Abstract
Although psychosocial stress has been linked to clinical asthma outcomes, controlled, laboratory paradigms that test associations between psychosocial stress and markers of airway inflammation in humans are lacking. There is also little known about how individual background characteristics may affect variability across individuals in asthma-relevant inflammatory and pulmonary responses to stress. The goals of this study were to investigate the effects of a laboratory stress paradigm on markers of airway inflammation and pulmonary function in children with asthma, and to determine why some children are more biologically responsive to stress. 38 children physician-diagnosed with asthma, and 23 healthy control children (M age = 15 years) engaged in a conflict discussion task with a parent. Pulmonary function (FEV1) was measured before and immediately after the task. Airway inflammation (indicated by exhaled nitric oxide, FeNO) was measured before and 45 minutes after the task (to minimize effects from spirometry). Parents were interviewed about family socioeconomic status (SES: income and occupation). In children with asthma only, there was an inverse association of SES with change in FeNO levels in response to the conflict task, meaning that as SES declined, greater increases in FeNO were observed No changes in FEV1 were found in response to the conflict task. This study suggests that lower SES children with asthma may be more vulnerable to heightened airway inflammation in response to stress.
Keywords: stress, exhaled nitric oxide, socioeconomic status, childhood asthma
INTRODUCTION
Stress has long been thought to contribute to a number of diseases, including asthma. Clinical evidence supports this hypothesis. For example, experiencing an acute negative life event (e.g., death of a close family member) in the context of chronic stress increased the risk of a subsequent asthma attack in children by nearly 3-fold (Sandberg et al., 2000). Parental stress also has been linked to more frequent symptoms, poorer daily functioning, and greater health care service utilization in children with asthma (Shalowitz et al., 2001; Weil et al., 1999)
Researchers have attempted to understand the biological mechanisms underlying these effects by studying real-world stress. For example, stressors such as school exams are associated with greater mobilization of eosinophils into sputum and blood following allergen challenge, greater in vitro production of the Th-2 cytokine IL-5 in patients with asthma, and a decreased Th-1/Th-2 ratio of cytokine production in atopic individuals (Hoglund et al., 2006; Kang et al., 1997; Liu et al., 2002). Children whose caregivers report high levels of stress have greater stimulated production of the pro-inflammatory cytokine TNF-a, as well as reduced production of the Th-1 cytokine IFN-γ (Wright et al., 2004). In addition, stressful experiences such as exposure to violence and parental conflict have been associated with decreased pulmonary function (Suglia et al., 2008). Furthermore, the relationship between daily stress and pulmonary function has been found to be mediated by airway inflammation (Kullowatz et al., 2008).
While these studies are clearly informative, there remain limitations to the conclusions that can be drawn. First, the naturalistic setting, while closer to the real world, can make alternative confounding explanations difficult to rule out. Second, variability in stress responsiveness across individuals with asthma remains less well-understood. For example, we know that social group characteristics, such as low socioeconomic status (SES), are associated with increased inflammatory signaling, as marked by higher levels of C reactive protein and IL-6, and greater stimulated production of Th-2 cytokines (Chen et al., 2003; Chen et al., 2006; Hemingway et al., 2003; Panagiotakos et al., 2005). SES refers to an individual's position within a social hierarchy, and can be defined both in terms of the social prestige ascribed to an individual, as well as in terms of the material resources an individual possesses, with both having been associated with health outcomes (Adler et al., 1993; Krieger et al., 1997). Hence social group characteristics such as SES may also determine variability in inflammatory responses in the context of asthma.
In asthma, the fractional concentration of exhaled nitric oxide (FeNO) is a non-invasive marker related to airway inflammation. FeNO is elevated in children with asthma (Kovesi and Dales, 2008; Pijnenburg and de, 2008; Strunk et al., 2003). FeNO levels are related to asthmatic airway inflammation indicated by bronchial wall inflammation (Payne et al., 2001), sputum eosinophila (Jatakanon et al., 1998), and airway hyper-responsiveness (Strunk et al., 2003). FeNO levels increase as asthma control deteriorates (Jones et al., 2001), and decrease when treatment reduces airway inflammation (Beck-Ripp et al., 2002; Bratton et al., 1999; Kharitonov et al., 2002; Montuschi et al., 2007; Sorkness et al., 2007; Straub et al., 2005a; Straub et al., 2005b).
The present study had two goals: 1) to document the effects of a standardized stressor on markers of airway inflammation (as reflected by FeNO) and pulmonary function in children with asthma vs. healthy children; and 2) to understand which background characteristics may account for variability in FeNO or pulmonary function changes in response to stress. Based on previous research reviewed above, we hypothesized that SES would be associated with changes in pulmonary function and FeNO following an acute laboratory stressor, such that as SES declined, pulmonary function would be reduced and FeNO would increase in children with asthma.
Method
Participants
38 children physician-diagnosed with asthma and 23 healthy control children were recruited from Vancouver, B.C. Children ranged in age from 10-20 years, were fluent in English, free of acute respiratory illness, had not received prednisone for at least 2 weeks, and had no other chronic illnesses (other than asthma). Asthma severity was determined from the NAEPP/EPR2 Guidelines based on symptoms and medication use, paralleling the approach of previous researchers (Bacharier et al., 2004). Atopic status was determined by the presence of both positive parental report of child allergic status and positive screening of serum IgE antibodies to common allergens (ImmunoCAP Phadiatop, Uppsala, Sweden). Details regarding sample characteristics are presented in Table 1. The protocol was approved by the UBC Research Ethics Board. Written consent was obtained from parents, and assent from children.
Table 1.
