Abstract
Sex-based differences in food intake related behaviors have been observed previously. The objective of this study was to examine sex-based differences in the behavioral and neuronal responses to food. 22 women and 21 men were studied. After 6 days of controlled eucaloric feeding, ad libitum energy intake (EI) was measured for three days. Appetite ratings using visual analog scales were obtained before and after each meal. Functional magnetic resonance imaging was performed in the overnight fasted state on the last day of eucaloric feeding while subjects were presented visual stimuli of food and neutral nonfood objects. While hunger and prospective consumption were not different between sexes, women had higher post-meal satiety ratings and dietary restraint than men. Images of hedonic foods resulted in significantly greater activation of lateral and dorsolateral prefrontal cortex (DLPFC) and parietal cortex in women as compared to men. No brain regions were more activated in men as compared to women. Men increased their EI during the ad libitum diet phase. While measures of appetite or feeding behaviors did not correlate with either neuronal activation or subsequent EI, DLPFC activation in response to hedonic foods was negatively correlated with EI. In summary, greater prefrontal neuronal responses to food cues in women may suggest increased cognitive processing related to executive function, such as planning, guidance or evaluation of behavior. Finally, increased DLPFC activation, perhaps relating to inhibitory cognitive control in response to food cues may be a better predictor of food intake than behavioral measures.
Keywords: fMRI, neuroimaging, obesity, food intake, gender, appetite
Introduction
The regulation of energy intake is a complex process requiring the integration of multiple internal as well as external signals. Much has been learned about the homeostatic regulation of energy balance and the effects of adiposity and gut signals on hunger and satiety[1, 2]. Ultimately, however, the decision to initiate food intake, how much to consume, and when to terminate a meal is affected by not only these homeostatic mechanisms but also by `non-homeostatic' mechanisms such as learned and motivated behaviors, cognitive factors, habits, social context, availability of food, and external sensory cues and the integration of these different sensory inputs[3–5].
There are clear sex-based differences in the regulation of body weight[6, 7] which may be due in part to differences in the biological regulation of food intake or due to social, cultural and environmental forces that differentially affect women and men. Animal studies have found marked sex-specific differences in central leptin and insulin action[6–9]. Human studies have also shown sex-based differences in serum levels of leptin as well as in other hormones important in energy intake regulation, such as PYY, ghrelin, and obestatin[10–12]. There are also important sex-dependent differences in eating and dietary behaviors such as restraint and weight concerns[13–17]. We have, for example, previously shown sex-based differences in the appetitive response to overfeeding with women appearing to be more sensitive to positive energy balance with significantly greater changes in hunger and satiety ratings as well as with reduced subsequent energy intake as compared to men[18]. These findings suggest that there may be fundamental differences in how men and women process information relating to food intake
With the use of neuroimaging techniques we have begun to better understand the neural circuitry associated with the processes involved in ingestive behavior. A number of studies have examined the neuronal response to visual food cues, finding a network of brain regions that respond to visual food cues, including the prefrontal cortex, orbitofrontal cortex, inferior temporal cortex, insula, striatum, amygdala, hippocampus, and hypothalamus[18–26] with the salience of the food-related stimulus and the nutritional state also being important[18, 20, 24, 26, 27]. Few neuroimaging studies, however, have reported data on both sexes. Sex-based differences have been found in the pattern of brain response to hunger, satiation, and food stimulation using positron emission tomography[28, 29]. A functional magnetic resonance imaging (fMRI) study found that women had greater response in the occipito-temporal cortex to food-related visual stimuli than men[21]. Sex-based differences in the neuronal response to taste stimuli, specifically chocolate, have also been observed[21, 30].
Based on these observations we hypothesized that women would have greater sensitivity to hunger and satiety responses to eating as compared to men, resulting in the ability to better maintain energy balance during an ad libitum diet setting. We also hypothesized that women would have a greater cognitive or frontal response to food-related visual stimuli than men which would be related to the behavioral measures. The present study was designed to examine these hypotheses.
Methods
Subjects
Healthy, right-handed men and women aged 25–45 were recruited and screened. The study was approved by the Colorado Multiple Institutional Review Board, and all subjects gave informed consent. Eligible subjects were free of metabolic and psychiatric disease and eating disorders and were not actively dieting (purposely restricting food intake for weight control) at the time studies were performed. Twenty-two women and 21 men were enrolled into the study (Table 1).
Table 1.
Subject characteristics (number or mean ± SD).
| Women | Men | |
|---|---|---|
| Number | 22 | 21 |
| Age (years) | 35.8 ± 5.4 | 34.2 ± 5.7 |
| BMI (kg/m2) | 24.3 ± 4.1 | 24.2 ± 3.0 |
| Body Fat (%) | 33.8 ± 4.6* | 18.5 ± 5.0 |
| Reported Intake: | ||
| Energy (kcal/day) | 1,714 ± 591 | 2,424 ± 1 419 |
| Protein (%) | 18.1 ± 4.3 | 18.2 ± 3.0 |
| Fat (%) | 33.8 ± 5.2 | 36.8 ± 9.7 |
| Carbohydrate (%) | 47.2 ± 5.0 | 43.6 ± 9.1 |
| Dietary Restraint | 8.0 ± 5.1# | 4.6 ± 3.4 |
| Dietary Disinhibition | 5.6 ± 3.8 | 5.0 ± 3.4 |
| Power of Food Scale | 46.4 ± 15.2 | 40.9 ± 17.5 |
p < 0.001 for women compared to men.
p < 0.05 for women compared to men.
