Abstract
Aging can have a profound effect on the neurobehavioral response to immune activation; aged subjects are predisposed to greater deficits in performance and cognitive function in conjunction with an exaggerated neuroinflammatory response. While increased reactivity to an immune insult has been well characterized in aged subjects, the alterations that may exist by middle-age have not been thoroughly investigated. The present study compared the reactions of young (4-month) and middle-age (12-month) male BALB/c mice to an acute or repeated lipopolysaccharide (LPS) challenge(s). The data suggest that in some respects middle-aged mice are more sensitive to endotoxin exposure, as they show enhanced weight loss, splenic cytokine levels, and c-fos expression in the brain following acute LPS administration compared to younger mice. However, acute LPS exposure led to comparable decreases in locomotor activity in young and middle-aged mice. Following repeated LPS administration both age groups showed diminished behavioral and neural reactions to the final LPS challenge, indicating tolerance development. However, the immune system of the middle-aged mice was still mildly responsive to the final LPS exposure, as splenic levels of IL-1β were significantly elevated. Collectively, the data suggest that middle-age subjects are more sensitive to an immune insult.
Keywords: Middle-age, cytokine, LPS, lipopolysaccharide, endotoxin tolerance, c-fos, microglia, behavior
1. Introduction
The general health of an organism often decreases as a function of age, with aged individuals showing increased frequency of infection, poorer outcomes, and increased mortality (Laupland et al., 2003; Miller, 1996). This increased propensity for illness likely results from a series of age-related changes in immune function, or immunosenescence, and may vary with the site and immune parameters assessed (Aw et al., 2007; Miller, 1996). Corresponding changes also develop in the central nervous system (CNS), as evidenced by exaggerated neuroinflammatory responses in aged subjects (Abraham and Johnson, 2009; Chen et al., 2008; Dilger and Johnson, 2008; Godbout et al., 2005; Henry et al., 2009; Terao et al., 2002).
Whereas the focus on altered immune function and subsequent neurobehavioral effects has largely been placed on elderly subjects (Dilger and Johnson, 2008), alterations in these domains occurs in middle age (Blalock et al., 2003; Izgut-Uysal et al., 2004; Popp and Francis, 1979; Rozovsky et al., 1998; Terao et al., 2002; Verbitsky et al., 2004). For example, even in the absence of an immune stimulus, middle-aged subjects show increased hippocampal expression of multiple inflammation-associated genes relative to younger subjects (Blalock et al., 2003; Terao et al., 2002; Verbitsky et al., 2004). Furthermore, whereas the behavioral response of middle-aged subjects did not differ from younger subjects following a single challenge with the endotoxin, lipopolysaccharide (LPS; Gram-negative bacteria cell wall) (Kinoshita et al., 2009; Kohman et al., 2007; Krzyszton et al., 2008; Sparkman et al., 2004), repeated LPS exposure, a model of chronic inflammation, produced greater cognitive and performance decrements in middle-aged subjects (Kohman et al., 2007; Sparkman et al., 2004). Therefore, while less apparent than the effects observed in elderly subjects, the findings suggest that under certain conditions middle-aged subjects are more vulnerable to the cognitive and behavioral effects of immune activation.
The exaggerated deficits observed in middle-aged subjects following repeated LPS exposure (Kohman et al., 2007; Sparkman et al., 2004) may indicate impairments in tolerance development. Endotoxin tolerance is a temporary decreased responsiveness to LPS, induced by prior exposure to LPS (Fan and Cook, 2004), and which is an adaptive response that can protect against the occurrence of septic shock. LPS administration stimulates immune activation through interactions with toll-like receptor-4 (TLR-4). Tolerance to LPS can develop through a reduction in TLR expression or signaling capacity, via the production of molecules such as interleukin-1 receptor associated kinase-M (IRAK-M) that inhibits TLR signaling (Fan and Cook, 2004; Li et al., 2009; Nomura et al., 2000). Li et al. (2009) demonstrated that peripheral blood mononuclear cells (PBMCs) from aged (i.e., 24 month-old) rats show impaired development of endotoxin tolerance, as pre-exposure to LPS failed to decrease cytokine production in response to a secondary LPS challenge. Further, the aged cells failed to increase levels of IRAK-M and maintained higher monocyte expression of TLR-4 (Li et al., 2009). Presently, whether middle-age subjects show similar deficits in endotoxin tolerance is unknown, though early work by Habicht et al. (1985) found evidence of impaired tolerance to another immune stimulant, deaggregated human immunoglobulin (DHGG), in aged and middle-aged subjects.
To determine if middle-aged subjects show alterations in endotoxin tolerance development, the present study compared the behavioral, neural, and immune responses of young and middle-aged mice following acute or repeated LPS exposure. This study chose to employ a lower dose of LPS than administered in prior work with middle-aged animals (Chorinchath et al., 1996; Kinoshita et al., 2009; Kohman et al., 2007; Sparkman et al., 2004). This allowed for the determination of possible differences in LPS sensitivity and tolerance development between young and middle-aged subjects, as well as limited the potential loss of middle-aged animals following repeated administration of higher doses of LPS. Indeed, research with aged (i.e., 24-month old) subjects has shown a lower threshold for cytokine expression and induction of endotoxin-induced shock syndrome as well as mortality (Chorinchath et al., 1996; Saito et al., 2003; Tateda et al., 1996). Though the lethal dose of LPS was shown to be similar between young and middle-aged subjects (Chorinchath et al., 1996), distinct reactions between these age groups may be observed at substantially lower doses of LPS. Therefore, in the present study, we examined whether young and middle-aged subjects would show differential reactions to a lower of dose LPS, and hypothesized that middle-aged mice would show delayed tolerance development as compared to young subjects.
