Abstract
The Iowa Gambling Task (IGT) is a sensitive test for the detection of decision‐making impairments in several neurological and psychiatric populations. Very few studies have employed the IGT in functional magnetic resonance imaging (fMRI) investigations, in part, because the task is cognitively complex. Here we report a method for exploring brain activity using fMRI during performance of the IGT. Decision‐making during the IGT was associated with activity in several brain regions in a group of healthy individuals. The activated regions were consistent with the neural circuitry hypothesized to underlie somatic marker activation and decision‐making. Specifically, a neural circuitry involving the dorsolateral prefrontal cortex (for working memory), the insula and posterior cingulate cortex (for representations of emotional states), the mesial orbitofrontal and ventromedial prefrontal cortex (for coupling the two previous processes), the ventral striatum and anterior cingulate/SMA (supplementary motor area) for implementing behavioral decisions was engaged. These results have implications for using the IGT to study abnormal mechanisms of decision making in a variety of clinical populations. Hum Brain Mapp, 2010. © 2009 Wiley‐Liss, Inc.
Keywords: decision making, fMRI, IGT, somatic marker hypothesis
INTRODUCTION
The Iowa Gambling Task (IGT) has been used extensively in clinical and research studies, and it has been shown to be a highly sensitive measure of impaired decision making in a variety of neurological and psychiatric conditions [Bechara, 2004; Brand et al., 2007]. Neurological patients with damage to the mesial orbitofrontal/ventromedial prefrontal cortex (mOFC/vmPFC), or to the bilateral amygdala, show severe decision‐making impairments as measured by the IGT [Bechara, 2004]. Poor IGT performance is also observed in patients with damage to the dorsolateral prefrontal cortex (dlPFC), mostly when the damage is on the right side, and especially in patients who exhibit poor working memory and impairments on other executive function tests like the Wisconsin Card Sorting Task [Bechara, 2004; Clark et al., 2004; Manes et al., 2002]. Damage to the parietal cortex that includes the insula, and more mesial structures, such as the posterior cingulate, especially on the right side, has also been associated with poor IGT performance [Bechara, 2004]. The IGT has also been used in older individuals who manifest decision‐making impairments, despite intact memory, intellect, and other cognitive functions [Denburg et al., 2005]. Disadvantageous decision making, especially as observed on the IGT, has been shown to characterize various psychopathological conditions, such as substance addiction [Bechara and Martin, 2004; Goldstein et al., 2007], pathological gambling [Cavedini et al., 2002], schizophrenia [Sevy et al., 2007], obsessive‐compulsive disorder [Whitney et al., 2004], anorexia nervosa [Cavedini et al., 2004], chronic pain [Apkarian et al., 2004], attention‐deficit/hyperactivity disorder [Malloy‐Diniz et al., 2007], psychopathy [van Honk et al., 2002], impulsive aggressive disorders [Best et al., 2002], obesity [Davis et al., 2004], affective disorders with suicide attempts [Jollant et al., 2005], and Huntington's disease [Campbell et al., 2004]. HIV‐positive substance‐dependent males [Martin et al., 2004b] and patients who have undergone psychosurgery for depression [Dalgleish et al., 2004] also exhibit poor decision‐making, as measured by the IGT. Thus, a wide range of disorders are characterized by some sort of impairments in decision‐making, as judged from clinical observations and real‐life behaviors, and which are detected by the IGT. At the neural level, all these disorders may share a common pathophysiology that involves the ventromedial region of the prefrontal cortex. However, impaired decision‐making may also arise from dysfunctions in other neural systems, namely neural systems concerned with memory (e.g., dorsolateral prefrontal cortex or hippocampus), or neural systems connected to the limbic system and concerned with processing affective and emotional information (e.g., insula, amygdala, cingulate cortex). Therefore, the use of the IGT in functional neuroimaging may help shed light on the neurobiological source of the impaired decision‐making manifested in the lives of individuals diagnosed with any of this wide range of neuropsychiatric disorders.
Although the IGT has been shown to be an ecologically valid and sensitive test for the detection of decision‐making impairments in several neurologic and psychiatric populations, very few studies have employed the IGT in functional magnetic resonance imaging (fMRI) investigations. In part, this has been related to the fact that the IGT is cognitively complex, and perhaps it is more difficult to tease out the underlying cognitive processes with fMRI. For this reason, several alternate decision‐making tasks have been developed, namely the Cambridge Gambler Task [Rogers et al., 1999], and subsequently the Game of Dice [Brand et al., 2005]. The key distinguishing feature of these tasks from that of the IGT is that all the information for making the decision is provided in an explicit manner. In contrast, in the IGT, especially the early part of the task, the information is implicit in nature. Nonetheless, using fMRI to identify the brain regions involved in the IGT would be extremely valuable for understanding the neuropathological mechanisms underlying the decision‐making impairment in a variety of neuropsychiatric conditions [Max et al., 2005; Roca et al., 2008; Torralva et al., 2007]. Indeed, studies with the IGT have been theoretically guided by the neural framework of the Somatic Marker Hypothesis (SMH) [Damasio, 1994, 1996]. Although a review study [Dunn, 2006] has criticized some aspects of the SMH, especially the role of peripheral processes in decision‐making, this study has also confirmed the soundness of this neural framework, especially in terms of its anatomical structures. Only one study has been used to undermine the role of somatic markers in decision‐making [Maia and McClelland, 2004]. However, this criticism is now questioned in light of a more recent study [Persaud et al., 2007]. Therefore, the primary goal of this study was to develop an effective method for using fMRI to explore brain activities during performance of the IGT in the normal population.
The SMH provides a systems‐level neuroanatomical and cognitive framework for decision‐making, and for choosing according to long‐term outcomes rather than short‐term ones, and it suggests that the process of decision‐making depends in many important ways on neural substrates that regulate homeostasis, emotion, and feeling [Damasio, 1994]. Several neural structures have been shown to be key components of the neural circuitry underlying somatic state activation. The amygdala and mOFC/vmPFC are critical structures for triggering somatic states, but the amygdala seems more important for triggering somatic states from emotional events that occur in the environment (i.e., primary inducers), whereas the mOFC/vmPFC seems more important for triggering somatic states from memories, knowledge, and cognition (i.e., secondary inducers) [Bechara and Damasio, 2005]. Decision making is a complex process that relies on the integrity of at least two sets of neural systems: (1) one set is important for memory (e.g., the hippocampus), and especially working memory (e.g., the dlPFC), to bring online knowledge and information used during the deliberation of a decision; (2) another set is important for triggering emotional responses. This includes effector structures such as the hypothalamus and autonomic brainstem nuclei that produce changes in internal milieu and visceral structures along with other effector structures such as the ventral striatum, periacqueductal gray (PAG), and other brainstem nuclei, which produce changes in facial expression and specific approach or withdrawal behaviors. It also includes cortical structures that receive afferent input from the viscera and internal milieu, such as the insular cortex and the posterior cingulate gyrus (or retrosplenial cortex). This notion is supported by several studies that brought to light the important role of the insula in decision‐making [Ernst and Paulus, 2005; Paulus, 2007; Paulus et al., 2003a; Paulus and Stein, 2006].
For somatic signals to influence cognition and behavior, they must act on the appropriate neural systems. One target for somatic state action is the striatum. A large number of channels convey body information (somatic signals) to the central nervous system (e.g., spinal cord, vagus nerve, humoral signals). Evidence suggests that the vagal route is especially critical for relaying somatic signals [Martin et al., 2004a]. We have proposed that the next link in this body‐brain channel involves neurotransmitter systems [Bechara and Damasio, 2005; Damasio 1994, 1996]. Indeed, the cell bodies of the neurotransmitter dopamine, serotonin, noreadrenaline, and acetylcholine are located in the brainstem; the axon terminals of these neurotransmitter neurons synapse on cells and/or terminals all over the cortex and striatum [Blessing, 1997]. When somatic state signals are transmitted to the cell bodies of dopamine or serotonin neurons, for example, the signaling influences the pattern of dopamine or serotonin release at the terminals. In turn, changes in dopamine or serotonin release will modulate synaptic activities of neurons subserving behavior and cognition within the cortex. This chain of neural mechanisms provides a way for somatic states to exert a biasing effect on decisions. At the cellular, and more recently the fMRI level, the pioneering work of Schultz [ 1997] on the role of dopamine in reward processing and error prediction provides a strong validity for the proposed neural framework. Thus, all the work related to dopamine and the ventral striatum is consistent with the somatic marker framework. The key difference is that the dopamine mechanism addresses only one specific component of a larger neural network that is important for implementing decisions. The SMH is a neural framework that incorporates all the different neural steps involved in decision‐making, including the dopamine link, such as the one initially studied by Schultz. This theoretical framework of the SMH guides our hypotheses about the neural regions that will be engaged during performance of the IGT.
