Abstract
Background
The lack of a standardized laboratory animal model that mimics key aspects of human shigellosis remains major obstacle to addressing questions on pathogenesis, screening therapeutics and evaluating vaccines.
Methods
We characterize a piglet model for Shigella dysenteriae type 1.
Results
Piglets developed acute diarrhea, anorexia, dehydration and often fatal, with severity depending on age and dose. Bacteria were apparent in the lumen and on surface epithelium throughout the gut initially, but severe mucosal damage and bacterial cellular invasion were most profound in the colon. Detached necrotic colonocytes were present in the lumen, with inflammatory cells outpouring from damaged mucosa. High levels of IL-8 and IL-12, were followed by other proinflammatory cytokines. Elevated TNF-α, IL-1β, IL-6, and IL-10 were detected in feces and in gut segments of infected animals. Bacteria were present inside epithelial cells and within colonic lamina propria. In contrast, an isogenic strain lacking Shiga toxin, induced similar but milder symptoms, with moderate mucosal damage and lower cytokine levels.
Conclusion
We conclude that piglets are highly susceptible to shigellosis providing a useful tool to compare vaccine candidates for immunogenicity, reactogenicity and response to challenge, investigate the role of virulence factors and test the efficacy of microbial agents.
Introduction
Shigellosis, or bacillary dysentery, is caused by a group of Gram-negative, facultative anaerobic bacilli belonging to several species which includes Shigella dysenteriae (group A, with 15 serotypes) S. flexneri (group B, with 14), S. boydii (group C, with 20), and S. sonnei (group D, with only one)1. S. dysenteriae type1 (SD1) strains produce Shiga toxin (Stx), and cause severe epidemics in developing countries with higher case fatality rates than other serotypes,2-4.
Shigellosis is a disease limited to humans, and to non-human primates (NHP) to a lesser degree since the dose required to induce infection experimentally is considerably higher than that required for humans 5-7. There is a lack of laboratory animal models that mimic some of the essential gastrointestinal characteristics seen in human shigellosis. Consequently, studies of mechanisms of disease including identification and determination of the role of virulence attributes and the nature of the host response are based on observations made on primates1, 5-8. In addition, evaluation of the many vaccine candidates currently being developed, depend entirely on the use of NHP or human volunteers9-12. There are several serious limitations to the study of shigellosis under these circumstances. Not least is the considerable variation among individual animals including a likely earlier natural exposure, the limited number of animals available, the need for specialized accommodation and handling and the associated expense, and the serious ethical considerations involved in the use of NHP. This limits the ability to test large number of live attenuated bacterial strains to define their exact immunogenicity and reactogenicity, and to the oral challenge in a comparative fashion using larger number of animals in an optimized model and under controlled experimental conditions. Other animal models have been described including the guinea pig challenged through the rectal route13, and the xenographed mouse with human intestinal cells14.
In this communication we show that newborn gnotobiotic (GB) piglets orally challenged with SD1 develop many of the clinical symptoms and the gastrointestinal manifestations which mimic human shigellosis. This includes severe diarrhea, dehydration, anorexia which can often be fatal, with bacterial colonization, cellular invasion, mucosal inflammatory reaction and damage to the mucosa targeting specifically the large bowel.
Materials and Methods
Bacterial inoculum
Shigella dysenteriae type 1 strain SD1617 (kindly provided by S. Formal, Walter Reed Army Institute of Research), was originally isolated from an epidemic outbreak of shigellosis in Guatemala in 1968-1969. The isogenic strain SD1617-StxAB (lacking the stx and the fnr genes with additional uncharacterized chromosomal deletion) was also used in this study. Bacteria were grown overnight on tryptic soy agar plates from which a single colony was further grown in tryptic soy broth overnight. Bacteria in mid-exponential growth phase (OD600 = 0.8) were concentrated, resuspended in PBS to contain 5×109 CFU per inoculum, and confirmed by OD and by plating dilutions on TSA plates. OD = 1 corresponded to 109 CFU/ml with a SD of 2.5%.
Experiments in gnotobiotic (GB) piglets
Animals were derived via cesarean section and maintained in plastic sterile microbiological isolators, fed 3 times daily with a total of 500ml of human infant milk replacer. Thirty five piglets aged 1 to 3 days were each orally inoculated with 3ml suspension containing SD1617 5×109 CFU, and 25 animals were inoculated with the SD1617-Stx strain. Six piglets were kept as uninoculated control animals. Clinical manifestations, such as diarrhea, vomiting, depression, anorexia and dehydration, were monitored and recorded 3 times daily and diarrhea was determined based on fecal consistency: 0 - normal; 1 - pasty; 2 - semi-liquid; and 3 - liquid. Fecal scores greater than 2 were considered diarrheic. Fecal scores of individual piglets were recorded and rectal swabs were collected daily for bacterial counts and cytokine analysis.
