Summary
Phosphatidylcholine transfer protein (PC-TP, a.k.a. StarD2) is abundantly expressed in liver and is regulated by PPARα. When fed the synthetic PPARα ligand fenofibrate, Pctp−/− mice exhibited altered lipid and glucose metabolism. Microarray profiling of livers from fenofibrate fed wild type and Pctp−/− mice revealed differential expression of a broad array of metabolic genes, as well as their regulatory transcription factors. PC-TP expression in cell culture controlled the activities of both PPARα and HNF4α, suggesting that the mechanism by which it modulates hepatic metabolism is at least in part via activation of transcription factors that govern nutrient homeostasis.
Keywords: START domain, fibrate drug, lipid, glucose, microarray, gene transcription
Introduction
Phosphatidylcholine transfer protein (PC-TP, a.k.a. StarD2) is a 25 kDa cytosolic lipid binding protein with exquisite specificity for phosphatidylcholines [1]. It is a member of the steroidogenic acute regulatory protein-related lipid transfer (START) domain superfamily of proteins that mediate intracellular lipid transport and metabolism, as well as cellular signaling and gene transcription [2–4]. PC-TP is robustly expressed in oxidative tissues [5] and studies of Pctp−/− mice have revealed key roles in the regulation of lipid and glucose metabolism [6, 7], potentially by regulating the balance of fatty acids and fatty acyl-CoAs within cells [8].
PPARα is a transcription factor that is enriched in oxidative tissues and regulates nutrient metabolism [9]. PPARα itself binds phosphatidylcholines [10] and is activated by endogenous polyunsaturated fatty acids [9]. The PC-TP gene promoter contains consensus DNA response elements for binding PPARα [11], and accumulating evidence suggests that PPARα controls PC-TP expression: Fibrate drugs, which are synthetic ligands that activate PPARα [9], upregulate hepatic PC-TP mRNA [12] and protein [13, 14] in mice.
The aim of the current study was to explore the contributions of PC-TP expression to PPARα-mediated regulation of lipid and glucose homeostasis. In mice administered a fenofibrate-supplemented diet, the absence of PC-TP expression altered lipid and glucose metabolism. Consistent with diverse roles for PC-TP in mediating the effects of PPARα, expression profiling in livers of fenofibrate fed Pctp−/− compared with wild type mice revealed a broad array of differentially expressed metabolic genes. The absence of PC-TP expression also led to differential expression of transcription factors that control hepatic lipid and glucose metabolism. Acute siRNA-mediated knockdown of PC-TP in culture cells altered the transcriptional activities of both PPARα and HNF4α, suggesting a mechanism for the broader impact of this specific lipid binding protein on nutrient metabolism within the liver.
Materials and Methods
Animal and diets
Wild type C57BL/6J mice, as well as PPARα−/− and wild type 129S3/svlmJ mice were obtained from The Jackson Laboratory (Bar Harbor, ME). Pctp−/− mice [15] and wild type littermate controls on an FVB/NJ genetic background were as previously described [16]. A fenofibrate-supplemented chow diet (0.2% wt/wt) was prepared (Bioserve, Frenchtown, NJ) by pelleting fenofibrate obtained from Sigma (St. Louis, MO) together with standard rodent diet 5001 (Purina, St. Louis, MO). Experiments were conducted using male mice at 8–12 w of age. Preliminary experiments revealed that Pctp−/− and wild type mice consumed the same amount of fenofibrate-supplemented chow. Mice were euthanized, blood collected and tissues harvested as described [7]. Protocols for animal use and euthanasia were approved by the institutional committee of Harvard Medical School.
Analytical techniques
Plasma concentrations of triglycerides, cholesterol, nonesterified fatty acids (NEFA) and β-hydroxybutyrate were determined enzymatically [7]. Hepatic triglyceride and cholesterol concentrations were measured following organic extraction [17]. Distributions of cholesterol among lipoprotein particles were determined by FPLC [17]. Plasma insulin concentrations were measured by ELISA [18]. Western and northern blot analyses were as described [11, 19]. Quantitative polymerase chain reaction (qPCR) was performed with RPL32 as an invariant control [7], using primers listed in Supplemental Table 1.
Hepatic triglyceride production rates
Rates of hepatic triglyceride production were measured after 4 h of fasting [7].
Glucose and insulin tolerance tests
After a 4 h fast, baseline glucose concentrations were measured using a OneTouch Ultra glucose monitor (LifeScan, Milpitas, CA). This was immediately followed by glucose or insulin tolerance tests [7].
Microarray analysis of gene expression
Total RNA was extracted from livers of mice fed the fenofibrate-supplemented diet for 7 d (wild type, n = 6; Pctp−/−, n = 6) using Trizol (Invitrogen). Following purification (RNeasy Mini kit, Qiagen, Valencia, CA), cDNA was synthesized, cleaned up and transcribed in vitro using appropriate Affymetrix kits (Affymetrix, Inc., Santa Clara, CA). cRNA samples (20 µg) were hybridized to GeneChip Mouse Genome 430A 2.0 Arrays (one mouse liver per GeneChip) at 45°C in an Affymetrix hybridization oven. Microarrays were prepared and scanned using a Model 450 Fluidics Station and a Model 3000 7G scanner, each controlled by GeneChip Operating Software (Affymetrix).