2. Methods
2.1. Experimental subjects
Subjects were 19 four-month-old and 17 twelve-month-old male BALB/c mice that were purchased from The Jackson Laboratory (Bar Harbor, ME) at 6-8 weeks and aged in our colony room at Rutgers University. All animals were housed in groups of 3–4 in a standard polycarbonate mouse cage with food and water available ad libitum. Lights were turned on at 0600 and then turned off at 1800 hrs. Animals were treated in compliance with the Guide for the Care and Use of Laboratory Animals and the experiment was conducted in accordance with a protocol approved by the Institutional Animal Care and Use Committee (IACUC) at Rutgers University.
2.2. Treatment conditions
Mice were divided by age into three treatment groups: LPS-acute (daily saline injections, followed by a single LPS injection on Day 4), LPS-repeated (LPS given all four days), and saline control (saline given all four days) for a total of six treatment conditions. LPS (Escherichia coli serotype 0111:B4, Sigma, St Louis, MO) was administered at a dose of 3μg/mouse in sterile 0.9% saline. Control animals received an equivalent volume injection of sterile saline. All the animals received a total of four injections, one per day, of either LPS or saline. Three-hours after the final injection subjects were tested for behavioral alterations and then sacrificed for tissue collection (as described below).
2.3. Behavioral testing procedure: open field and novel object test
Three hours after the fourth injection of LPS or saline, subjects were tested for locomotor activity in an open field (56cm × 63.5cm) for 10 min. Dependent measures included total distance travelled (cm) and the time spent in the center and outer portions of the maze. After 10 min, a novel object (i.e., a silver cylinder) was placed into the center of the arena and the mice were allowed 5 min to explore the object. Dependent measures included total distance travelled (cm), number of contacts with novel object, and percent time spent in area surrounding the novel object. Behavior was recorded and analyzed by a computerized animal tracking system (Panlab Smart software, San Diego Instruments, San Diego, CA).
2.4. Perfusion and sectioning for immunohistochemistry
Immediately following behavioral testing, mice were given a lethal dose of sodium pentobarbital (7.5mg/kg) and sacrificed by transcardial perfusion of saline (5 min), followed by 4% paraformaldehyde (10 min), and a saline wash (5 min). Brains were post-fixed in 4% paraformaldehyde overnight and then transferred to 30% sucrose solution until sectioning. Brains were sliced into 30μm coronal sections on a freezing microtome and cryopreserved at -20°C until the staining was performed.
2.5. Immunohistochemistry: c-fos detection
Brain sections were incubated for 48hrs with rabbit anti-rat c-fos (1:15,000, Calbiochem PC38, San Diego, CA) antibody in 0.4% Triton X-100 in potassium phosphate buffered saline (KPBS, pH 7.4; Sigma, St. Louis, MO). Following a series of KPBS washes the sections were then incubated for 2hrs at room temperature with biotinylated goat anti-rabbit antibody (1:500; Vector Laboratories, Burlingame, CA) in 0.4% Triton X-100 in KPBS. After washing out the secondary antibody, sections were incubated for 1hr at room temperature with an avidin-biotin-peroxidase complex solution from Vector Elite ABC kit (Vector Laboratories), followed by a series of washes in KPBS and 0.175M sodium acetate (NaOAc). The enzyme-substrate reaction was generated by adding a 3,3-diaminobenzadine (DAB) substrate solution of 0.2mg/ml DAB in 0.175M NaOAc with hydrogen peroxide (H2O2). The enzyme-substrate reaction was terminated by a series of washes in 0.175M NaOAc solution and then in KPBS. Tissue was mounted on Superfrost Plus slides (Fischer Scientific) allowed to dry and then counter stained with 0.5% methyl green and coverslipped using Permount (Fischer Scientific). Confirmation of non-specific reactivity was determined by omission of the primary antibody. Furthermore, this staining procedure has consistently yielded positive c-fos results in this laboratory (Shurin et al, 1997; Rossi-George et al, 2005; Cooper & Kusnecov, 2007), with confidence in the non-specificity of this staining was initially confirmed using an isotype specific control (Rabbit IgG), which produced no subsequent reactions. Images of c-fos slides were captured at 20× magnification with the average of three sections per region per subject used for analysis. C-fos levels were assessed in paraventricular hypothalamic nucleus (PVN), central nucleus of the amygdala (CeA), paraventricular thalamic nucleus (PV), and the granular cell layer of the dentate gyrus (GrDG). Brain regions were identified based on the mouse brain atlas of Paxinos and Franklin (2003). C-fos was quantified with ImageJ 1.4 software (available at http://rsb.info.nih.gov/ij/ NIH, USA) and counts were confirmed by spot checking with hand counts.
2.6. Splenic cytokine levels
Immediately prior to the infusion of saline during the perfusion procedure, spleens were removed and frozen for analysis of cytokine levels. Spleens were homogenized in 1mM phenylmethanesulfonyl fluoride (PMSF) in 0.1M phosphate buffer, centrifuged (4000rpm for 30min at 4°C), and supernatants collected. Splenic cytokine levels were measured by enzyme-linked immunosorbant assays (ELISA), according to the manufacturer's instructions (BD Biosciences, San Diego, CA). Total protein content for the spleen samples was determined by BCA protein assay (Pierce, Rockford, IL), according to the manufacturer's instructions. Cytokine data are expressed as pg/mg of protein.
2.7. Statistical analyses
The data were analyzed with Age (middle-aged and young) × Treatment (LPS-acute, LPS-repeated, and saline) factorial ANOVAs. Since body weight was measured daily the data were analyzed by a repeated measure ANOVA with Test day as the repeated measure. To determine if any differences in initial body weight existed between the age groups a one-way ANOVA was conducted on Day 1 (prior to treatment) body weights. An alpha level of 0.05 was the criterion for rejection of the null hypothesis. Differences between Treatment and Age groups were assessed by Fischer PLSD post hoc test only if the omnibus F was significant.