Piglets were euthanized (n= 3-8/time point) 8 hr, and on days 1, 2, 3, 5, and 8 after oral challenge with SD1617, and 3 and 8 days for the control group. They were heavily sedated by intramuscular injection of Ketamine (100 mg/piglet)/Xylazine (5 mg/piglet) mixture, followed by intracardiac injection of Pentobarbital (120 mg/kg). These animal studies were carried out under a GB piglet protocol approved by the Institutional Animal Care and Use Committee (IACUC) at Tufts Cummings School of Veterinary Medicine (TCSVM). At euthanasia, gut tissues (stomach, jejunum, ileum, and colon) were collected for histology, immunohistochemistry (IHC), electron microscopy, bacterial counts and cytokine analysis, all of which are standard procedures performed in this laboratory.
Cytokine profiles in the feces and gut contents
Rectal swabs were diluted 10-fold, while gut contents were diluted 5-fold in PBS, and stored at −70°C. ELISA was performed to measure TNF-α, IL-1β, IL-6, IL-8 (pro-inflammatory cytokines); IL-12, or IFN-γ (Th1 cytokines); IL-10 (Th2 cytokine). Briefly, 96-well plates were coated with anti-porcine cytokine antibodies at a final concentrations of 1∼2 μg/ml. Rectal swabs and spun gut supernatants (60 μl) were loaded in duplicate wells filled with 40 μl of the PBS-BSA, along with 2-fold serial dilutions of standard recombinant proteins (0∼8000 pg/ml) of respective porcine cytokines loaded in each plate. After 2 hr incubation, plates were loaded with biotinylated detection antibodies for 2 hr at RT: IL-8 (0.04 μg/ml), IL-6 (0.15 μg/ml), IL-1β, IFN-γ and IL-10 (0.2 μg/ml), IL-12 (0.25 μg/ml), or TNF-α (0.3 μg/ml). Subsequently, plates were incubated for 30 min with horseradish peroxidase-conjugated streptavidin (Biosource; Camarillo, CA) at a concentration of 0.1 - 0.2 μg/ml. After washing 4 times with buffer, the plates were incubated with substrate solution (SureBlue Reserve; KPL, Gaithersburg, MD). Optical density was read in a microplate reader at 450 nm. Cytokine concentrations (pg/ml) in the rectal swabs and GI supernatants were calculated using standard curves (SOFTmax Pro; Molecular Devices Corp., Sunnyvale, CA, USA) of respective standard recombinant cytokines. The capture and detection antibodies and standard recombinant cytokines were purchased from BioSource.
Statistical analysis
Analyzed data are presented as means ± SE (Fig. 1-4). Significant differences in bacterial counts among the various gut segments and between the two bacterial strains were calculated using the student t test, and cytokine-ELISA data were compared among the tissues (stomach, jejunum, ileum, and colon) in relation to different time-points and between the 2 strains using the Kruskal-Wallis nonparametric test. Significant differences in fecal scores and logarithmic transformed bacterial counting data were identified among the different time-points with Student t test. Data were analyzed with SAS (SAS Institute, Cary, NC, USA), and a P-value of < 0.05 was considered significant.
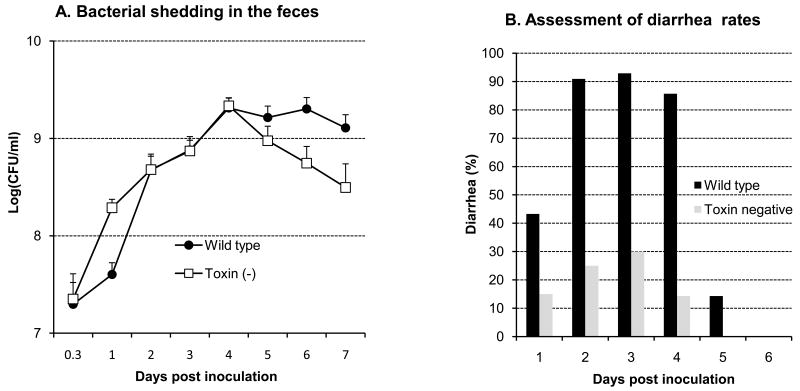
Fig. 1.
Bacterial excretion in feces (A) and diarrheal rates (B) over 7 days after an oral challenge with 5×109 CFU/animal of wild type SD1 strain 1617 (total 35 animals) and its isogenic Stx minus strain (total 25 animals). 1A shows that significant differences (p<0.05) in the rate of bacterial excretion occurred on days 5-7 after challenge. Each bar in 1B indicates the daily percentage of piglets with diarrhea with #representing significantly higher values between the 2 bacterial strains at each time points (p<0.05).