Studies that have looked at neural activation while participants performed the IGT remain relatively scarce. One study had individuals perform the IGT while situated in a positron emission tomography (PET) scanner [Ernst et al., 2002]. The control task in this experiment involved the examiner signaling the participant to select cards from the four decks in a specified order, instead of allowing the participant to actually select decks. A predominantly right‐sided network of prefrontal and posterior cortical regions was activated, which included the mOFC/vmPFC region, adjacent anterior cingulate cortex, dorsolateral prefrontal cortex, insula, and adjacent inferior parietal cortex. This neural network overlaps considerably with that known from lesion studies to interfere with IGT performance, as outlined earlier. Abnormal activity in this neural circuitry affecting IGT performance was revealed in a subsequent PET study, using the same IGT protocol, on cocaine users, who showed impaired performance on the IGT [Bolla et al., 2003]. Cocaine use was associated with increased activation in the right mOFC and decreased activation in the right dlPFC, relative to the performance of healthy participants. In a different study, which involved patients in a more acute phase of cocaine abstinence, IGT performance was negatively correlated with activity in the anterior cingulate gyrus, which is a part of the mOFC/vmPFC region, and the middle frontal gyrus, medial frontal gyrus, and superior frontal gyrus, all of which are parts of the dlPFC [Tucker et al., 2004].
Similar neural correlates underlying IGT performance were revealed using fMRI. Fifteen healthy volunteers performed the IGT while having their brain activity scanned using event‐related fMRI [Fukui et al., 2005]. When the neural activity occurring during selections from the advantageous decks was compared with the neural activity occurring during selections from the disadvantageous decks, it was found that activity during the anticipatory period (i.e., the time spent pondering which deck to choose) engaged the superior part of the anterior cingulate and the neighboring medial frontal gyrus. This activity occurred in an area that is relatively superior to the mOFC/vmPFC area, though it still lies within the overall region known for housing decision‐making impairments in patients with prefrontal cortex lesions. It is unclear whether the mOFC/vmPFC area was precluded in this fMRI study because of signal dropout due to distortion artifacts. Another study in different groups of polysubstance‐dependent individuals and in pathological gamblers using fMRI [Tanabe et al., 2007] showed, relative to controls, reduced activities in the ventromedial prefrontal region (vmPFC), and in the right frontopolar and superior frontal cortex regions (these areas are part of the dlPFC) during performance of the IGT. A study by Windmann et al. [ 2006] used the original and inverted versions of the IGT in healthy controls and suggested that the high outcome from the bad decks in the original task tended to make subjects stay on bad decks for longer and activated the medial OFC more than in the inverted task, which is consistent with the notion that the medial OFC is involved in maintaining a behavioral strategy. Conversely, the inverted task generated higher activations in the lateral OFC subregions, consistent with the notion that the ability to shift from the initially preferred choice option to alternative options is the relevant variable determining lateral OFC activation, as well as performance on the IGT, and not the ability to look into the future. The Windmann et al. study was the first one to use variant versions of the IGT, such as the ones used in this study. However, they did not use a control task such as the one we used here, and they primarily focused on the contrasts that the different versions of the IGT bring. In a recent paper, using a modified IGT, Lawrence et al. [ 2008] studied the distinct roles of the prefrontal regions in IGT using event related fMRI, and they found that choices from disadvantageous versus advantageous decks produced activation in the medial frontal gyrus, lateral orbitofrontal cortex, and insula. These regions, along with the pre‐supplementary motor area and secondary somatosensory cortex, were positively correlated with task performance. This study is highly consistent with aims of the current study and provides a strong independent support for the role of the somatic marker circuitry under study in decision making. The key distinction is that Lawrence et al. used a modified version of the IGT to render it more suitable for use in event related fMRI. As this modified version has not yet been validated in clinical populations, our study employs versions of the IGT that were tested in a variety of clinical samples with decision‐making deficits. Finally, a more recent study by Frangou et al. [ 2008] examined patients with bipolar disorder and a healthy control group performing the IGT inside an fMRI scanner. They found that while healthy controls showed significant activation in both the ventral and dorsal prefrontal cortex, this activation was attenuated in bipolar disorder patients. Again, this is consistent with prior studies conducted with PET in clinical populations, and it suggests that patients who may suffer from decision making impairments may reveal abnormal activity in certain key components of the somatic marker neural circuitry.
In light of these functional neuroimaging results and the theoretical framework of the SMH, we tested the hypothesis that performance of the IGT during fMRI will engage a neural circuitry consisting of the dlPFC (for working memory), the insula and posterior cingulate/retrosplenial cortex (for representations of emotional states), the mOFC/vmPFC (for coupling the two previous processes), the ventral striatum and anterior cingulated/SMA (supplementary motor area) for implementing behavioral decisions.
METHODS
Participants
Ten healthy undergraduate or graduate students (five females and five males), all right‐handed, with a mean age of 23.1 years (SD = 3.3 years), with normal or corrected‐to‐normal vision, were recruited from the University of Southern California (USC). They all gave fully informed consent prior to the participation. All experimental procedures had prior approval by the Institutional Review Board at USC.
Tasks
In addition to the original version of the IGT, we used three variant versions to increase the number of trials during the fMRI session. The original version (with decks A′B′C′D′) is widely used in the literature [Bechara et al., 2000b]. Another alternate version of the task (with decks K′L′M′N′) was also used. This K′L′M′N′ version has been developed and tested in prior behavioral studies for the purpose of enabling repeated testing by countering the practice effects using two manipulations [Preston et al., 2007]: (1) reducing the percentage of times that advantageous selections yielded lower initial rewards than disadvantageous selections, and (2) reducing the magnitude of increases in net gains and losses within the good and bad decks. The third version of the IGT (with decks E′F′G′H′), identical to the one that has been used in some studies [Bechara and Damasio, 2002; Bechara et al., 2000b, 2002] was also used. The key feature of this variant version is that the delivery of gains and losses are opposite to those in the original version: in the original version (A′B′C′D′), the gains are immediate and the losses are delayed; in the variant version (E′F′G′H′), the losses are immediate, and the gains are delayed. Similar to the original version, an alternate version of the E′F′G′H′ with decks I′J′O′P′ was developed for the same purpose. Because the alternate I′J′O′P′ version was specifically developed to mitigate the learning and practice effect of the parent version (E′F′G′H′), this alternate version must always follow the administration of the parent version. Thus, the K′L′M′N′ task was a parallel version to the original A′B′C′D′ task, whereas the I′J′O′P′ task was a parallel version to the E′F′G′H′ task. These parallel tasks (K′L′M′N′ and I′J′O′P′) have been tested for their reliability and validity on a large sample of healthy subjects and neurological patients in a separate study (manuscript in preparation).
Figure 1A shows a computer snapshot of the original IGT. The subject sees four decks of cards, labeled A′, B′, C′, and D′. The subject can choose a card from any of the four decks. The computer tracks the sequence of the cards selected from the various decks. Every time the subject picks a card from a deck, a message is displayed on the screen indicating the amount of money the subject has won or lost. Specifically, after selecting a deck with a reward, the following message is displayed: “Win $ X!.” When the gain is followed by a loss/punishment, the following message is displayed: “Win $ X! but lose $ Y.” Different audio feedbacks are also given for gains and losses. A green bar at the top of the screen displayed the cumulative monetary reward. A gain is indicated by a proportionate increase in the length of the green bar, and a loss is indicated by a proportionate decrease in the length of the bar. Each deck includes 60 cards. The backs of the cards, as they appear on the screen, all look the same.
Figure 1.
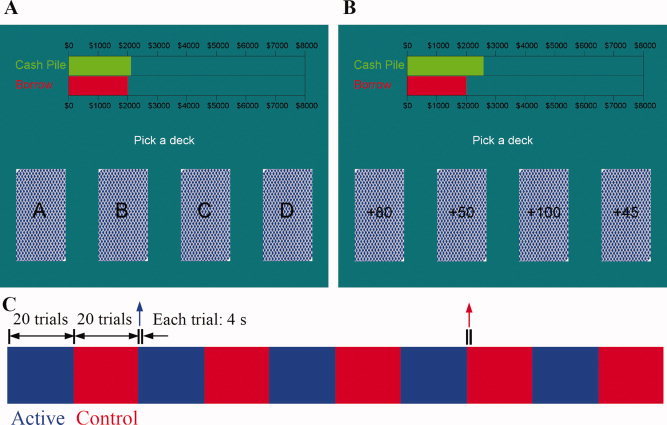
Tasks and design. (A) A computer screen snapshot of the IGT. (B) A computer screen snapshot of the control task. (C) Diagram of an fMRI run. Each run contained five blocks of the IGT (blue) and five blocks of the control task (red). There were 20 trials in each block. Each trial lasted 4 s. The instruction “Pick a deck” at the center of the screen indicated the beginning of a trial.