3. Results
3.1. Body weight
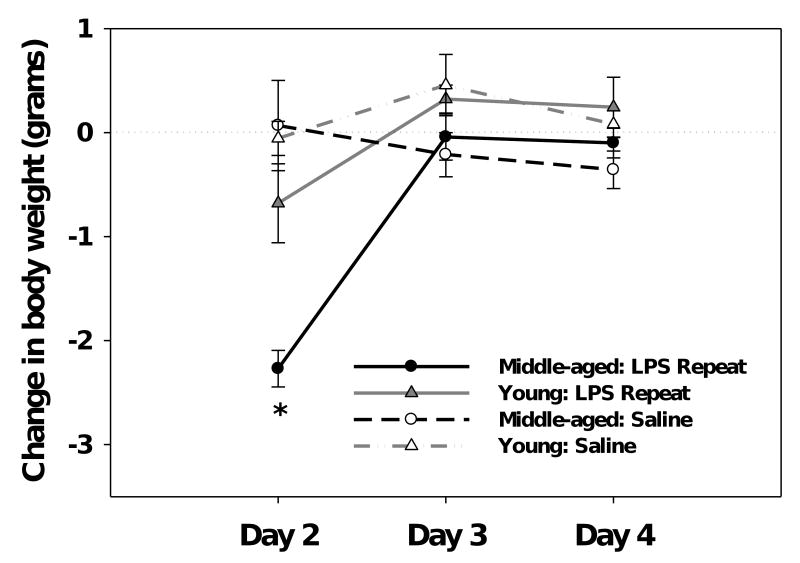
Comparison of the subjects body weight prior to treatment, on Day 1, revealed there was no significant difference between the age groups, both young and middle-aged mice weighed an average of 30 grams (F(1,30)=2.1, ns). Change in body weight following LPS administration was analyzed by repeated measures ANOVA that included the mice given repeated LPS or saline injections. Mice in the LPS-acute condition were not included given that LPS-induced weight loss is not observed until the following day. A significant Age × Treatment × Test day interaction (F(1,40)=4.16, p<0.05, see Figure 1) revealed that middle-aged mice showed a significant decrease in their weight 24hrs after the first LPS injection (p<0.05). Although the young mice showed some reduction in weight 24hrs after the initial LPS challenge, they did not differ from saline controls. No differences in weight were observed in either age group following the 2nd or 3rd LPS injection (i.e., Day 3 and Day 4, respectively).
Figure 1.
Middle-aged, but not young, mice showed a significant decrease in body weight 24hrs after a single LPS challenge. Subsequent LPS injections had no effect on weight, as there were no differences between LPS- and saline-treated mice on Days 3 or 4. Data are expressed as mean change in body weight ±SEM. * indicates a significant difference from saline controls.
3.2. Open field and novel object tests
Open field
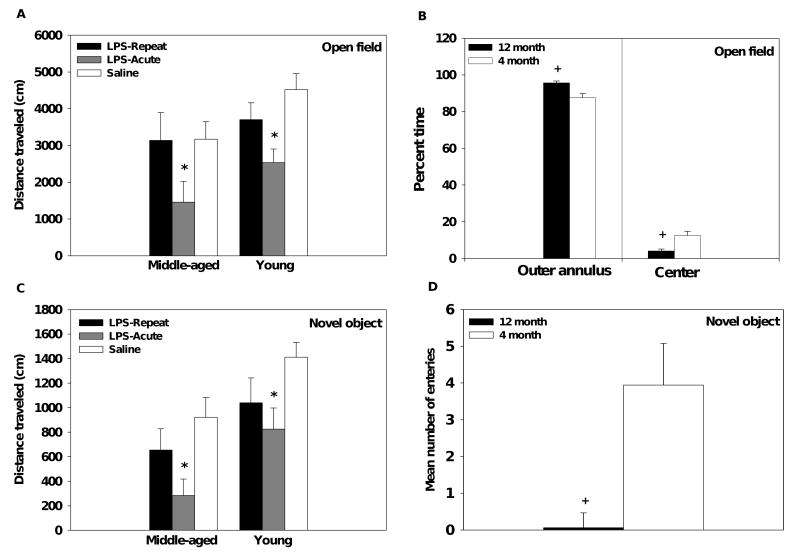
Mice were observed for 10min in the open field following acute or repeated LPS administration. Middle-aged mice traveled a shorter distance than young mice (main effect of Age, F(1,30)=5.54, p<0.05, see Figure 2A). Additionally, middle-aged mice spent more time in the outer ring and consequently less time in the center compared to young mice (main effects of Age, F(1,30)= 13.19, p<0.005; F(1,30)=13.95, p<0.005, respectively, see Figure 2B). Regardless of age, acute, but not repeated, LPS administration decreased total distance traveled in both young and middle-aged mice (main effect of Treatment, F(2,30)=7.12, p<0.005, see Figure 2A). A single LPS challenge increased time spent in the outer ring and decreased time spent in the center of the field (main effects of Treatment, F(2,30)= 4.12, p<0.05; F(2,30)=4.19, p<0.05, respectively, data not shown).
Figure 2.
Acute LPS administration decreased activity in both the open field (A) and novel object test (C) the reduction was comparable in the young and middle-aged mice. Middle-aged mice, regardless of their treatment condition, spent more time in the outer annulus and less time in the center of the open field (B; data collapsed across treatment condition). Additionally, middle-age mice made fewer entries into the area immediately surrounding the novel object (D; data collapsed across treatment condition). Data are expressed as means ±SEM. * indicates a significant difference from saline controls. + indicates a significant difference from young mice.
Novel object
Following the open field, a novel object (i.e., a silver cylinder) was placed in the center of the field and the subject's behavior was recorded for an additional 5min. Similar to the open field test, the middle-aged mice traveled a significantly shorter distance than young mice (main effect of Age, F(1,30)=12.61, p<0.005, see Figure 2C). In addition, the middle-age mice, regardless of their treatment condition, made fewer entries into and spent less time in the area immediately surrounding the novel object than younger subjects (main effects of Age, F(1,30)=8.35, p<0.01; F(1,30)=4.78, p<0.05, respectively, see Figure 2D). Following acute or repeated LPS administration mice showed a significant decrease in total distance traveled relative to saline-treated subjects (main effect of Treatment, F(2,30)=7.38, p<0.005, see Figure 2C), this response was not influenced by the subject's age.