Study Design and Measurements
Subjects first underwent baseline assessments, including a 3-day diet diary, completion of the Three Factor Eating Inventory[31], Power of Food scale[32], measurements of resting metabolic rate (RMR) by hood indirect calorimetry (2900 metabolic cart, Sensormedics, Yorba Linda, CA), and body composition measurement by dual-energy x-ray absorptiometry (DPX whole-body scanner, Lunar Radiation Corp., Madison, WI).
Subjects then started a 9-day study period which included a 6-day “controlled” eucaloric diet phase and a 3-day ad libitum diet phase. In women, the study period was performed in the follicular phase of their menstrual cycle. The first three days of controlled diet (study days 1–3) were planned as a run-in diet phase to allow the participants to habituate to the diet. The controlled eucaloric diet was designed to ensure energy and macronutrient balance. Estimates of daily energy needs were made using several factors: 1) usual intake via 3-day food diary, 2) the Harris-Benedict equation, 3) baseline RMR plus an activity factor, and 4) lean body mass. The macronutrient composition of the diet was 50% carbohydrate, 30% fat, and 20% protein. The saturated, mono-unsaturated, and poly-unsaturated fat ratio was set at 1:1:1. All food was prepared and provided by the University of Colorado Denver Clinical Translational Research Center (CTRC) kitchen. Subjects presented to the CTRC every morning. They were weighed, ate breakfast, and picked up the remainder of their daily meals in coolers. They were asked to return any uneaten food, which was then measured and incorporated into their next day of food.
Energy intake during the ad libitum diet phase (study days 7–9) was measured using weigh and measure methods to quantify unrestricted food and macronutrient intake. Specifically, a research dietitian worked with the subjects to provide a complete diet that replicated, to the extent practical/possible, the usual diet consumed by the subject. Subjects consumed breakfast on the CTRC offered in a `buffet' style with 15% more food than predicted requirements and the option to get more food as desired. The other meals and a snack were carried out for consumption off-site. Again, 15% more food than predicted was offered. Subjects were instructed to eat what they wanted, but subjects were required to return uneaten food and packages to be weighed. Subjects were told in advance that they could request more of any food. During the 9-day study period subjects were asked to maintain their usual pattern of physical activity and were regularly questioned regarding activity and compliance. Subjects were also specifically asked to not consume any alcoholic or extra calorie-containing beverages during the study period.
Behavioral Measurements
Measures of appetite were performed during days 4–6 of the controlled diet phase and during the three days of the ad libitum diet phase. Before and after each meal, subjects rated their hunger, fullness, and prospective consumption on visual analogue scales (VAS) as described by Rolls[33]. Hunger was rated on a 100-mm line preceded by the question, “How hungry do you feel right now?” and anchored on the left by “not at all hungry” and “extremely hungry” on the right. Fullness was rated by the question, “How full do you feel right now?” with the anchors “not at all” and “extremely.” Prospective food consumption was rated using the question, “How much food do you think you could eat right now?” anchored by “nothing at all” and “a large amount.”
Functional Magnetic Resonance Imaging (fMRI)
Subjects presented to the Brain Imaging Center at the University of Colorado Denver the morning of the sixth day of the controlled diet phase (study day 6) in the overnight fasted state. Imaging studies were performed using a GE 3.0 T MR scanner equipped with high speed gradients (300μs rise time and maximum gradient strength 24mT/m). Anatomical imaging was first performed. fMRI data were then acquired using EPI T2* BOLD (Blood Oxygen Level Dependent) contrast technique (TR=2000,TE=30, 642 matrix, 240 mm2 FOV, 28 axial slices angled parallel to the planum sphenoidale, 4mm thick, 0 mm gap). Functional imaging was performed while the subjects were presented visual stimuli using a projector and screen system. Visual stimuli consisted of three different categories: neutral nonfood-related objects, foods of high hedonic value, and foods of neutral hedonic value. Examples of nonfood objects included images of animals, trees, books, furniture, and buildings. Examples of hedonic foods included images of waffles with whipped cream and syrup, chocolate cake, cookies, plate of eggs and bacon, and pastries. Because previous studies have shown that comparisons involving neutral food objects to be qualitatively similar but less sensitive[18], the primary analysis examined differences between hedonic and nonfood objects. Two runs each lasting 8 minutes were performed with each run consisting of a pseudo randomized block design with 8 blocks of pictures of each category. Each block consisted of 10 stimuli shown for 2 seconds each for a total of 20 seconds per block or 240 scans per run. Subjects were asked lie quietly and to view the images.
