Abstract
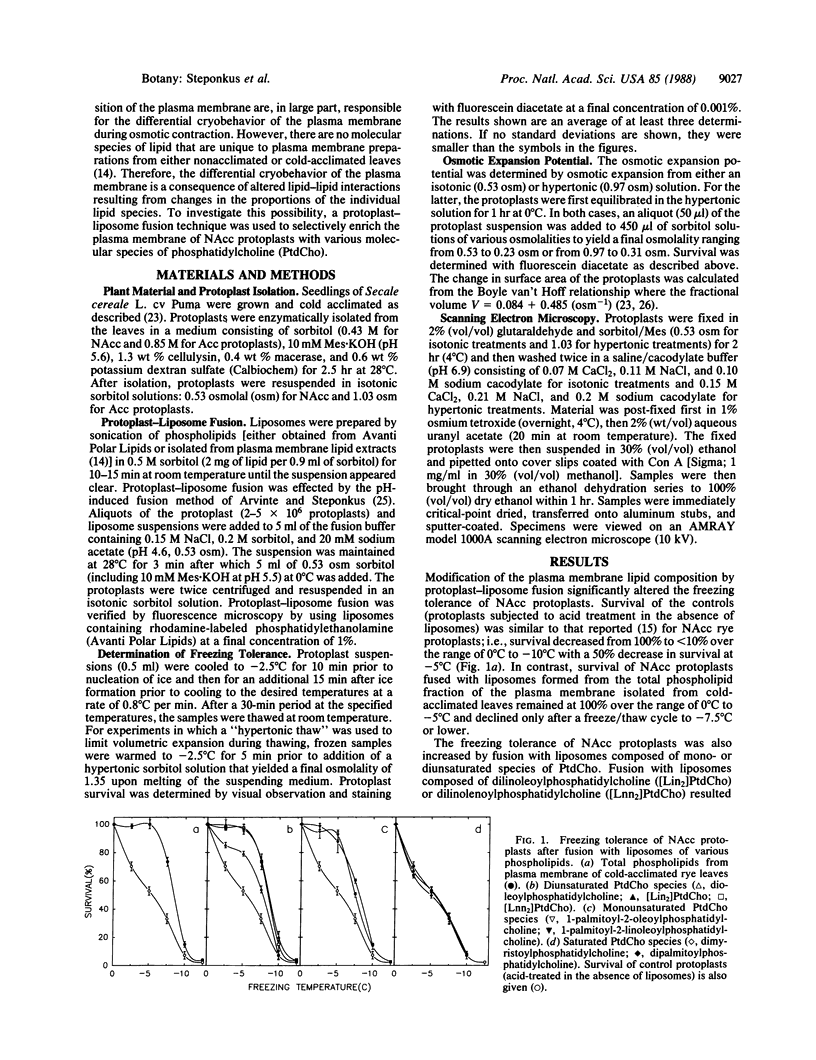
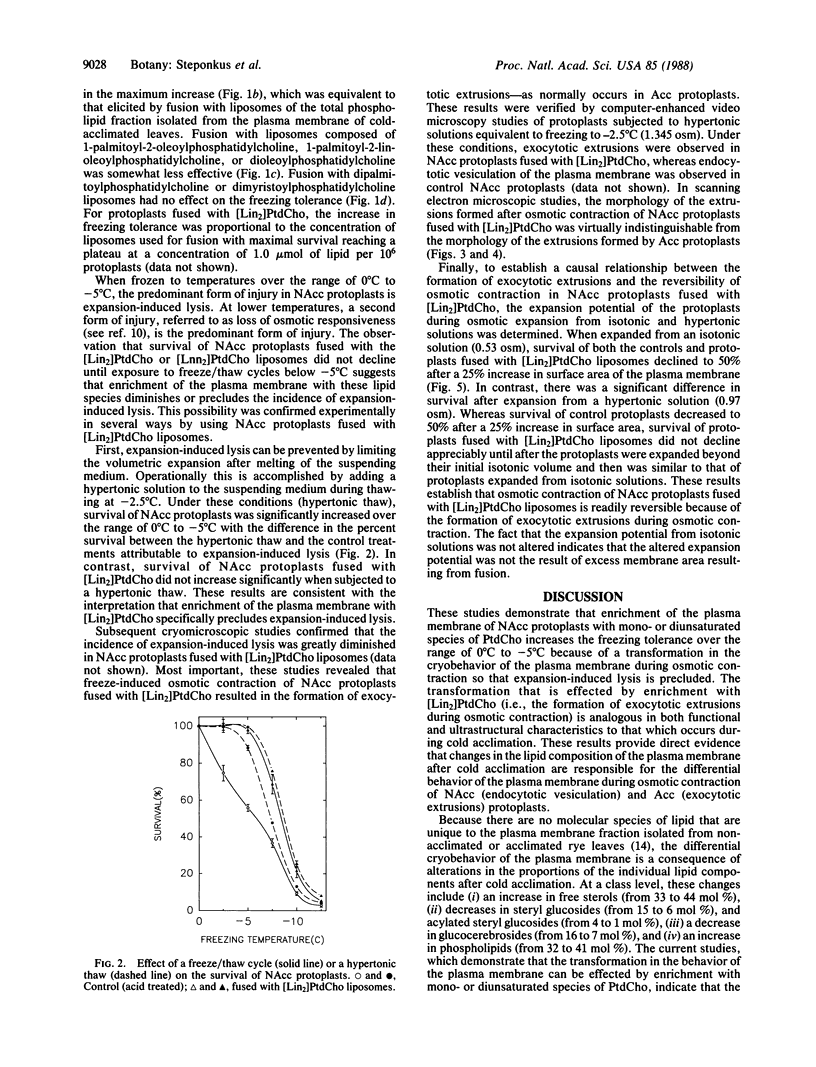
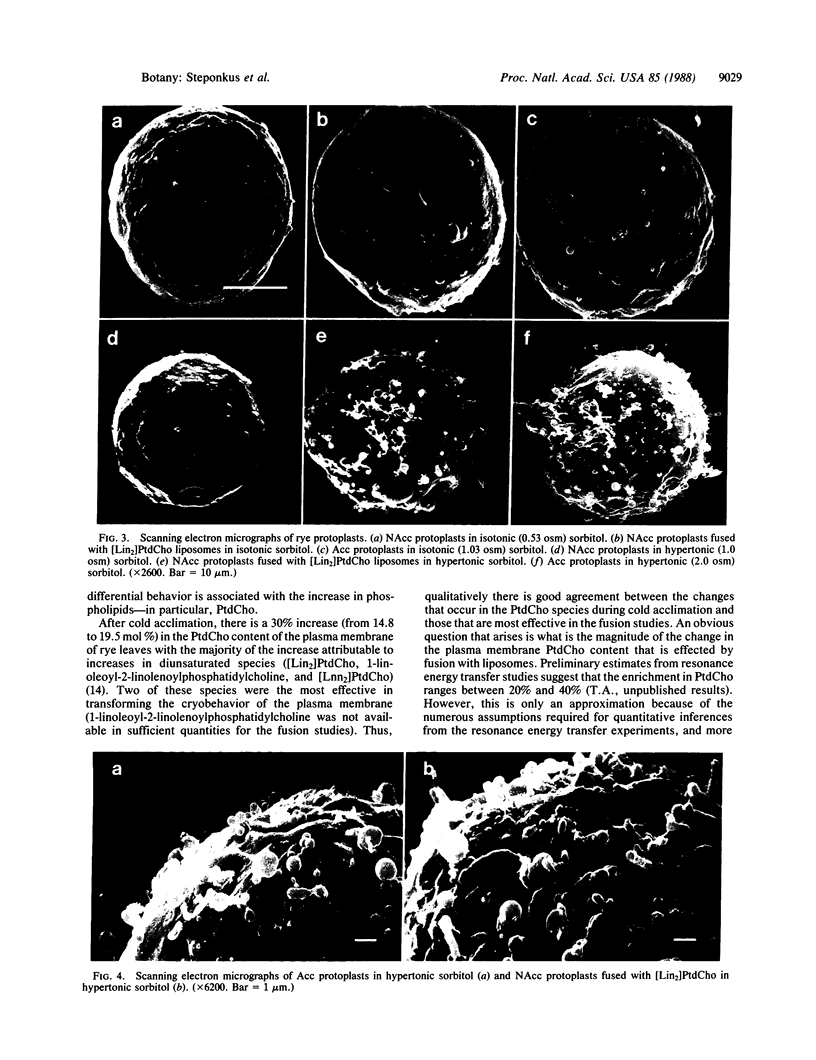
The freezing tolerance of protoplasts isolated from nonacclimated rye leaves (Secale cereale L. cv Puma) was significantly altered by using a pH-induced protoplastliposome fusion technique to modify the lipid composition of the plasma membrane. The increase in freezing tolerance was elicited by fusion with liposomes composed of either the total phospholipid fraction isolated from the plasma membrane of cold-acclimated leaves or single mono- or diunsaturated species of phosphatidylcholine (PtdCho). Of the PtdCho species tested, dilinoleoylphosphatidylcholine ([Lin2]PtdCho) and dilinolenoylphosphatidylcholine ([Lnn2]PtdCho) liposomes were the most effective; 1-palmitoyl-2-oleoylphosphatidylcholine, 1-palmitoyl-2-linoleoylphosphatidylcholine, or dioleoylphosphatidylcholine liposomes were somewhat less effective; dimyristoylphosphatidylcholine or dipalmitoylphosphatidylcholine liposomes had no effect. The increased freezing tolerance was the result of a transformation in the cryobehavior of the plasma membrane during freeze-induced osmotic contraction. In control nonacclimated protoplasts, osmotic contraction resulted in endocytotic vesiculation of the plasma membrane which was irreversible and resulted in lysis during osmotic expansion after melting of the suspending medium. In nonacclimated protoplasts fused with mono- or diunsaturated