3.3. Immunohistochemistry: c-fos
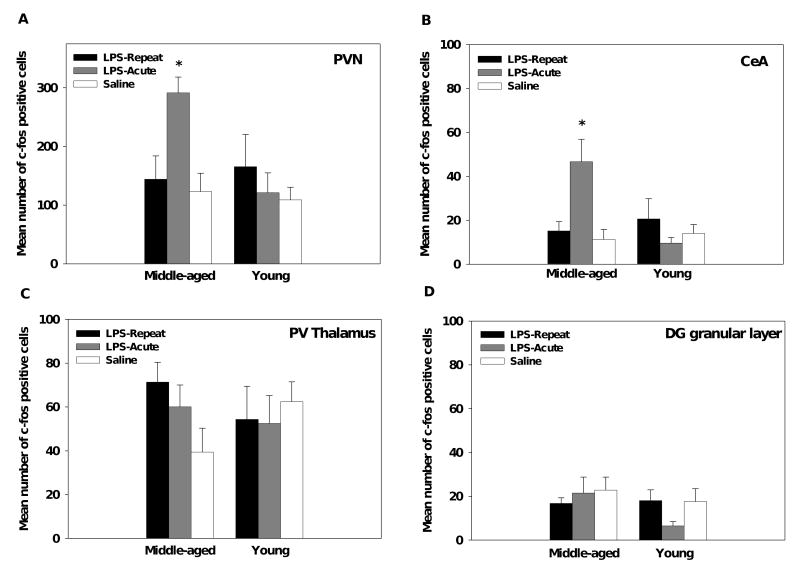
The number of c-fos positive cells were assessed in paraventricular hypothalamic nucleus (PVN), central nucleus of the amygdala (CeA), paraventricular thalamic nucleus (PV), and the granular cell layer of the dentate gyrus (GrDG). No significant age or treatment differences were found in the PV or GrDG (see Figures 3C and 3D). However, for both the PVN and CeA the middle-aged mice had significantly higher c-fos levels in response to acute, but not repeated, LPS administration as compared to saline controls (Age × Treatment interactions, F(2,30)=4.13, p<0.05; F(2,30)=8.39, p<0.005, respectively, see Figures 3A, 3B, and 4). In the young mice, acute or repeated LPS administration failed to induce c-fos expression in the regions assessed; young LPS-treated mice did not differ from saline controls.
Figure 3.
In response to acute LPS administration, middle-aged showed an increase in c-fos positive cells in the PVN (A) and CeA (B) as compared to saline controls, whereas younger mice failed to show a response in these regions following LPS administration. No differences were observed in the PV of the thalamus (C) or the granular cell layer of the dentate gyrus (D) for either age group. Data are expressed as means ±SEM. * indicates a significant difference from saline controls.
Figure 4.
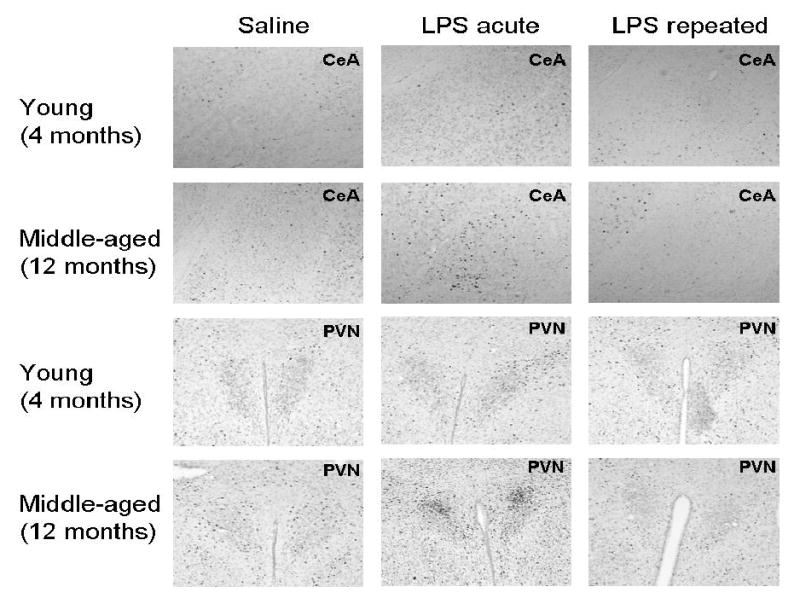
Representative photomicrographs of c-fos levels in the central nucleus of the amygdala (CeA) and the paraventricular nucleus of the hypothalamus (PVN).
3.4. Splenic cytokine levels
Interleukin-1β
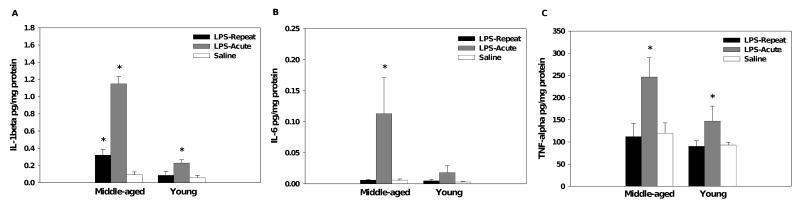
A single LPS injection significantly increased splenic levels of IL-1β in both age groups, but the increase was greater in the middle-aged mice (Age × Treatment interaction, F(2,30)=43.13, p<0.001, respectively, see Figure 5A). Repeated LPS injections increased splenic IL-1β levels in the middle-aged, but not the young, mice relative to saline-treated mice (p<0.05), though this effect was attenuated relative to that seen with an acute LPS challenge.
