Abstract
Methamphetamine (METH) is a psychostimulant that can cause long-lasting neurodegenerative effects in humans and animals. These toxic effects appear to occur, in part, via activation of dopamine (DA) D1 receptors. This paper assessed the possibility that the DA D1 receptor antagonist, SCH23390, might inhibit METH-induced changes in the expression of several members of immediate early genes (IEGs) which are known to control more delayed expression of other genes. We found that injections of METH (4×10 mg/kg, given at 2 hr intervals) caused significant increases in c-fos and fra-2 expression which lasted from 30 min to 4 hr. Pre-treatment with SCH23390, given 30 min before each METH injection, completely blocked METH-induced expression of c-fos, but only partially inhibited fra-2 mRNA expression. These results were confirmed by western blot analysis which showed METH-induced changes in c-Fos protein expression that were blocked by pretreatment with SCH23390. There were also delayed METH-induced DA D1 receptor-dependent effects on fosB mRNA expression. Even though fra-1 expression was not affected by pretreatment with METH alone, the repeated injections of SCH23390 caused substantial decreases in fra-1 mRNA expression in both the presence and absence of METH. The repeated injections of METH caused no changes in the mRNAs for c-jun, junB or junD. However, there were significant increases in the phosphorylation of c-Jun protein (ser63). Phosphorylation of c-Jun occurred in a delayed fashion (16 and 24 hours after the last METH injections) and was attenuated by SCH23390 pretreatment. Interestingly, SCH23390 given alone caused significant decreases in phospho-c-Jun at all time-points. The METH injections also caused delayed induction in the expression of members of the Egr family of transcription factors in a DA D1 receptor-dependent fashion. Repeated injections of SCH23390 caused substantial suppression of basal striatal egr-1 and egr-2 mRNA expression but not of that of egr-3. Both crem and arc mRNA levels were induced by METH in a SCH23390-sensitive fashion. Moreover, multiple injections of SCH23390 given alone caused marked inhibition of basal arc expression. These results show that multiple injections of METH can differentially affect the expression of several IEGs, some of which occurred in a DA D1 receptor dependent fashion. The SCH23390-mediated suppression of basal fra-1, egr-1, and egr-2 mRNA levels suggests that their basal expression in the striatum might be dependent on tonic stimulation of the DA D1 receptor.
Keywords: IEG, RT-PCR, SCH23390, signal transduction
Introduction
Methamphetamine (METH) is an addictive drug that causes long-term motor and cognitive abnormalities (Volkow et al., 2001; Gold et al., 2009). Imaging studies using positron emission tomography and post-mortem studies of the brains of METH-abusers have provided evidence for loss of striatal dopamine (DA) nerve terminals (Wilson et al., 1996; Volkow et al., 2001). Studies in rodents have shown that administration of toxic doses of METH, either as single or multiple injections, results in long-term changes in the brain (Krasnova and Cadet, 2009). These abnormalities include decreases in the levels of striatal DA and serotonin (5-HT) and of their metabolites as well as decreases in the activity of their biosynthetic enzymes (Kita et al., 2003). The use of toxic doses of METH can also cause neuronal apoptosis in the striata and cortices of rodents (Deng et al., 2002; Jayanthi et al., 2005; Thiriet et al., 2005; Cadet et al., 2005).
The pharmacological and neurotoxic effects of METH depend on the release of DA from striatal DA terminals and stimulation of DA receptors in the striatum which contains high densities of DA D1 and D2 receptors (Russell et al., 1992, O'Dell et al., 1991, 1993). The toxic effects of the drug depend, in part, on activation of D1 receptors because inhibition of DA D1 receptors protects against METH-induced toxicity in the striatum (Jayanthi et al., 2005; Xu et al., 2005). These protective effects of DA D1 antagonism might occur through inhibition of DA D1 receptor-mediated induction of genes that might be involved in pro-toxic cascades (Cadet et al., 2005; Jayanthi et al., 2005, 2009). To test the idea, we measured the effects of multiple METH injections on the expression of immediate early genes (IEGs), including several members of AP-1 and Egr families of transcription factors, in the absence and presence of the DA D1 receptor antagonist, SCH23390 (Iorio et al., 1983). We also measured the effects of SCH23390 on METH-induced changes in arc and crem mRNA levels because Arc is an effector gene involved in synaptic plasticity (Bramham et al., 2008) and because Crem participates in the regulation of neuroadaptive processes (Hughes and Dragunow, 1995). Our experiments show that multiple injections of METH doses which are known to cause long-term DA depletion and neuronal apoptosis (Deng et al., 1999; Ladenheim et al., 2000; Jayanthi et al., 2005) are associated with marked increases in the expression of several IEGs in a SCH23390-sensitive fashion.
2. Experimental procedures
2.1. Animals and Drug Treatment
Male Sprague-Dawley rats (Charles River Labs, Raleigh, NC, USA), weighing 250-300g were maintained in a room at temperature of 22 °C and had free access to food and water. They were divided into four groups of animals: [i) saline (C), ii) SCH23390 (0.5 mg/kg × 4 injections every 2 hr) (S), iii) METH (10 mg/kg × 4 injections every 2 hr) (M), and iv) METH plus SCH23390 (M + S)]. All injections were given intraperitoneally. The SCH23390 injections were given 30 min before each METH injection. For quantitative PCR, rats were euthanized at 30 min, 2, and 4 hr after drug injections. For Western blot analysis, animals were euthanized at 30 min, 2, 4, 16, and 24 hr after the last drug injection. All animal use procedures were according to the NIH Guide for the Care and Use of Laboratory Animals and were approved by the local Animal Care Committee.
2.2. Isolation of RNA
After animals were euthanized, striatal tissues were rapidly dissected, placed on dry ice and stored at −80°C (n = 6 per group). Total RNA for the 30 min, 2 hr and 4 hr samples was extracted with Qiagen RNeasy Midi kit (Qiagen, Valencia, CA, USA) according to the manufacturer's protocol. Analysis of samples for quality and quantity was assessed using an Agilent 2100 Bioanalyzer 2 (Agilent, Palo Alto, CA, USA).