Calculations and Statistical Analyses
Functional images were analyzed with SPM5 (Wellcome Dept. of Imaging Neuroscience, London.). After discarding the first four scans from each run for saturation effects, images were motion corrected, normalized to standard space, spatially smoothed with a 6mm FWHM kernel. After accounting for reslicing during preprocessing steps, the final smoothness of the data was approximately 3 times the acquisition voxel size. Data were then evaluated using the General Linear Model (GLM) in a random effects analysis. To generate the random effects model in SPM5, statistical parametric maps were first generated for each subject using the GLM to describe the variability of the data on a voxel by voxel basis. Hypotheses expressed in terms of model parameters were assessed at each voxel with univariate statistics, yielding an image whose voxel values comprise a statistical parametric map[34]. The model consisted of an HRF-convolved boxcar function. Additionally, a 128 s high pass filter was applied to remove low-frequency fluctuation in the BOLD signal. A second level analysis was performed to incorporate both within subject and between subject variance, allowing inference to the population. Accordingly, each individual subject's data for each condition of interest were summarized with one parametric map (SPM contrast image), and then assessed across subjects, thereby implementing a random effects model. Second level comparisons of interest (t-tests) were used to evaluate specific hypotheses, mainly the effect of sex on the neuronal response to hedonic food images. Group differences were considered significant at the whole-brain level if clusters exceeded an extent threshold of p < 0.05, corrected (130 voxels), with a height threshold of t = 2.4.
In addition to the whole-brain analysis, a supplementary region of interest (ROI) analysis was performed to examine possible sex-based differences in response of the temporal visual cortex. For the analysis, a small volume correction (SVC) was applied using 10 mm radius spheres centered on the right (x=29, y=−78, z=−13) and left (x=−33, y=−63, z= −18) fusiform gyri. These coordinates correspond to the location of sex-based differences in the response to visual food cues previously reported by Uher et al[21].
Non-imaging analyses were performed using SigmaStat software (Jandel Scientific, San Rafael, CA, USA). The effects of diet (controlled eucaloric and ad libitum) and sex were analyzed using two-way repeated measures analysis of variance (ANOVA). Significance tests were two-sided with significance set at level 0.05. Interactions were used to assess differences among the groups in the change from eucaloric to overfeeding. Finally, the Pearson Product Correlation between the fMRI (local maxima), behavioral, and intake measures was examined.
Results
Study Participants
Subject characteristics are summarized in Table 1. Women had greater percent body fat compared to men. Women also had greater dietary restraint scores on the Three-Factor Eating Questionnaire than men. Reported total energy intake tended to be greater in men (p=0.1), as would be expected, but macronutrient intake did not differ between sexes.
Appetite
Ratings of appetite as measured by visual analog scales were obtained before and after each meal during the controlled eucaloric and ad libitum diet phases. During the controlled eucaloric diet phase no sex-based difference was seen in ratings of pre-meal hunger or prospective food consumption (Table 2). Despite eating fewer calories with each meal, post-meal satiety ratings were significantly greater in women as compared to men (Women: 82.8 ± 3.0 mm, Men: 69.5 ± 3.9 mm; p = 0.01). Pre-meal hunger and prospective food consumption ratings were lower during the ad libitum diet phase as compared to ratings observed during the controlled eucaloric diet phase. Satiety ratings, however, were not significantly different during the ad libitum diet phase, and sex-based differences in appetite ratings were no longer apparent.
Table 2.
Appetite Ratings. Mean ± SEM for pre-meal hunger, pre-meal prospective food consumption (PFC), and post-meal satiety as measured by visual analog scales (mm).
| Total | Women | Men | ||||
|---|---|---|---|---|---|---|
| Eucaloric | Ad Libitum | Eucaloric | Ad Libitum | Eucaloric | Ad Libitum | |
| Hunger | 78.8 ± 2.1 | 71.3 ± 2.3* | 81.7 ± 2.8 | 73.9 ± 2.8 | 75.8 ± 3.1 | 68.8 ± 3.6 |
| PFC | 77.9 ± 2.1 | 73.7 ± 2.1* | 78.9 ± 2.9 | 73.4 ± 3.0 | 77.1 ± 3.1 | 73.9 ± 3.1 |
| Satiety | 75.8 ± 2.7 | 77.9 ± 2.5 | 82.8 ± 3.0# | 81.7 ± 3.5 | 69.5 ± 3.9 | 74.3 ± 3.6 |
p < 0.05 for eucaloric compared to ad libitum diet phase
p < 0.05 for women compared to men
Energy and Macronutrient Intake
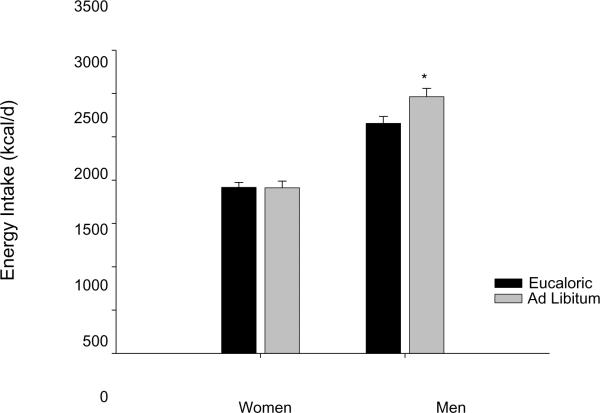
Ad libitum energy intake (EI) was measured over 3 days and compared to controlled eucaloric EI (Table 3). As would be expected, because of their larger mass, men had greater energy and macronutrient intake than women at all time points. Adjusting for lean body mass did not alter the interactions, thus for ease of interpretation results are expressed as total energy or macronutrient intake. Overall, mean EI was greater in the ad libitum phase (eucaloric: 2 278 ± 74, ad libitum: 2 426 ± 101, kcal/d, p = 0.01), and there was a significant interaction between sex and diet phase (p = 0.01). As shown in Figure 1, men had significantly greater EI in the ad libitum as compared to the controlled eucaloric diet phases (eucaloric: 2 656 ± 79 kcal/d, ad libitum: 2 964 ± 96 kcal/d) while women held their EI intake stable (eucaloric: 1 917 ± 57 kcal/d, ad libitum: 1 912 ± 77 kcal/d). In other words, the main effect of over consumption during the ad libitum diet phase was driven by the men. No differences in EI were seen across the 3 days of ad libitum diet. As seen in Table 3, protein and fat intake did not change during the ad libitum diet phase, but carbohydrate intake was significantly greater (266 ± 8 to 296 ± 12 g/d, p < 0.001) which again was driven by the men (p = 0.022).