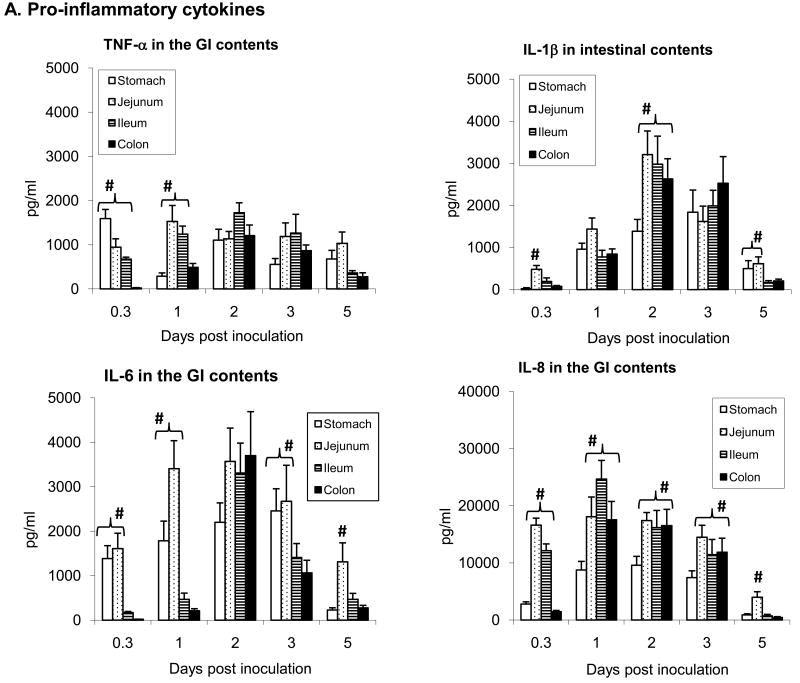
Fig. 4.
Cytokine responses measured by ELISA in the luminal contents of GI tissues (stomach, jejunum, ileum, and colon). The error bars represent standard errors of the means of data (n=3 ∼ 5) time-points. Cytokine concentrations (pg/ml) in the GI contents were calculated by regression based on the standard curves of respective standard recombinant cytokines. # represent statistically significantly higher values (p<0.05).
Results
Clinical symptoms
Cultured rectal swabs showed that the wild type and the Stx-minus strains of SD1 rapidly colonized the gut maintaining high levels for the duration of the experiment (Fig. 1A). In particular, wild type SD1 were excreted in higher numbers throughout compared to the Stx-minus strain in which shedding gradually declined from the peak at 4 days postinoculation (. Animals infected with wild type developedwith infected animals developing serious symptoms of watery diarrhea, anorexia, dehydration (85∼93%) within 24 hours over a three day period (Fig. 1B). Approximately 60% of the piglets had to be euthanized within 3 days after oral challenge due to the severity of their symptoms, characterized by marked dehydration, depression, anorexia and considerable loss of body weight. In contrast, piglets infected with the Stx-minus strain exhibited lower rates of diarrhea (14-30%) and moderate symptoms. Control animals remained healthy and continued to gain weight. Animals which either died or were euthanized with severe illness including respiratory distress were not included in any of the analyses.
Bacterial colonization in GI segments
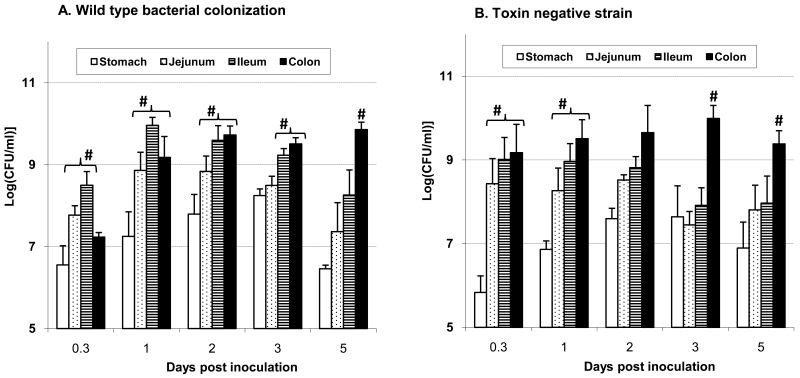
Gut contents cultured for bacteria showed presence of extensive colonization of both SD1 strains (Fig. 2). At 8hr after challenge bacteria were more abundant in the jejunum and ileum than in other gut sections. By day one bacteria in the jejunum, ileum and colon increased 10- 100 fold, gradually increasing in the colon over 5 days, and decreasing in the proximal segments at the same time (Fig. 2).
Fig. 2.
Bacterial colonization in the gastrointestinal (GI) tract. Luminal contents were collected from stomach, jejunum, ileum, and colon from piglets euthanized 8 hr and 1, 2, 3, and 5 days after challenge. The error bars represent standard errors of the means of data (n=5 ∼ 18) at each time-points. #, represents significantly higher values than other GI segments (p<0.05).