2.3. Reverse transcription and mRNA quantitation
The expression levels of several immediate genes were studied by real-time quantitative polymerase chain reaction (qPCR). Total RNA for each sample was reverse-transcribed using oligo (dT) into cDNA using Advantage RT for PCR kit (Clontech, Mountain View, CA, USA). For PCR amplification of cDNA, primer Sequences for rat were generated by the LightCycler probe design software v. 2.0 (Roche, Indianapolis, IN, USA) and purchased from Synthesis and Sequencing Facility of Johns Hopkins University (Baltimore, MD, USA). The primer list is shown in Table 1. PCR experiments were performed on Lightcycler 480 II (Roche, Indianapolis, IN, USA), using iQ SYBR Green Supermix (BioRad, Hercules, CA, USA). The relative amounts of messenger RNA were normalized to clathrin mRNA and then quantified.
Table 1.
List of primer sequences used for quantitative RT-PCR.
| Forward | Reverse | |
|---|---|---|
| c-fos | TGA GGT GGT CTG AAT G | TTG ACA ATG TCT TGG AAC |
| fosB | GAA GCT GGA GTT CAT GC | ATG GGC TTG ATG ACA GA |
| fra-1 | GAT GAG GAA GGT CAA AGT | TTT CCT TCA GCC TGT AGT |
| fra-2 | AGC AAT GCT AAT GGG C | CTG TGT GCA CCC TCA GT |
| c-jun | TCT CAG GAG CGG ATC AA | TGT TAA CGT GGT TCA TGA C |
| junB | TCT TTC TCT TCA CGA CTA CA | CTA GCT TCA GAG ATG CG |
| junD | GTG TGT TTC CTT GTG TTG | TTT GGC GTA ACG AAG AC |
| egr-1 | AGG TCT CCC TGT TGT TGT GG | TGC ACC CAC CTT TCC TAC TC |
| egr-2 | CAG ATC CGA CAC TGG AA | CCT GAG ACC TCG AAA GTA |
| egr-3 | AAA GTT CGC TTT CGA C | GCC GAT TGG TAA TCC T |
| crem | CCA ACT TAC CAG ATC CGA | TTC TTT AGC AGC TTC CCT |
| arc | CTG GGT GGA GTT CAA GA | CGT CCA CAT ACA GTG TC |
2.4. Western Blot
Protein for striatal tissues were homogenized in a buffer containing 320 mM sucrose, 5 mM HEPES, 1 μg/ml leupeptin, 1 μg/ml aprotinin, and 1 μg/ml pepstatin. Homogenates were centrifuged at 5000g for 5 min, and the supernatant fractions were subsequently centrifuged at 30,000g for 30 min. The resulting pellet was resuspended in the sample buffer (62.5 mM Tris-HCl, 10% glycerol, 2% SDS, 0.1% bromophenol blue, and 50 mM dithiothreitol). Protein concentration was quantified with the BCA protein assay kit (Thermo scientific, Rockford, IL, USA). The lysates were denatured in sample buffer at 100 °C, and separated by SDS-PAGE. After the proteins were electrophoretically transferred on PVDF membranes, and membrane blocking, primary and secondary antibody incubations, and chemiluminescence reactions were carried out according to the protocol described by individual antibody suppliers. The membranes were incubated with c-Fos, c-Jun (Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA), and phospho-c-Jun (Ser63) (New England Biolabs, Beverly, MA, USA) (1:1000) antibodies, at 4 °C overnight. The blots were re-probed with α-Tubulin antibody (1:4000; Sigma, 2 hr at room temperature). For quantification, the signal intensity was normalized over the signal intensity of α-Tubulin. Signal intensity was measured densitometrically with LabWorks version 4.5 (BioImaging Systems analysis software, BioImaging System, UVP Inc., Upland, CA, USA).
2.5. Statistical Analysis
For analysis of the qPCR data, the values used consist of a ratio of the fluorescence values, normalized to the values of the endogenous gene clathrin. Values represent means ± SE (6 animals/ group). The fold changes in gene expression were generated from normalized data from the various in comparison to the control group. Statistical analysis for the q-PCR and western blot data was carried by a one-way ANOVA followed by Fisher's protected least square difference (PLSD) test using StatView (SAS Institute, Cary, NC, USA). The null hypothesis was rejected at p<0.05.