Figure 5.
As expected, acute LPS administration increased splenic levels of IL-1β in both age groups, but the increase was greater in middle-aged mice (A). Though attenuated relative to acute treatment, middle-aged mice showed increased levels of IL-1β following repeated LPS administration. Only middle-aged mice showed significantly elevated splenic levels of IL-6 (B), whereas both age groups showed similar levels of TNF-α following acute LPS administration (C). Data are expressed as means ±SEM. * indicates a significant difference from saline controls.
Interleukin-6
Acute LPS increased splenic levels of IL-6 in the middle-aged, but not the young, mice (Age × Treatment interaction, F(2,30)=3.51, p<0.05, see Figure 5B).
Tumor necrosis factor-α
A single LPS injection, but not repeated injections, increased splenic levels of TNF-α (main effect of Treatment, F(2,30)=7.53, p<0.005, see Figure 5C). Analysis did not reveal an Age effect.
Discussion
Age-related alterations in the inflammatory and neurobehavioral responses to an immune stimulus have been well characterized in elderly subjects (Abraham and Johnson, 2009; Aw et al., 2007; Barrientos et al., 2006; Godbout et al., 2005; Godbout et al., 2008; Krabbe et al., 2005; Miller, 1996). However, the onset and trajectory of these age-related changes in sensitivity to immune activation have not been fully elucidated. As an attempt to identify earlier age-associated changes that may precede the effects observed in elderly subjects, the present study compared the responses of young and middle-aged subjects following LPS administration. The results suggest that differential reactivity exists between these age groups, as middle-aged subjects show increased sensitivity to acute LPS administration. Specifically, middle-aged subjects mounted enhanced neural and immune responses to acute LPS administration compared to the younger subjects. In addition, body weight was monitored as an index of sickness, and in agreement with Kinoshita et al. (2009), middle-aged subjects showed greater weight loss compared to younger subjects following acute LPS administration. These data provide evidence of enhanced sensitivity to immune activation in middle-aged subjects.
With respect to neuronal c-fos expression, it was noted that after a single injection of LPS, the numbers of c-fos immunoreactive cells in the PVN of the hypothalamus and the central nucleus of the amygdala (CeA) were significantly augmented in 12-month-old middle-aged mice relative to young mice. This is consistent with a previous report showing that much older 22-24 month-old BALB/c mice showed greater overall c-fos positive numbers of cells in the PVN and CeA in response to LPS (Gaykema et al, 2006). Interestingly, in this latter study a higher dose of LPS (10μg/mouse) was used, which activated strongly neural structures in young mice. In contrast, young mice in the present study failed to show an increase in the number of c-fos expressing PVN and CeA neurons. This was likely due to the lower LPS dose of 3μg/mouse, and highlights the apparent development of increased neuronal sensitivity to LPS challenge by middle age.
Overall, the present data stand in contrast to prior reports that have shown limited differences between young and middle-aged subjects following acute LPS treatment (Chorinchath et al., 1996; Kinoshita et al., 2009; Kohman et al., 2007; Krzyszton et al., 2008; Sparkman et al., 2004; Terao et al., 2002). The difference in findings likely results from the higher dose of LPS employed in prior reports (Chorinchath et al., 1996; Kinoshita et al., 2009; Kohman et al., 2007; Krzyszton et al., 2008; Sparkman et al., 2004; Terao et al., 2002). Whereas, in the present study, the use of a lower dose discriminated between younger and middle-aged subjects, revealing that the latter animals have a lower threshold for activation of the immune system. As a result of this heightened sensitivity, the frequency of inflammatory episodes may increase in middle-age which has the potential to adversely impact neural function and general well being.
In terms of the behavioral response, prior reports have found limited age-related differences in sickness associated behaviors between young and middle-aged subjects following a single challenge with LPS (Kinoshita et al., 2009; Kohman et al., 2007; Krzyszton et al., 2008; Sparkman et al., 2004). In agreement, we report that acute LPS administration produced a similar decrease in locomotor behavior in both age groups (Kinoshita et al., 2009). This lack of a difference is not surprising, given prior findings (Kinoshita et al., 2009; Kohman et al., 2007; Krzyszton et al., 2008; Sparkman et al., 2004). Further, adult mice show reductions in activity in an open field and burrowing behavior following administration of very low doses of LPS (e.g., 0.5 and 1μg/kg) (Teeling et al., 2007). Given that behavior can be suppressed in response to a sub-pyrogenic dose of LPS (Teeling et al., 2007), alterations in activity levels may not be useful in identifying changes in sensitivity to an immune stimulus. Rather, direct measures of inflammation may better dissociate the age-related differences that exist by middle-age.
Though the behavioral response to a single LPS challenge is comparable in young and middle-aged subjects, divergent results on the effects of repeated LPS exposure on activity have been reported (Kinoshita et al., 2009; Kohman et al., 2007; Sparkman et al., 2004). For example, repeated LPS caused a prolonged decrease in swim speed in middle-aged female mice in the water maze (Sparkman et al., 2004), whereas no difference was found in activity in an open field (Kinoshita et al., 2009, Figure 2). The present data confirm prior reports that show development of tolerance to the behavioral effects of LPS administration (Engeland et al., 2001; Kinoshita et al., 2009). Additionally, our data suggest that tolerance to the LPS-induced reductions in locomotor/exploratory behavior develops in both young and middle-aged subjects.
A central feature of endotoxin tolerance is a reduction in the inflammatory response to subsequent endotoxin administration (Fan and Cook, 2004). Assessment of splenic cytokine levels revealed that middle-aged subjects retain some immunoreactivity to the last (i.e., fourth) LPS exposure, with the IL-1β response showing an apparent slower dissipation. Prior work in adult animals has shown a similar cytokine profile in response to repeated high dose LPS exposure, wherein tolerance develops more quickly with TNF-α and IL-6 production than with IL-1β (Nagano et al., 1999; Pacheco-Lopez et al., 2008). However, at a lower LPS dose, young mice appear to develop tolerance faster than middle-aged subjects, as demonstrated in the present study. This difference in rate of tolerance development may be related to the initial reaction to LPS exposure. Given that the middle-aged subjects showed augmented cytokine production in response to acute LPS, as compared to the young mice, the response may be slower to dissipate.