Cytokine response
Cytokine responses from rectal swabs show that IL-8 excretion in feces was the leading pro-inflammatory cytokine at an early phase of the infection (days 1 and 2), while IL-12 (Th1) response was seen later, from day 3 to 8 as illustrated in Fig. 3. Cytokine levels measured at different gut segments after necropsy are presented in Fig. 4. Cytokine response measured at 8 hr after challenge was clearly elevated in the stomach through to mid ileum but not in the colon as yet. Even at this early time-point however, a significant amount of IL-8 (>10000 pg/ml) was measured in the contents of jejunum and ileum, and substantial levels of TNF-α and IL-6 were detected in the stomach (Fig 4A). At day 1 and 3 the levels of pro-inflammatory cytokines undergone transition along the gut, with IL-8 as the dominant cytokine on day 1, and TNF-α, IL-1β, and IL-6 reached peaks on day 2 after challenge. On day 5 however, these cytokines had abruptly decreased in each gut segment except for TNF-α. Secretions of IFN-γ and IL-12, representing Th1 cytokine response, steadily increased from 8 hr to day 3, typically in the jejunum and ileum, with IL-12 as the primary Th1 cytokine, and were still high on day 5 (Fig 4B). A slight increase of IL-10, one of Th2 cytokines, was present from 8 hr to ∼ 3 days after challenge in gut contents (Fig 4C). In contrast, cytokines levels in rectal swabs and in gut contents of the control piglets were minimal (< 250 pg/ml).
Fig. 3.
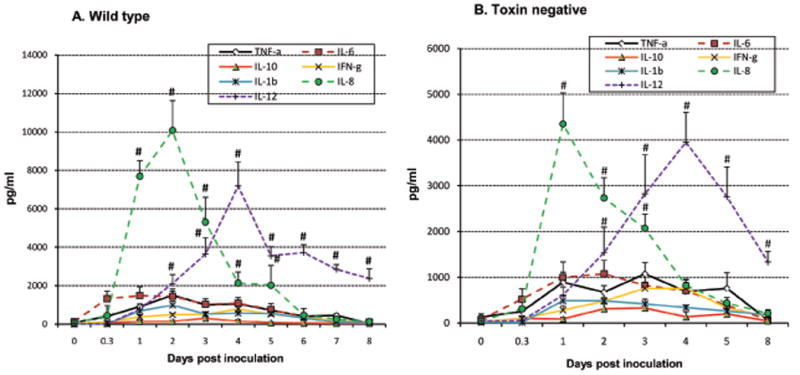
Cytokine excretion in the feces. Seven cytokine profiles were measured by ELISA over 8 days after challenge. The error bars represent standard errors of the means of data (n=5 ∼ 18) time-points. Cytokine concentrations (pg/ml) in rectal swabs were calculated by regression based on the standard curves of respective standard recombinant cytokines. # represents statistically significantly higher values (p<0.05).
Histopathology and immunohistochemistry (IHC)
The impact of strain SD1617 infection on mucosal integrity was evaluated in 1 (8 hr) to 3 animals per time point. Progressive tissue damage such as cell necrosis, erosion, hemorrhage, and inflammatory infiltration of cells were apparent in tissues collected at 8 hr and on days 1, 2, 3, and 5, with bacteria localized in gut sections as observed with IHC staining. At 8 hr mucosal lesions were minimal in the intestines with the exception of focal mucosal erosions in the stomach as shown in H&E stained sections. With IHC staining bacterial antigen was present within the epithelium in the stomach and throughout the small intestine with limited, mostly in the luminal contents, of the large bowel at this early time-point. In the jejunum, staining pattern was unique in that bacterial cells were visualized as ovoid to rod shapes localized mostly in cytoplasm of enterocytes. In the ileum however, most of the staining products were found on the villous surface rather than inside the enterocytes, and in gut contents.
On day 1 (Fig. 5), moderate tissue damage was observed throughout the GI tract, showing a multi-focal epithelial necrosis and erosion accompanied by frequent congestion and hemorrhage. The lamina propria was heavily infiltrated with inflammatory cells. In the colon epithelial detachment was observed mostly in the surface. The difference in IHC staining pattern showed antigen products frequently localized heavily in the lamina propria through a disrupted epithelial layer of stomach through to the ileum, in addition to within the epithelial cells lining the gut lumen. In contrast, milder and less frequent IHC staining was observed in the colon.
Fig. 5.
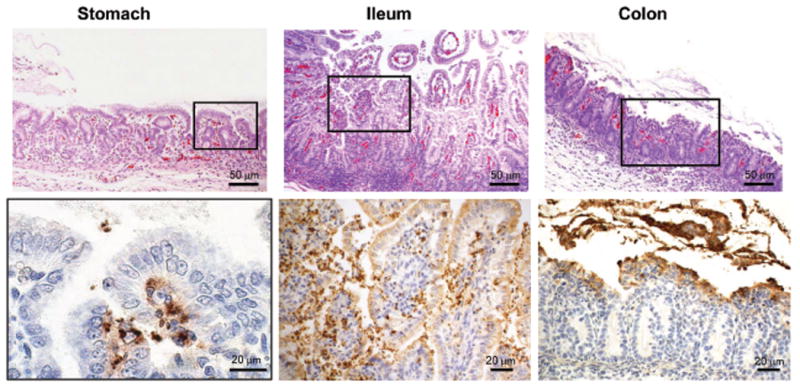
Micrographs of gut sections of a piglet euthanized one day after challenge: Upper panel shows H&E stained sections, while the lower panel shows bacterial antigen localization by IHC in the corresponding section. Areas marked by the rectangles in the upper panels are presented at higher magnification in the lower panels.