Descriptive information about sample
| Asthma | Healthy | |||||
|---|---|---|---|---|---|---|
| % | M | SD | % | M | SD | |
| Age (range 10-20 yearsa) | 15.0 | 2.8 | 15.9 | 2.0 | ||
| Gender (% male) | 68 | 57 | ||||
| Ethnicity | ||||||
| Caucasian | 58 | |||||
| Asian | 26 | 17 | ||||
| Other | 16 | 9 | ||||
| Severity | ||||||
| Mild intermittent | 13 | |||||
| Mild persistent | 45 | |||||
| Moderate persistent | 29 | |||||
| Severe | 13 | |||||
| Atopic | 55 | 13 | ||||
| FEV1/FVC | .76 | .12 | .84 | .08 | ||
| ICSa | 6.0 | 6.3 | 0 | 0 | ||
| ICS dose | ||||||
| Low | 73 | |||||
| Medium | 27 | |||||
| High | 0 | |||||
| Beta agonistsb | 5.1 | 6.2 | 0 | 0 | ||
| Baseline FEV1% | 90.6 | 16.6 | 99.0 | 8.4 | ||
| Post-task FEV1% | 90.0 | 14.1 | 97.0 | 9.1 | ||
| Baseline FeNO (ppb) | 58.5 | 57.5 | 22.8 | 24.4 | ||
| Post-task FeNO (ppb) | 53.5 | 51.5 | 21.4 | 23.5 | ||
ICS=inhaled corticosteroids. FEV1%= forced expiratory volume in 1 second percent predicted. FeNO= fractional concentration of exhaled nitric oxide in parts per billion. FEV1/FVC=ratio of FEV1 to forced vital capacity.
age range for asthma group: 10-20 years; age range for healthy group: 11-18 years.
=number of days taken in previous 2 weeks, range for both medications in the asthma group was from 0 to 14 days.
Procedures
All visits occurred in the afternoon. Baseline FeNO and then spirometry measures were first taken on children. Children and parents were then brought together and participated in a family conflict task. Immediately after the task, spirometry was re-assessed in children. 45 minutes later, post-task exhaled nitric oxide was assessed in children. This timing of post-task measures was meant to capture changes in spirometry, which occur rapidly following an acute stimulus, while at the same time minimizing the ability of spirometry to reduce FeNO readings (Kissoon et al., 2002; Silkoff et al., 1999; Terada et al., 2001). To achieve both of these goals, we opted to assess spirometry immediately after the stressor task, and then collect FeNO measures 45 min later. The FeNO protocol is also consistent with the time frame of other studies in healthy populations of acute stressors and inflammation (Miller et al., 1999; Schedlowski et al., 1993).
Measures and Task
Acute stressor task
Families participated in a standardized laboratory family conflict task. First, parents and children independently completed the Potential Parent Child Conflict scale (Donenberg and Weisz, 1997), a questionnaire that asks respondents to rate the amount of disagreement they have with their parent/child in a number of domains (e.g., household chores, friendships, etc.). A research assistant selected the topic that on average, parents and children disagreed the most about. Families were then asked to spend 8 minutes discussing their disagreements related to this topic and trying to reach a resolution. Conflict discussions between parents and children have been shown to be an ecologically valid yet controlled stressor that alters physiological parameters (Granger et al., 1994; Granger et al., 1996; Klimes-Dougan et al., 2001). To test effects on physiology in the present study paradigm, we assessed blood pressure (BPM-100, VSM MedTech, Coquitlam, BC) in children during a 10 minute rest (3 readings spaced 1 minute at the end of rest) and as well during the conflict discussion (5 readings spaced 1 minute apart at the beginning of conflict). Blood pressure increases in response to conflict discussions between family members have been established in previous research (Ewart et al., 1991).
Pulmonary function
Pulmonary function was assessed via spirometry (Vmax/Spectra, SensorMedics, Yorba Linda, California), according to American Thoracic Society guidelines (American Thoracic Society, 1995). Measures were taken at least 4 hours after the last use of a short-acting beta agonist and at least 24 hours after the last use of a long-acting beta agonist. Measurement included forced expiratory volume in the first second (FEV1) percent predicted.
Airway inflammation
As a marker of airway inflammation, exhaled nitric oxide was measured via a chemiluminescence analyzer (Aerocrine AB, Stockholm, Sweden), according to American Thoracic Society guidelines (American Thoracic Society/European Respiratory Society, 2005). The NIOX system was used (Aerocrine AB, Stockhold, Sweden), which uses a resistive device that provides a constant low expiratory flow rate and vellum closure. Participants were seated, exhaled to residual volume, inserted a mouthpiece, inhaled to total lung capacity, and then exhaled for 10 seconds at a constant flow rate of 0.05L/s ±10%. Visual cues on the computer provided guidance about maintaining constant flow rate. The measurement ended when a plateau of 4 seconds was observed (variance criterion of 10% or 5ppb). Repeated exhalations were conducted until 3 values were obtained that varied by less than 10%. No caffeine, food, or exercise was allowed for one hour prior to the start of testing. The measure reported is the average of three acceptable readings, expressed as fraction of exhaled nitric oxide (FeNO).
Family socioeconomic status (SES)
SES has been conceptualized both in terms of the material resources an individual possesses, as well as the social prestige that an individual has within a particular social context (Krieger et al., 1997). To capture each of these aspects currently, two measures of SES were used: annual family income, and parent occupational status. As an indicator of material resources, parents reported on the family's total gross income for the past 12 months before taxes. As an indicator of social prestige, parent report of occupations were coded according to the Socioeconomic Index for US Census occupations, a validated index that assigns scores to each Census occupation, with a range from 7 to 80 (Hauser and Warren, 1997). In two-parent families, the higher occupational status of the two parents was used. These types of measures are recommended by the MacArthur Foundation Research Network on Socioeconomic Status and Health (www.macses.ucsf.edu).
Covariates
Covariates included demographic characteristics (child age, gender, ethnicity), asthma severity, atopic status, the number of days the child used inhaled corticosteroids in the past 14 days, and the number of days the child used a beta agonist over the past 14 days.
Data analyses
Multiple linear regression analyses using SPSS 15.0 were conducted to test study hypotheses. Change scores for FEV1 and FeNO (post minus pre-stressor task) were calculated as dependent variables. Our primary hypothesis was that change in FEV1 and FeNO would vary by SES in children with asthma. Because change scores may vary as a function of baseline levels, we first regressed each dependent variable change score onto the baseline levels of FEV1 or FeNO. Next each dependent variable was regressed onto 1) group status (asthma vs. healthy); 2) SES (either family income or parent occupation); and 3) the interaction between group and SES. This allowed us to test the hypothesis that SES would be associated with change in FEV1/FeNO among children with asthma, but not healthy children. Significant interactions were plotted and interpreted following the recommendations of Aiken and West (Aiken and West, 1991).