Table 3.
Daily intake during eucaloric and ad libitum diet phases. Mean ± SEM.
| Total | Women | Men | ||||
|---|---|---|---|---|---|---|
| Eucaloric | Ad Libitum | Eucaloric | Ad Libitum | Eucaloric | Ad Libitum | |
| Energy (kcal) | 2,278 ± 74 | 2,426 ± 101* | 1,917 ± 57 | 1,912 ± 77 | 2,656 ± 79 | 2,964 ± 96*,# |
| Carbohydrate (g) | 266 ± 8 | 296 ± 12* | 224 ± 6 | 235 ± 10 | 309 ± 9 | 360 ± 121,2 |
| Fat (g) | 87 ± 3 | 89 ± 4 | 73 ± 2 | 69 ± 3 | 102 ± 3 | 110 ± 5 |
| Protein (g) | 115 ± 4 | 119 ± 5 | 97 ± 3 | 95 ± 4 | 135 ± 4 | 144 ± 5 |
p < 0.05 for eucaloric compared to ad libitum diet phase
p < 0.05 for women compared to men
Figure 1.
fMRI
The neuronal response to visual stimuli as measured by fMRI was examined in the fasted state on the third day of controlled eucaloric diet. Similar to a previous report which included a sub-group of the current subjects [18], responses to hedonic foods across all participants were observed in the inferior visual cortex (right: t=8.08, p < 0.001, left: t=9.00, p< 0.001), insula (right: t=6.39, p < 0.001, left: t=7.67, p< 0.001), parietal cortex (right: t=7.52, p< 0.001, left: t=5.74, p < 0.001), somatosensory cortex (right: t=4.05, p = 0.004, left: t=6.62, p < 0.001), lateral prefrontal cortex (left: t=4.65, p= 0.001, right: 3.62, p = 0.010), and posterior cingulate cortex (right: t=3.43, p< 0.014) at a whole-brain FDR corrected threshold of p<0.05.
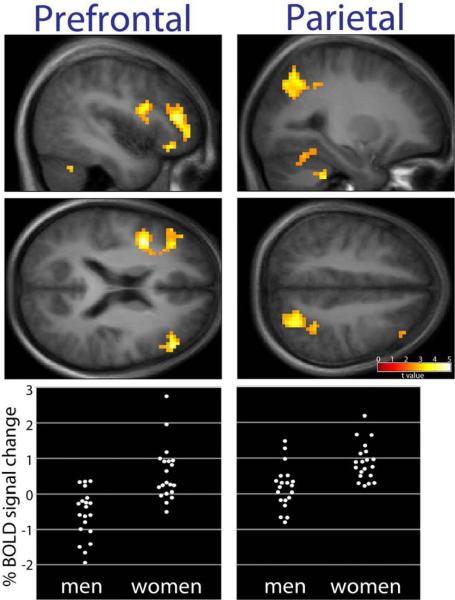
As seen in Figure 2 and Table 4, however, significantly greater activation of lateral and dorsolateral prefrontal cortex (DLPFC) and parietal cortex was observed in women as compared to men. Interestingly, no brain regions were more activated in men as compared to women. A supplementary region of interest analysis also revealed greater responses in the left fusiform gyrus in women, compared to men (t=3.80, p = 0.033).
Figure 2.
Table 4.
Regions of increased neuronal activation in women as compared to men in response to hedonic (H) compared to non-food (O) images, from whole-brain analyses.
| Local Maxima Coordinates* |
||||||
|---|---|---|---|---|---|---|
| x | y | z | t value# | cluster size | p value** | |
| Lateral Prefrontal (R) | 48 | 39 | 15 | 4.97 | 305 | 0.001 |
| 36 | 36 | 12 | 3.54 | |||
| 45 | 33 | −12 | 3.68 | |||
| Lateral Prefrontal (R) | 39 | 9 | 21 | 5.09 | 154 | 0.056 |
| 48 | 12 | 9 | 3.03 | |||
| 30 | 15 | 21 | 2.59 | |||
| Lateral Prefrontal (L) | −51 | 33 | 21 | 4.52 | 159 | 0.048 |
| −33 | 33 | 12 | 3.23 | |||
| −51 | 27 | 36 | 3.04 | |||
| Parietal (R) | 27 | −69 | 48 | 4.64 | 130 | 0.042 |
| 36 | −57 | 45 | 3.67 | |||
| Parietal (L) | −30 | −60 | 45 | 3.99 | 233 | 0.0006 |
| −33 | −45 | 42 | 3.53 | |||
| −24 | −63 | 36 | 2.98 | |||
Stereotactic Coordinates in MNI space
T values reported for local maxima within clusters
P values for significant clusters, corrected for multiple comparisons across the entire brain volume
To confirm that group differences were not caused by sex-related differences in evaluating food versus non-food objects, such as image complexity, item familiarity or typicality, a comparison also was made between foods of high hedonic value to foods of neutral hedonic value. This comparison revealed a group difference that was very similar to the hedonic food > non-food objects comparison, with women exhibiting greater response than men in the parietal cortex (right: t=5.63, p= 0.001, x= 27, y = −69, z = 51)) and lateral prefrontal cortex (right: t=4.44, p = 0.03, x = 39, y = 36, z = 15).