On day 2 and 3, tissue damage was more prominent in the colon than in the proximal gut, with numerous necrotic epithelial cells detached from the mucosa, with inflammatory cells outpouring into the lumen (Fig 6A). Bacterial invasion of colonocytes was evident throughout, with bacterial cells can be seen deep in the submucosa causing severe multi-focal necrosis (Fig 6B-D). On day 5 the tissue damage became substantially milder, compared to earlier time points, however with profound flattening of the villi, depletion of goblet cells, and numerous lymphocytes and other inflammatory cells accumulation in the submucosa, lamina propria and the gut lumen (data not shown). Unexpectedly, in some piglets which rapidly developed severe symptoms and needed to be euthanized within 2 days, lungs had evidence of suppurative bronchopneumonia with apparent bacterial antigen seen by IHC staining.
Fig. 6.
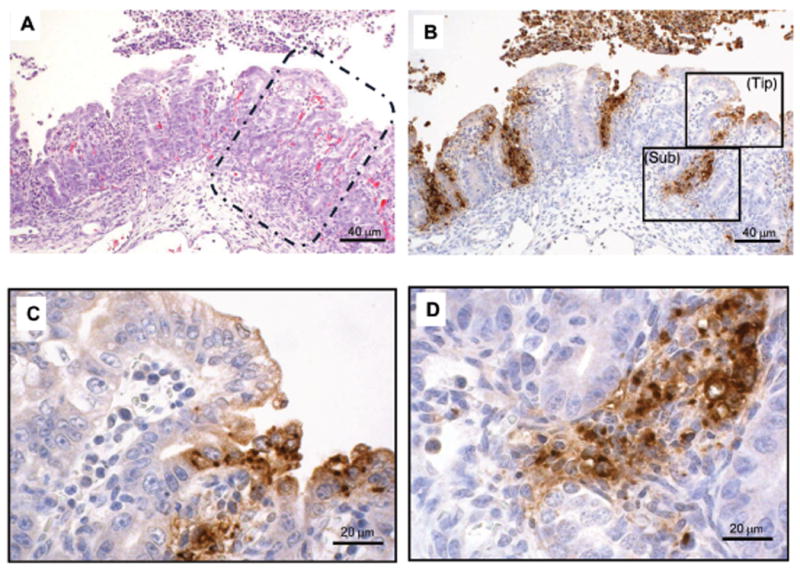
Micrographs of colon sections taken from a piglet euthanized on day 2 after bacterial challenge. Serial sections of colon were stained with H&E (A), and with IHC (B-D) to illustrate the location of bacterial antigen in the mucosa. The areas marked by the 2 rectangles in B are presented at higher magnification in C (luminal regions) and D (crypt-submucosal junction).
Electron microscopic findings
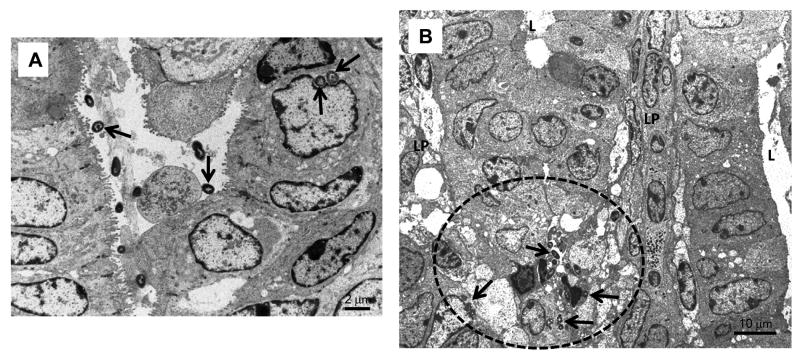
Colonic tissue of infected animals euthanized on day 1 and processed for electron microscopy, are presented in Fig. 7. Bacteria can be seen bound tightly to epithelial luminal surface as well as within cells (Fig. 7A), causing moderate to severe tissue damage, depending on the number of days after challenge. Typically, loss of microvilli, cell necrosis and detachment from the basement membrane can be seen. Presence of bacteria deep in the lamina propria surrounded by inflammatory cells was also observed (Fig. 7B).
Fig. 7.
Electron micrographs of sections taken from the colon one day after bacterial challenge; A showing organisms (arrowed) closely adherent to colonocyte surfaces or inside cells; and B showing deeply embedded in the lamina propria (B) surrounded by inflammatory cells (arrowed bacteria is enclosed by dotted line).