In subsequent analyses, we addressed the possibility that any relationships of group status or SES with FEV1/FeNO were due to other confounding variables. Demographic (age, gender, ethnicity) and biomedical variables (atopic status, asthma severity, days of inhaled corticosteroid use, and days of beta agonist use) were thus entered alongside SES in the above regression analyses. This allowed us to test whether effects of group status, SES, or their interaction on FEV1/FeNO change remained significant above and beyond the influence of these factors.
Results
Participant characteristics
Children with asthma and healthy children did not differ in terms of age, sex, or ethnicity (all p's>.15). Children with asthma had a range of severity (13% mild intermittent, 45% mild persistent, 29% moderate, 13% severe), and 71% were currently being prescribed inhaled corticosteroid medication. Further details on the characteristics of this sample can be found in Table 1.
There was a main effect of group on FEV1%, such that children with asthma had lower FEV1% predicted both at baseline and post-task compared to healthy children (t=2.26,p<.05, and t=2.11,p<.05, respectively). There was also a main effect of group on FeNO. As expected, children with asthma had higher FeNO levels, both at baseline and post-task compared to healthy children (t=4.02,p<.001, and t=4.12,p<.001, respectively). There was no main effect of time, meaning that on average across the sample, there was no significant change in FEV1 or FeNO from pre-task to post-task (p's>.25). See Table 2.
Table 2.
Exhaled nitric oxide values (ppb) in relation to the acute stressor
| Asthma | Healthy | |
|---|---|---|
| Baseline | ||
| Mean | 58.48 | 22.78 |
| SD | 57.51 | 24.45 |
| Median | 34.80 | 14.45 |
| 25%ile | 23.50 | 10.20 |
| 75%ile | 74.85 | 24.68 |
| Post-task | ||
| Mean | 53.51 | 21.35 |
| SD | 51.54 | 23.51 |
| Median | 34.15 | 13.30 |
| 25%ile | 20.68 | 8.70 |
| 75%ile | 71.75 | 19.30 |
Family conflict as a stressor
To test whether the family conflict task was experienced as stressful, we examined differences in blood pressure and heart rate from baseline to task. Across all children, all physiological measures showed significant increases from baseline to task (SBP: t=2.09,p<.05, DBP: t=3.78,p<.001, HR: t=3.41,p<.01), indicating that the task was stressful for children.
Effect of psychosocial stress on exhaled nitric oxide
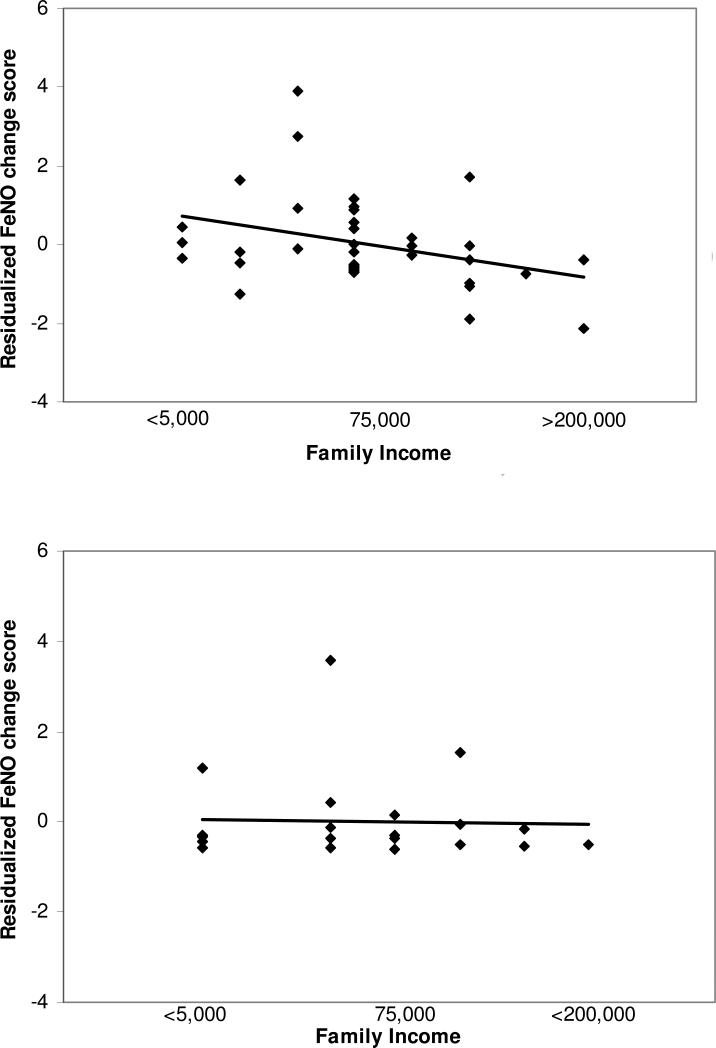
There were significant interactions of family SES with group status in predicting FeNO responses to the psychosocial stressor, both for family income (b=−2.74, SE=1.36, p=.05) and parent occupation (b=−3.93, SE=1.46, p=.01). Figure 1 presents a graphical illustration of these interaction effects, in which the linear association between SES and FeNO change is graphed separately for children with asthma and healthy children. Among children with asthma, there was an inverse association such that the lower the family SES, the greater children's increase in FeNO from pre-stressor to post-stressor, or conversely, the higher the family SES, the greater the decrease in FeNO from pre- to post-stressor (b=−2.07, SE=1.01, p=.05). Among healthy children, there was no significant relationship between SES and FeNO change (See Figure 1).
Figure 1.
Relationship between family income and change in exhaled nitric oxide (FeNO) in response to the acute psychosocial stressor. Top panel depicts data for children with asthma. Bottom panel depicts data for healthy children. Because measurements of change in response to a stressor need to take into account baseline values, change scores were regressed onto baseline values. The residualized score represents the variance in pre- to post-FeNO scores that is left over once the variability due to baseline FeNO levels has been removed. These residuals are scaled such that the mean for the sample equals 0 and the standard deviation of the residuals equals 1. Hence Figure 1 depicts residualized FeNO values on the y-axis, and the graph indicates the relationship between family income and the change in FeNO not due to baseline FeNO levels. Associations between FeNO and family income are significant in children with asthma (b=−2.97, SE=1.07, p=.01), but not among healthy children (b=.51, SE=.61, p=.42).