3. Results
3.1 Multiple injections of METH caused differential changes in the expression of fos and jun families of IEGs
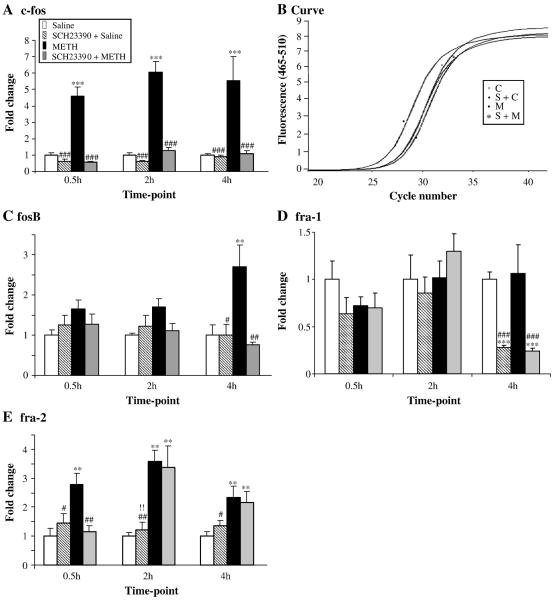
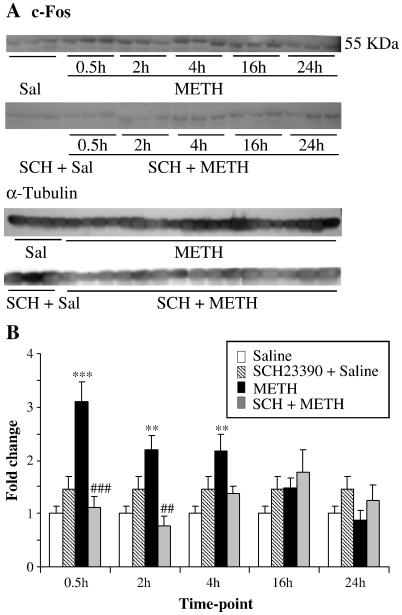
Fig. 1 shows the effects of METH and SCH23390 on members of the fos family of transcription factors. Repeated injections of SCH23390 alone caused no changes in c-fos expression (Fig. 1A). METH injections caused rapid and substantial increases in c-fos expression which were apparent at 30 min and lasted for the 4 hr duration of the study. Injections of saline before each of the four METH injections gave identical results to the injections of METH alone (data not shown). Injections of SCH23390 before each METH administration caused total inhibition of METH-induced c-fos expression (Fig. 1A). mRNA levels were measured according to a standard curve for each gene. We used 6 replicates for each reaction. These reactions yielded a standard curve with a slope of −3.3 and the efficiency value was ≈ 2. The amplification curve for a replicate of c-fos mRNA is shown in Fig. 1B. Similar curves were generated for each gene at all time points and are available on request. The protein level of c-Fos was also assessed and showed also increased at 30 min, 2 hr and 4 hr (Fig. 2). In contrast to the rapid METH-induced changes in c-fos expression, METH caused somewhat more delayed increases in fosB expression occurring at the 4 hr time point (Fig. 1C). Pretreatment of the animals with SCH23390 also blocked the METH effects of fosB expression (Fig. 1C). Unexpectedly, repeated injections of METH caused no changes in fra-1 expression in the rat striatum (Fig. 1D). In contrast, repeated injections of SCH23390 caused significant decreases in fra-1 expression at the 4 hr time point in both the absence and presence of METH, suggesting that the latter effects were due solely to DA D1 receptor antagonism. Fig. 1D shows that the METH-induced effects on fra-2 expression are somewhat similar to those observed for c-fos expression. Specifically, fra-2 expression was rapidly induced by METH, peaked at 2 hr then started to revert towards normal by the 4 hr time point (Fig. 1E). Interestingly, SCH23390 pretreatment blocked the effects of METH on fra-2 expression only at the 30-min time point, suggesting that METH might cause the more delayed increases via mechanisms other than stimulation of DA D1 receptors.
Fig. 1. Effects of METH and SCH23390 on the expression of the fos family genes.
METH administration caused induction of (A) c-fos, (C) fosB and (E) fra-2, but did not influence fra-1 expression (D). Amplification curve for c-fos gene expression for the 30 min time-point is shown in fig. 1B and was reproducible. The levels of mRNA were normalized to clathrin mRNA levels. Data were obtained from RNA isolated from six animals per group and quantitation determined individually. Statistical significance was determined by ANOVA followed by protected least-squares difference (PLSD). Key to statistics: *, **, *** = p < 0.05, 0.01, 0.001, respectively in comparison to the control (C) group. #, ##, ### = p < 0.05, 0.01, 0.001, respectively in comparison to the METH (M) group.
Fig. 2. Effects of METH and SCH23390 on c-Fos protein level.
(A) Representative photomicrograph of results showing the effects of METH and SCH23390 pre-treatment on striatal c-Fos protein levels at different time-points. (B) The quantitative data of the western blots represent means ± SEM (n = 3). The experiments were repeated three times for each protein. For quantification, the signal intensity was normalized over the signal intensity of α-tubulin. (B) METH caused significant DA D1 receptor-dependent increases in c-Fos protein. Statistics are as described in Fig. 1. Key to statistics: *, **,*** = p < 0.05, 0.01, 0.001, respectively in comparison to the control (C) group. #, ##, ### = p < 0.05, 0.01, 0.001, respectively in comparison to the METH (M) group.
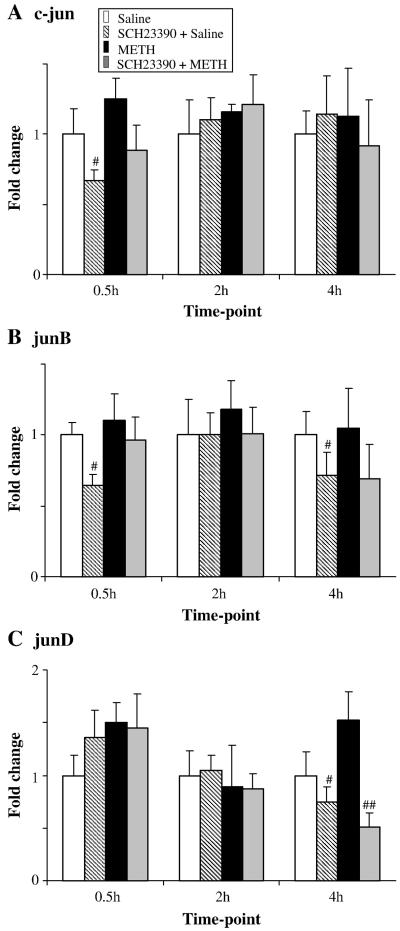
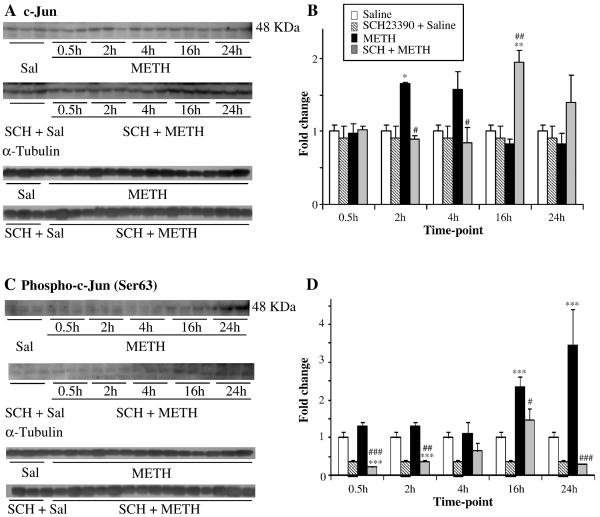
The repeated injections of METH caused no significant changes in the expression of c-jun (Fig. 3A), junB (Fig. 3B) and junD (Fig. 3C). Repeated injections of saline before each METH injection did not affect these results (not shown). Injections of SCH23390 alone caused no significant decreases in basal expression of these genes when compared to controls. As previously reported, c-Jun protein exerts its action via phosphorylation at serine-63 or -73 through the actions of c-Jun N-terminal kinases (JNKs) (Raivich, 2008), a process that seems to be involved in METH-induced toxicity since a single large toxic dose of METH can cause increased c-Jun phosphorylation at ser-63 (Jayanthi et al., 2002). Similarly, repeated injections of METH used in the present study also caused cJun phosphorylation at 16- and 24-hr after the last METH injection (Fig. 4). In contrast, SCH23390 caused significant decreases in the basal levels of phosphorylated c-Jun. These changes are not due to increased c-Jun protein expression since, similar to the PCR results, METH caused no changes in total c-Jun protein expression.