Correlations
Neither appetite ratings (hunger, prospective consumption, satiety) nor dietary behaviors (restraint, disinhibition) correlated with ad libitum energy intake. There was also no association between these behavioral measures and the neuronal response to food cues. Right DLPFC activation in response to hedonic food images, however, was negatively correlated with ad libitum EI (r=−0.53, p<0.001).
Discussion
The present study was performed to examine sex-based differences in appetitive behavior as well as in the neuronal response to food-related visual cues. The results of this study demonstrate that across sexes, food-related visual cues result in activation of brain regions known to be important in energy intake regulation. Women, however, have a more robust prefrontal and parietal response to food-related visual cues than men. In addition, women appear to be more sensitive to food intake as indicated by increased post-meal satiety ratings. Women are more likely to maintain isocaloric intake during ad libitum feeding, whereas men are more likely to overeat. Furthermore, although measures of feeding and dietary behaviors do not predict subsequent energy intake, dorsolateral prefrontal cortical (DLPFC) response to visual food cues correlates with subsequent energy intake.
As we have previously shown, the neuronal response to food-related visual cues is complex associated with the activation of a network of brain regions, including the insula, inferior temporal visual cortex, posterior parietal cortex, ventral striatum, posterior cingulate, hippocampus, sensory cortex, and lateral prefrontal cortex[18]. The activation of a number of these regions is consistent with increased attention to food cues and enhanced motivation to eat and implicates these regions as important in the regulation of food intake.
Similar to the findings of Uher et al[21] we found a pattern of increased neuronal response to visual food stimuli in women as compared to men, supporting the hypothesis that women are more sensitive or `reactive' to food-related cues than men. Our findings suggest that women have greater attention (parietal response) and cognitive processing (prefrontal response) related to food stimuli. The greater DLPFC activation in women may also suggest a greater inhibitory response to the food cues. A more sensitive region of interest analysis also revealed greater response in women in the fusiform gyrus at the location reported by Uher et al[21]. The present fusiform finding, along with Uher's finding of a fusiform difference during visual, but not taste food cues, is consistent with the notion that response differences in this region may be sensory modality- specific, relating to the visual processing of food cues[21]. Other differences between our study and the Uher et al study may be due to the greater salience of the food cues in our study.
Wang et al found that while food stimulation (sight, smell, taste) as measured by PET was associated with higher whole brain metabolism in both sexes, women were unable to suppress, using cognitive inhibition, brain regions important in emotional regulation, conditioning and motivation to the same degree as men[29]. Other neuroimaging studies have also found sex-based differences in the response to general cognitive and emotional tasks[35–37]. These sex-based differences in neuronal responses could be due to differences in brain organization and/or to the use of different cognitive or emotional strategies.
What mechanisms underlie these observed differences in responses to visual food stimuli in women as compared to men? Certainly differences in sex steroids could be an important mechanism. Animal studies have shown that sex steroids have direct effects on specific brain regions such as the prefrontal cortex[38, 39]. Cortical responses during cognitive tasks in humans appear to also be associated with estradiol levels[36, 40]. In addition, sex-based differences in leptin action could be important. Animal studies have shown that estradiol alters leptin and ghrelin sensitivity[8, 41] as well as altering the anorexic/orexigenic responses to other mediators such as NPY and melanin concentrating hormone[42, 43]. Although one might postulate that these central effects would primarily impact homeostatic-related brain regions, fMRI studies examining leptin deficiency and replacement have shown that it can alter higher brain responses to food stimuli[44, 45]. Certainly these sex-based differences deserve further investigation and emphasize the importance of potentially studying men and women as separate groups.
We also found sex-based differences in appetitive behavior. As has been previously reported[14], we found that women had greater dietary restraint than men. There was no sex-based difference in disinhibition, typically a better predictor of weight gain. We also found a sex-based difference in appetite ratings. When the diet was controlled, women reported greater post-meal satiety than men, suggesting greater sensitivity or reactivity to feeding. When “control” over the diet was removed this sex-based difference disappeared. Of greatest interest, though, was the sex-based difference in ad libitum intake. Women were able to self-select a diet to match their energy needs. Men, on the other hand, overate by over 300 kcal per day once study control was removed from diet. While these findings are consistent with the fact that women had greater dietary restraint we found no correlation between restraint scores and food intake. These findings could relate to women having higher levels of body weight and shape concerns as well as greater social and cultural influences[17, 46]. The subjects in this study were not dieting and were screened to be free of eating disorders.