Microarray data were analyzed by two independent methods. The first utilized DNA-Chip Analyzer (dChip) [20]. Data for individual GeneChips were normalized using the “invariant normalization” function of dChip. Normalized intensity values for individual genes expression were filtered out if a strong signal for the gene was not detected on greater than 30% of the GeneChips. Fold changes (Pctp−/− vs. wild type) of at least 1.2 were considered to be significant for P < 0.05 by two-tailed Student’s t-test. Microarray data were also analyzed using Bayesian Analysis of Differential Gene Expression (BADGE) version 1.0 [21]. This method computes the posterior probability that each gene was regulated more than one fold in Pctp−/− compared with wild type mice. Differentially regulated genes were classified according to biological functions using GenMAPP [22].
Cell culture and knockdown of endogenous PC-TP
Human embryonic kidney (HEK) 293T cells in culture [19] were plated at densities of 3 × 104 cells/well in BD Falcon 12-well plates (BD Biosciences, San Jose, CA). After 24 h, cells were transfected (Lipofectamine, Invitrogen) with 20 nM siRNA (5'-CCAGUAUGUUAAAGAACUC-dTdT-3') to knockdown endogenous PC-TP expression in HEK 293T cells [19] or scrambled Negative siRNA Control #1 (Applied Biosystems/Ambion, Austin, TX). Following 6 h of transfection, fresh media was added for 48 h.
Transcriptional activities of PPARα and HNF4α
To measure PPARα activity, HEK 293T cells were cotransfected with 70 ng of a PPARα promoter-firefly luciferase reporter plasmid (PPRE3-tk-luc; gift from Dr. Jorge Plutzky, Harvard Medical School, Boston, MA), 10 ng of the PPARα expression plasmid pcDNA3.1-hPPARα (gift from Dr. John Chiang, Northeastern Ohio University College of Medicine, Rootstown, OH) plus 1.4 ng of an expression plasmid encoding renilla luciferase (pRL-tk, Promega, Madison, WI) as a control for transfection efficiency. Because in preliminary experiments fenofibrate concentrations ranging up to 100 µM failed to activate PPARα, this was instead accomplished using bovine serum albumin (BSA)/docosahexaenoic acid (DHA) (1:5 mol:mol, 0.6 mM DHA) complexes [23]. BSA/DHA or BSA alone was added to the media for 4 h at the end of the 24 h transfection period. HNF4α activity was similarly measured using 70 ng of HNF4α promoter-firefly luciferase (HNF4-tk-luc) and 70 ng of the HNF4α expression plasmid pCMX-HNF4α (gifts from Dr. Chiang). After 24 h incubation in OPTI-MEM (Invitrogen), cells were harvested using Passive Lysis Buffer (Promega). Luciferase activity was measured using a Dual-Luciferase reporter assay system (Promega).
Statistics
Data are reported as means ± SEM. Differences between groups were analyzed using a two-tailed unpaired Student’s t-test or ANOVA.
Results
PPARα-mediated regulation of PC-TP expression
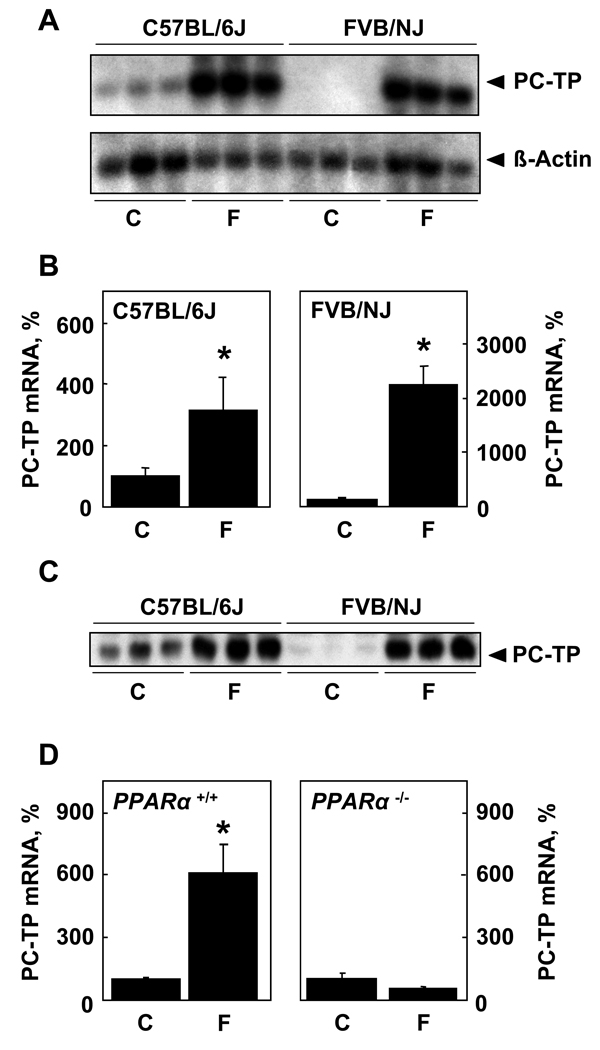
Figure 1 demonstrates the regulation of PC-TP expression by PPARα. In keeping with our prior observation that PC-TP is expressed more robustly in livers of C57BL/6J compared with FVB/NJ mice [16], PC-TP mRNA was readily detected in livers of chow fed C57BL/6J mice by northern blot analysis, but was not apparent in FVB/NJ mice at similar X-ray film exposure times (Figure 1A). There was marked upregulation of mRNA by fenofibrate feeding, with transcript levels reaching similar levels in the two mouse strains. Figure 1B quantifies this finding by qPCR, which revealed a 3-fold upregulation of PC-TP mRNA in C57BL/6J mice and 20-fold upregulation in FVB/NJ mice. Consistent with transcriptional regulation, Figure 1C shows that variations in protein expression reflected changes in mRNA levels. Figure 1D demonstrates that, in the absence of PPARα, upregulation of PC-TP in response to fenofibrate did not occur. Based upon the more robust fenofibrate-mediated upregulation of PC-TP, FVB/NJ mice were utilized for subsequent studies.