Fig. 3. Effects of METH and SCH23390 on the levels of jun transcripts.
The expression of (A) c-jun, (B) junB and (C) junD mRNA expression was not affected by METH treatment although SCH23390 pretreatment caused significant decreases in junD at 4 hr post drug treatment (C). Normalization, quantification, and statistics are as described in Fig. 1. Key to statistics: #, ## = p < 0.05, 0.01, respectively in comparison to the METH (M) group.
Fig. 4. Effects of METH and SCH23390 on c-Jun and phospho-c-Jun protein levels.
(A and C) show representative photomicrographs of results showing the effects of METH and SCH23390 pre-treatment on the levels of c-Jun and phospho-c-Jun in rat striatal tissues et different time-points. (B and D) show the quantitative data of the western blots represent means ± SEM (n = 3). The experiments were repeated three times for each protein. For quantification, the signal intensity was normalized over the signal intensity of α-tubulin. (C) METH-induced changes in c-Jun were seen only at the 2-hr time point and this was blocked by SCH23390 pre-treatment. (D) Phospho-c-Jun showed significant increases at 16 hr and 24 hr after METH treatment. Pretreatment of SCH23390 blocked METH-induced changes in phospho-c-Jun. Normalization, quantification, and statistics are as described in Fig. 1. Key to statistics: *, **,*** = p < 0.05, 0.01, 0.001, respectively in comparison to the control (C) group. #, ##, ### = p < 0.05, 0.01, 0.001, respectively in comparison to the METH (M) group.
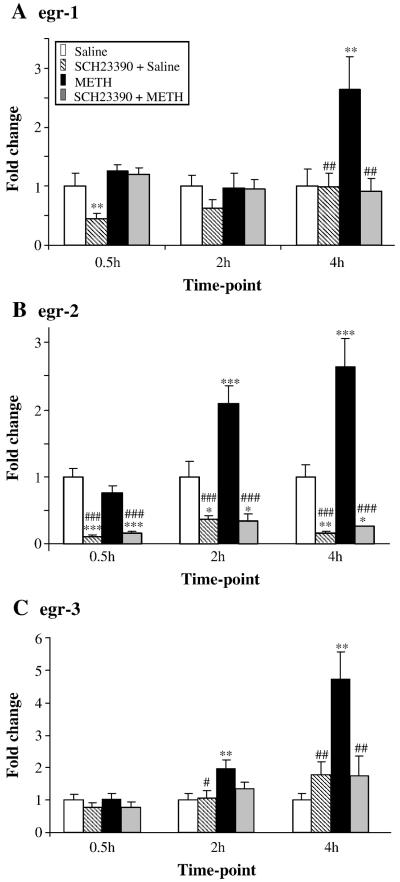
3.2 Effects of toxic doses of METH on egr expression
The effects of METH on members of the Egr family of transcription factors are shown in Fig. 5. Repeated injections of SCH23390 alone caused significant suppression of the basal egr-1 expression (Fig. 5A). SCH23390 caused decreases in basal egr-1 expression at 60 min after the last injection of SCH23390 (30 min after the last METH injection) in both the absence and presence of METH. METH caused significant increases in egr-1 expression at the 4-hr time point. Injections of saline before each METH injection caused identical changes to those observed in the METH alone group (not shown). SCH23390 pretreatment blocked the METH-induced changes. Repeated injections of SCH23390 also suppressed basal egr-2 expression at all the time points studied. METH caused time-dependent increases in egr-2 expression which become significant at the 2-hr time point (Fig. 5B). Pretreatment with SCH23390 also blocked METH-mediated increases in egr-2 expression. Injections of SCH23390 alone caused no significant changes in egr-3 expression (Fig. 5C). METH caused time-dependent increases in egr-3 expression. These increases were of greater magnitude than the changes observed in egr-1 and egr-2 expression (compare Fig. 5C to 5A and 5B). SCH23390 pretreatment also blocked the METH-induced increases in egr-3 expression.
Fig. 5. METH caused induction of striatal egr mRNA levels.
(A, B, C) Time-dependent induction of egr-1, egr-2 and egr-3 was observed after METH injections. SCH23390 blocked METH-induces changes in these genes. Normalization, quantification, and statistics are as described in Fig. 1. Key to statistics *, **, *** = p < 0.05, 0.01, 0.001, respectively in comparison to the control (C) group. #, ##, ### = p < 0.05, 0.01, 0.001, respectively in comparison to the METH (M) group.
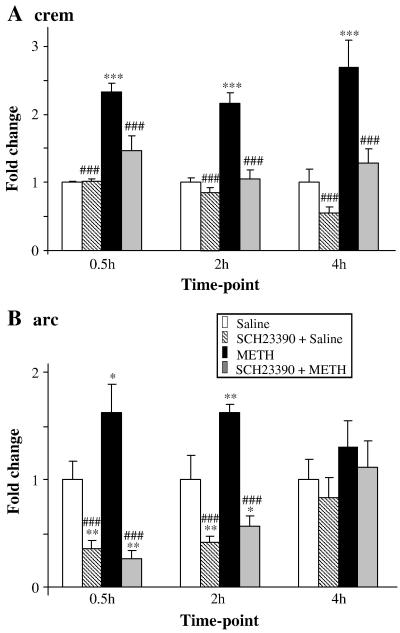
3.3 Effects of METH and SCH23390 on arc and crem expression in the striatum
Fig. 6 shows the effects of METH and SCH23390 on arc and crem mRNA levels. Administration of SCH23390 alone caused suppression of basal arc mRNA (Fig. 6A). METH injections caused small increases in arc expression at the 30-min and 2-hr time points, with reversal towards normal at the 4-hr time point. SCH23390 pretreatment blocked the METH-induced increases in arc expression (Fig. 6A). Interestingly, the levels of arc mRNA in the groups that got the combined SCH23390 and METH treatments were comparable to the levels observed in the SCH23390 alone groups (compare the S groups to the M+S groups in Fig. 6A).