A control task (Fig. 1B) was designed in such a way that it matched the IGT on its sensorimotor components, but without the process that involves making a complex decision. More specifically, on each trial, the win/loss amount was displayed on each deck. The win/loss amount of the cards was randomly chosen from the same four decks so that the overall win/loss amount was similar to the real IGT. There were no advantageous or disadvantageous decks in this control task. Subjects were simply asked to choose the card with the maximum gain or minimum loss. Thus, the control task contained all the visual and feedback characteristics of the experimental tasks, and a simple decision such as choosing a high or low number, but without the requirement to make a complex decision, as required in the IGT, involving the selection of an advantageous choice.
Design and Procedures
The IGT program used in this study was written in Matlab based on the Psychtoolbox extensions [Brainard, 1997]. Several technical changes from the original IGT were implemented to suit the fMRI environment. First, the duration for each trial was set at 4 s. If subjects did not make their choice after 3.5 s had passed, the computer would randomly make a selection. We tried several interval settings and found that the 4 s interval is long enough for subject to make their selection, and yet it allowed us to have the same number of trials as in the original IGT, without exceeding the typical length of an fMRI run. Second, blocks of trials from the “control” task were interspersed with blocks of trials from the IGT. Third, instead of using computer mouse to select a deck, subjects used 4 buttons of an MRI‐compatible response box.
Prior to the fMRI experiment, subjects were given instructions on the IGT. Details of these instructions have been published previously [Bechara et al., 2000b]. Subjects were given a chance to practice a few trials on a dummy IGT to get familiar with screen instructions, audio feedback, and the use of the buttons to make their choices. The dummy IGT included a feedback just like the actual task, except that subjects did not make decisions based on prior outcomes; subjects were simply instructed to select each of the four decks once, starting from left to right. The gains and losses associated with each selection were similar to the real IGT, thus providing subjects certain familiarity with the procedure.
In each fMRI run, there were five blocks of the IGT and five blocks of the control task, interleaved (Fig. 1C). There were 20 trials in each block, so the total number of trials was 100 for each version of the IGT. The scores for the IGT and control task were displayed and recorded separately.
We did four runs for each subject. Each run was a different version of the IGT (A′B′C′D′, K′L′M′N′, E′F′G′H′, and I′J′O′P′) described earlier. Half of the subjects did A′B′C′D′ and K′L′M′N′ first, followed by E′F′G′H′ and I′J′O′P′, and the other half did E′F′G′H′ and I′J′O′P′ first.
Image Acquisition
MRI recording was performed using a standard birdcage head‐coil on a Siemens 3T MAGNETON Trio MRI system housed in the Dana and David Dornsife Cognitive Neuroscience Imaging Center at USC. Subjects lay supine on a scanner bed, and viewed visual stimuli back‐projected onto a screen through a mirror built into the head coil. Foam pads were used to minimize head motion.
For each subject, sagittal images (256 × 256 × 192) of 1 mm3 isotropic spatial resolution were obtained with a T1‐weighted 3D MPRAGE sequence (TI = 900 ms, TR = 2,070 ms, TE = 4.13 ms, flip angle = 7°). Blood‐oxygenation‐level‐dependent (BOLD) signal were measured with a T2*‐weighted echo‐planar imaging (EPI) sequence (TR = 2,000 ms, TE = 25 ms, flip angle = 90°, FOV = 192 mm × 192 mm, in‐plane resolution = 64 × 64 pixels or 3 mm × 3 mm). Thirty‐five interlaced coronal slices, with thickness of 3.5 mm (no gap), were acquired. For each subject, four functional runs were collected, each of which lasted 13.6 min. Each session lasted about 1.5 h.
Image Analysis
All MRI‐ and fMRI‐related data analyses were performed using BrainVoyager QX 1.9 (Brain Innovation, Maastricht, The Netherlands). The anatomical data for each subject were corrected for image intensity inhomogeneity, and transformed into Talairach space [Talairach and Tournoux, 1988]. The gray‐white matter boundaries resulted from gray‐white matter segmentation that were used to create a 3D surface model of the brain, which was then inflated to display both sulci and gyri on the smooth surfaces (Fig. 2A,B) of the two hemispheres. Twelve possibly involved brain regions in each hemisphere (Table I) were defined as region‐of‐interests (ROIs) based on anatomical features [Damasio, 2005]. Regions on the cortical surface, e.g., the frontal gyri, were defined on the inflated hemispheres and converted back to their anatomical images. For regions that are not on the cortical surface, e.g., amygdala and striatum, ROIs were drawn directly on the anatomical images (Fig. 2C). The average limits for amygdala are 5.6 to −7.8 (anterior to posterior) and 10.9 to 27.9 (medial to lateral), and those for striatum are 23.5 to −22.8 and 3.4 to 30.9. An anatomy expert identified each anatomical region directly on the scan, as opposed to relying on coordinates (e.g., Talairach coordinates) to identify anatomical regions. Signal loss in the EPI images in the left lOFC, right lOFC, left mOFC, and right mOFC, was about 4, 14, 31, and 30%, respectively.
Figure 2.
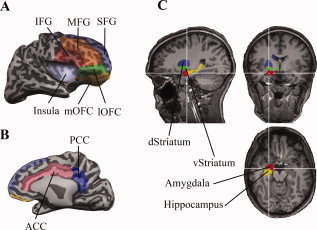
ROI definitions based on anatomical features. (A) Lateral view of an inflated right hemisphere, showing the ROIs on the surface. These surface‐defined ROIs were converted into 3D volume for ROI analysis. (B) Medial view of the hemisphere, showing the cingulate gyrus. (C) Non‐surface‐based ROIs were drawn directly in 3D volume.
Table I.
Region‐of‐interests used in the study
| Short name or abbreviation | Full name or description |
|---|---|
| Amygdala | Amygdala |
| ACC | Cingulate cortex anterior to ascending branch of cingulate sulcus |
| PCC | Cingulate cortex posterior to ascending branch of cingulate sulcus |
| SFG | Superior frontal gyrus |
| MFG | Middle frontal gyrus |
| IFG | Inferior frontal gyrus |
| dStriatum | Striatum above the anterior commissure in Talairach coordinate |
| vStriatum | Striatum below the anterior commissure in Talairach coordinate |
| HC | Hippocampus |
| Insula | Insula cortex |
| lOFC | Lateral orbitofrontal cortex |
| mOFC | Medial orbitofrontal cortex |
The fMRI data were first preprocessed to correct for slice timing and head motion, followed by high‐pass temporal filtering. The 2D functional images were aligned to the 3D structural images in the same session and constructed into a 3D volume in the Talairach space for each time point.
Although functional areas among subjects do not precisely follow cortical landmarks, it has been shown that the cortical matching approach substantially improves statistical group results by reducing anatomical variability, at least for some of the cortical areas [Fischl et al., 1999]. We therefore aligned each hemisphere of all subjects in BrainVoyager, and converted the 3D time course into the surface‐based time course for each hemisphere of each subject. This allows us to show the averaged activation on the surface of a hemisphere.
General Linear Model was used for both the ROI analysis, volume‐based analysis and cortical surface‐based analysis. The BOLD responses during the IGT relative to the control task were used to do statistical comparison.
Regression Analyses
Our regression analysis focuses on reward and risk processing, and prediction error [D'Acremont et al., 2009; McClure et al., 2003; O'Doherty et al., 2004; Preuschoff and Bossaerts, 2007; Xue et al., 2009]. Following the literature [Holt and Laury, 2002; Preuschoff et al., 2006; Tobler et al., 2007], risk in this study is defined as the variance of the outcome. Prediction error is defined as the difference between the actual pay and the expected value.