Figure 1. Upregulation of PC-TP expression in response to fenofibrate is mediated by PPARα activation.
Mice were fed chow (C) a fenofibrate-supplemented diet (F) for 7 d prior to A) northern blot analysis, B) qPCR (C57BL/6J, n = 6/group; FVB/NJ, n = 8/group) and C) western blot analysis. D) qPCR of PC-TP mRNA harvested from PPARα−/−(n = 4/group) and wild type 129S3/svlmJ (n = 6/group) mice. *P < 0.05, wild type vs. Pctp−/− mice.
Influence of PC-TP expression on lipid metabolism in fenofibrate fed mice
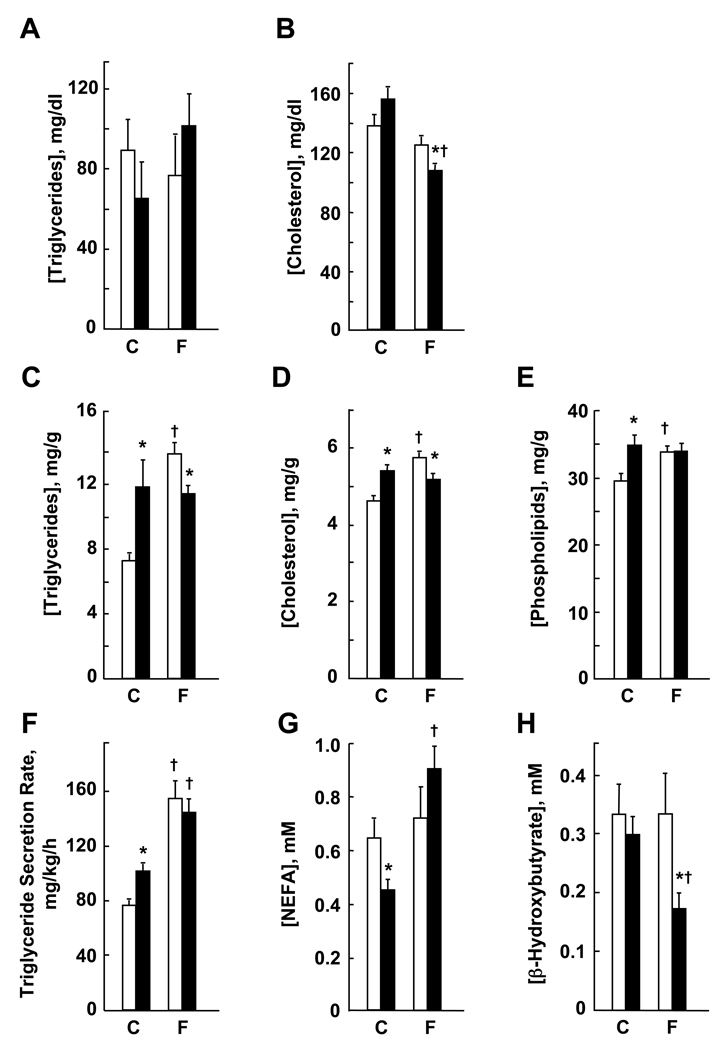
Figure 2 shows the influence of PC-TP on key parameters of lipid metabolism in mice fed chow or a fenofibrate supplemented diet. Plasma triglycerides were not different in chow fed Pctp−/− and wild type mice and were not influenced significantly by fenofibrate feeding (Figure 2A). Plasma cholesterol concentrations were not different on a chow diet (Figure 2B). Whereas fenofibrate feeding did not affect plasma cholesterol concentrations in wild type mice, values for Pctp−/− mice were decreased. Not shown is that the lipoprotein profile by FPLC did not differ for fenofibrate fed mice. Figure 2C shows that hepatic triglyceride concentrations were higher in chow fed mice lacking PC-TP. Whereas fenofibrate feeding increased hepatic triglycerides in wild type mice, values for Pctp−/− mice were unchanged, so that hepatic triglycerides were lower in Pctp−/− mice after fenofibrate treatment. Hepatic concentrations of cholesterol were increased in chow fed Pctp−/− mice (Figure 2D). Whereas fenofibrate increased hepatic cholesterol concentrations in wild type mice, there was no change in Pctp−/− mice. As a result, hepatic cholesterol concentrations were lower in fenofibrate fed Pctp−/− mice. Hepatic phospholipid concentrations were higher in chow fed Pctp−/− mice (Figure 2E). Because they increased in wild type but not Pctp−/− mice, hepatic phospholipid concentrations were the same in both genotypes following fenofibrate feeding.
Figure 2. PC-TP expression regulates the response of lipid metabolism to fenofibrate.
Wild type mice (open bars) and Pctp−/− mice (solid bars) were fed 7 d with chow (C: wild type, n = 7 – 8; Pctp−/−, n = 6 – 8) or fenofibrate supplemented (F; wild type, n = 6 – 8; Pctp−/−, n = 6 – 8) diets prior to measuring plasma concentrations of (A) triglycerides and (B) cholesterol; hepatic concentrations of (C) triglycerides, (D) cholesterol and (E) phospholipids; (F) hepatic triglyceride secretion rates (please note that values for chow fed Pctp−/− and wild type mice in this panel were replotted from reference [7]); and plasma concentrations of (G) NEFA and (H) β-hydroxybutyrate. *P < 0.05, wild type vs. Pctp−/− mice; †P < 0.05, C vs. F.