Discussion
In this communication we describe the response of GB piglets to infection with SD1 wild type and with its isogenic Stx-minus strain. Other animal models have been described previously which provide useful tools to investigate specific aspects of pathogenesis13 and the immune response14. We place the GB piglet model closely behind the NHP in its response to infection with shigellosis including SD1. We obtained similar responses from piglets infected with S. flexneri 2a and S. sonnei (data not shown). Evaluation of control measures and therapeutic agents against shigellosis will greatly benefit from the availability of an optimized and standardized animal model that mimic key characteristics of human shigellosis, namely diarrheal symptoms and nature and location of the mucosal damage, mediated by intense inflammatory reaction and bacterial invasion, as shown in the figures and charts included in this communication. After challenge with SD1 piglets developed watery diarrhea at first, progressing to intermittent and darkly colored feces. Infection was often fatal and depended largely on age at inoculation and on the bacterial dose (data not shown). Symptoms lasted 5-6 days, with SD1 initially colonizing the entire gastrointestinal tract, becoming localized subsequently in the distal ilium and large bowel where SD1 was able to induce most of the mucosal damage. With the availability of specific immune reagents including a comprehensive panel for a quantitative analysis of cytokines and other markers of inflammation, we believe this model will help close the knowledge gap which exists in relation to defining the roles of intestinal sIgA and serum IgG antibodies in mediating protection against shigella species – an unresolved issue1 - as well as the nature of the antigens involved such as surface antigens, virulence attributes, immunodominant molecules, and key metabolic enzymes.
Unexpectedly, we observed by IHC foci of deep bacterial invasion in the glandular portion of the stomach and surface colonization of the entire small intestine in the initial phase. Whether this is due to the absence of other competing microflora in the gut or proximal gut colonization does occur briefly in the early phase in humans too, is not clear. If it does, it may provide an initial bacterial amplification phase in the upper gut which explains the small infectious dose required for humans to eventually establish fulminating infection in the large bowel. It can also explain the early watery diarrhea phase observed in children and in piglets, which is attributed to a Shigella enterotoxin ShET215-17 whose activity most likely is exerted in the small intestine. Such speculations certainly can be investigated in this model.
Proinflammatory and Th1 cytokines were markedly elevated in infected piglets, with animals challenged with the Stx-minus strain having considerably lower levels. This clearly indicated that Stx-excreting strains of Shigella contribute to more intense inflammatory reaction by the mucosa than do species and serotypes lacking this toxin. These subtle differences between the SD1 wild type and the Stx-minus strain in symptomatic manifestation, cytokine profile, and degree of mucosal injury reflect the sensitivity of the piglet model. It should however be pointed out that this particular Stx-minus strain of SD1 also lacked an uncharacterized portion of the chromosome including fnr, the cytoplasmic ferritin gene (M. Venkatesan, Walter Reed Army Institute of Research). The impact of this deletion on the virulence of the toxin-minus SD1 is unclear, and may have contributed to a much reduced virulence.
In human shigellosis higher numbers of cytokine producing cells were detected in rectal biopsies including IL-1α, IL-1β, TNF-α, IL-6, IL-8, IL-4, IL-10, IFN-γ, TNF-β, and transforming growth factor β1-3, than in healthy individuals18. In piglets an onset of high level of IL-8 was measured in feces within 2 days after challenge. IL-8, considered a major mediator for recruiting polymorphonuclear leukocytes (PMNs), is largely produced by the epithelial cells themselves. Neutralization of IL-8 function was shown to decrease the amount of PMNs streaming through the lamina propria and the epithelium, thus significantly attenuating the severity of epithelial lesions in areas of bacterial invasion19, and therefore is thought to play an indirect role in the control of bacterial translocation. IL-12 excretion peaked on day 4 after oral challenge, and two days after IL-8. IL-12, an immunoregulatory cytokine that plays a central role in the control of Th1 immune response, selectively induces the differentiation of T cells into Th1 lymphocytes but suppresses Th2-dependent functions, and acts as a potent inducer of proinflammatory cytokines, such as IFNγ and TNFα20, indicating an ability to promote Th1 mediated immunity.
The absence of gut microflora in these piglets permitted a rapid colonization by Shigella, bacteria which are known to only readily colonize primates. Despite the ability to colonize the germfree piglet gut, high doses (>108) were required to induce symptoms of diarrhea, indicating that, apart from gut microflora, other factors including physiological and anatomical differences among the species, play a role in colonization by Shigella. The piglet model should help identify these unique requirements.
The model is ideally suited for evaluating the contribution of attributes involved in disease, and in immune responsiveness and protection. It is also a likely useful model to test drug efficacy and mechanisms of emergence of drug resistance. There are several vaccines currently being developed and tested by several groups1, and this model can provide a useful comparative screening tool to rank their relative efficacy in relation to their safety and immunogenicity, duration of protection, extent of side-effect in those that are attenuated, as well as resistance to challenge after immunization. Since piglets are derived by cesarian section, they are agammaglobulinemic, and are completely devoid of maternal interfering antibodies. However they can be fed immune colostrum containing specific antibodies after birth to evaluate specific passive immunity in infants. We have recently exploited the GB piglet model to identify key novel proteins which were upregulated in the gastrointestinal environment as compared with proteins derived from broth cultured SD1. Proteomic analysis of SD1 collected from the large bowel of infected piglets revealed that bacterial cells switched to an anaerobic energy metabolism in vivo. In addition, there was a marked increased expression of the outer membrane protein OmpA, the heat shock protein HtpG and OspC2 indicating that proteomic changes reflect an adaptive response of SD1 cells to host environment. This can help identify a new set of targets for subunit vaccines and drug development21.