species of PtdCho, osmotic contraction resulted in the reversible formation of exocytotic extrusions of the plasma membrane—as normally occurs in protoplasts isolated from cold-acclimated leaves (acclimated protoplasts). In scanning electron micrographs, the morphology of the extrusions of nonacclimated protoplasts fused with [Lin2]PtdCho was virtually indistinguishable from that of the extrusions formed in acclimated protoplasts. These studies provide direct evidence that changes in the lipid composition of the plasma membrane are causally related to one facet of the cold-acclimation process.
Keywords: cold acclimation, freezing injury, osmotic behavior, protoplast-liposome fusion, phosphatidylcholine
Full text
PDF
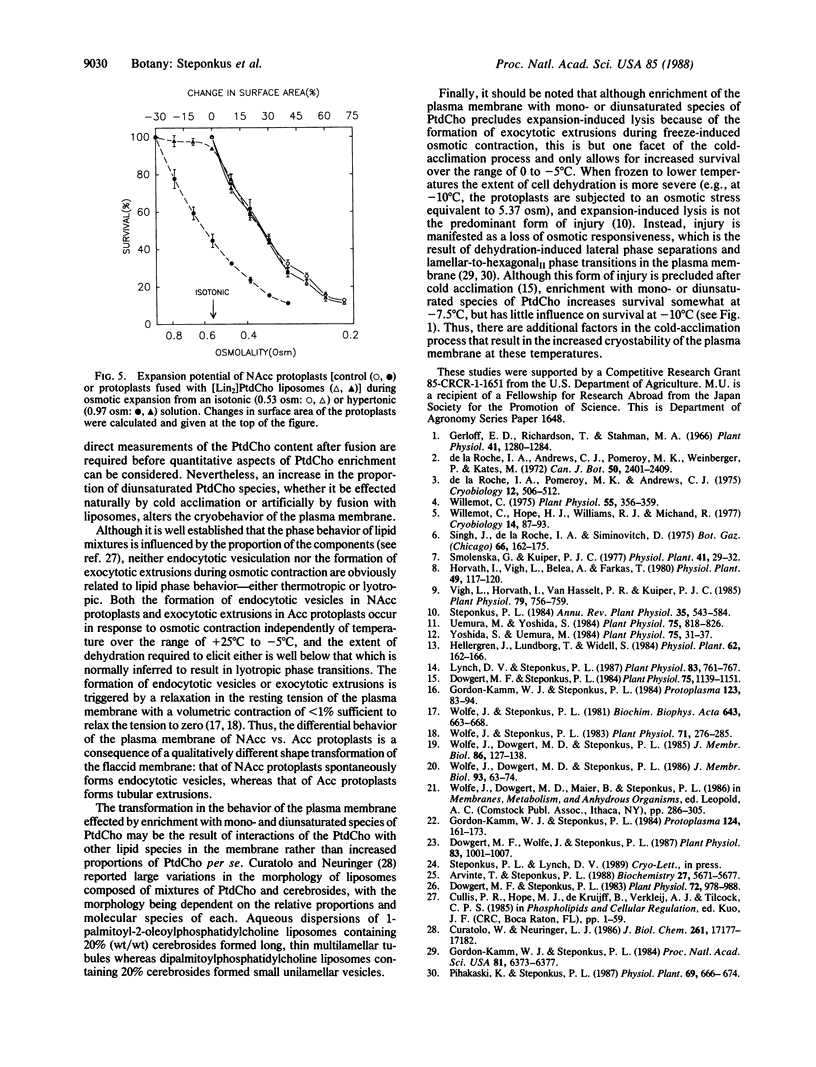
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Curatolo W., Neuringer L. J. The effects of cerebrosides on model membrane shape. J Biol Chem. 1986 Dec 25;261(36):17177–17182. [PubMed] [Google Scholar]
- Dowgert M. F., Steponkus P. L. Behavior of the Plasma Membrane of Isolated Protoplasts during a Freeze-Thaw Cycle. Plant Physiol. 1984 Aug;75(4):1139–1151. doi: 10.1104/pp.75.4.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowgert M. F., Steponkus P. L. Effect of cold acclimation on intracellular ice formation in isolated protoplasts. Plant Physiol. 1983 Aug;72(4):978–988. doi: 10.1104/pp.72.4.978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowgert M. F., Wolfe J., Steponkus P. L. The Mechanics of Injury to Isolated Protoplasts following Osmotic Contraction and Expansion. Plant Physiol. 