As previously reported [7], hepatic triglyceride production rates were higher in chow fed Pctp−/− mice (Figure 2F). Fenofibrate increased production rates to similar levels in both genotypes. Plasma NEFA concentrations were lower in chow fed Pctp−/− mice (Figure 2G). Whereas values remained the same in wild type mice following fenofibrate feeding, NEFA concentrations increased in the absence of PC-TP expression. As also reported previously [7], plasma concentrations of β-hydroxybutyrate were the same in chow fed Pctp−/− and wild type. Whereas there was no change in wild type mice, fenofibrate feeding decreased β-hydroxybutyrate concentrations in Pctp−/− mice (Figure 2H).
PC-TP expression regulates glucose metabolism in fenofibrate treated mice
The effects of PC-TP expression on glucose homeostasis in chow and fenofibrate fed mice are illustrated in Figure 3. Fasting plasma glucose concentrations were reduced in Pctp−/− mice (Figure 3A) in the absence of changes in plasma insulin (Figure 3B). Indicative of delayed clearance, glucose tolerance tests (Figure 3C) revealed that peak plasma glucose concentrations were achieved at 30 min in wild type mice and at 60 min in Pctp−/− mice. The absence of PC-TP expression was associated with an increase in the area under the curve (AUC, inset to Figure 3C). Consistent with these findings, insulin tolerance tests in Pctp−/− mice (Figure 3D) revealed decreased glucose clearance at each point in time and an increase in AUC (inset).
Figure 3. Influence of PC-TP on the response of glucose metabolism to fenofibrate.
(A) Wild type mice (open bars) and Pctp−/− mice (solid bars) were fed 7 d with a fenofibrate supplemented diet (wild type, n = 8; Pctp−/−, n = 6) prior to measuring plasma concentrations of (A) glucose and (B) insulin. The same mice (wild type, ○; Pctp−/−, ●) were then subjected to (C) glucose and (D) insulin tolerance tests. Inserts provide AUC values (wild type, open bars; Pctp−/−, closed bars). *P < 0.05, wild type vs. Pctp−/− mice.
Differentially regulated hepatic genes in fenofibrate fed Pctp−/− mice
Microarray analysis was utilized to examine the influence of PC-TP expression on hepatic gene expression in response to PPARα activation. On the GeneChip, 14,000 genes were represented by 22,690 probes. According to dChip analysis, 727 hepatic genes met the pre-specified criteria for altered regulation. In Pctp−/− compared with wild type mice, 414 genes were upregulated and 313 genes were downregulated. For analysis by BADGE, an expected false positive rate of 0.30% selected the genes with more than 99.85% and less than 0.15% chance of being more expressed in wild type mice. Under these conditions, 757 genes were differentially expressed, with 89 upregulated and 668 downregulated genes in Pctp−/− mice. Comparison of the two approaches demonstrated that 263 genes were differentially expressed according to both algorithms. Table 1 shows the 53 differentially regulated genes related to lipid and glucose metabolism grouped using GenMapp. Differential expression of selected genes was validated by qPCR and consistently demonstrated higher fold changes than observed by microarray analysis. Testing of selected genes using chow fed mice revealed that these differences did not pre-exist fenofibrate treatment.
Table 1.
Genes Involved in Lipid and Glucose Metabolism that are Differentially Expressed in Fenofibrate Fed Pctp−/− vs. Wild Type Micea
| Gene | Abbreviationb | Affymetrix ID | dChipc | BADGEc | qPCRd |
|---|---|---|---|---|---|
| Triglyceride Metabolism | |||||
| Fibroblast growth factor 21e | FGF21 | 1422916_at | 3.00 | 2.95 | 11.22 |
| Lipase, endotheliale | 1421262_at, 1450188_s_at |
2.09 | 2.17 | ||
| Phosphatidic acid phosphatase 2A |
1422620_s_at | 1.56 | 1.49 | ||
| Stearoyl-Coenzyme A desaturase 2 |
1415823_at | 1.55 | |||
| Aquaporin 9 | 1421605_a_at | 1.47 | 1.62 | ||
| ATP-binding cassette, sub- family D (ALD), member 2 |
1419748_at | 1.45 | 1.72 | ||
| Glycerol-3-phosphate acyltransferase, mitochondrial |
1425834_a_at | 1.39 | 1.54 | ||
| Acyl-CoA synthetase long- chain family member 1e |
1422526_at | 1.22 | 5.80 | ||
| Fatty acid synthase | FAS | 1423828_at | 1.07f | 4.30 | |
| Apolipoprotein M | 1419095_a_at, 1419096_at |
−1.41 | −1.40 | ||
| Lipin 2 | 1452837_at | −1.47 | |||