Fig. 6. METH caused rapid induction of crem and arc mRNA levels.
(A) METH induced rapid increases in the crem transcript levels. (B) arc expression was also induced by METH. Normalization, quantification, and statistics are as described in Fig. 1. Key to statistics: *, **, *** = p < 0.05, 0.01, 0.001, respectively in comparison to the control (C) group. #, ##, ### = p < 0.05, 0.01, 0.001, respectively in comparison to the METH (M) group.
Repeated injections of SCH23390 had no effects on basal crem expression in comparison to the control group (Fig. 6B). Repeated injections of METH caused increases in crem mRNA levels which were observable at the 30-min time-point and lasted for the duration of the study. Administration of SCH23390 prior to the injections of METH also blocked METH-mediated increases in crem expression (Fig. 6B).
4. Discussion
The main findings of this paper are that (i) multiple injections of METH caused substantial increases in the expression of multiple transcription factors in the rat striatum; (ii) the DA D1 receptor antagonist, SCH23390, blocked these METH-induced changes in a time-dependent fashion; and that (iii) repeated injections of SCH23390 can suppress the basal levels of egr-1, egr-2 and arc mRNAs. Unexpectedly, the repeated injections of METH caused no changes in the levels of c-jun, junB or junD mRNAs. The observations of METH-induced increases in IEG mRNA levels and their dependency, for the most part, on stimulation on DA D1 receptors are consistent with the report that four injections of METH, given according to a schedule similar to the one used in the present study, can cause increases in DA efflux that stayed elevated for 16 hours after the last METH injection (O'Dell et al., 1991). The results of the present study are also consonant with and extend those of previous papers that have reported that single injections of METH (40 mg/kg) caused significant increases in c-fos, fosB, fra-2, c-jun, junB and junD in the mouse striatum (Cadet et al., 2001; Jayanthi et al., 2009). We also extended our previous results by showing that the effects of multiple METH injections occur mostly via stimulation of DA D1 receptor. These results are consistent with previous observations that acute administration of cocaine (Berretta et al., 1992) and amphetamine (Wang et al., 1995) can cause increases in a number of IEGs in a DA D1 receptor-dependent fashion.
The early induction of c-fos observed within 30 min of the last METH injection was not surprising, given the fact that c-fos is an IEG that is activated within minutes of neuronal stimulation (Hughes and Dragunow, 1995). This observation is consistent with those of other investigators who have reported that single injections of variable doses of METH can cause rapid increases in the expression of c-fos in the rodent brain (Wang et al., 1995, Thiriet et al., 2001). Because METH toxicity is exacerbated in c-fos knock-out mice (Deng et al., 1999), we had proposed previously that METH-induced increases in c-fos expression might constitute an attempt for the organism to protect striatal neurons against METH-induced neuronal apoptosis. The observation that the repeated injections of the DA D1 receptor antagonist, SCH23390, can block METH-induced increases in c-fos is consistent with observations of other authors who have reported that cocaine and METH-induced c-fos protein expression occurs via DA D1 receptor-mediated mechanisms (Young et al., 1991, Yoshida et al., 1995). Our observations of METH-induced increases in fosB and fra-2 expression are also consistent with previous demonstrations that cocaine can also affect the expression of other fos-related proteins (Nye et al., 1995) and that METH can cause increases in fosB expression in mice (Cadet et al., 2002). Similar to c-fos, the METH-induced increases in fosB might be involved in a neuroprotective loop since METH toxicity is exacerbated in fosB knockout mice (Kuroda et al., 2009).
Our observations of METH-induced increases in fra-2 expression are consistent with the previous demonstration that administration of METH doses, similar to those used in our study, caused substantial increases in Fra-2 protein in the mouse striatum at 3 days post-drug (Pennypacker et al., 2000). The timing in protein expression reported in the Pennypacker paper was, however, more delayed than the changes in mRNA levels as might be expected. The current study extends those results further by showing that the early but not the delayed effects of METH on fra-2 mRNA are dependent on stimulation of striatal DA D1 receptors. The lack of inhibiting effects of SCH23390 on METH-induced changes on fra-2 at the 2-hr and 4-hr time points suggests that METH might induce fra-2 via other mechanisms which involve oxidative stress (Kranova and Cadet, 2009). When taken together, these results suggest the possibility that several members of the fos family of transcription factors might work in tandem to protect the brain against METH toxicity.
Fos proteins are known partners of Jun members of the AP-1 family of transcription factors (Raivich, 2008). These transcription factors participate in the regulation of neurotransmission via their influence on genes that code for various neurotransmitters (Hughes and Dragunow., 1995). Although our PCR results did not show any effects of METH on the expression of c-jun, junB and junD, we found that repeated injections of METH did cause c-Jun phosphorylation at ser-63 which is an important activating mechanism for that protein (Derijard et., 1994; Raivich, 2008). We identified significant increases in phospho-c-Jun (Ser63) at the 16- and 24-hr time points, which were dependent on DA D1 receptor stimulation since pretreatment with SCH23390 blocked these changes. The manner by which stimulation of DA D1 receptors can cause phosphorylation of c-Jun might occur via its effects on the cAMP/PKA cascade which is known to interact with the SAPK pathway to activate JNK (Disa et al., 2000; Zhen et al., 1998).