For each ROI, the double gamma function of BrainVoyager was used as the hemodynamic response template to extract the response amplitude of each trial, for both IGT trials and control trials1. The amplitudes of the IGT trials from all four versions were used in a regression analysis that assessed the correlation between the BOLD responses in each ROI with three parameters: (1) the average gain for a particular choice in trials prior to t, EV(t – 1), which reflects what a subject expects the outcome is going to be before deciding; (2) the standard deviation of the gain for a particular option in trials prior to t, Risk(t – 1), which reflects what the subject may experience as a subjective feeling of what the risk is; and (3) the difference between the actual and expected pay, Diff(t) = pay(t) – EV(t – 1), which reflects the neuronal activity after the person finds out what the outcome was, and whether it matched the expected outcome.
For each ROI, the relationship between the BOLD response and the three variables was expressed as
where β0,…,β3 are unknown regression coefficients. Ordinary least squares were used to estimate the coefficients:
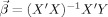 , where
, where
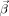 is the vector of coefficients, X represents all the regressors, and Y is the response variable BOLD(t) represented as a vector. One concern with using the ordinary least squares was that the BOLD responses might be autocorrelated. In other words, the error term of the model might not be independent, which violated the basic assumption of ordinary least squares. We examined the use of regression models with ARMA errors, where the error term is modeled by the Box‐Jenkins method. Nonetheless, the overall improvement of fit yielded by such a procedure was minimal. Therefore, the classic ordinary least squares approach was adopted.
is the vector of coefficients, X represents all the regressors, and Y is the response variable BOLD(t) represented as a vector. One concern with using the ordinary least squares was that the BOLD responses might be autocorrelated. In other words, the error term of the model might not be independent, which violated the basic assumption of ordinary least squares. We examined the use of regression models with ARMA errors, where the error term is modeled by the Box‐Jenkins method. Nonetheless, the overall improvement of fit yielded by such a procedure was minimal. Therefore, the classic ordinary least squares approach was adopted.
To examine the association between the BOLD responses with each of the variables, Student's t‐test was applied in each of the 24 regression models to test whether individual coefficient was significantly different from zero (i.e. H 0:βj = 0, j = 1,2,3). As a result, a total of 72 significance tests were carried out. The Bonferroni method was used to adjust the critical level and to ensure that the family‐wise error rate was less than 0.05. In this case, the adjusted critical level was 0.000694. A stepwise regression analysis based on AIC was also performed.
RESULTS
Behavioral Performance
For each version of the IGT, we counted the total number of card selections from the disadvantageous decks, and the total number of card selections from the advantageous decks for each block of 20 cards [Bechara et al., 2000b]. Then, we derived a net score for each block (the number from advantageous decks minus the number from disadvantageous decks); net scores below zero indicate that the subjects were selecting disadvantageously, whereas net scores above zero indicate that subjects were selecting advantageously. By fitting a line to each learning curve (net score of each block vs. number of blocks), we divided performers for each task into learners (positive slope) and nonlearners (negative slope). The missed trials by participants were only 7 of 4,000, so it is unlikely to have an impact on the participant's learning.
Figure 3 shows the behavioral performance for all subjects, learners, and nonlearners. Ten subjects performed 4 tasks each, so we have 40 tasks in total. Subjects failed to figure out the good decks for nearly half (17 of 40) of the tasks. One possible reason for the poor performance may be that the young college students have relatively immature prefrontal cortex [Bechara, 2007; Casey et al., 2000; Crone and van der Molen, 2004; Diamond, 2002; Eshel et al., 2007; Giedd et al., 1999; Steinberg and Morris, 2001; Zelazo et al., 2003]. Another possible reason is that the interference from the control task may affect the decision in IGT in some subjects.
Figure 3.
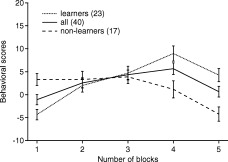
Behavioral performance during the IGT. The learning curves consist of the net score (the number of advantageous selection minus the number of disadvantageous selection) within each IGT block of cards for all subjects and tasks (solid line), learners (dotted line) and non‐learners (dashed line). The error bars are standard error.
As far as gender differences are concerned, we did not detect differences in this particular study (F‐test, F(1,4) = 4.0, P = 0.12), but it is possible that this null result is due to limited number of subjects (five for each group).
Brain Activity During fMRI
We conducted analyses comparing each of the four IGT versions, A′B′C′D′, K′L′M′N′, E′F′G′H′, and I′J′O′P′ to their respective controls. Different versions yielded a very similar response pattern in terms of activated brain regions, although there were slight differences. It is feasible to localize activation using one version of the IGT, but we combined them here to increase the statistical power. Also, our within‐subject comparison between IGT and control blocks makes the number of subjects less of a problem.
Figure 4A shows the brain activity during the active versus control conditions from all 10 subjects, using all four versions of the task. The activations on the cortical surfaces are the result after cortex‐based alignment among 10 subjects. Because some activated brain structures are not on the surface, we showed them in the Talairach space (Fig. 4B). The statistical maps are t‐maps with Bonferroni correction (P < 0.05). The same statistical criterion was applied to all the subsequent statistics, unless noted otherwise. A neural circuitry consistent with the somatic marker neural framework (Fig. 4C) was engaged. Specifically, significant activities were detected in (1) neural systems critical for memory, namely the dorsolateral prefrontal cortex in both hemispheres, although no significant activity was detected in the hippocampus; (2) neural systems critical for processing emotions, namely the anterior insula in both hemispheres, the posterior cingulate cortex in both hemispheres, although no significant activity was detected in the amygdala; (3) neural systems critical for coupling these two systems, namely the mesial orbitofrontal cortex and ventromedial prefrontal cortex in both hemispheres; (4) neural systems critical for behavioral actions, namely the dorsal striatum and supplementary motor area in both hemispheres. The dorsal striatal activity extended to the ventral striatum in the right hemisphere (left side on Fig. 4B), whereas the supplementary motor area activity extended to the ventral striatum in the right hemisphere. Both the anterior cingulate and ventral striatum have been implicated in reward processing and conflict monitoring, both of which are characteristics of the IGT used in this study [Premkumar et al., 2008].
Figure 4.
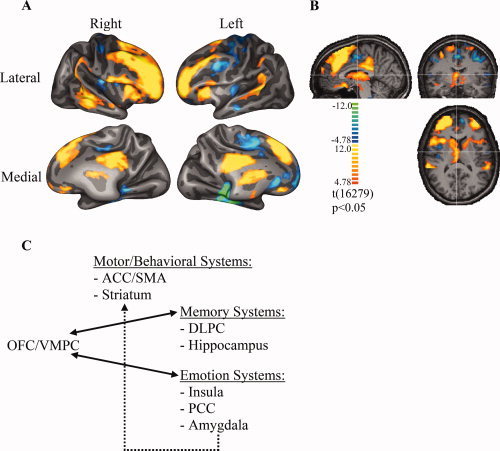
Brain activation during IGT. (A) BOLD activation associated with performance of the IGT, in comparison to its control task, from all 10 subjects. Cortical surfaces of all the subjects were aligned based on their curvatures. Functional maps were transformed to the aligned surface and shown on one partially inflated surface. The target regions include the OFC/vmPFC, the dlPFC, the anterior regions of the insula, the PCC, the striatum (including the ventral striatum), the supracallosal part of the anterior cingulate and adjacent SMA. The threshold for the activation is P < 0.05 with Bonferroni correction. (B) BOLD activations of brain structures that are not on the cortical surface are shown in the Talairach coordinate system. The threshold is the same as in (A). No significant activity was detected in the amygdala and/or hippocampus. (C) A schematic of all the brain regions involved in decision‐making according to the somatic marker hypothesis.
We also conducted ROI analysis on each subject, and then averaged the result across subjects. Figure 5 shows the BOLD amplitude of each possible region for both hemispheres. The ROI analysis confirmed the results based on cortical alignment. It is worthy to note that among all the designated ROI's, the right hemisphere showed stronger activation than the left hemisphere. In some regions, such as the ACC, PCC, ventral striatum, and mOFC, only activation in the right hemisphere reached statistical significance (corrected t‐test by dividing P with number of comparisons, P < 0.05). The overall BOLD amplitudes are small (lower than 0.2%), because the anatomically defined ROI's (see Fig. 2) might be much larger than the actual activation regions (Fig. 4A).
Figure 5.
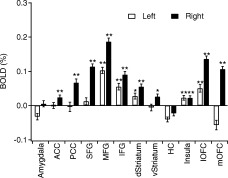
The IGT‐related BOLD response amplitudes of the 12 brain regions defined in Figure 2, shown for each hemisphere separately. The error bars are standard error. **P < 0.01; *P < 0.05; m, 0.05 < P < 0.1.
