81)90344-2. [DOI] [PubMed] [Google Scholar]
- Schulten Z., Schulten K. Proton conduction through proteins: an overview of theoretical principles and applications. Methods Enzymol. 1986;127:419–438. doi: 10.1016/0076-6879(86)27033-0. [DOI] [PubMed] [Google Scholar]
- Selwyn M. J. Membrane biophysics. Surface conduction of protons. Nature. 1986 Aug 21;322(6081):685–686. doi: 10.1038/322685a0. [DOI] [PubMed] [Google Scholar]
- Váró G., Keszthelyi L. Photoelectric signals from dried oriented purple membranes of Halobacterium halobium. Biophys J. 1983 Jul;43(1):47–51. doi: 10.1016/S0006-3495(83)84322-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang P. H., Rupley J. A. Protein--water interactions. Heat capacity of the lysozyme--water system. Biochemistry. 1979 Jun 12;18(12):2654–2661. doi: 10.1021/bi00579a035. [DOI] [PubMed] [Google Scholar]