We next tested whether demographic or biomedical confounding variables might explain these associations of SES with FeNO change in children with asthma. After controlling for baseline FeNO values, atopic status, asthma severity, inhaled corticosteroid use, beta agonist use, age, sex, and ethnicity, the interaction between group status and SES predicting FeNO change in response to the stressor remained significant (see Table 3), suggesting that it could not be attributed to the demographic or biomedical confounders in the model. As with the analyses described above, among children with asthma only, there was an inverse association such that the lower the family SES, the greater children's increase in FeNO from pre-stressor to post-stressor, or conversely, the higher the family SES, the greater the decrease in FeNO from pre- to post-stressor (b=−2.97, SE=1.07, p=.05, and b=−2.50, SE=1.04, p=.02 for income and occupation respectively).
Table 3.
Regression analyses of effects of group status, family SES, and their interaction on exhaled nitric oxide (FeNO) responses to an acute psychosocial stressor
| Variable | b | SE | t | p |
|---|---|---|---|---|
| Baseline FeNO | −0.08 | 0.02 | 5.49 | <.001 |
| Atopy | 4.90 | 1.40 | 3.51 | .001 |
| Severity | −2.82 | 1.34 | 2.10 | .04 |
| ICS use | 0.17 | 0.19 | 0.87 | .39 |
| Beta agonist use | −0.04 | 0.19 | 0.21 | .84 |
| Age | −0.04 | 0.26 | 0.17 | .86 |
| Sex | 0.12 | 1.28 | 0.09 | .93 |
| Ethnicity | −0.51 | 0.39 | 1.30 | .20 |
| Family income | 0.04 | 1.00 | 0.04 | .96 |
| Group (asthma vs. healthy) | 2.55 | 2.50 | 1.02 | .31 |
| Income × Group |
−3.06 |
1.26 |
2.44 |
.02 |
| Baseline FeNO | −0.09 | 0.01 | 6.44 | <.001 |
| Atopy | 5.54 | 1.40 | 3.96 | <.001 |
| Severity | −1.56 | 1.51 | 1.03 | .31 |
| ICS use | 0.18 | 0.20 | 0.87 | .39 |
| Beta agonist use | −0.14 | 0.18 | 0.82 | .42 |
| Age | 0.01 | 0.27 | 0.05 | .96 |
| Sex | 0.43 | 1.26 | 0.34 | .74 |
| Ethnicity | 0.94 | 0.56 | 1.70 | .10 |
| Parent occupation | 2.07 | 1.11 | 1.87 | .07 |
| Group (asthma vs. healthy) | −0.13 | 2.75 | 0.05 | .96 |
| Occupation × Group | −4.46 | 1.38 | 3.24 | .002 |
Note. Regression coefficients are presented first for the block of covariates controlled in all analyses (baseline FeNO, atopic status, asthma severity, ICS use, beta agonist use, age, sex ethnicity), second for the main effects of family SES and group status, and third for the interaction between family SES and group. The top half of the table reports coefficients when family income was included as the SES variable. The bottom half of the table reports coefficients when parent occupation was used as the SES variable. The dependent variable was change in FeNO from pre-stressor to post-stressor.
To give an indication of the magnitude of this association, the percent of variance accounted for by SES, as well as an r statistic, was calculated for the effect in children with asthma. For r, values of .1 indicate small effects, values of .3 indicate medium effects, and values of .5 indicate large effects (Cohen, 1988). Net of baseline FeNO values and other covariates (which together explained 60% of the variance), family income accounted for an additional 8.8% of the variance in FeNO change scores in children with asthma, with r=−.30, indicating a medium effect size. Similarly, parent occupation accounted for an additional 6.6% of the variance in FeNO change scores, with an r=−.26, above and beyond the effects of baseline FeNO values and other covariates (which themselves explained 65% of the variance).
Effect of psychosocial stress on pulmonary function
There was no interaction of either family income or parent occupation with group status in predicting change in FEV1% after the psychosocial stressor (b=.46, SE=1.44, p=.75, and b=−1.41, SE=1.53, p=.36, respectively).
Discussion
This is the first study that we are aware of to use a laboratory stress paradigm to investigate changes in a marker of airway inflammation (FeNO) in children with asthma. It is also the first to document inverse associations of SES with FeNO change in children with asthma, such that lower SES children showed greater increases in FeNO, whereas higher SES children showed greater decreases in FeNO, after a conflict task with their parents. In contrast, healthy children did not show these patterns. The fact that these associations persisted in children with asthma after controlling for atopic status, asthma severity, medication-taking behaviors, and demographic factors such as age, suggests that the FeNO effects cannot be attributed to these alternative explanations. Moreover, SES accounted for 6.6-8.8% of the variance in FeNO change, and represented a medium-size effect, according to effect size conventions (Cohen, 1988).
Previous research has documented that naturalistically occurring stressors (such as exam stress) are associated with greater airway inflammation in induced sputum samples, as well as greater production of Th-2 cytokines and a decreased Th-1/Th-2 ratio (Hoglund et al., 2006; Kang et al., 1997; Kullowatz et al., 2008; Liu et al., 2002). However, because of the naturalistic setting, alternative confounding explanations can be difficult to rule out. Hence the present study took a different approach of utilizing a standardized laboratory stressor to assess changes in a marker airway inflammation. Furthermore, in order to create a laboratory paradigm that was more representative of the real world, we had parents and children engage in a conflict discussion. Thus the present study was able to show that the experience of conflict with a parent acutely alters FeNO levels in children with asthma, with effects varying depending on SES background.
There are no well-established guidelines for judging the clinical significance of a change in FeNO levels. However, the goal of the present study was not to demonstrate that a single, acute stressor would have clinically significant effects on FeNO. Rather, we sought to determine whether a mild and brief stressor, administered under standardized laboratory conditions, could be shown to have effects on FeNO in children with asthma. If so, we speculate that repeated, frequent experiences with such psychosocial stressors might, over time, worsen symptoms by exacerbating airway inflammation.