In conclusion, it has been demonstrated that numerous age-related changes in immune function occur around middle-age and likely continue to progress with further aging (Aw et al., 2007; Miller, 1996). One implication of this appears to be increased sensitivity to immune activation. Though at higher doses of LPS no differences exist between young and middle-aged subjects (Kinoshita et al., 2009; Kohman et al., 2007; Krzyszton et al., 2008; Sparkman et al., 2004); although the current data should be extended to a direct dose-response comparison, they nonetheless suggest that middle-aged subjects are more responsive to a lower dose of endotoxin. These data also suggest that tolerance development to repeated LPS exposure may be slower in middle-age subjects when using a low dose of endotoxin. Additional investigation of the immune changes present in middle-age subjects will help clarify the progression of age-associated immune alterations that exist during later stages of life.
Acknowledgments
Work was supported by grants MH60706, NIEHS P30 ES05022, and NIEHS Training grant 5T32 E507148.
Footnotes
Conflict of Interest Statement: All authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abraham J, Johnson RW. Central inhibition of interleukin-1beta ameliorates sickness behavior in aged mice. Brain behav immun. 2009;23:396–401. doi: 10.1016/j.bbi.2008.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aw D, Silva AB, Palmer DB. Immunosenescence: emerging challenges for an ageing population. Immunol. 2007;120:435–446. doi: 10.1111/j.1365-2567.2007.02555.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrientos RM, Higgins EA, Biedenkapp JC, Sprunger DB, Wright-Hardesty KJ, Watkins LR, Rudy JW, Maier SF. Peripheral infection and aging interact to impair hippocampal memory consolidation. Neurobiol aging. 2006;27:723–732. doi: 10.1016/j.neurobiolaging.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Blalock EM, Chen KC, Sharrow K, Herman JP, Porter NM, Foster TC, Landfield PW. Gene microarrays in hippocampal aging: statistical profiling identifies novel processes correlated with cognitive impairment. J Neurosci. 2003;23:3807–3819. doi: 10.1523/JNEUROSCI.23-09-03807.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruunsgaard H, Andersen-Ranberg K, Jeune B, Pedersen AN, Skinhoj P, Pedersen BK. A high plasma concentration of TNF-alpha is associated with dementia in centenarians. J gerontol. 1999;54:M357–364. doi: 10.1093/gerona/54.7.m357. [DOI] [PubMed] [Google Scholar]
- Chen J, Buchanan JB, Sparkman NL, Godbout JP, Freund GG, Johnson RW. Neuroinflammation and disruption in working memory in aged mice after acute stimulation of the peripheral innate immune system. Brain behav immun. 2008;22:301–311. doi: 10.1016/j.bbi.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JH, Lee CH, Hwang IK, Won MH, Seong JK, Yoon YS, Lee HS, Lee IS. Age-related changes in ionized calcium-binding adapter molecule 1 immunoreactivity and protein level in the gerbil hippocampal CA1 region. J veterin med sci / the Japanese Society of Veterinary Sci. 2007;69:1131–1136. doi: 10.1292/jvms.69.1131. [DOI] [PubMed] [Google Scholar]
- Chorinchath BB, Kong LY, Mao L, McCallum RE. Age-associated differences in TNF-alpha and nitric oxide production in endotoxic mice. J Immunol. 1996;156:1525–1530. [PubMed] [Google Scholar]
- Cooper JF, Kusnecov AW. Methylmercuric chloride induces activation of neuronal stress circuitry and alters exploratory behavior in the mouse. Neurosci. 2008;148:1046–1064. doi: 10.1016/j.neuroscience.2007.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham C, Wilcockson DC, Campion S, Lunnon K, Perry VH. Central and systemic endotoxin challenges exacerbate the local inflammatory response and increase neuronal death during chronic neurodegeneration. J Neurosci. 2005;25:9275–9284. doi: 10.1523/JNEUROSCI.2614-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng XH, Bertini G, Xu YZ, Yan Z, Bentivoglio M. Cytokine-induced activation of glial cells in the mouse brain is enhanced at an advanced age. Neurosci. 2006;141:645–661. doi: 10.1016/j.neuroscience.2006.04.016. [DOI] [PubMed] [Google Scholar]
- Dilger RN, Johnson RW. Aging, microglial cell priming, and the discordant central inflammatory response to signals from the peripheral immune system. J leuk biol. 2008;84:932–939. doi: 10.1189/jlb.0208108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engeland CG, Kavaliers M, Ossenkopp KP. Influence of the estrous cycle on tolerance development to LPS-induced sickness behaviors in rats. Psychoneuroendocrin. 2006;31:510–525. doi: 10.1016/j.psyneuen.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Engeland CG, Nielsen DV, Kavaliers M, Ossenkopp KP. Locomotor activity changes following lipopolysaccharide treatment in mice: a multivariate assessment of behavioral tolerance. Physiol behav. 2001;72:481–491. doi: 10.1016/s0031-9384(00)00436-4. [DOI] [PubMed] [Google Scholar]
- Fan H, Cook JA. Molecular mechanisms of endotoxin tolerance. J endotoxin res. 2004;10:71–84. doi: 10.1179/096805104225003997. [DOI] [PubMed] [Google Scholar]