Finally, we hypothesized that an individual's baseline feeding and dietary behavior might impact subsequent energy intake, and therefore examined the relationship between baseline measures of restraint, disinhibition, appetite and subsequent ad libitum energy intake. Interestingly, none of these measures correlated with subsequent intake, nor did any of these measures correlate with each other. In other words, these `qualitative' measures of food intake and behavior were poor predictors of energy intake. Some preload studies have shown that qualitative measures of appetite do not predict subsequent energy intake[47, 48]. Other longer-term diet studies have also shown a lack of correlation between appetite ratings and intake[18, 49, 50]. Additionally, it has been shown that dietary restraint is not a consistent predictor of energy intake and body weight[51–53]. In contrast, we found that neuronal activation in response to food cues, specifically in the right DLPFC was negatively correlated with subsequent energy intake. This suggests that food intake may be partially under inhibitory cognitive control and that a more objective measure such as neuronal response is a better predictor of energy intake. We are not aware of any other studies examining such a relationship.
In conclusion, the results of this study demonstrate that there are important sex-based differences in the appetitive responses to food. Women have a much more robust neuronal response to food-related visual cues in prefrontal and parietal cortex than men, suggesting greater cognitive processing related to executive function, such as planning, guidance or evaluation of behavior. Women have a heightened satiety response to meals as compared to men, and men are more likely to overeat during ad libitum feeding. Finally, increased DLPFC response to food cues, perhaps relating to inhibitory cognitive control may be a better predictor of food intake than more subjective behavioral measures. These findings emphasize the importance of considering sex when designing and interpreting feeding-related behavioral and neuroimaging studies and suggest that sex should be considered when behavioral interventions related to food intake are implemented.
Acknowledgements
We acknowledge and thank Debra Singel and Yiping Du for their assistance with the fMRI studies. We also thank the dietary services and metabolic kitchen of the University of Colorado Denver CTRC. This publication was supported by NIH/NCRR Colorado CTSI Grant Number UL1 RR025780, NIH/NIDDK Clinical Nutrition Research Unit Grant Number DK48520, and NIH/NCRR Grant Number RR016185. Its contents are the authors' sole responsibility and do not necessarily represent official NIH views.
This publication was supported by NIH/NCRR Colorado CTSI Grant Number UL1 RR025780, NIH/NIDDK Clinical Nutrition Research Unit Grant Number DK48520, and NIH/NCRR Grant Number RR016185. Its contents are the authors' sole responsibility and do not necessarily represent official NIH views.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Baskin DG, et al. Insulin and leptin: dual adiposity signals to the brain for the regulation of food intake and body weight. Brain Res. 1999;848(1–2):114–23. doi: 10.1016/s0006-8993(99)01974-5. [DOI] [PubMed] [Google Scholar]
- 2.Schwartz MW. Central nervous system regulation of food intake. Obesity (Silver Spring) 2006;14(Suppl 1):1S–8S. doi: 10.1038/oby.2006.275. [DOI] [PubMed] [Google Scholar]
- 3.Berthoud HR. Mind versus metabolism in the control of food intake and energy balance. Physiol Behav. 2004;81(5):781–93. doi: 10.1016/j.physbeh.2004.04.034. [DOI] [PubMed] [Google Scholar]
- 4.Watts AG. Understanding the neural control of ingestive behaviors: helping to separate cause from effect with dehydration-associated anorexia. Horm Behav. 2000;37(4):261–83. doi: 10.1006/hbeh.2000.1581. [DOI] [PubMed] [Google Scholar]
- 5.Zheng H, et al. Appetite control and energy balance regulation in the modern world: reward-driven brain overrides repletion signals. Int J Obes (Lond) 2009;33(Suppl 2):S8–13. doi: 10.1038/ijo.2009.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Woods SC, Gotoh K, Clegg DJ. Gender differences in the control of energy homeostasis. Exp Biol Med (Maywood) 2003;228(10):1175–80. doi: 10.1177/153537020322801012. [DOI] [PubMed] [Google Scholar]
- 7.Shi H, Clegg DJ. Sex differences in the regulation of body weight. Physiology & Behavior. 2009;97(2):199–204. doi: 10.1016/j.physbeh.2009.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clegg DJ, et al. Gonadal hormones determine sensitivity to central leptin and insulin. Diabetes. 2006;55(4):978–87. doi: 10.2337/diabetes.55.04.06.db05-1339. [DOI] [PubMed] [Google Scholar]