Egr family transcription factors are expressed in several brain regions including the striatum and can be induced in responses to various stimuli to promote memory formation, tolerance to the effects of drugs, and to promote synaptic plasticity (Alberini, 2009). As shown above, SCH23390 treatment alone caused significant decreases in basal egr-1 and egr-2 expression, findings that are consistent with those of Mailleux et al (1992) who had used in situ hybridization technique and reported that SCH23390 can cause significant decreases in basal egr-1 expression in the rat striatum. These observations, taken together, indicate that basal levels of egr-1 and egr-2 mRNA are dependent on baseline stimulation of DA D1 receptors. Our observations that repeated injections of METH caused significant time-dependent increases in egr-1, egr-2 and egr-3 expression are compatible with those of earlier studies that had reported METH-induced egr-1 expression after single injections of METH (Thiriet et al., 2001, Wang et al., 1995). Jayanthi et al (2005) had previously reported that a single large dose of METH can also cause changes in the expression of these IEGs and that they were involved in the activation of the FasL/Fas death pathway in the rat striatum. Our present observations that multiple injections of METH can also result in egr expression are consistent with these results and support the idea that these transcription factors might indeed play a role in mediating METH-induced degeneration of intrinsic striatal cells (Jayanthi et al., 2005). Nevertheless, because these IEGs are known to influence processes that lead to synaptic plasticity and memory formation (Bozon et al., 2002; Ko et al., 2005, Li et al., 2007), it is possible that these transcription factors might also be involved in METH-induced neuroadaptive changes in the brain. This discussion is also consistent with the fact that Egr-1 and Egr-3, which are induced by METH, can regulate the expression of Arc (Li et al., 2005), an METH-inducible effector gene that is involved in long-term potentiation (LTP) and neuronal plasticity (Bramham at al., 2008). It is thus not farfetched to suggest that the METH-induced increases in striatal arc mRNA expression might play a role in the development of structural plasticity observed after chronic exposure to psychostimulant (Robinson and Kolb., 2004).
The regulation of the expression of several transcription factors that are influenced by direct and indirect DA agonists is mediated, in part, by phosphorylation of the cAMP Response Element Binding (CREB) protein (Hyman et al., 1995, Hughes and Dragunow., 1995) although there are many IEGs whose expression is independent of stimulation of DA D1 receptors (Jayanthi et al., 2009). CREB function is regulated, in part, by ICER, which is an inducible product of the CREM gene (Borlikova and Endo., 2009, Mioduszewska et al., 2003). ICER is a powerful repressor which is important for the transient nature of cAMP- induced gene expression (Mioduszewska et al., 2003). Thus, the observed METH-induced changes in CREM expression might serve to maintain cellular homeostasis by suppressing CREB-mediated gene expression.
In summary, the present data show that administration of METH caused differential changes in the expression of several IEGs, for the most part, in a DA D1 receptor-dependent fashion. The time course of these changes and their dependency, for the most part, on stimulation of DA D1 receptors, are consistent with the fact that repeated injections of METH can cause prolonged elevation (up to 16 hr after the last METH injection) of the levels of DA in the extracellular space (O'Dell et al., 1991). In any case, the observations that SCH23390 can block the METH-induced increases in the expression of the majority of the mRNAs measured in the present study provide a partial explanation for the protective effects that pretreatment with the DA antagonist, SCH23390, affords against METH-induced cell death in the striatum (Xu et al., 2005). Finally, these observations further implicate DA D1 receptor-mediated mechanisms as important targets for therapeutic interventions against METH addiction and toxicity.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alberini CM. Transcription factors in long-term memory and synaptic plasticity. Physiol. Rev. 2009;89:121–145. doi: 10.1152/physrev.00017.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berretta S, Robertson HA, Graybiel AM. Dopamine and glutamate agonists stimulate neuron-specific expression of Fos-like protein in the striatum. J. Neurophysiol. 1992;68:767–777. doi: 10.1152/jn.1992.68.3.767. [DOI] [PubMed] [Google Scholar]
- Borlikova G, Endo S. Inducible cAMP early repressor (ICER) and brain functions. Mol Neurobiol. 2009;40:73–86. doi: 10.1007/s12035-009-8072-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozon B, Davis S, Laroche S. Regulated transcription of the immediate-early gene Zif268: mechanisms and gene dosage-dependent function in synaptic plasticity and memory formation. Hippocampus. 2002;12:570–577. doi: 10.1002/hipo.10100. [DOI] [PubMed] [Google Scholar]
- Bramham CR, Worley PF, Moore MJ, Guzowski JF. The immediate early gene arc/arg3.1: regulation, mechanisms, and function. J. Neurosci. 2008;28:11760–11767. doi: 10.1523/JNEUROSCI.3864-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadet JL, Jayanthi S, Deng X. Methamphetamine-induced neuronal apoptosis involves the activation of multiple death pathways. Neurotox Res. 2005;8:199–206. doi: 10.1007/BF03033973. [DOI] [PubMed] [Google Scholar]