- Frank MG, Barrientos RM, Biedenkapp JC, Rudy JW, Watkins LR, Maier SF. mRNA up-regulation of MHC II and pivotal pro-inflammatory genes in normal brain aging. Neurobiol aging. 2006;27:717–722. doi: 10.1016/j.neurobiolaging.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Gaykema RP, Balachandran MK, Godbout JP, Johnson RW, Goehler LE. Enhanced neuronal activation in central autonomic network nuclei in aged mice following acute peripheral immune challenge. Auton Neurosci. 2007;131:137–142. doi: 10.1016/j.autneu.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Godbout JP, Chen J, Abraham J, Richwine AF, Berg BM, Kelley KW, Johnson RW. Exaggerated neuroinflammation and sickness behavior in aged mice following activation of the peripheral innate immune system. Faseb J. 2005;19:1329–1331. doi: 10.1096/fj.05-3776fje. [DOI] [PubMed] [Google Scholar]
- Godbout JP, Moreau M, Lestage J, Chen J, Sparkman NL, OC J, Castanon N, Kelley KW, Dantzer R, Johnson RW. Aging exacerbates depressive-like behavior in mice in response to activation of the peripheral innate immune system. Neuropsychopharm. 2008;33:2341–2351. doi: 10.1038/sj.npp.1301649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habicht GS. Acquired immunological tolerance in aged mice. II. The cellular basis of the loss of tolerance sensitivity. Mech ageing dev. 1985;30:23–36. doi: 10.1016/0047-6374(85)90056-9. [DOI] [PubMed] [Google Scholar]
- Hauss-Wegrzyniak B, Vraniak PD, Wenk GL. LPS-induced neuroinflammatory effects do not recover with time. Neuro report. 2000;11:1759–1763. doi: 10.1097/00001756-200006050-00032. [DOI] [PubMed] [Google Scholar]
- Henry CJ, Huang Y, Wynne A, Hanke M, Himler J, Bailey MT, Sheridan JF, Godbout JP. Minocycline attenuates lipopolysaccharide (LPS)-induced neuroinflammation, sickness behavior, and anhedonia. J neuroinflamm. 2008;5:15. doi: 10.1186/1742-2094-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry CJ, Huang Y, Wynne AM, Godbout JP. Peripheral lipopolysaccharide (LPS) challenge promotes microglial hyperactivity in aged mice that is associated with exaggerated induction of both pro-inflammatory IL-1beta and anti-inflammatory IL-10 cytokines. Brain behav immun. 2009;23:309–317. doi: 10.1016/j.bbi.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes C, El-Okl M, Williams AL, Cunningham C, Wilcockson D, Perry VH. Systemic infection, interleukin 1beta, and cognitive decline in Alzheimer's disease. J neurol neurosurg psychiat. 2003;74:788–789. doi: 10.1136/jnnp.74.6.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izgut-Uysal VN, Ozkaya YG, Ozdemir S, Yargicoglu P, Agar A. Effect of L-arginine on age-related changes in macrophage phagocytic activity. Immun investigat. 2004;33:287–293. doi: 10.1081/imm-120037276. [DOI] [PubMed] [Google Scholar]
- Kinoshita D, Cohn DW, Costa-Pinto FA, de Sa-Rocha LC. Behavioral effects of LPS in adult, middle-aged and aged mice. Physiol behav. 2009;96:328–332. doi: 10.1016/j.physbeh.2008.10.018. [DOI] [PubMed] [Google Scholar]
- Kohman RA, Tarr AJ, Byler SL, Boehm GW. Age increases vulnerability to bacterial endotoxin-induced behavioral decrements. Physiol behav. 2007;91:561–565. doi: 10.1016/j.physbeh.2007.03.032. [DOI] [PubMed] [Google Scholar]
- Krabbe KS, Bruunsgaard H, Hansen CM, Moller K, Fonsmark L, Qvist J, Madsen PL, Kronborg G, Andersen HO, Skinhoj P, Pedersen BK. Ageing is associated with a prolonged fever response in human endotoxemia. Clin diagnostic lab immun. 2001;8:333–338. doi: 10.1128/CDLI.8.2.333-338.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krabbe KS, Reichenberg A, Yirmiya R, Smed A, Pedersen BK, Bruunsgaard H. Low-dose endotoxemia and human neuropsychological functions. Brain behav immun. 2005;19:453–460. doi: 10.1016/j.bbi.2005.04.010. [DOI] [PubMed] [Google Scholar]
- Krzyszton CP, Sparkman NL, Grant RW, Buchanan JB, Broussard SR, Woods J, Johnson RW. Exacerbated fatigue and motor deficits in interleukin-10-deficient mice after peripheral immune stimulation. American J physiol. 2008;295:R1109–1114. doi: 10.1152/ajpregu.90302.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laupland KB, Church DL, Mucenski M, Sutherland LR, Davies HD. Population-based study of the epidemiology of and the risk factors for invasive Staphylococcus aureus infections. J infect diseases. 2003;187:1452–1459. doi: 10.1086/374621. [DOI] [PubMed] [Google Scholar]
- Letiembre M, Hao W, Liu Y, Walter S, Mihaljevic I, Rivest S, Hartmann T, Fassbender K. Innate immune receptor expression in normal brain aging. Neurosci. 2007;146:248–254. doi: 10.1016/j.neuroscience.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Li Y, Howell EA, Lagoo AS, Kuchibhatla M, Pan H, Cohen HJ, Lagoo SA. Shock. Vol. 31. Augusta, Ga: 2009. Differential gene expression of interleukin-1 receptor associated kinase-1 and interleukin-1 receptor associated kinase-M in peripheral blood mononuclear cells of young and aged rats following preconditioning with endotoxin; pp. 55–63. [DOI] [PubMed] [Google Scholar]
- Liu B, Hong JS. Role of microglia in inflammation-mediated neurodegenerative diseases: mechanisms and strategies for therapeutic intervention. J pharmac experim therap. 2003;304:1–7. doi: 10.1124/jpet.102.035048. [DOI] [PubMed] [Google Scholar]