- 9.Edelsbrunner ME, Herzog H, Holzer P. Evidence from knockout mice that peptide YY and neuropeptide Y enforce murine locomotion, exploration and ingestive behaviour in a circadian cycle- and gender-dependent manner. Behav Brain Res. 2009;203(1):97–107. doi: 10.1016/j.bbr.2009.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim BJ, et al. Peptide YY is secreted after oral glucose administration in a gender-specific manner. J Clin Endocrinol Metab. 2005;90(12):6665–71. doi: 10.1210/jc.2005-0409. [DOI] [PubMed] [Google Scholar]
- 11.Beasley JM, et al. Characteristics associated with fasting appetite hormones (obestatin, ghrelin, and leptin) Obesity (Silver Spring) 2009;17(2):349–54. doi: 10.1038/oby.2008.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carroll JF, et al. Influence of BMI and gender on postprandial hormone responses. Obesity (Silver Spring) 2007;15(12):2974–83. doi: 10.1038/oby.2007.355. [DOI] [PubMed] [Google Scholar]
- 13.Rolls BJ, Fedoroff IC, Guthrie JF. Gender differences in eating behavior and body weight regulation. Health Psychol. 1991;10(2):133–42. doi: 10.1037//0278-6133.10.2.133. [DOI] [PubMed] [Google Scholar]
- 14.Klem ML, et al. A psychometric study of restraint: the impact of race, gender, weight and marital status. Addict Behav. 1990;15(2):147–52. doi: 10.1016/0306-4603(90)90018-s. [DOI] [PubMed] [Google Scholar]
- 15.Stoeckel LE, et al. Motivational state modulates the hedonic value of food images differently in men and women. Appetite. 2007;48(2):139–44. doi: 10.1016/j.appet.2006.07.079. [DOI] [PubMed] [Google Scholar]
- 16.Provencher V, et al. Eating Behaviors and Indexes of Body Composition in Men and Women from the Quebec Family Study. Obesity. 2003;11(6):783–792. doi: 10.1038/oby.2003.109. [DOI] [PubMed] [Google Scholar]
- 17.Paeratakul S, et al. Americans on diet: results from the 1994–1996 Continuing Survey of Food Intakes by Individuals. J Am Diet Assoc. 2002;102(9):1247–51. doi: 10.1016/s0002-8223(02)90276-2. [DOI] [PubMed] [Google Scholar]
- 18.Cornier MA, et al. Effects of short-term overfeeding on hunger, satiety, and energy intake in thin and reduced-obese individuals. Appetite. 2004;43(3):253–9. doi: 10.1016/j.appet.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 19.Porubska K, et al. Subjective feeling of appetite modulates brain activity: an fMRI study. NeuroImage. 2006;32(3):1273–80. doi: 10.1016/j.neuroimage.2006.04.216. [DOI] [PubMed] [Google Scholar]
- 20.Simmons WK, Martin A, Barsalou LW. Pictures of appetizing foods activate gustatory cortices for taste and reward. Cereb Cortex. 2005;15(10):1602–8. doi: 10.1093/cercor/bhi038. [DOI] [PubMed] [Google Scholar]
- 21.Uher R, et al. Cerebral processing of food-related stimuli: Effects of fasting and gender. Behavioural Brain Research. 2006;169(1):111–119. doi: 10.1016/j.bbr.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 22.Fuhrer D, Zysset S, Stumvoll M. Brain activity in hunger and satiety: an exploratory visually stimulated FMRI study. Obesity (Silver Spring) 2008;16(5):945–50. doi: 10.1038/oby.2008.33. [DOI] [PubMed] [Google Scholar]
- 23.LaBar KS, et al. Hunger selectively modulates corticolimbic activation to food stimuli in humans. Behav Neurosci. 2001;115(2):493–500. doi: 10.1037/0735-7044.115.2.493. [DOI] [PubMed] [Google Scholar]
- 24.Killgore WD, et al. Cortical and limbic activation during viewing of high- versus low-calorie foods. Neuroimage. 2003;19(4):1381–94. doi: 10.1016/s1053-8119(03)00191-5. [DOI] [PubMed] [Google Scholar]
- 25.St-Onge MP, et al. Human cortical specialization for food: a functional magnetic resonance imaging investigation. J Nutr. 2005;135(5):1014–8. doi: 10.1093/jn/135.5.1014. [DOI] [PubMed] [Google Scholar]
- 26.Beaver JD, et al. Individual differences in reward drive predict neural responses to images of food. J Neurosci. 2006;26(19):5160–6. doi: 10.1523/JNEUROSCI.0350-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schur EA, et al. Activation in brain energy regulation and reward centers by food cues varies with choice of visual stimulus. Int J Obes. 2009;33(6):653–661. doi: 10.1038/ijo.2009.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Del Parigi A, et al. Sex differences in the human brain's response to hunger and satiation. Am J Clin Nutr. 2002;75(6):1017–22. doi: 10.1093/ajcn/75.6.1017. [DOI] [PubMed] [Google Scholar]
- 29.Wang GJ, et al. Evidence of gender differences in the ability to inhibit brain activation elicited by food stimulation. Proc Natl Acad Sci U S A. 2009;106(4):1249–54. doi: 10.1073/pnas.0807423106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smeets PA, et al. Effect of satiety on brain activation during chocolate tasting in men and women. Am J Clin Nutr. 2006;83(6):1297–305. doi: 10.1093/ajcn/83.6.1297. [DOI] [PubMed] [Google Scholar]
- 31.Stunkard AJ, Messick S. The three-factor eating questionnaire to measure dietary restraint, disinhibition and hunger. J Psychosom Res. 1985;29(1):71–83. doi: 10.1016/0022-3999(85)90010-8. [DOI] [PubMed] [Google Scholar]
- 32.Lowe MR, et al. The Power of Food Scale. A new measure of the psychological influence of the food environment. Appetite. 2009;53(1):114–8. doi: 10.1016/j.appet.2009.05.016. [DOI] [PubMed] [Google Scholar]