Finally, we examined whether activity within the somatic marker circuitry changes over time, i.e., examined the activation difference between the first and last block of the IGT. As Figure 3 indicates, many of our subjects surprisingly did not perform as expected on the IGT, but there were several subjects who demonstrated learning. Figure 6 shows the activation map using the cortex‐based alignment method from the subsample of subjects who showed learning. By combining all the four versions of IGT, the results reveal that in the last block, the learners showed more activation in the left OFC and in the right insula/SII region of the somatosensory cortex. This suggests that executing decisions relies on these two key components of the somatic marker circuitry. The right superior temporal gyrus also shows some differential activation, which may be a result of the different auditory feedback in different IGT blocks.
Figure 6.
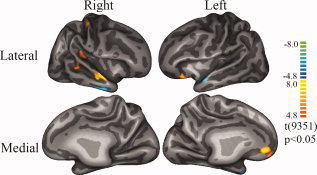
The contrast in brain activations between the first and last block of the IGT among subjects who showed learning in their performance. The analysis method was the same as that used in Figure 4A. The warm color (red) means more activation during the last block, while the cold color (blue) means more activation in the first block.
The lack of robust effects in this contrast (and especially in the nonlearner subgroup) may reflect the fact that in the early stage of the IGT the decisions are completely ambiguous; when learning does not take place by the time we get to the last block of the IGT, the decisions are still ambiguous, thus resulting in an absent or very weak changes in activity due to comparisons of very similar brain states. We note that these same regions were implicated in a study by Lawrence et al. [ 2008], where they examined how brain activity changes over time during performance of the IGT. However, in the Lawrence et al. study, there were linear decreases, as opposed to increases, in activity over time within these same regions. This is intriguing in light of some of the regression results that we found later. We found that when the expectancy for gain gets higher (or when the decider becomes more certain of their decision), the key regions of the somatic marker circuitry begins to shut down. Given in the Lawrence et al. study that some of the subjects showed much more robust learning (i.e., reaching a higher conceptual stage where the decider becomes more certain about their decision), it is possible that the need for the somatic marker circuitry becomes less important. In other words, engaging the somatic circuitry is especially important when making decisions under ambiguity, as opposed to decisions involving more certainty.
Regression Results
Table II shows the coefficients of regression analysis. Very similar results were obtained in the stepwise regression analysis. The regression analysis provides more detail about the possible role during the IGT of each ROI. The expected gain seems to engage a neural circuitry that considerably overlaps with what we have described as the Somatic Marker neural circuitry. Interestingly, this circuitry seems to be more lateralized to the right side, as originally proposed by Damasio [ 1994]. Specifically, this neural circuitry involves regions important for (1) processing of memory, working memory, and its executive processes. In support, the regression analysis showed significant correlation between the expected gain and the BOLD responses in the hippocampus bilaterally, SFG bilaterally, and the right MFG, IFG, and lOFC; (2) the processing of emotions, and in support we found significant correlation between the expected gain and the BOLD responses in the amygdala on the right, and the insula bilaterally; and (3) regions that couple or link the two, namely the OFC/vmPFC region, which we have considered it to include the ACC region below the genu of the corpus callosum, and all the cortex anterior to it to the frontal pole, and all the way inferiorly to include the mOFC region [Bechara et al., 2000a]. In this study, we found significant correlation between expected gain and the BOLD responses in the ACC (on the left) and mOFC (on the right), which is consistent with the outlined neural framework. It is interesting to note, however, that the significant correlation coefficients were in the negative direction, meaning that the higher the expectancy of a gain is, the lower the BOLD signal becomes. This is intriguing as it may suggest that when expectancy gets high (or as one almost becomes certain), the somatic maker circuitry that is important for decision‐making shuts down (i.e., no longer needed for pondering a decision).
Table II.
Regression coefficients (10− 3) for each ROI on both hemispheres
| ROIs | Left hemisphere | Right hemisphere | ||||
|---|---|---|---|---|---|---|
| EV | Risk | Diff | EV | Risk | Diff | |
| Amygdala | 2.1 | 3.1* | −0.24 | −3.1* | −2.0* | −0.52 |
| ACC | −2.5* | 2.6* | 0.44 | −0.67 | 1.7* | 0.66* |
| PCC | −2.1 | 2.2* | 0.18 | −0.63 | 4.2* | 0.36 |
| SFG | −3.0* | 0.15 | 0.15 | −2.5* | −0.72 | 0.27 |
| MFG | −0.78 | −0.027 | 0.70* | −3.8* | −1.2 | 0.78* |
| IFG | −1.8 | 0.83 | −0.76* | −4.4* | −1.2 | −0.12 |
| dStriatum | −1.7** | −0.36 | 0.32 | −0.78 | 0.017 | 0.68* |
| vStriatum | −0.91 | 0.30 | 1.2* | −0.048 | −0.19 | 0.51** |
| HC | −2.1* | 0.33 | 0.23 | −3.0* | 1.3 | −0.30 |
| Insula | −3.1* | 1.1 | −0.63* | −1.8* | 1.6* | 0.20 |
| lOFC | 0.37 | −0.18 | 0.58 | −3.9* | 1.3 | 0.24 |
| mOFC | −0.067 | 3.7* | 0.21 | −1.8* | −0.61 | 0.66* |
Significance levels with Bonferroni correction: *P < 0.05; **0.05 < P < 0.1.
In relation to the risk parameter, two regions have been implicated in the emergence of such a feeling. One is the insula [Craig, 2002; Damasio, 1999; Ernst and Paulus, 2005; Paulus, 2007; Paulus et al., 2003a, b; Paulus and Stein, 2006], and the other is the PCC and surrounding regions, i.e., the precuneus and retrosplenial region [Damasio, 1999; Damasio et al., 2000]. The PCC activation, especially on the right, was exclusively associated with the risk parameter. Insula activation on the right side was also observed, but this activity was also observed in connection with the other two parameters. Although some lesion studies have shown that the insula is critical for risk adjustment [Clark et al., 2008], other lesion studies have shown that both the amygdala and the OFC/vmPFC region play a role in risk estimation [Weller et al., 2007]. More specifically, it was suggested that the amygdala operates in System 1 fashion, in that it instinctively, automatically, and subconsciously sends a signal to the OFC/vmPFC about the presence of a risk. Once the OFC/vmPFC is signaled, then it operates in System 2 fashion, in that it consciously engages the person in the deliberation and evaluation of the risk. The regression analysis revealed that both areas were engaged: the amygdala bilaterally, and the ACC bilaterally, as well as the mOFC on the left side, which are included in what we have called OFC/vmPFC region. We note here that except for one correlation coefficient (right amygdala for risk), all the significant correlation coefficients were in the positive direction, suggesting that when risk prospects get higher, and risk evaluation is needed, the somatic maker circuitry for decision‐making becomes engaged, as reflected by an increase in the BOLD signal.
Finally, the regression analyses revealed that the difference between the actual and expected gain engages a neural circuitry that involves the mesolimbic dopamine system [Glimcher et al., 2005; Schultz, 1997]. The key element of that neural circuitry is the ventral striatum. In support, our analysis revealed significant ventral striatum correlation between difference of actual and expected gain and the BOLD responses on the left side. However, this can also involve the dorsal striatum, which was also significantly correlated on the right side. The activation of these striatum dopamine target sites was exclusive to the “difference” parameter, and these regions were not active in connection with any of the other two parameters. Additional neural regions were engaged in connection with, but not exclusive to, the “difference” parameter. These regions included the MFG bilaterally, and the IFG on the left side. These areas are known to be important for working memory and executive functions, especially the ability to shift attention from one dimension to the other. Other regions that were engaged included the ACC (on the right) and mOFC (on the right), areas known to be important for making decisions. These results are consistent with the hypothesis that when an error is detected (between expected and experienced outcome) and dopamine is released, this signal is transmitted to other neural regions, such as the MFG and IFG, as well as the vmPFC (ACC and mOFC) to adjust learning and decision‐making, and to shift attention away from a previously unsuccessful strategy. Another region that was significantly active in association with the difference parameter was the insula on the left side. Given the more recent evidence from lesion studies showing that insula is important for risk adjustment [Clark et al., 2008], we suggest that the dopamine error signal would also be transmitted to the insula in order to adjust the “feeling” of risk associated with the choice. We note, however, that most of the difference correlation coefficients were positive, indicating that the greater error signal is, the higher the BOLD signal is in the correspondent region, except for the left inferior frontal gyrus (IFG) and left insula. It is unclear why these regions would dis‐engage during error signal detection, but it is possible that in order to correct for learning, some neural regions will increase its activity at the expense of decreased activity in other regions.