1987 Apr;83(4):1001–1007. doi: 10.1104/pp.83.4.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerloff E. D., Richardson T., Stahmann M. A. Changes in Fatty acids of alfalfa roots during cold hardening. Plant Physiol. 1966 Oct;41(8):1280–1284. doi: 10.1104/pp.41.8.1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon-Kamm W. J., Steponkus P. L. Lamellar-to-hexagonalII phase transitions in the plasma membrane of isolated protoplasts after freeze-induced dehydration. Proc Natl Acad Sci U S A. 1984 Oct;81(20):6373–6377. doi: 10.1073/pnas.81.20.6373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch D. V., Steponkus P. L. Plasma Membrane Lipid Alterations Associated with Cold Acclimation of Winter Rye Seedlings (Secale cereale L. cv Puma). Plant Physiol. 1987 Apr;83(4):761–767. doi: 10.1104/pp.83.4.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uemura M., Yoshida S. Involvement of Plasma Membrane Alterations in Cold Acclimation of Winter Rye Seedlings (Secale cereale L. cv Puma). Plant Physiol. 1984 Jul;75(3):818–826. doi: 10.1104/pp.75.3.818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigh L., Horvàth I., van Hasselt P. R., Kuiper P. J. Effect of frost hardening on lipid and Fatty Acid composition of chloroplast thylakoid membranes in two wheat varieties of contrasting hardiness. Plant Physiol. 