Previous animal studies have demonstrated that experimental manipulations of stress alter airway inflammation in mice (Forsythe et al., 2004; Joachim et al., 2003). Our study extends this work to humans, and as well, highlights the importance of understanding social group differences in humans, given that inflammatory responses to an acute stressor were greater as SES declined. This finding suggests that in order to anticipate the magnitude of an individual's biological response to stress, it is useful to understand broader social context variables (Chen and Miller, 2007). For example, the socioeconomic background a child comes from may shape perceptions of stress and coping responses in ways that affect biological responses to stress (Chen et al., 2004; Chen et al., 2006).
Our study findings are also consistent with human work that has documented physiological effects of acute psychological stressors on airway resistance, pulmonary function, cholinergic pathways, and leukocyte counts in patients with asthma (Kang and Fox, 2000; Miller and Wood, 1994; Miller and Wood, 1997; Rietveld et al., 2000; Ritz et al., 2001; Ritz, 2004). Our study provides another plausible biological pathway through which stress may impact asthma outcomes, in drawing links to markers of airway inflammation. In addition, the present study is consistent with previous research that has shown that acute stress can influence immune markers, although this research tends to be conducted in healthy adults without disease-specific measures (Segerstrom and Miller, 2004). We note that our study did not find effects of acute stress on pulmonary function. However, it has become increasingly clear that there is poor correlation between different measures of asthma activity and severity (e.g., spirometry and FeNO levels (Strunk et al., 2003)), thus allowing for a disconnect between FEV1 and FeNO changes in response to stress. Other potential explanations for these findings may be limitations in the timing of collection of these measures, or in the type of measure collected (forced exhalation spirometry, rather than respiratory resistance or airway responsiveness measures).
How would an acute stressor come to affect FeNO in children with asthma? One speculative pathway involves the induction of nitric oxide (NO) production by the hormonal endproducts of the autonomic nervous system, epinephrine and norepinephrine. These hormones can be released within minutes into circulation during acute psychological stressors like the one used here (Malarkey et al., 1994), and they can induce NO production in macrophages stimulated with lipopolysaccharide (Chi et al., 2003; Lin et al., 2005). This process is partially dependent on catecholamine-mediated upregulation of inducible nitric oxide synthase (iNOS) (Chi et al., 2003), a gene whose promoter bears a cyclic AMP binding protein response element that can transduce adrenergic signals in leukocytes (Kohm and Sanders, 2000). In the present study, the increases in blood pressure and heart rate seen during the task are consistent with the notion that the stressor may have increased epinephrine and norepinephrine, but it remains unclear whether such effects were of sufficient magnitude or duration to affect iNOS expression.
Limitations of this study include the preliminary nature of the protocol time frame. Because this is the first study that we are aware of to investigate the effects of a standardized, laboratory psychosocial stressor on FeNO, guidelines have not been established regarding optimal time frames for assessing FeNO post-stressor. In order to both capture acute effects of the stressor on lung function and to minimize effects of spirometry on FeNO, we collected spirometry measures immediately post-task, and FeNO readings 45 minutes later. We were unable to do repeated assessments given the established effects of spirometry on FeNO (Kissoon et al., 2002; Silkoff et al., 1999). The present study provides some preliminary suggestion of SES differences in acute stress effects on FeNO; however, future studies would need to replicate this findings focusing on repeated FeNO measures (without spirometry) over a longer period of time in order to establish the optimal time frame for detecting effects on FeNO. A second limitation is the lack of psychological measures collected regarding the stressor task. Other limitations include the infeasibility of manipulating socioeconomic status for causal conclusions, and the relatively small sample size and wide age range. Age during childhood may have significant effects on immune and pulmonary measures, although in this study, age was controlled in all analyses to account for these potential effects as much as possible. We also note that this sample consisted of a high percentage of minorities, primarily Asian. Finally, although FeNO is becoming increasingly accepted as a marker of airway inflammation, there is debate about whether it plays a causal role in disease pathogenesis (Sanders, 1999).
The strengths of this study include the acute, laboratory stress paradigm combined with the collection of a marker of airway inflammation in both children with asthma and healthy children. While the design of our study involved an acute laboratory stressor, the findings suggest the possibility that interventions to reduce real-life stress may protect against the relatively greater increases in airway inflammation among lower SES children with asthma. Previous research has found that an intervention involving written disclosure and processing of stressful life experiences improved pulmonary function in a sample of patients with asthma (Smyth et al., 1999); this or other stress-management interventions could have similar benefits for airway inflammation. Overall, the present study indicates that SES is negatively associated with FeNO response to an acute stressor in children with asthma. Given that lower SES individuals are more likely to experience stressors (Brady and Matthews, 2002), this suggests that they may also be more vulnerable biologically to the effects of stress relative to their higher SES counterparts. Hence we need to better understand the stressors and the responses that they evoke in order to begin to address the greater asthma morbidity that many low SES children experience in our society (Miller, 2000; Simon et al., 2003).
Acknowledgments
This study was supported by NIH grant HL073975 and the William T. Grant Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement: All authors declare that there are no conflicts of interest.