Some piglets which rapidly developed severe illness and needed to be euthanized within 2 days, their lungs had evidence of suppurative bronchopneumonia with apparent bacterial antigen seen by IHC staining. Yet, bacteremia was not evident in orally challenged piglets. This could, but less likely, to have resulted from the oral inoculation resulted from the oral inoculation. It is not clear whether the pneumonia was the cause of the rapid deterioration, or severely ill piglets were more prone to bacterial invasion and localization in the lung tissue. Infant pneumonia directly linked to shigellosis was not convincingly confirmed through a literature search.
GB piglets are clinically susceptible to shigellosis and to other enteric bacteria such as Clostridium difficile and E. coli O157:H7 during a short window of 1-5 days22,23. Therefore a balance between dose and age must be maintained, as well as other factors such as the maturity status of the animals at birth, how quickly they begin to feed, and their overall size. We routinely infect piglets 24-48 hr after birth, when they appear healthy and consume all of their milk diet. A dose of ∼109 induces symptoms and characteristic gut lesions that mimic those seen in humans. Higher doses will often lead to rapid onset of symptoms and often death in some, while a lower dose will result in mild diarrhea and symptoms. While this is a serious limitation of the model, piglets challenged at >5 days or with a dose <107 remain asymptomatically infected for probably several weeks. This is probably due to the fact that digestion and absorption of the milk diet at the neonatal phase in piglets is completed in the small intestine and mild to moderate injury to the large bowl has little clinical impact. There are several other drawbacks to this model which include the need for specialized isolators, environmentally controlled accommodation, highly skilled animal handlers and the system is labor-intensive, all of which contribute to higher costs. However, a cost ratio of ∼ 20 GB piglets to one NHP makes this optimized and highly controlled animal model worth considering for some studies. This is more so as human volunteer studies are getting more difficult to perform and primate research has serious limitations which includes regulatory, ethical, limited availability, variability and cost. While it is not suggested that this model can replace entirely NHP or human studies, it can be used to generate preclinical information that should reduce the number of studies in NHP and in humans substantially.
Acknowledgments
The authors would like to thank Donald Girouard, Patty Boucher and Cathy Lane for the care of animals; Donald Girouard for performing the surgery; and Michelle Debatis for technical assistance.
Grant Support: This project has been funded in whole with Federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services, under Contract Number N01-AI-30050.
Footnotes
Disclosures: No conflicts of interest exist for each author of this manuscript.
Kwang-il Jeong and Quanshun Zhang were involved in this manuscript by performing the animal lab and technical work associated with this study. John Nunnari performed the electron microscopy and interpretation of micrographs. Saul Tzipori was involved with the study concept and design, drafting of the manuscript, obtaining funding and study supervision.
References
- 1.Levine MM, Kotloff KL, Barry EM, et al. Clinical trials of Shigella vaccines: two steps forward and one step back on a long, hard road. Nat Rev Microbiol. 2007;5:540–53. doi: 10.1038/nrmicro1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rahaman MM, Khan MM, Aziz KMS, et al. An outbreak of dysentery caused by Shigella dysenteriae type 1 on a Coral Island in the Bay of Bengal. Journal of Infectious Diseases. 1975;132:15–19. doi: 10.1093/infdis/132.1.15. [DOI] [PubMed] [Google Scholar]
- 3.Ebright JR, Moore EC, Sanborn WR, et al. Epidemic Shiga bacillus dysentery in Central Africa. The American Journal of Tropical Medicine and Hygiene. 1984;33:1192–7. doi: 10.4269/ajtmh.1984.33.1192. [DOI] [PubMed] [Google Scholar]
- 4.Aragon M, Barreto A, Chambule J, et al. Shigellosis in Mozambique: the 1993 outbreak rehabilitation - a follow-up study. Tropical Doctor. 1995;25:159–162. doi: 10.1177/004947559502500405. [DOI] [PubMed] [Google Scholar]
- 5.Formal SB, Kent TH, May HC, et al. Protection of monkeys against experimental shigellosis with a living attenuated oral polyvalent dysentery vaccine. Journal of Bacteriology. 1966;92:17–22. doi: 10.1128/jb.92.1.17-22.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Formal SB, Maenza RM, Austin S, et al. Proceedings of the Society for Experimental Biology and Medicine. Vol. 125. Society for Experimental Biology and Medicine; 1967. Failure of parenteral vaccines to protect monkeys against experimental shigellosis; pp. 347–349. [DOI] [PubMed] [Google Scholar]