- Cadet JL, Jayanthi S, McCoy MT, Vawter M, Ladenheim B. Temporal profiling of methamphetamine-induced changes in gene expression in the mouse brain: Evidence from cDNA array. Synapse. 2001;41:40–48. doi: 10.1002/syn.1058. [DOI] [PubMed] [Google Scholar]
- Cadet JL, McCoy MT, Ladenheim B. Distinct gene expression signatures in the striata of wild-type and heterozygous c-fos knockout mice following methamphetamine administration: evidence from cDNA array analyses. Synapse. 2002;44:211–226. doi: 10.1002/syn.10074. [DOI] [PubMed] [Google Scholar]
- Chen K, Vita JA, Berk BC, Keaney JF., Jr. c-Jun N-terminal kinase activation by hydrogen peroxide in endothelial cells involves src-dependent epidermal growth factor receptor transactivation. J Biol Chem. 2001;276:16045–16050. doi: 10.1074/jbc.M011766200. [DOI] [PubMed] [Google Scholar]
- Deng X, Cai NS, McCoy MT, Chen W, Trush MA, Cadet JL. Methamphetamine induces apoptosis in an immortalized rat striatal cell line by activating the mitochondrial cell death pathway. Neuropharmacology. 2002;42:837–845. doi: 10.1016/s0028-3908(02)00034-5. [DOI] [PubMed] [Google Scholar]
- Deng X, Ladenheim B, Tsao LI, Cadet JL. Null mutation of c-fos causes exacerbation of methamphetamine-induced neurotoxicity. J. Neurosci. 1999;19:10107–10115. doi: 10.1523/JNEUROSCI.19-22-10107.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derijard B, Hibi M, Wu IH, Barrett T, Su B, Deng T, Karin M, Davis RJ. JNK1: a protein kinase stimulated by UV light and Ha-Ras that binds and phosphorylates the c-Jun activation domain. Cell. 1994;76:1025–1037. doi: 10.1016/0092-8674(94)90380-8. [DOI] [PubMed] [Google Scholar]
- Disa J, Parameswaran N, Nambi P, Aiyar N. Involvement of cAMP-dependent protein kinase and pertussis toxin-sensitive G-proteins in CGRP mediated JNK activation in human neuroblastoma cell line. Neuropeptides. 2000;34:229–233. doi: 10.1054/npep.2000.0810. [DOI] [PubMed] [Google Scholar]
- Fei J, Viedt C, Soto U, Elsing C, Jahn L, Kreuzer J. Endothelin-1 and smooth muscle cells: induction of jun amino-terminal kinase through an oxygen radical-sensitive mechanism. Arterioscler. Thromb. Vasc. Biol. 2000;20:1244–1249. doi: 10.1161/01.atv.20.5.1244. [DOI] [PubMed] [Google Scholar]
- Gold MS, Kobeissy FH, Wang KK, Merlo LJ, Bruijnzeel AW, Krasnova IN, Cadet JL. Methamphetamine- and trauma-induced brain injuries: comparative cellular and molecular neurobiological substrates. Biol. Psychiatry. 2009;66:118–127. doi: 10.1016/j.biopsych.2009.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert MA, O'Callaghan JP. Protein phosphorylation cascades associated with methamphetamine-induced glial activation. Ann N Y Acad. Sci. 2000;914:238–262. doi: 10.1111/j.1749-6632.2000.tb05200.x. [DOI] [PubMed] [Google Scholar]
- Hughes P, Dragunow M. Induction of immediate-early genes and the control of neurotransmitter-regulated gene expression within the nervous system. Pharmacol. Rev. 1995;47:133–178. [PubMed] [Google Scholar]
- Hyman SE, Cole RL, Konradi C, Kosofsky BE. Dopamine regulation of transcription factor-target interactions in rat striatum. Chem. Senses. 1995;20:257–260. doi: 10.1093/chemse/20.2.257. [DOI] [PubMed] [Google Scholar]
- Iorio LC, Barnett A, Leitz FH, Houser VP, Korduba CA. SCH 23390, a potential benzazepine antipsychotic with unique interactions on dopaminergic systems. Pharmacol Exp. Ther. 1983;226:462–468. [PubMed] [Google Scholar]
- Jayanthi S, Deng X, Ladenheim B, McCoy MT, Cluster A, Cai NS, Cadet JL. Calcineurin/NFAT-induced up-regulation of the Fas ligand/Fas death pathway is involved in methamphetamine-induced neuronal apoptosis. Proc. Natl, Acad, Sci, U S A. 2005;102:868–873. doi: 10.1073/pnas.0404990102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayanthi S, Ladenheim B, Cadet JL. Methamphetamine-induced changes in antioxidant enzymes and lipid peroxidation in copper/zinc-superoxide dismutase transgenic mice. Ann. N Y Acad. Sci. 1998;844:92–102. [PubMed] [Google Scholar]
- Jayanthi S, McCoy MT, Beauvais G, Ladenheim B, Gilmore K, Wood W, 3rd, Becker K, Cadet JL. Methamphetamine induces dopamine D1 receptor-dependent endoplasmic reticulum stress-related molecular events in the rat striatum. PLoS One. 2009;4:e6092. doi: 10.1371/journal.pone.0006092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayanthi S, McCoy MT, Ladenheim B, Cadet JL. Methamphetamine Causes Coordinate Regulation of Src, Cas, Crk, and the Jun N-Terminal Kinase-Jun Pathway. Mol. Pharmacol. 2002;61:1124–1131. doi: 10.1124/mol.61.5.1124. [DOI] [PubMed] [Google Scholar]
- Kita T, Wagner GC, Nakashima T. Current research on methamphetamine-induced neurotoxicity: animal models of monoamine disruption. J. Pharmacol. Sci. 2003;92:178–195. doi: 10.1254/jphs.92.178. [DOI] [PubMed] [Google Scholar]
- Ko SW, Ao HS, Mendel AG, Qiu CS, Wei F, Milbrandt J, Zhuo M. Transcription factor Egr-1 is required for long-term fear memory and anxiety. Sheng Li Xue Bao. 2005;57:421–432. [PubMed] [Google Scholar]