References
- Adler NE, Boyce WT, Chesney MA, Folkman S, Syme SL. Socioeconomic inequalities in health: No easy solution. Journal of the American Medical Association. 1993;269:3140–3145. [PubMed] [Google Scholar]
- Aiken LS, West SG. Multiple regression: Testing and interpreting interactions. Sage Publications; London: 1991. [Google Scholar]
- American Thoracic Society Standardization of spirometry, 1994 update. American Journal of Respiratory and Critical Care Medicine. 1995;152:1107–1136. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- American Thoracic Society/European Respiratory Society ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. American Journal of Respiratory and Critical Care Medicine. 2005;171:912–930. doi: 10.1164/rccm.200406-710ST. [DOI] [PubMed] [Google Scholar]
- Bacharier LB, Strunk RC, Mauger D, White D, Lemanske RF, Sorkness CA. Classifying asthma severity in children: Mismatch between symptoms, medication use, and lung function. American Journal of Respiratory and Critical Care Medicine. 2004;170:426–432. doi: 10.1164/rccm.200308-1178OC. [DOI] [PubMed] [Google Scholar]
- Beck-Ripp J, Griese M, Arenz S, Koring C, Pasqualoni B, Bufler P. Changes of exhaled nitric oxide during steroid treatment of childhood asthma. European Respiratory Journal. 2002;19:1015–1019. doi: 10.1183/09031936.02.01582001. [DOI] [PubMed] [Google Scholar]
- Brady SS, Matthews KA. The effect of socioeconomic status and ethnicity on adolescents’ exposure to stressful life events. Journal of Pediatric Psychology. 2002;27:575–583. doi: 10.1093/jpepsy/27.7.575. [DOI] [PubMed] [Google Scholar]
- Bratton DL, Lanz MJ, Miyazawa N, White CW, Silkoff PE. Exhaled nitric oxide before and after montelukast sodium therapy in school-age children with chronic asthma: A preliminary study. Pediatr. Pulmonol. 1999;28:402–407. doi: 10.1002/(sici)1099-0496(199912)28:6<402::aid-ppul3>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Chen E, Fisher EB, Jr., Bacharier LB, Strunk RC. Socioeconomic status, stress, and immune markers in adolescents with asthma. Psychosom. Med. 2003;65:984–992. doi: 10.1097/01.psy.0000097340.54195.3c. [DOI] [PubMed] [Google Scholar]
- Chen E, Hanson MD, Paterson LQ, Griffin MJ, Walker HA, Miller GE. Socioeconomic status and inflammatory processes in childhood asthma: The role of psychological stress. Journal of Allergy and Clinical Immunology. 2006;117:1014–1020. doi: 10.1016/j.jaci.2006.01.036. [DOI] [PubMed] [Google Scholar]
- Chen E, Langer DA, Raphaelson YE, Matthews KA. Socioeconomic status and health in adolescents: The role of stress interpretations. Child Development. 2004;75:1039–1052. doi: 10.1111/j.1467-8624.2004.00724.x. [DOI] [PubMed] [Google Scholar]
- Chen E, Miller GE. Social context as an individual difference in psychoneuroimmunology_. In: Irwin MR, editor. Psychoneuroimmunology 4th edition. Elsevier; Boston, MA: 2007. pp. 497–508. [Google Scholar]
- Chi DS, Qui M, Krishnaswamy G, Li CF, Stone W. Regulation of nitric oxide production from macrophages by lipopolysaccharide and catecholamines. Nitric Oxide. 2003;8:127–132. doi: 10.1016/s1089-8603(02)00148-9. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Lawrence Erlbaum; New Jersey: 1988. [Google Scholar]
- Donenberg GR, Weisz JR. Experimental task and speaker effects on parent-child interactions of aggressive and depressed/anxious children. J. Abnorm. Child Psychol. 1997;25:367–387. doi: 10.1023/a:1025733023979. [DOI] [PubMed] [Google Scholar]
- Ewart CK, Taylor CB, Kraemer HC, Agras WS. High blood pressure and marital discord: Not being nasty matters more than being nice. Health Psychology. 1991;10:155–163. doi: 10.1037//0278-6133.10.3.155. [DOI] [PubMed] [Google Scholar]
- Forsythe P, Ebeling C, Gordon JR, Befus AD, Vliagoftis H. Opposing effects of short- and long-term stress on airway inflammation. American Journal of Respiratory and Critical Care Medicine. 2004;169:220–226. doi: 10.1164/rccm.200307-979OC. [DOI] [PubMed] [Google Scholar]
- Granger DA, Weisz JR, Kauneckis D. Neuroendocrine reactivity, internalizing behavior problems, and control-related cognitions in clinic-referred children and adolescents. J. Abnorm. Psychol. 1994;103:267–276. doi: 10.1037//0021-843x.103.2.267. [DOI] [PubMed] [Google Scholar]
- Granger DA, Weisz JR, McCracken JT, Ikeda SC, Douglas P. Reciprocal influences among adrenocortical activation, psychosocial processes, and the behavioral adjustment of clinic-referred children. Child Development. 1996;67:3250–3262. [PubMed] [Google Scholar]
- Hauser RM, Warren JR. Socioeconomic indexes for occupations: A review, update, and critique. Sociological Methodology. 1997;27:177–298. [Google Scholar]
- Hemingway H, Shipley M, Mullen MJ, Kumari M, Brunner E, Taylor M, Donald AE, Deanfield JE, Marmot M. Social and psychosocial influences on inflammatory markers and vascular function in civil servants (The Whitehall II study). Am. J. Cardiol. 2003;92:984–987. doi: 10.1016/s0002-9149(03)00985-8. [DOI] [PubMed] [Google Scholar]
- Hoglund CO, Axen J, Kemi C, Jernelov S, Grunewald J, Muller-Suur C, Smith Y, Gronneberg R, Eklund A, Stierna P, Lekander M. Changes in immune regulation in response to examination stress in atopic and healthy individuals. Clinical and Experimental Allergy. 2006;36:982–992. doi: 10.1111/j.1365-2222.2006.02529.x. [DOI] [PubMed] [Google Scholar]