- 33.Rolls BJ. Carbohydrates, fats, and satiety. Am J Clin Nutr. 1995;61(4 Suppl):960S–967S. doi: 10.1093/ajcn/61.4.960S. [DOI] [PubMed] [Google Scholar]
- 34.Friston KJ, et al. Analysis of fMRI time-series revisited. Neuroimage. 1995;2(1):45–53. doi: 10.1006/nimg.1995.1007. [DOI] [PubMed] [Google Scholar]
- 35.Klein S, et al. The influence of gender and emotional valence of visual cues on FMRI activation in humans. Pharmacopsychiatry. 2003;36(Suppl 3):S191–4. doi: 10.1055/s-2003-45129. [DOI] [PubMed] [Google Scholar]
- 36.Schöning S, et al. Functional anatomy of visuo-spatial working memory during mental rotation is influenced by sex, menstrual cycle, and sex steroid hormones. Neuropsychologia. 2007;45(14):3203–3214. doi: 10.1016/j.neuropsychologia.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 37.Bell EC, et al. Males and females differ in brain activation during cognitive tasks. NeuroImage. 2006;30(2):529–538. doi: 10.1016/j.neuroimage.2005.09.049. [DOI] [PubMed] [Google Scholar]
- 38.Handa RJ, Hejna GM, Lorens SA. Androgen inhibits neurotransmitter turnover in the medial prefrontal cortex of the rat following exposure to a novel environment. Brain Research. 1997;751(1):131–138. doi: 10.1016/s0006-8993(96)01394-7. [DOI] [PubMed] [Google Scholar]
- 39.Barker JM, Galea LAM. Sex and regional differences in estradiol content in the prefrontal cortex, amygdala and hippocampus of adult male and female rats. General and Comparative Endocrinology. 2009;164(1):77–84. doi: 10.1016/j.ygcen.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 40.Castellanos EH, et al. Obese adults have visual attention bias for food cue images: evidence for altered reward system function. Int J Obes. 2009;33(9):1063–1073. doi: 10.1038/ijo.2009.138. [DOI] [PubMed] [Google Scholar]
- 41.Clegg DJ, et al. Estradiol-Dependent Decrease in the Orexigenic Potency of Ghrelin in Female Rats. Diabetes. 2007;56(4):1051–1058. doi: 10.2337/db06-0015. [DOI] [PubMed] [Google Scholar]
- 42.Santollo J, Eckel LA. Estradiol decreases the orexigenic effect of neuropeptide Y, but not agouti-related protein, in ovariectomized rats. Behavioural Brain Research. 2008;191(2):173–177. doi: 10.1016/j.bbr.2008.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Messina MM, et al. Estradiol decreases the orexigenic effect of melanin-concentrating hormone in ovariectomized rats. Physiology & Behavior. 2006;88(4–5):523–528. doi: 10.1016/j.physbeh.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 44.Baicy K, et al. Leptin replacement alters brain response to food cues in genetically leptin-deficient adults. Proc Natl Acad Sci U S A. 2007;104(46):18276–9. doi: 10.1073/pnas.0706481104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rosenbaum M, et al. Leptin reverses weight loss-induced changes in regional neural activity responses to visual food stimuli. J Clin Invest. 2008;118(7):2583–91. doi: 10.1172/JCI35055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Field AE, et al. Peer, parent, and media influences on the development of weight concerns and frequent dieting among preadolescent and adolescent girls and boys. Pediatrics. 2001;107(1):54–60. doi: 10.1542/peds.107.1.54. [DOI] [PubMed] [Google Scholar]
- 47.Harper A, et al. Increased satiety after intake of a chocolate milk drink compared with a carbonated beverage, but no difference in subsequent ad libitum lunch intake. Br J Nutr. 2007;97(3):579–83. doi: 10.1017/S0007114507339846. [DOI] [PubMed] [Google Scholar]
- 48.Almiron-Roig E, Drewnowski A. Hunger, thirst, and energy intakes following consumption of caloric beverages. Physiology & Behavior. 2003;79(4–5):767–773. doi: 10.1016/s0031-9384(03)00212-9. [DOI] [PubMed] [Google Scholar]
- 49.Doucet E, et al. Relation between appetite ratings before and after a standard meal and estimates of daily energy intake in obese and reduced obese individuals. Appetite. 2003;40(2):137–143. doi: 10.1016/s0195-6663(02)00143-5. [DOI] [PubMed] [Google Scholar]
- 50.Levitsky DA. The non-regulation of food intake in humans: Hope for reversing the epidemic of obesity. Physiology & Behavior. 2005;86(5):623–632. doi: 10.1016/j.physbeh.2005.08.053. [DOI] [PubMed] [Google Scholar]
- 51.Bellisle F, et al. Influence of dietary restraint and environmental factors on meal size in normal-weight women. A laboratory study. Appetite. doi: 10.1016/j.appet.2009.07.006. In Press, Uncorrected Proof. [DOI] [PubMed] [Google Scholar]
- 52.Klesges RC, et al. A longitudinal evaluation of dietary restraint and its relationship to changes in body weight. Addict Behav. 1991;16(5):363–8. doi: 10.1016/0306-4603(91)90030-l. [DOI] [PubMed] [Google Scholar]
- 53.Smith CF, et al. Association of dietary restraint and disinhibition with eating behavior, body mass, and hunger. Eat Weight Disord. 1998;3(1):7–15. doi: 10.1007/BF03354907. [DOI] [PubMed] [Google Scholar]