DISCUSSION
Our results indicate that the pattern of brain activities during performance of the IGT support the general theoretical framework of the SMH, that a neural circuitry involving the dorsolateral prefrontal cortex (for working memory), the insula and posterior cingulate cortex (for representations of emotional/somatic states), the mesial orbitofrontal and ventromedial prefrontal cortex (for coupling the two previous processes), the ventral striatum and anterior cingulate/SMA for implementing behavioral decisions were engaged during performance of the IGT. We note that lesion studies have shown that the amygdala is also a key structure in the operation of the somatic marker circuitry and in decision‐making as measured by the IGT [Bechara, 2004; Bechara and Damasio, 2005], but the current results did not reveal any significant activity in this region. This finding does not exempt the amygdala from playing a critical role in triggering somatic states and in decision‐making, but rather it suggests that the lack of activity may reflect a combination of the functional properties of the amygdala (e.g., rapid neuronal firing and habituation) and the experimental procedures designed to circumvent this issue in fMRI [Buchel et al., 1998]. Another region thought to be important for decision‐making is the hippocampus, because complex decisions require the ability to remember certain information for more than 40 s, which is the limit of the working memory capacity of the dlPFC [Eldridge et al., 2000]. We failed to detect significant activity in the hippocampus during performance of the IGT. Again this lack of activity does not necessarily preclude the importance of memory and the hippocampus in decision‐making, but similar to the case of the amygdala, it could be the result of the experimental procedure that was used.
These findings are significant because they validate with fMRI the neural circuitry thought to be engaged during decision‐making as measured by performance on the IGT. Because several clinical conditions associated with poor decision‐making in real‐life have also been associated with poor performance on the IGT, the current results open a new window to understand the underlying decision‐making deficits among different clinical conditions. Decision‐making is a complex process that relies on multiple neural systems, including systems for memory, emotion/affect, and feeling; damage to any of these systems compromises the ability to make decisions that are advantageous in the long‐term. The OFC/vmPFC region links these systems together, and therefore, when dysfunctional there are many manifestations, including alterations of emotional/affective experience, poor decision‐making and impulse control, and abnormal social functioning. However, dysfunction within any of the other neural systems that feed into the OFC/vmPFC system could also lead to the same decision‐making impairments. Thus different clinical conditions could have similar behavioral or clinical manifestations, but the underlying neural dysfunction can be different. The links established here between performance on the IGT, and a neural circuitry that encompasses different neural systems involved in decision‐making will facilitate determination of more specific neural dysfunction underlying the poor decision‐making behavior observed in different clinical conditions. Specific questions would include, for example, what underlies the poor decision‐making behavior observed in substance abusers? Is it hyperactivity in the affect/emotion related systems linked to reward, is it abnormality in their working memory system, or is it a problem at the level where motivation gets translated into motor responses and behavioral actions? This systemic approach to understand the underlying neural dysfunction of a given clinical condition can be effective in informing proper strategy for treatment or management.
It is important to note that the IGT has encountered numerous criticisms for its complexity, and the implication of several auxiliary processes in successful performance. Therefore, many investigators have opted for studies aimed at dissecting the neural mechanisms of decision‐making using simpler tasks where more specific cognitive processes of decision‐making can be targeted. However, there are disadvantages to the use of simple decision tasks as well. Specifically, although the use of simple tasks is advantageous for targeting more specific processes, the drawback is that such tasks are not clinically valid. Indeed, the rationale for developing the IGT was capturing the decision‐making deficit characteristic of a certain group of neurological patients with vmPFC damage. Simpler decision‐making tasks failed to capture the deficit in these patients. Therefore, adopting experimental decision‐making tasks that are not clinically valid may also have disadvantages. Thus, there is a tradeoff in designing tasks that focus on process specificity versus clinical validity. We argue that the use of simpler decision tasks in fMRI may be questionable in terms of clinical and real‐life validity, and it is important to tackle down the methodological complexities associated with using complex decision‐making tasks, such as the IGT, which have established clinical validity. We believe that the long line of studies testing predictions from the SMH using the IGT provides a strong theoretical foundation on which we can separate the more specific neurocognitive processes involved in decision‐making as measured by performance on the IGT. Furthermore, we suggest that the time is ripe to use other approaches for deciphering the complexity of decision‐making tasks such as the IGT, using modeling approaches such as those previously used to decompose behavioral performance on the IGT into more specific cognitive processes [Yechiam et al., 2005], including the relative impact of rewards and punishments on evaluations, the rate that the contingent payoffs are learned, and the consistency between learning and responding. In this study, we have used regression analyses approaches that also attempt to decompose the same behavioral performance on the IGT into simpler constructs of moment‐to‐moment observer state, with focus on reward and risk processing, and prediction error [Hollander et al., 2005; McClure et al., 2003; O'Doherty et al., 2004; Preuschoff and Bossaerts, 2007; Xue et al., 2009]. Compared to the constructs of cognitive models, the moment‐by‐moment expected value, uncertainty, and prediction error reflect the state of the subject and may be more easily related to brain activations. The analyses revealed evidence that is consistent with prior literature addressing these parameters with more specific behavioral tasks, thus supporting the validity of this approach.
In conclusion, this study corroborates findings of prior functional neuroimaging studies in terms of engaging certain neural regions during performance of the IGT. The key difference here is fitting neural activities that emerge from multiple neural systems into a cohesive neural framework on decision‐making that is rooted in theory and empirical evidence, and which can eventually be applied in a systemic way to understand the decision‐making impairments of various clinical conditions.
Acknowledgements
We wish to thank Jiancheng Zhuang for help in fMRI data collection.
Footnotes
The relatively short trial duration (4 s) is not ideal for extracting trial‐by‐trial BOLD response amplitudes. However, we could not afford longer trial durations, primarily because we wanted to include all the 100 trials of the original IGT in each run. In fact, each run in this study is nearly 14 min, which is on the long side for an fMRI run. Furthermore, based on many years of experience with the IGT, using longer trial durations renders the task too boring for the subject. The way we extract the trial‐by‐trial BOLD amplitude by the use of a HRF template can therefore be viewed as an approximation. Traditional jittered delay between trials would not help because we need to extract the amplitude of every single trial.