| Carnitine palmitoyltransferase 1a, liver |
CPT1a | 1434866_x_at | −1.53 | ||
| Lipin 1 | 1418288_at, 1426516_a_at |
−2.40 | −2.08 | ||
| Acetyl-coenzyme A carboxylase alpha |
ACCα | not present | 2.39 | ||
|
Cholesterol and Phospholipid Metabolism |
|||||
| NAD(P) dependent steroid dehydrogenase-like |
1451799_at | 2.78 | |||
| Acetoacetyl-CoA synthetase | 1423797_at, 1456081_a_at |
2.63 | 3.08 | ||
| 3-hydroxy-3-methylglutaryl- Coenzyme A reductasee |
1427229_at | 1.78 | 2.07 | 2.34 | |
| Low density lipoprotein receptore |
LDLr | 1450383_at, 1421821_at |
1.75 | 1.96 | |
| ATP-binding cassette, sub- family A (ABC1), member 1e |
ABCA1 | 1421839_at, 1450392_at |
1.75 | 2.03 | |
| Phosphatidylserine synthase 1 |
PSS1 | 1441866_s_at | 1.53 | ||
| Mevalonate (diphospho) decarboxylase |
1417303_at, 1448663_s_at |
1.68 | |||
| Squalene epoxidasee | 1415993_at | 1.39 | 2.33 | ||
| Sterol-C4-methyl oxidase-like | 1423078_a_at | 1.38 | |||
| Phosphate cytidylyltransferase 1, choline, alpha isoform |
CTα | 1424453_at | 1.34 | 1.44 | |
| Phosphatidylinositol transfer protein, cytoplasmic 1 |
1452940_x_at, 1455204_at |
−1.51 | |||
| ATP-binding cassette, sub- family G (WHITE), member 8 |
ABCG8 | 1420656_at | −1.69 | −1.55 | |
| Glucose Metabolism | |||||
| Glucokinasee | GK | 1419146_a_at, 1425303_at |
2.76 | 2.43 | |
| Ribose 5-phosphate isomerase A |
1418337_at | 1.57 | 1.49 | ||
| Dihydrolipoamide S- acetyltransferase (E2 component of pyruvate dehydrogenase complex) |
1452005_at | 1.43 | |||
| Isocitrate dehydrogenase 3 (NAD+) alpha |
1432016_a_at | 1.40 | |||
| Phosphoglucomutase 1 | 1453283_at | 1.37 | |||
| Pyruvate dehydrogenase E1 alpha 1 |
1449137_at | 1.34 | |||
| 6-phosphofructo-2- kinase/fructose-2,6- biphosphatase 1 |
1427213_at | −1.41 | |||
| Solute carrier family 2 (facilitated glucose transporter), member 1 |
1434773_a_at | −1.55 | |||
| Insulin receptor substrate 2 | IRS2 | 1443969_at | −1.81 | ||
| Phosphoenolpyruvate carboxykinase 1, cytosolice |
PEPCK | 1423439_at | −1.88 | −2.63 | |
| Glutamate oxaloacetate transaminase 1, soluble |
1450970_at | −2.67 | |||
| Transcriptional Factors | |||||
| Nuclear receptor subfamily 0, group B, member 2e |
SHP | 1449854_at | 2.26 | ||
| Sterol regulatory element binding factor 1 |
SREBP1c | 1426690_a_at | 1.62 | 1.67 | 14.06 |
| Nuclear receptor subfamily 3, group C, member 1(glucocorticoid receptor) |
1421866_at | 1.59 | 1.49 | ||
| Retinoic acid receptor, beta | 1454906_at | 1.53 | 1.44 | ||
| Hypoxia inducible factor 1, alpha subunit |
HIF-1α | 1448183_a_at | 1.50 | 1.74 | |
| Hepatic nuclear factor 4, alphae |
HNF4α | 1421983_s_at | 1.49 | 1.60 | 6.84 |
| Sterol regulatory element binding factor 2e |
SREBP2 | 1426744_at | 1.47 | 9.60 | |
| Nuclear receptor subfamily 2, group F, member 2 (COUP-TFII) |
1416158_at | 1.34 | 1.78 | ||
| Peroxisome proliferator activated receptor alphae |
PPARα | 1449051_at | −1.36 | ||
| Nuclear receptor subfamily 5, group A, member 2e |
LRH-1 | 1449706_s_at | −1.41 | ||
| CCAAT/enhancer binding protein (C/EBP), delta |
1423233_at | −1.56 | |||
| Forkhead box O1e | FoxO1 | 1416982_at, 1416983_s_at |
−1.60 | −1.51 | |
| Forkhead box O3a | 1434831_a_at, 1434832_at |
−1.68 | −1.61 | ||
| Peroxisome proliferative activated receptor, gamma, coactivator 1 beta |
PCG-1β | 1449945_at | −2.35 | −1.85 | |
| Peroxisome proliferative activated receptor, gamma, coactivator 1 alphae |
PCG-1α | 1437751_at, 1456395_at, 1460336_at |
−3.15 | −2.21 | |
Data are expressed as ratios of hepatic gene expression values for Pctp−/− mice/wild type mice after feeding a fenofibrate-supplemented chow diet for 7 d as determined by dChip or BADGE algorithms. Selected genes were tested by quantitative real-time PCR (qPCR) for differential expression. Genes were assigned to categories using GenMAPP.
Common gene abbreviation used in the text.
Where more than one Affymetrix ID is listed, values represent averages of fold changes.
qPCR was performed (wild type, n = 6 – 7; Pctp−/−, n = 4 – 6) and differences were determined to be significant at P < 0.05.
qPCR was performed using cDNA prepared from livers of chow fed mice (wild type, n = 4; Pctp−/−, n = 4), which revealed no significant difference for these genes.
Falls below the threshold for significance by dChip algorithm.