1985 Nov;79(3):756–759. doi: 10.1104/pp.79.3.756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willemot C., Hope H. J., Williams R. J., Michaud R. Changes in fatty acid composition of winter wheat during frost hardening. Cryobiology. 1977 Feb;14(1):87–93. doi: 10.1016/0011-2240(77)90126-2. [DOI] [PubMed] [Google Scholar]
- Willemot C. Stimulation of Phospholipid Biosynthesis during Frost Hardening of Winter Wheat. Plant Physiol. 1975 Feb;55(2):356–359. doi: 10.1104/pp.55.2.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe J., Steponkus P. L. Mechanical properties of the plasma membrane of isolated plant protoplasts : mechanism of hyperosmotic and extracellular freezing injury. Plant Physiol. 1983 Feb;71(2):276–285. doi: 10.1104/pp.71.2.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe J., Steponkus P. L. The stress-strain relation of the plasma membrane of isolated plant protoplasts. Biochim Biophys Acta. 1981 May 20;643(3):663–668. doi: 10.1016/0005-2736(81)90363-1. [DOI] [PubMed] [Google Scholar]
- Yoshida S., Uemura M. Protein and Lipid Compositions of Isolated Plasma Membranes from Orchard Grass (Dactylis glomerata L.) and Changes during Cold Acclimation. Plant Physiol. 1984 May;75(1):31–37. doi: 10.1104/pp.75.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Roche I. A., Pomeroy M. K., Andrews C. J. Changes in fatty acid composition in wheat cultivars of contrasting hardiness. Cryobiology. 1975 Oct;12(5):506–512. doi: 10.1016/0011-2240(75)90032-2. [DOI] [PubMed] [Google Scholar]