- 7.Formal SB, Oaks EV, Olsen RE, et al. Effect of prior infection with virulent Shigella flexneri 2a on the resistance of monkeys to subsequent infection with Shigella sonnei. Journal of Infectious Diseases. 1991;164:533–537. doi: 10.1093/infdis/164.3.533. [DOI] [PubMed] [Google Scholar]
- 8.Birmingham ME, Lee LA, Ntakibirora M, et al. A household survey of dysentery in Burundi: implications for the current pandemic in sub-Saharan Africa. Bull World Health Organization. 1997;75:45–53. [PMC free article] [PubMed] [Google Scholar]
- 9.Coster TS, Hoge CW, VanDeVerg LL, et al. Vaccination against shigellosis with attenuated Shigella flexneri 2a strain SC602. Infection and Immunity. 1999;67:3437–3443. doi: 10.1128/iai.67.7.3437-3443.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cohen D, Ashkenazi S, Green MS, et al. Double-blind vaccine-controlled randomised efficacy trial of an investigational Shigella sonnei conjugate vaccine in young adults. Lancet. 1997;349:155–159. doi: 10.1016/S0140-6736(96)06255-1. [DOI] [PubMed] [Google Scholar]
- 11.Ashkenazi S, Passwell JH, Harlev E, et al. Safety and immunogenicity of Shigella sonnei and Shigella flexneri 2a O-specific polysaccharide conjugates in children. Journal of Infectious Diseases. 1999;179:1565–1568. doi: 10.1086/314759. [DOI] [PubMed] [Google Scholar]
- 12.Passwell JH, Ashkenazi S, Harlev E, et al. Safety and immunogenicity of Shigella sonnei-CRM9 and Shigella flexneri type 2arEPAsucc conjugate vaccines in one- to four-year-old children. The Pediatric Infectious Disease Journal. 2003;22:701–706. doi: 10.1097/01.inf.0000078156.03697.a5. [DOI] [PubMed] [Google Scholar]
- 13.Shim DH, Suzuki T, Chang SY, et al. New animal model of shigellosis in the Guinea pig: its usefulness for protective efficacy studies. Journal of Immunology. 2007;178:2476–82. doi: 10.4049/jimmunol.178.4.2476. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Z, Jin L, Champion G, et al. Shigella infection in a SCID mouse-human intestinal xenograft model: role for neutrophils in containing bacterial dissemination in human intestine. Infection and Immunity. 2001;69:3240–7. doi: 10.1128/IAI.69.5.3240-3247.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nataro JP, Seriwatana J, Fasano A, et al. Identification and cloning of a novel plasmid-encoded enterotoxin of enteroinvasive Escherichia coli and Shigella strains. Infection and Immunity. 1995;63:4721–4728. doi: 10.1128/iai.63.12.4721-4728.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yavzori M, Cohen D, Orr N. Prevalence of the genes for Shigella enterotoxins 1 and 2 among clinical isolates of Shigella in Israel. Epidemiology and Infection. 2002;128:533–535. doi: 10.1017/s0950268802006866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roy S, Thanasekaran K, Dutta Roy AR, et al. Distribution of Shigella enterotoxin genes and secreted autotransporter toxin gene among diverse species and serotypes of Shigella isolated from Andaman Islands, India. Tropical Medicine & International Health. 2006;11:1694–1698. doi: 10.1111/j.1365-3156.2006.01723.x. [DOI] [PubMed] [Google Scholar]
- 18.Raqib R, Lindberg AA, Wretlind B, et al. Persistence of local cytokine production in shigellosis in acute and convalescent stages. Infection and Immunity. 1995;63:289–96. doi: 10.1128/iai.63.1.289-296.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mukhopadhaya A, Mahalanabis D, Chakrabarti MK. Role of Shigella flexneri 2a 34 kDa outer membrane protein in induction of protective immune response. Vaccine. 2006;14:6028–36. doi: 10.1016/j.vaccine.2006.03.026. [DOI] [PubMed] [Google Scholar]
- 20.Sansonetti PJ, Arondel J, Huerre M, et al. Interleukin-8 controls bacterial transepithelial translocation at the cost of epithelial destruction in experimental shigellosis. Infection and Immunity. 1999;67:1471–80. doi: 10.1128/iai.67.3.1471-1480.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pieper R, Zhang Q, Parmar PP, et al. The Shigella dysenteriae serotype 1 proteome, profiled in the host intestinal environment, reveals major metabolic modifications and increased expression of invasive proteins. doi: 10.1002/pmic.200900196. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Steele J, Hanping Feng, Nicola Parry, Saul Tzipori. Piglet Models for Acute or Chronic Clostridium difficile Illness (CDI) Journa of Infectious Diseases. doi: 10.1086/649799. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tzipori S, Gunzer F, Donnenberg MS, de Montigny L, Kaper JB, Donohue-Rolfe A. The role of the eaeA gene in diarrhea and neurological complications in a gnotobiotic piglet model of enterohemorrhagic Escherichia coli infection. Infect Immun. 1995;63:3621–7. doi: 10.1128/iai.63.9.3621-3627.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]