For genes related to triglyceride metabolism (Figure 2), there was variable regulation in response to fenofibrate. In livers of Pctp−/− mice, some lipogenic genes were differentially upregulated, whereas others were suppressed. Whereas SREBP1c, which controls genes of fatty acid synthesis, was upregulated, anticipated target genes [24] were not identified by the microarray analysis. These included ACCα, which was not represented on the GeneChip, and FAS, which fell below the threshold for upregulation by dChip analysis. Testing by qPCR (Table 1) demonstrated that both of these mRNA transcripts were expressed at higher levels in Pctp−/− compared with wild type mice. The relative suppression observed for Lipin-1 and CPT1a in fenofibrate fed mice lacking PC-TP are both consistent with downregulation of PPARα [9, 25]. In contrast to the mixed regulation of genes governing triglyceride metabolism, Table 1 demonstrates more consistent upregulation of mRNA transcripts for proteins that mediate synthesis of cholesterol (e.g. 3-hydroxy-3-methylglutaryl-Coenzyme A reductase and squalene epoxidase) and phospholipids (i.e. CTα and PSS1), as well as lipoprotein turnover (i.e. LDLr and ABCA1). Upregulation of cholesterol metabolic genes is in line with increased mRNA levels of the transcription factor SREBP2 [24]. In keeping with reduced fasting plasma glucose concentrations but impaired glucose and insulin tolerance in Pctp−/− mice (Figure 3), the associated pattern of differentially regulation genes appeared to be more complex. This involved altered expression of mRNAs encoding several key transcription factors that play critical roles in regulating glucose metabolism (e.g. HNF4α, PGC-1α, FoxO1 and HIF-1α).
Regulation of PPARα and HNF4α activity by PC-TP in vitro
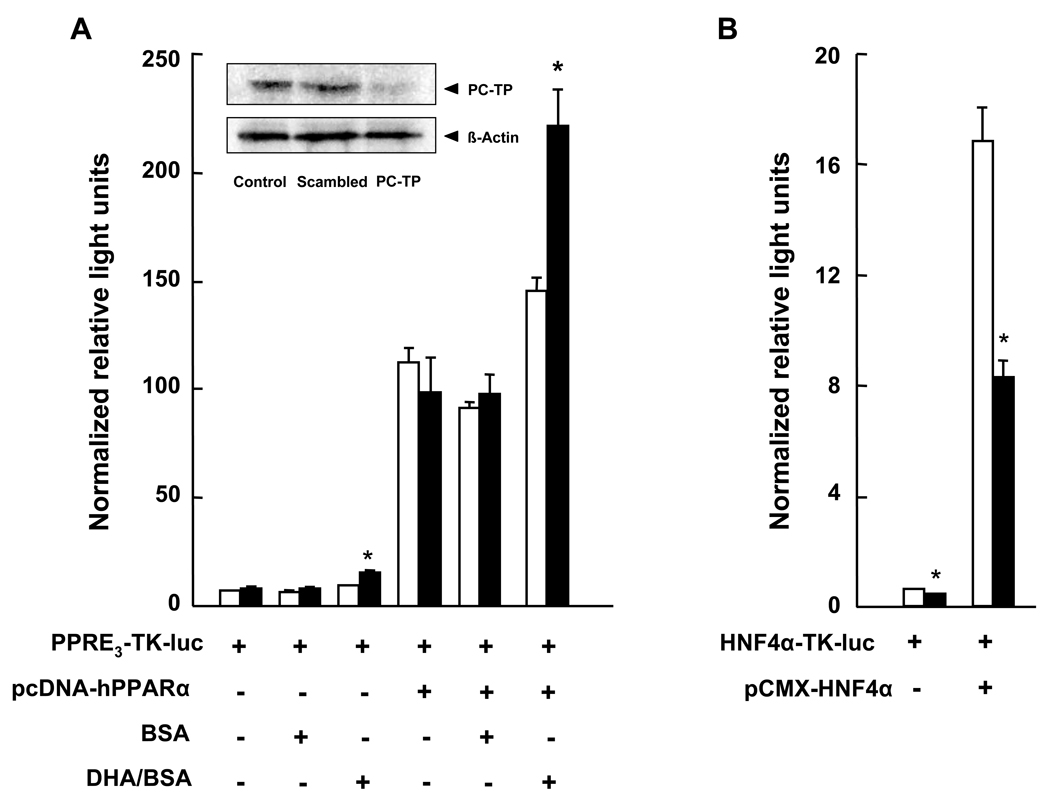
Potential regulation of PPARα and HNF4α activities by PC-TP expression was examined using HEK 293T cells. PC-TP siRNA treatment led to >90% reductions in PC-TP expression compared to scrambled siRNA treatment and non-transfected cells (inset to Figure 4A). PC-TP knockdown did not affect transcriptional activity of PPARα that was attributable to endogenous or overexpressed protein (Figure 4A). However, in the presence of the PPARα ligand DHA [9], the transcriptional activities of endogenous and overexpressed PPARα were both increased further in the setting of PC-TP knockdown. Although fenofibrate was not effective in HEK 293T cells under these conditions, the more potent fibrate WY-14,643 (1 µM) similarly increased the activity of overexpressed PPARα following knockdown of PC-TP (data not shown). Endogenous HNF4α activity was reduced with PC-TP knockdown (Figure 4B), an effect that was also observed when HNF4α was overexpressed.
Figure 4. Regulation of PPARα and HNF4α transcriptional activity by PC-TP in vitro.