- Jatakanon A, Lim S, Kharitonov SA, Chung KF, Barnes PJ. Correlation between exhaled nitric oxide, sputum eosinophils, and methacholine responsiveness in patients with mild asthma. Thorax. 1998;53:91–95. doi: 10.1136/thx.53.2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joachim RA, Quarcoo D, Arck PC, Herz U, Renz H, Klapp BF. Stress enhances airway reactivity and airway inflammation in an animal model of allergic bronchial asthma. Psychosom. Med. 2003;65:811–815. doi: 10.1097/01.psy.0000088582.50468.a3. [DOI] [PubMed] [Google Scholar]
- Jones SL, Kittelson J, Cowan JO, Flannery EM, Hancox RJ, McLachlan CR, Taylor DR. The predictive value of exhaled nitric oxide measurements in assessing changes in asthma control. American Journal of Respiratory and Critical Care Medicine. 2001;164:738–743. doi: 10.1164/ajrccm.164.5.2012125. [DOI] [PubMed] [Google Scholar]
- Kang D, Coe C, McCarthy DO, Jarjour NN, Kelly EA, Rodriguez RR, Busse WW. Cytokine profiles of stimulated blood lymphocytes in asthmatic and healthy adolescents across the school year. J. Interferon Cytokine Res. 1997;17:481–487. doi: 10.1089/jir.1997.17.481. [DOI] [PubMed] [Google Scholar]
- Kang D, Fox C. Neuroendocrine and leukocyte responses and pulmonary function to acute stressors. Annals of Behavioral Medicine. 2000;22:276–285. doi: 10.1007/BF02895663. [DOI] [PubMed] [Google Scholar]
- Kharitonov SA, Donnelly LE, Montuschi P, Corradi M, Collins JV, Barnes PJ. Dose-dependent onset and cessation of action of inhaled budesonide on exhaled nitric oxide and symptoms in mild asthma. Thorax. 2002;57:889–896. doi: 10.1136/thorax.57.10.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kissoon N, Duckworth LJ, Blake KV, Murphy SP, Lima JJ. Effect of beta(2)-agonist treatment and spirometry on exhaled nitric oxide in healthy children and children with asthma. Pediatr. Pulmonol. 2002;34:203–208. doi: 10.1002/ppul.10154. [DOI] [PubMed] [Google Scholar]
- Klimes-Dougan B, Hastings PD, Granger DA, Usher BA, Zahn-Waxler C. Adrenocortical activity in at-risk and normally developing adolescents: Individual differences in salivary cortisol basal levels, diurnal variation, and responses to social challenges. Development and Psychopathology. 2001;13:695–719. doi: 10.1017/s0954579401003157. [DOI] [PubMed] [Google Scholar]
- Kohm AP, Sanders VM. Norepinephrine: A messenger from the brain to the immune system. Immunol. Today. 2000;21:539–542. doi: 10.1016/s0167-5699(00)01747-3. [DOI] [PubMed] [Google Scholar]
- Kovesi T, Dales R. Exhaled nitric oxide and respiratory symptoms in a community sample of school aged children. Pediatr. Pulmonol. 2008;43:1198–1205. doi: 10.1002/ppul.20927. [DOI] [PubMed] [Google Scholar]
- Krieger N, Williams DR, Moss NE. Measuring social class in US public health research: Concepts, methodologies, and guidelines. Annual Review of Public Health. 1997;18:341–378. doi: 10.1146/annurev.publhealth.18.1.341. [DOI] [PubMed] [Google Scholar]
- Kullowatz A, Rosenfield D, Dahme B, Magnussen H, Kanniess F, Ritz T. Stress effects on lung function in asthma are mediated by changes in airway inflammation. Psychosom. Med. 2008;70:468–475. doi: 10.1097/PSY.0b013e31816f9c2f. [DOI] [PubMed] [Google Scholar]
- Lin WC, Tsai PS, Huang CJ. Catecholamines’ enhancement of inducible nitric oxide synthase-induced nitric oxide biosynthesis involves CAT-1 and CAT-2A. Anesthesia and Analgesia. 2005;101:226–232. doi: 10.1213/01.ANE.0000153860.71992.29. [DOI] [PubMed] [Google Scholar]
- Liu LY, Coe CL, Swenson CA, Kelly EA, Kita H, Busse WW. School examinations enhance airway inflammation to antigen challenge. American Journal of Respiratory and Critical Care Medicine. 2002;165:1062–1067. doi: 10.1164/ajrccm.165.8.2109065. [DOI] [PubMed] [Google Scholar]
- Malarkey WB, KiecoltGlaser JK, Pearl D, Glaser R. Hostile behavior during marital conflict alters pituitary and adrenal hormones. Psychosom. Med. 1994;56:41–51. doi: 10.1097/00006842-199401000-00006. [DOI] [PubMed] [Google Scholar]
- Miller BD, Wood BL. Psychophysiologic reactivity in asthmatic children: A cholinergically mediated confluence of pathways. J Am Acad Child Adolesc Psychiatry. 1994;33:1236–1244. doi: 10.1097/00004583-199411000-00004. [DOI] [PubMed] [Google Scholar]
- Miller BD, Wood BL. Influence of specific emotional states on autonomic reactivity and pulmonary function in asthmatic children. J Am Acad Child Adolesc Psychiatry. 1997;36:669–677. doi: 10.1097/00004583-199705000-00018. [DOI] [PubMed] [Google Scholar]
- Miller GE, Dopp JM, Myers HF, Felton SY, Fahey JL. Psychosocial predictors of natural killer mobilization during marital conflict. Health Psychology. 1999;18:262–271. doi: 10.1037//0278-6133.18.3.262. [DOI] [PubMed] [Google Scholar]
- Miller JE. The effects of race/ethnicity and income on early childhood asthma prevalence and health care use. American Journal of Public Health. 2000;90:428–430. doi: 10.2105/ajph.90.3.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montuschi P, Mondino C, Koch P, Ciabattoni G, Barnes PJ, Baviera G. Effects of montelukast treatment and withdrawal on fractional exhaled nitric oxide and lung function in children with asthma. Chest. 2007;132:1876–1881. doi: 10.1378/chest.07-1587. [DOI] [PubMed] [Google Scholar]
- Panagiotakos DB, Pitsavos C, Manios Y, Polychronopoulos E, Chrysohoou CA, Stefanadis C. Socio-economic status in relation to risk factors associated with cardiovascular disease, in healthy individuals from the ATTICA study. European Journal of Cardiovascular Prevention & Rehabilitation. 2005;12:68–74. [PubMed] [Google Scholar]
- Payne DNR, Adcock IM, Wilson NM, Oates T, Scallan M, Bush A. Relationship between exhaled nitric oxide and mucosal eosinophilic inflammation in children with difficult asthma, after treatment with oral prednisolone. American Journal of Respiratory and Critical Care Medicine. 2001;164:1376–1381. doi: 10.1164/ajrccm.164.8.2101145. [DOI] [PubMed] [Google Scholar]