REFERENCES
- Apkarian AV, Sosa Y, Krauss BR, Thomas PS, Fredrickson BE, Levy RE, Harden RN, Chialvo DR ( 2004): Chronic pain patients are impaired on an emotional decision‐making task. Pain 108: 129–136. [DOI] [PubMed] [Google Scholar]
- Bechara A ( 2004): Disturbances of emotion regulation after focal brain lesions. Int Rev Neurobiol 62: 159–193. [DOI] [PubMed] [Google Scholar]
- Bechara A ( 2007): Iowa Gambling Task Professional Manual. Lutz, FL: Psychological Assessment Resources, Inc. [Google Scholar]
- Bechara A, Damasio H ( 2002): Decision‐making and addiction (part I): Impaired activation of somatic states in substance dependent individuals when pondering decisions with negative future consequences. Neuropsychologia 40: 1675–1689. [DOI] [PubMed] [Google Scholar]
- Bechara A, Martin E ( 2004): Impaired decision‐making related to working memory deficits in substance addicts. Neuropsychology 18: 152–162. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio AR ( 2005): The somatic marker hypothesis: A neural theory of economic decision. Game Econ Behav 52: 336–372. [Google Scholar]
- Bechara A, Damasio H, Damasio AR ( 2000a): Emotion, decision making and the orbitofrontal cortex. Cereb Cortex 10: 295–307. [DOI] [PubMed] [Google Scholar]
- Bechara A, Tranel D, Damasio H ( 2000b): Characterization of the decision‐making deficit of patients with ventromedial prefrontal cortex lesions. Brain 123: 2189–2202. [DOI] [PubMed] [Google Scholar]
- Bechara A, Dolan S, Hindes A ( 2002): Decision‐making and addiction (part II): Myopia for the future or hypersensitivity to reward? Neuropsychologia 40: 1690–1705. [DOI] [PubMed] [Google Scholar]
- Best M, Williams JM, Coccaro EF ( 2002): Evidence for a dysfunctional prefrontal circuit in patients with an impulsive aggressive disorder. Proc Natl Acad Sci USA 99: 8448–8453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blessing WW 1997. Anatomy of the Lower Brainstem. The Lower Brainstem and Bodily Homeostasis. New York, Oxford: Oxford University Press; pp 29–99. [Google Scholar]
- Bolla KI, Eldreth DA, London ED, Kiehl KA, Mouratidis M, Contoreggi CS, Matochik JA, Kurian V, Cadet JL, Kimes AS, Funderburk FR, Ernst M ( 2003): Orbitofrontal cortex dysfunction in abstinent cocaine abusers performaing a decision‐making task. NeuroImage 19: 1085–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brainard DH ( 1997): The psychophysics toolbox. Spat Vis 10: 433–436. [PubMed] [Google Scholar]
- Brand M, Fujiwara E, Borsutzky S, Kalbe E, Kessler J, Markowitsch HJ ( 2005): Decision‐making deficits of korsakoff patients in a new gambling task with explicit rules: Associations with executive functions. Neuropsychology 19: 267–277. [DOI] [PubMed] [Google Scholar]
- Brand M, Grabenhorst F, Starcke K, Vandekerckhove MM, Markowitsch HJ ( 2007): Role of the amygdala in decisions under ambiguity and decisions under risk: Evidence from patients with Urbach‐Wiethe disease. Neuropsychologia 45: 1305–1317. [DOI] [PubMed] [Google Scholar]
- Buchel C, Morris J, Dolan RJ, Friston KJ ( 1998): Brain systems mediating aversive conditioning: An event‐related fMRI study. Neuron 20: 947–957. [DOI] [PubMed] [Google Scholar]
- Campbell MC, Stout JC, Finn PR ( 2004): Reduced autonomic responsiveness to gambling task losses in Huntington's disease. J Int Neuropsych Soc 10: 239–245. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Giedd JN, Thomas KM ( 2000): Structural and functional brain development and its relation to cognitive development. Biol Psychol 54: 241–257. [DOI] [PubMed] [Google Scholar]
- Cavedini P, Riboldi G, Keller R, D'Annucci A, Bellodi L ( 2002): Frontal lobe dysfunction in pathological gambling patients. Biol Psychiat 51: 334–341. [DOI] [PubMed] [Google Scholar]
- Cavedini P, Bassi T, Ubbiali A, Casolari A, Giordani S, Zorzi C, Bellodi L ( 2004): Neuropsychological investigation of decision‐making in anorexia nervosa. Psychiat Res 127: 259–266. [DOI] [PubMed] [Google Scholar]
- Clark L, Cools R, Robbins T ( 2004): The neuropsychology of ventral prefrontal cortex: Decision‐making and reversal learning. Brain Cogn 55: 41–53. [DOI] [PubMed] [Google Scholar]
- Clark L, Bechara A, Damasio H, Aitken MR, Sahakian BJ, Robbins TW ( 2008): Differential effects of insular and ventromedial prefrontal cortex lesions on risky decision‐making. Brain 131: 1311–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AD ( 2002): How do you feel? Interoception: The sense of the physiological condition of the body. Nat Rev Neurosci 3: 655–666. [DOI] [PubMed] [Google Scholar]
- Crone EA, van der Molen MW ( 2004): Developmental changes in real life decision making: Performance on a gambling task previously shown to depend on the ventromedial prefrontal cortex. Dev Neuropsychol 25: 251–279. [DOI] [PubMed] [Google Scholar]
- D'Acremont M, Lu Z‐L, Li X, Van der Linden M, Bechara A ( 2009): Neural correlates of risk prediction error during reinforcement learning in humans. Neuroimage 47: 1929–1939. [DOI] [PubMed] [Google Scholar]
- Dalgleish T, Yiend J, Bramham J, Teasdale JD, Ogilvie AD, Malhi G, Howard R ( 2004): Neuropsychological processing associated with recovery from depression after stereotactic subacute tractotomy. Am J Psychiatry 161: 1913–1916. [DOI] [PubMed] [Google Scholar]
- Damasio AR ( 1994): Descartes' Error: Emotion, Reason, and the Human Brain. New York: Grosset/Putnam. [Google Scholar]
- Damasio AR ( 1996): The somatic marker hypothesis and the possible functions of the prefrontal cortex. Philos Trans R Soc Lond B Biol Sci 351: 1413–1420. [DOI] [PubMed] [Google Scholar]
- Damasio AR ( 1999): The Feeling of What Happens: Body and Emotion in the Making of Consciousness. New York: Harcourt Brace & Company. [Google Scholar]
- Damasio H ( 2005): Human Brain Anatomy in Computerized Images. New York: Oxford University Press. [Google Scholar]
- Damasio AR, Grabowski TJ, Bechara A, Damasio H, Ponto LL, Parvizi J, Hichwa RD ( 2000): Subcortical and cortical brain activity during the feeling of self‐generated emotions. Nat Neurosci 3: 1049–1056. [DOI] [PubMed] [Google Scholar]
- Davis C, Levitan RD, Muglia P, Bewell C, Kennedy JL ( 2004): Decision‐making deficits and overeating: A risk model for obesity. Obes Res 12: 929–935. [DOI] [PubMed] [Google Scholar]
- Denburg NL, Tranel D, Bechara A ( 2005): The ability to decide advantageously declines prematurely in some normal older persons. Neuropsychologia 43: 1099–1106. [DOI] [PubMed] [Google Scholar]
- Diamond A ( 2002): Normal development of prefrontal cortex from birth to young adulthood: Cognitive functions, anatomy, and biochemistry. In: Stuss DT, Knight RT, editors. New York: Oxford University Press; pp 466–503. [Google Scholar]
- Dunn BD ( 2006): The somatic marker hypothesis: A critical evaluation. Neurosci Biobehav Rev 30: 239–271. [DOI] [PubMed] [Google Scholar]
- Eldridge LL, Knowlton BJ, Furmanski CS, Bookheimer SY, Engel SA ( 2000): Remembering episodes: A selective role for the hippocampus during retrieval. Nat Neurosci 3: 1149–1152. [DOI] [PubMed] [Google Scholar]
- Ernst M, Paulus MP ( 2005): Neurobiology of decision making: A selective review from a neurocognitive and clinical perspective. Biol Psychiatry 58: 597–604. [DOI] [PubMed] [Google Scholar]
- Ernst M, Bolla K, Moratidis M, Contoreggi CS, Matochick JA, Kurian V, Cadet JL, Kimes AS, London ED ( 2002): Decision‐making in a risk taking task. Neuropsychopharmacol 26: 682–691. [DOI] [PubMed] [Google Scholar]
- Eshel N, Nelson EE, Blair RJ, Pine DS, Ernst M ( 2007): Neural substrates of choice selection in adults and adolescents: Development of the ventrolateral prefrontal and anterior cingulate cortices. Neuropsychologia 45: 1270–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM ( 1999): Cortical surface‐based analysis. II: Inflation, flattening, and a surface‐based coordinate system. NeuroImage 9: 195. [DOI] [PubMed] [Google Scholar]
- Frangou S, Kington J, Raymont V, Shergill SS ( 2008): Examining ventral and dorsal prefrontal function in bipolar disorder: A functional magnetic resonance imaging study. European Psychiatry 23: 300–308. [DOI] [PubMed] [Google Scholar]
- Fukui H, Murai T, Fukuyama H, Hayashi T, Hanakawa T ( 2005): Functional activity related to risk anticipation during performance of the Iowa Gambling Task. NeuroImage 24: 253–259. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL ( 1999): Brain development during childhood and adolescence: A longitudinal MRI study. Nat Neurosci 2: 861–863. [DOI] [PubMed] [Google Scholar]