(A) Influence of PC-TP expression on PPARα transcriptional activity. HEK 293T cells were treated with scrambled siRNA (open bars) or PC-TP siRNA (closed bars). PPARα transcriptional activity was determined according to firefly luciferase activity expressed by PPRE3-tk-luc in the absence (−) or presence (+) of exogenous PPARα expression (pcDNA-hPPARα), as well as BSA or DHA complexed to BSA (DHA/BSA). The inset western blot shows the effects of PC-TP siRNA treatment compared with scrambled siRNA or non-transfected control cells. (B) Influence of PC-TP expression on HNF4α transcriptional activity. HEK 293T cells were treated with siRNA as described in panel A. HNF4α transcriptional activity was determined according to firefly luciferase activity expressed by HNF4α-TK-luc in the absence (−) or presence (+) of exogenous HNF4α expression (pCMX-HNF4α). Data are representative of three independent experiments, each experiment was performed in triplicate. *P < 0.05, PC-TP vs. scrambled siRNA.
Discussion
The objective of this study was to explore the contribution of PC-TP to the metabolic response of lipids and glucose to activation of PPARα. The response of PC-TP to fenofibrate feeding both confirms and extends observations in the literature that PPARα regulates PC-TP [13, 14, 26]. The demonstration in two different strains of mice that levels of expression of mRNA and protein are tightly coupled suggests that transcriptional control is the main mechanism that governs PC-TP levels in the liver.
Our observation that hepatic triglyceride concentrations increased by 91% in FVB/NJ wild type mice fed 0.2% fenofibrate for 7 d is consistent with published reports concerning the effects of fibrate drugs. When fed 0.2% fenofibrate for 14 d, C57BL/6 mice exhibited a 58% increase in hepatic triglyceride concentrations [27], whereas a much more modest, non-significant increase was observed for Sv/129 mice gavage daily for 7 d with 10 mg/kg of WY-14,643 [28]. Unlike the C57BL/6 mice in which fenofibrate reduced plasma triglyceride concentrations by 80% [27], we observed a much more modest 19% decrease, which did not achieve significance. These differences may have been attributable to duration or methods of feeding, type of fibrate drug or mouse strain.
We previously reported that hepatic triglyceride concentrations were lower in chow fed Pctp−/− compared with wild type mice [7], whereas in the current study values for Pctp−/− mice were higher. This was due to variable hepatic triglyceride concentrations for the wild type mice because there was no difference in values for Pctp−/− mice in the two studies. The higher values previously published for livers of wild type mice were attributable to the longer duration of fasting (16 h compared vs 4 h), which would be expected to increase hepatic triglyceride concentrations [28]. Considering the central regulatory role of PPARα during fasting [9], the absence of changes in Pctp−/− mice during fasting or following fenofibrate feeding suggests that PC-TP contributes to the metabolic response that follows PPARα activation. This notion is further supported by the influence of PC-TP expression on fenofibrate-mediated control of hepatic triglyceride production rates, as well as plasma concentrations of NEFA and β-hydroxybutyrate.
We have provided evidence that NEFA from adipose tissue provided the source for increased hepatic triglyceride secretion rates in chow fed Pctp−/− mice [7]. This occurred in the setting of marked reductions in hepatic fatty acid synthesis, which were accompanied by downregulation of SREBP1c and its target genes. By contrast in fenofibrate fed mice, the absence of PC-TP was associated with differential upregulation of SREBP1c, which was accompanied in Pctp−/− mice by relative increases in fatty acid synthetic genes, including ACCα and FAS. The relative reduction in lipin-1 [25] appears to have contributed to the reduced hepatic triglyceride concentrations in fenofibrate fed Pctp−/− mice compared with fenofibrate fed wild type controls. The marked decrease in plasma β-hydroxybutyrate concentrations in response to fenofibrate feeding of Pctp−/− mice is in keeping with the reduction in CPT1a expression, which was most likely attributable to relative downregulation of PPARα [9], PGC-1α and PGC-1β [29]. Our data do not provide insights into the relative upregulation in Pctp−/− mice of FGF21, a PPARα regulated gene that promotes fatty acid oxidation and ketogenesis [30].
In fenofibrate fed Pctp−/− mice, the relative increases in the expression of SREPB2 and its target genes, cholesterol biosynthetic enzymes and the LDL receptor [24], appear to represent a compensatory response to the observed reduction in hepatic cholesterol concentrations. This may have occurred secondary to increased expression of ABCA1, which promotes apolipoprotein AI-mediated cholesterol efflux from hepatocytes during HDL formation [31]. It is noteworthy that the decrease in hepatic cholesterol was not accompanied by a decrease in hepatic phospholipids in Pctp−/− mice. It is possible that an increase in phospholipid synthesis associated with hepatic upregulation of CTα and PSS1 [32] contributed to increased SREBP2 processing by reducing the relative concentration of cholesterol concentration in the endoplasmic reticulum [33]. The relative reduction in ABCG8 argues against the possibility that the increase in biliary cholesterol excretion decreased hepatic cholesterol contents [34], and the decreased relative expression of SHP and increased LRH-1 mRNA make it unlikely that conversion of cholesterol to bile salts was increased [35].
In contrast to chow fed mice [7], fenofibrate fed Pctp−/− mice exhibited reduced fasting plasma glucose concentrations, but decreased glucose clearance and insulin responsiveness. The reduction in fasting plasma glucose concentrations was presumably explained by the reduced relative expression levels of PGC-1α and FoxO1, leading to decreased PEPCK expression [36, 37]. The relative increase in expression of GK may have contributed and was likely due to the higher expression of both HNF4α and HIF-1α [38]. Downregulation of IRS2 may have played a role in reducing both glucose clearance and the sensitivity of plasma glucose to insulin [39]. However, additional experiments will be required to dissect the influence of PC-TP on insulin signaling in the context of PPARα activation.