- Pijnenburg MWH, de J, J.C. Exhaled nitric oxide in childhood asthma: A review. Clinical and Experimental Allergy. 2008;38:246–259. doi: 10.1111/j.1365-2222.2007.02897.x. [DOI] [PubMed] [Google Scholar]
- Rietveld S, Everaerd W, Creer TL. Stress-induced asthma: a review of research and potential mechanisms. Clinical and Experimental Allergy. 2000;30:1058–1066. doi: 10.1046/j.1365-2222.2000.00809.x. [DOI] [PubMed] [Google Scholar]
- Ritz T, Claussen C, Dahme B. Experimentally induced emotions, facial muscle activity, and respiratory resistance in asthmatic and non-asthmatic individuals. Br J Med Psychol. 2001;74:167–182. [PubMed] [Google Scholar]
- Ritz T. Probing the psychophysiology of the airways: Physical activity, experienced emotion, and facially expressed emotion. Psychophysiology. 2004;41:809–821. doi: 10.1111/j.1469-8986.2004.00247.x. [DOI] [PubMed] [Google Scholar]
- Sandberg S, Paton JY, Ahola S, McCann DC, McGuinness D, Hillary CR. The role of acute and chronic stress in asthma attacks in children. Lancet. 2000;356:982–987. doi: 10.1016/S0140-6736(00)02715-X. [DOI] [PubMed] [Google Scholar]
- Sanders SP . Nitric oxide in asthma: Pathogenic, therapeutic, or diagnostic? Am. J. Respir. Cell Mol. Biol. 1999;21:147–149. doi: 10.1165/ajrcmb.21.2.f158. [DOI] [PubMed] [Google Scholar]
- Schedlowski M, Jacobs R, Stratmann G, Richter S, Hadicke A, Tewes U, Wagner TOF, Schmidt RE. Changes of natural killer cells during acute psychological stress. J. Clin. Immunol. 1993;13:119–126. doi: 10.1007/BF00919268. [DOI] [PubMed] [Google Scholar]
- Segerstrom SC, Miller GE. Psychological stress and the human immune system: A meta-analytic study of 30 years of inquiry. Psychological Bulletin. 2004;130:601–630. doi: 10.1037/0033-2909.130.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalowitz MU, Berry CA, Quinn KA, Wolf RL. The relationship of life stressors and maternal depression to pediatric asthma morbidity in a subspecialty practice. Ambulatory Pediatrics. 2001;1:185–193. doi: 10.1367/1539-4409(2001)001<0185:trolsa>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Silkoff PE, Wakita S, Chatkin J, Ansarin K, Gutierrez C, Caramori M, McClean P, Slutsky AS, Zamel N, Chapman KR. Exhaled nitric oxide after beta(2)-agonist inhalation and spirometry in asthma. American Journal of Respiratory and Critical Care Medicine. 1999;159:940–944. doi: 10.1164/ajrccm.159.3.9805044. [DOI] [PubMed] [Google Scholar]
- Simon PA, Zeng ZW, Wold CM, Haddock W, Fielding JE. Prevalence of childhood asthma and associated morbidity in Los Angeles County: Impacts of race/ethnicity and income. J. Asthma. 2003;40:535–543. doi: 10.1081/jas-120018788. [DOI] [PubMed] [Google Scholar]
- Smyth JM, Stone AA, Hurewitz A, Kaell A. Effects of writing about stressful experiences on symptom reduction in patients with asthma or rheumatoid arthritis: A randomized trial. JAMA. 1999;281:1304–1309. doi: 10.1001/jama.281.14.1304. [DOI] [PubMed] [Google Scholar]
- Sorkness CA, Lemanske RF, Mauger DT, Boehmer SJ, Chinchilli VM, Martinez FD, Strunk RC, Szefler SJ, Zeiger RS, Bacharier LB, Bloomberg GR, Covar RA, Guilbert TW, Heldt G, Larsen G, Mellon MH, Morgan WJ, Moss MH, Spahn JD, Taussig LM. Long-term comparison of 3 controller regimens for mild-moderate persistent childhood asthma: The Pediatric Asthma Controller Trial. Journal of Allergy and Clinical Immunology. 2007;119:64–72. doi: 10.1016/j.jaci.2006.09.042. [DOI] [PubMed] [Google Scholar]
- Straub DA, Minocchieri S, Moeller A, Hamacher J, Wildhaber JH. The effect of montelukast on exhaled nitric oxide and lung function in asthmatic children 2 to 5 years old. Chest. 2005a;127:509–514. doi: 10.1378/chest.127.2.509. [DOI] [PubMed] [Google Scholar]
- Straub DA, Moeller A, Minocchieri S, Hamacher J, Sennhauser FH, Hall GL, Wildhaber JH. The effect of montelukast on lung function and exhaled nitric oxide in infants with early childhood asthma. European Respiratory Journal. 2005b;25:289–294. doi: 10.1183/09031936.05.00031904. [DOI] [PubMed] [Google Scholar]
- Strunk RC, Szefler SJ, Phillips BR, Zeiger RS, Chinchilli VM, Larsen G, Hodgdon K, Morgan W, Sorkness CA, Lemanske RF. Relationship of exhaled nitric oxide to clinical and inflammatory markers of persistent asthma in children. Journal of Allergy and Clinical Immunology. 2003;112:883–892. doi: 10.1016/j.jaci.2003.08.014. [DOI] [PubMed] [Google Scholar]
- Suglia SF, Ryan L, Laden F, Dockery DW, Wright RJ. Violence exposure, a chronic psychosocial stressor, and childhood lung function. Psychosom. Med. 2008;70:160–169. doi: 10.1097/PSY.0b013e318160687c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terada A, Fujisawa T, Katsumata H, Atsuta J, Iguchi K, Kamiya H, Togari H. Exhaled nitric oxide decreases during exercise-induced bronchoconstriction in children with asthma. Journal of Allergy and Clinical Immunology. 2001;107:S48–S48. doi: 10.1164/ajrccm.164.10.2009105. [DOI] [PubMed] [Google Scholar]
- Weil CM, Wade SL, Bauman LJ, Lynn H, Mitchell H, Lavigne J. The relationship between psychosocial factors and asthma morbidity in inner-city children with asthma. Pediatrics. 1999;104:1274–1280. doi: 10.1542/peds.104.6.1274. [DOI] [PubMed] [Google Scholar]
- Wright RJ, Finn P, Contreras JP, Cohen S, Wright RO, Staudenmayer J, Wand M, Perkins D, Weiss ST, Gold DR. Chronic caregiver stress and IgE expression, allergen-induced proliferation, and cytokine profiles in a birth cohort predisposed to atopy. Journal of Allergy and Clinical Immunology. 2004;113:1051–1057. doi: 10.1016/j.jaci.2004.03.032. [DOI] [PubMed] [Google Scholar]