- Glimcher PW, Dorris MC, Bayer HM ( 2005): Physiological untility theory and the neuroeconomics of choice. Behavior 52: 1–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Alia‐Klein N, Tomasi D, Zhang L, Cottone LA, Maloney T, Telang F, Caparelli EC, Chang L, Ernst T, Samaras D, Squires NK, Volkow ND ( 2007): Is decreased prefrontal cortical sensitivity to monetary reward associated with impaired motivation and self‐control in cocaine addiction? Am J Psychiatry 164: 43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollander E, Pallanti S, Baldini Rossi N, Sood E, Baker BR, Buchsbaum MS ( 2005): Imaging monetary reward in pathological gamblers. World J Biol Psychiatry 6: 113–120. [DOI] [PubMed] [Google Scholar]
- Holt CA, Laury SK ( 2002): Risk aversion and incentive effects. Am Econ Rev 92: 1644–1655. [Google Scholar]
- Jollant F, Bellivier F, Lobyer M, Astruc B, Torres S, Verdier R, Castelnau D, Malafosse A, Courtet P ( 2005): Impaired decision‐making in scuicide attempters. Am J Psychiatry 162: 304–310. [DOI] [PubMed] [Google Scholar]
- Lawrence NS, Jollant F, O'Daly O, Zelaya F, Phillips ML ( 2008): Distinct roles of prefrontal cortical subregions in the Iowa Gambling Task. Cereb Cortex 154: 1134–1143. [DOI] [PubMed] [Google Scholar]
- Maia TV, McClelland JL ( 2004): A reexamination of the evidence for the somatic marker hypothesis: What participants really know in the Iowa gambling task. Proc Natl Acad Sci USA 101: 16075–16080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malloy‐Diniz L, Fuentes D, Borges‐Leite W, Correa H, Bechara A. ( 2007): Impulsive behavior in adults with attention deficit/hyperactivity disorder: Characterization of attentional, motor and cognitive impulsiveness. J Int Neuropsych Soc 13: 693–698. [DOI] [PubMed] [Google Scholar]
- Manes F, Sahakian B, Clark L, Rogers R, Antoun N, Aitken M, Robbins T ( 2002): Decision‐making processes following damage to the prefrontal cortex. Brain 125: 624–639. [DOI] [PubMed] [Google Scholar]
- Martin C, Denburg N, Tranel D, Granner M, Bechara A ( 2004a): The effects of vagal nerve stimulation on decision‐making. Cortex 40: 1–8. [DOI] [PubMed] [Google Scholar]
- Martin EM, Pitrak DL, Weddington W, Rains NA, Nunnally G, Nixon H, Grbesic S, Vassileva J, Bechara A ( 2004b): Cognitive impulsivity and HIV serostatus in substance dependent males. J Int Neuropsych Soc 10: 931–938. [DOI] [PubMed] [Google Scholar]
- Max JE, Manes FF, Robertson BA, Mathews K, Fox PT, Lancaster J ( 2005): Prefrontal and executive attention network lesions and the development of attention‐deficit/hyperactivity symptomatology. J Am Acad Child Adolesc Psychiatry 44: 443–450. [DOI] [PubMed] [Google Scholar]
- McClure SM, Berns GS, Montague PR ( 2003): Temporal prediction errors in a passive learning task activate human striatum. Neuron 38: 339–346. [DOI] [PubMed] [Google Scholar]
- O'Doherty J, Dayan P, Schultz J, Deichmann R, Friston K, Dolan RJ ( 2004): Dissociable roles of ventral and dorsal striatum in instrumental conditioning. Science 304: 452–454. [DOI] [PubMed] [Google Scholar]
- Paulus MP ( 2007): Decision‐making dysfunctions in psychiatry—Altered homeostatic processing? Science 318: 602–606. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Stein MB ( 2006): An insular view of anxiety. Biol Psychiatry 60: 383–387. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Hozack N, Frank L, Brown GG, Schuckit MA ( 2003a): Decision making by methamphetamine‐dependent subjects is associated with error‐rate‐independent decrease in prefrontal and parietal activation. Biol Psychiatry 53: 65–74. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Rogalsky C, Simmons A, Feinstein JS, Stein MB ( 2003b): Increased activation in the right insula during risk‐taking decision making is related to harm avoidance and neuroticism. NeuroImage 19: 1439–1448. [DOI] [PubMed] [Google Scholar]
- Persaud N, McLeod P, Cowey A ( 2007): Post‐decision wagering objectively measures awareness. Nat Neurosci 10: 257–261. [DOI] [PubMed] [Google Scholar]
- Premkumar P, Fannon D, Kuipers E, Simmons A, Frangou S, Kumari V ( 2008): Emotional decision‐making and its dissociable components in schizophrenia and schizoaffective disorder: A behavioural and MRI investigation. Neuropsychologia 46: 2002–2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston SD, Buchanan TW, Stansfield RB, Bechara A ( 2007): Effects of anticipatory stress on decision making in a gambling task. Behav Neurosci 121: 257–263. [DOI] [PubMed] [Google Scholar]
- Preuschoff K, Bossaerts P ( 2007): Adding prediction risk to the theory of reward learning. Ann N Y Acad Sci 1104: 135–146. [DOI] [PubMed] [Google Scholar]
- Preuschoff K, Bossaerts P, Quartz SR ( 2006): Neural differentiation of expected reward and risk in human subcortical structures. Neuron 51: 381–390. [DOI] [PubMed] [Google Scholar]
- Roca M, Torralva T, Oacute, Pez P, Cetkovich M, Clark L, Manes F ( 2008): Executive functions in pathologic gamblers selected in an ecologic setting. Cogn Behav Neurol 21: 1–4. [DOI] [PubMed] [Google Scholar]
- Rogers RD, Everitt BJ, Baldacchino A, Blackshaw AJ, Swainson R, Wynne K, Baker NB, Hunter J, Carthy T, Booker E, London M, Deakin JF, Sahakian BJ, Robbins TW ( 1999): Dissociable deficits in the decision‐making cognition of chronic amphetamine abusers, opiate abusers, patients with focal damage to prefrontal cortex, and tryptophan‐depleted normal volunteers: Evidence for monoaminergic mechanisms. Neuropsychopharmacol 20: 322–339. [DOI] [PubMed] [Google Scholar]
- Schultz W ( 1997): A neural substrate of prediction and reward. Science 275: 1593–1599. [DOI] [PubMed] [Google Scholar]
- Sevy S, Burdick K, Visweswaraiah H, Abdelmessih S, Lukin M, Yechiam E, Bechara A ( 2007): Iowa Gambling Task in schizophrenia: A review and new data in patients with schizophrenia and co‐occurring cannabis use disorder. Schizophr Res 92: 74–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L, Morris AS ( 2001): Adolescent development. Annu Rev Psychol 52: 83–110. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P ( 1988): Co‐Planar Stereotaxic Atlas of the Human Brain. 3‐Dimensional Proportional System: An Approach to Cerebral Imaging. New York: Thieme Medical Publishers. [Google Scholar]
- Tanabe J, Thompson L, Claus E, Dalwani M, Hutchison K, Banich MT ( 2007): Prefrontal cortex activity is reduced in gambling and nongambling substance users during decision‐making. Hum Brain Mapp 28: 1276–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobler PN, O'Doherty JP, Dolan RJ, Schultz W ( 2007): Reward value coding distinct from risk attitude‐related uncertainty coding in human reward systems. J Neurophysiol 97: 1621–1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torralva T, Kipps CM, Hodges JR, Clark L, Bekinschtein T, Roca M, Calcagno ML, Manes F ( 2007): The relationship between affective decision‐making and theory of mind in the frontal variant of fronto‐temporal dementia. Neuropsychologia 45: 342–349. [DOI] [PubMed] [Google Scholar]
- Tucker KA, Potenza MN, Beauvals JE, Browndyke JN, Gottschalk PG, Kosten TR ( 2004): Perfusion abnormalities and decision‐making in cocaine dependence. Biol Psychiat 56: 527–530. [DOI] [PubMed] [Google Scholar]
- van Honk J, Hermans EJ, Putman P, Montague B, Schutter D ( 2002): Defective somatic markers in sub‐clinical psychopathy. Neuroreport 13: 1025–1027. [DOI] [PubMed] [Google Scholar]
- Weller JA, Levin IP, Shiv B, Bechara A ( 2007): Neural correlates of adaptive decision making for risky gains and losses. Psychol Sci 18: 958–964. [DOI] [PubMed] [Google Scholar]
- Whitney KA, Fastnau PS, Evans JD, Lysaker PH ( 2004): Comparative neuropsychological function in obsessive‐compulsive disorder and schizophrenia with and without obsessive‐compulsive symptoms. Schizophr Res 69: 75–83. [DOI] [PubMed] [Google Scholar]
- Windmann S, Kirsch P, Mier D, Stark R, Walter B, Gunturkun O, Vaitl D ( 2006): On framing effects in decision making: Linking lateral versus medial orbitofrontal cortex activation to choice outcome processing. J Cogn Neurosci 18: 1198–1211. [DOI] [PubMed] [Google Scholar]
- Xue G, Lu Z, Levin IP, Weller JA, Li X, Bechara A ( 2009): Functional dissociations of risk and reward processing in the medial prefrontal cortex. Cereb Cortex 19: 1019–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yechiam E, Busemeyer JR, Stout JC, Bechara A ( 2005): Using cognitive models to map relations between neuropsychological disorders and human decision‐making deficits. Psychol Sci 16: 973–978. [DOI] [PubMed] [Google Scholar]
- Zelazo PD, Müller U, Frye D, Marcovitch S, Argitis G, Boseovski J, Chiang JK, Hongwanishkul D, Schuster BV, Sutherland A ( 2003): The development of executive function in early childhood. Monogr Soc Res Child Dev 68: vii–137. [DOI] [PubMed] [Google Scholar]