- Krasnova IN, Cadet JL. Methamphetamine toxicity and messengers of death. Brain Res. Rev. 2009;60:379–407. doi: 10.1016/j.brainresrev.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroda KO, Ornthanalai VG, Kato T, Murphy NP. FosB Null Mutant Mice Show Enhanced Methamphetamine Neurotoxicity: Potential Involvement of FosB in Intracellular Feedback Signaling and Astroglial Function. Neuropsychopharmacology. 2009 doi: 10.1038/npp.2009.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Carte J, Gao X, Whitehead J, Tourtellotte WG. The neuroplasticity-associated arc gene is a direct transcriptional target of early growth response (Egr) transcription factors. Mol. Cell. Biol. 2005;25:10286–10300. doi: 10.1128/MCB.25.23.10286-10300.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Yun SH, Keblesh J, Trommer BL, Xiong H, Radulovic J, Tourtellotte WG. Egr3, a synaptic activity regulated transcription factor that is essential for learning and memory. Mol. Cell. Neurosci. 2007;35:76–88. doi: 10.1016/j.mcn.2007.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mailleux P, Zhang F, Vanderhaeghen J-J. The dopamine D1 receptor antagonist SCH-23390 decreases the mRNA levels of the transcription factor zif268 (krox-24) in adult rat intact striatum - an in situ hybridization study. Neurosci. Lett. 1992;147:182–184. doi: 10.1016/0304-3940(92)90590-4. [DOI] [PubMed] [Google Scholar]
- Mioduszewska B, Jaworski J, Kaczmarek L. Inducible cAMP early repressor (ICER) in the nervous system--a transcriptional regulator of neuronal plasticity and programmed cell death. J Neurochem. Review. 2003;87:1313–1320. doi: 10.1046/j.1471-4159.2003.02116.x. [DOI] [PubMed] [Google Scholar]
- Nye HE, Hope BT, Kelz MB, Iadarola M, Nestler EJ. Pharmacological studies of the regulation of chronic FOS-related antigen induction by cocaine in the striatum and nucleus accumbens. J. Pharmacol. Exp. Ther. 1995;275:1671–1680. [PubMed] [Google Scholar]
- O'Dell SJ, Weihmuller FB, Marshall JF. Methamphetamine-induced dopamine overflow and injury to striatal dopamine terminals: attenuation by dopamine D1 or D2 antagonists. J Neurochem. 1993;60:1792–1799. doi: 10.1111/j.1471-4159.1993.tb13405.x. [DOI] [PubMed] [Google Scholar]
- O'Dell SJ, Weihmuller FB, Marshall JF. Multiple methamphetamine injections induce marked increases in extracellular striatal dopamine which correlate with subsequent neurotoxicity. Brain Res. 1991;564:256–260. doi: 10.1016/0006-8993(91)91461-9. [DOI] [PubMed] [Google Scholar]
- Pennypacker KR, Yang X, Gordon MN, Benkovic S, Miller D, O'Callaghan JP. Long-term induction of Fos-related antigen-2 after methamphetamine, methylenedioxymethamphetamine-, 1-methyl-4-phenyl-1,2,3, 6-tetrahydropyridine- and trimethyltin-induced brain injury. Neuroscience. 2000;101:913–919. doi: 10.1016/s0306-4522(00)00381-x. [DOI] [PubMed] [Google Scholar]
- Raivich G. c-Jun expression, activation and function in neural cell death, inflammation and repair. J. Neurochem. 2008;107:898–906. doi: 10.1111/j.1471-4159.2008.05684.x. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Kolb B. Structural plasticity associated with exposure to drugs of abuse. Neuropharmacology. 2004;47:33–46. doi: 10.1016/j.neuropharm.2004.06.025. [DOI] [PubMed] [Google Scholar]
- Russell VA, Allin R, Lamm MC, Taljaard JJ. Regional distribution of monoamines and dopamine D1- and D2-receptors in the striatum of the rat. Neurochem. Res. 1992;17:387–95. doi: 10.1007/BF00974582. [DOI] [PubMed] [Google Scholar]
- Thiriet N, Deng X, Solinas M, Ladenheim B, Curtis W, Goldberg SR, Palmiter RD, Cadet JL. Neuropeptide Y protects against methamphetamine-induced neuronal apoptosis in the mouse striatum. J. Neurosci. 2005;25:5273–5279. doi: 10.1523/JNEUROSCI.4893-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiriet N, Zwiller J, Ali SF. Induction of the immediate early genes egr-1 and c-fos by methamphetamine in mouse brain. Brain Res. 2001;919:31–40. doi: 10.1016/s0006-8993(01)02991-2. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Leonido-Yee M, Franceschi D, Sedler MJ, Gatley SJ, Hitzemann R, Ding YS, Logan J, Wong C, Miller EN. Association of dopamine transporter reduction with psychomotor impairment in methamphetamine abusers. Am. J. Psychiatry. 2001;158:377–82. doi: 10.1176/appi.ajp.158.3.377. [DOI] [PubMed] [Google Scholar]
- Wang JQ, Smith AJ, McGinty JF. A single injection of amphetamine or methamphetamine induces dynamic alterations in c-fos, zif/268 and preprodynorphin messenger RNA expression in rat forebrain. Neuroscience. 1995;68:83–95. doi: 10.1016/0306-4522(95)00100-w. [DOI] [PubMed] [Google Scholar]
- Watson A, Eilers A, Lallemand D, Kyriakis J, Rubin LL, Ham J. Phosphorylation of c-Jun is necessary for apoptosis induced by survival signal withdrawal in cerebellar granule neurons. J. Neurosci. 1998;18:751–762. doi: 10.1523/JNEUROSCI.18-02-00751.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson JM, Kalasinsky KS, Levey A,I, Bergeron C, Reiber G, Anthony RM, Schmunk GA, Shannak K, Haycock JW, Kish SJ. Striatal dopamine nerve terminal markers in human, chronic methamphetamine users. Na.t Med. 1996;2:699–703. doi: 10.1038/nm0696-699. [DOI] [PubMed] [Google Scholar]
- Xu W, Zhu JP, Angulo JA. Induction of striatal pre- and postsynaptic damage by methamphetamine requires the dopamine receptors. Synapse. 2005;58:110–121. doi: 10.1002/syn.20185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida H, Ohno M, Watanabe S. Roles of dopamine D1 receptors in striatal fos protein induction associated with methamphetamine behavioral sensitization in rats. Brain Res. Bull. 1995;38:393–397. doi: 10.1016/0361-9230(95)02005-c. [DOI] [PubMed] [Google Scholar]
- Young ST, Porrino LJ, Iadarola MJ. Cocaine induces striatal c-fosimmunoreactive proteins via dopaminergic D1 receptors. Proc. Natl. Acad. Sci. U S A. 1991;88:1291–1295. doi: 10.1073/pnas.88.4.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhen X, Uryu K, Wang HY, Friedman E. D1 dopamine receptor agonists mediate activation of p38 mitogen-activated protein kinase and c-Jun amino-terminal kinase by a protein kinase A-dependent mechanism in SK-N-MC human neuroblastoma cells. Mol. Pharmacol. 1998;54:453–458. doi: 10.1124/mol.54.3.453. [DOI] [PubMed] [Google Scholar]