- Marchalant Y, Brothers HM, Wenk GL. Inflammation and aging: can endocannabinoids help? Biomed pharmacother. 2008;62:212–217. doi: 10.1016/j.biopha.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RA. Science. Vol. 273. New York, N.Y: 1996. The aging immune system: primer and prospectus; pp. 70–74. [DOI] [PubMed] [Google Scholar]
- Nagano I, Takao T, Nanamiya W, Takemura T, Nishiyama M, Asaba K, Makino S, De Souza EB. Differential effects of and repeated endotoxin treatment on pituitary-adrenocortical hormones in the mouse: role of interleukin-1 and tumor necrosis factor-α. Neuroimmunomod. 1998;6:284–292. doi: 10.1159/000026386. [DOI] [PubMed] [Google Scholar]
- Nomura F, Akashi S, Sakao Y, Sato S, Kawai T, Matsumoto M, Nakanishi K, Kimoto M, Miyake K, Takeda K, Akira S. Cutting edge: endotoxin tolerance in mouse peritoneal macrophages correlates with down-regulation of surface toll-like receptor 4 expression. J Immunol. 2000;164:3476–3479. doi: 10.4049/jimmunol.164.7.3476. [DOI] [PubMed] [Google Scholar]
- Ogura K, Ogawa M, Yoshida M. Effects of ageing on microglia in the normal rat brain: immunohistochemical observations. Neuroreport. 1994;5:1224–1226. doi: 10.1097/00001756-199406020-00016. [DOI] [PubMed] [Google Scholar]
- Pacheco-Lopez G, Niemi MB, Engler H, Reither C, Doenlen R, Espinosa E, Oberbeck R, Schedlowski M. Weaken taste-LPS association during endotoxin tolerance. Physiol behav. 2008;93:261–266. doi: 10.1016/j.physbeh.2007.08.022. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates. 2nd. 2003. [Google Scholar]
- Popp DM, Francis M. Age-associated changes in T-B cell cooperation demonstrated by the allogeneic effect. Mech ageing devel. 1979;10:341–353. doi: 10.1016/0047-6374(79)90046-0. [DOI] [PubMed] [Google Scholar]
- Roy A, Fung YK, Liu X, Pahan K. Up-regulation of microglial CD11b expression by nitric oxide. J biolog chem. 2006;281:14971–14980. doi: 10.1074/jbc.M600236200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi-George A, Urbach D, Colas D, Goldfarb Y, Kusnecov AW. Neuronal, endocrine, and anorexic responses to the T-cell superantigen staphylococcal enterotoxin A: dependence on tumor necrosis factor-alpha. J Neurosci. 2005;25:5314–5322. doi: 10.1523/JNEUROSCI.0687-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozovsky I, Finch CE, Morgan TE. Age-related activation of microglia and astrocytes: in vitro studies show persistent phenotypes of aging, increased proliferation, and resistance to down-regulation. Neurobiol aging. 1998;19:97–103. doi: 10.1016/s0197-4580(97)00169-3. [DOI] [PubMed] [Google Scholar]
- Saito H, Sherwood ER, Varma TK, Evers BM. Effects of aging on mortality, hypothermia, and cytokine induction in mice with endotoxemia or sepsis. Mech ageing devel. 2003;124:1047–1058. doi: 10.1016/j.mad.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Semmler A, Frisch C, Debeirm T, Ramanathan M, Okulla T, Klockgether T, Heneka MT. Long-term cognitive impairment, neuronal loss and reduced cortical cholinergic innervation after recovery from sepsis in a rodent model. Exper Neurol. 2007;204:733–740. doi: 10.1016/j.expneurol.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Shurin G, Shanks N, Nelson L, Hoffman G, Huang L, Kusnecov AW. Hypothalamic-pituitary-adrenal activation by the bacterial superantigen staphylococcal enterotoxin B: role of macrophages and T cells. Neuroendocrin. 1997;65:18–28. doi: 10.1159/000127161. [DOI] [PubMed] [Google Scholar]
- Sparkman NL, Martin LA, Calvert WS, Boehm GW. Effects of intraperitoneal lipopolysaccharide on Morris maze performance in year-old and 2-month-old female C57BL/6J mice. Behav brain res. 2004;159:145–151. doi: 10.1016/j.bbr.2004.10.011. [DOI] [PubMed] [Google Scholar]
- Tambuyzer BR, Ponsaerts P, Nouwen EJ. Microglia: gatekeepers of central nervous system immunology. J leuk biol. 2009;85:352–370. doi: 10.1189/jlb.0608385. [DOI] [PubMed] [Google Scholar]
- Tateda K, Matsumoto T, Miyazaki S, Yamaguchi K. Lipopolysaccharide-induced lethality and cytokine production in aged mice. Infect immun. 1996;64:769–774. doi: 10.1128/iai.64.3.769-774.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teeling JL, Felton LM, Deacon RM, Cunningham C, Rawlins JN, Perry VH. Sub-pyrogenic systemic inflammation impacts on brain and behavior, independent of cytokines. Brain behav immun. 2007;21:836–850. doi: 10.1016/j.bbi.2007.01.012. [DOI] [PubMed] [Google Scholar]
- Terao A, Apte-Deshpande A, Dousman L, Morairty S, Eynon BP, Kilduff TS, Freund YR. Immune response gene expression increases in the aging murine hippocampus. J neuroimmun. 2002;132:99–112. doi: 10.1016/s0165-5728(02)00317-x. [DOI] [PubMed] [Google Scholar]
- Urbach-Ross D, Kusnecov AW. Effects of acute and repeated exposure to lipopolysaccharide on cytokine and corticosterone production during remyelination. Brain behav immun. 2007;21:962–974. doi: 10.1016/j.bbi.2007.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbitsky M, Yonan AL, Malleret G, Kandel ER, Gilliam TC, Pavlidis P. Learn mem. Vol. 11. Cold Spring Harbor, N.Y: 2004. Altered hippocampal transcript profile accompanies an age-related spatial memory deficit in mice; pp. 253–260. [DOI] [PMC free article] [PubMed] [Google Scholar]