Because many of the differentially expressed genes were themselves regulated by PPARα [9], we explored in vitro the possibility that PC-TP expression per se could influence PPARα activity. Appreciating that the reductionist system in which PC-TP was acutely silenced in HEK 293T cells using siRNA does not reflect the complex changes that occur in livers of fenofibrate fed Pctp−/− mice, the observed changes in ligand-activated PPARα activity and intrinsic activity of HNF4α suggest that PC-TP may modulate the activity of these transcription factors. In this connection, we have reported that PC-TP enhances the acyl-CoA thioesterase activity of thioesterase superfamily member 2 (Them2) [8]. This could modulate the intracellular balance of fatty acids and fatty acyl-CoAs, which contributes to the regulation of PPARα and HNF4α [26]. Taken together, our findings are in keeping with a primary role for PC-TP in fatty acid metabolism [7], which secondarily regulates transcription factors that govern nutrient homeostasis.
Supplementary Material
Acknowledgements
This study was supported by NIH Grants DK56626 and DK48873 (DEC). The authors acknowledge support for the microarray studies from the Harvard Medical School-Partners HealthCare Center for Genetics and Genomics. The authors thank Drs. Marco Ramoni, Jorge Plutzky, John Chiang and Gabriela Orasanu for helpful discussions.
Abbreviations
- ABC
ATP-binding cassette
- ACC
Acetyl-coenzyme A carboxylase
- BADGE
Bayesian Analysis of Differential Gene Expression
- CPT
Carnitine palmitoyltransferase
- CT
CTP:phosphocholine cytidylyltransferase
- DHA
docosahexaenoic acid
- FAS
fatty acid synthase
- FGF
fibroblast growth factor
- FOX
forkhead box
- GK
glucokinase
- IRS
insulin receptor substrate
- LDLr
low density lipoprotein receptor
- LRH
liver receptor homolog
- HIF
hypoxia inducible factor
- HNF
hepatocyte nuclear factor
- NEFA
non-esterified fatty acid
- PEPCK
phosphoenolpyruvate carboxykinase
- PGC
peroxisome proliferator activated receptor, gamma, coactivator
- PC-TP
phosphatidylcholine transfer protein
- PPAR
peroxisome proliferator activated receptor
- PSS
phosphatidylserine synthase
- SHP
short heterodimer partner
- SREBP
sterol regulatory element binding protein
- START
steroidogenic acute regulatory protein-related lipid transfer
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wirtz KW. Phospholipid Transfer Proteins. Annu. Rev. Biochem. 1991;60:73–99. doi: 10.1146/annurev.bi.60.070191.000445. [DOI] [PubMed] [Google Scholar]
- 2.Ponting CP, Aravind L. START: a lipid-binding domain in StAR, HD-ZIP and signalling proteins. Trends Biochem. Sci. 1999;24:130–132. doi: 10.1016/s0968-0004(99)01362-6. [DOI] [PubMed] [Google Scholar]
- 3.Schrick K, Nguyen D, Karlowski WM, Mayer KF. START lipid/sterol-binding domains are amplified in plants and are predominantly associated with homeodomain transcription factors. Genome Biol. 2004;5:R41. doi: 10.1186/gb-2004-5-6-r41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Soccio RE, Breslow JL. StAR-related lipid transfer (START) proteins: mediators of intracellular lipid metabolism. J. Biol. Chem. 2003;278:22183–22186. doi: 10.1074/jbc.R300003200. [DOI] [PubMed] [Google Scholar]
- 5.Kang HW, Ribich S, Kim BW, Hagen SJ, Bianco AC, Cohen DE. Mice lacking phosphatidylcholine transfer protein/StarD2 exhibit increased adaptive thermogenesis and enlarged mitochondria in brown adipose tissue. J. Lipid Res. 2009 doi: 10.1194/jlr.M900013-JLR200. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kanno K, Wu MK, Scapa EF, Roderick SL, Cohen DE. Structure and function of phosphatidylcholine transfer protein (PC-TP)/StarD2. Biochim. Biophys. Acta. 2007;1771:654–662. doi: 10.1016/j.bbalip.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scapa EF, Pocai A, Wu MK, Gutierrez-Juarez R, Glenz L, Kanno K, Li H, Biddinger S, Jelicks LA, Rossetti L, Cohen DE. Regulation of energy substrate utilization and hepatic insulin sensitivity by phosphatidylcholine transfer protein/StarD2. FASEB J. 2008;22:2579–2590. doi: 10.1096/fj.07-105395. [DOI] [PubMed] [Google Scholar]
- 8.Wei J, Kang HW, Cohen DE. Thioesterase superfamily member 2 (Them2)/acyl-CoA thioesterase 13 (Acot13): A homotetrameric hotdog fold thioesterase with selectivity for long chain fatty acyl-CoAs. Biochem. J. 2009;421:311–322. doi: 10.1042/BJ20090039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mandard S, Muller M, Kersten S. Peroxisome proliferator-activated receptor alpha target genes. Cell. Mol. Life Sci. 2004;61:393–416. doi: 10.1007/s00018-003-3216-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chakravarthy MV, Lodhi IJ, Yin L, Malapaka RR, Xu HE, Turk J, Semenkovich CF. Identification of a physiologically relevant endogenous ligand for PPARalpha in liver. Cell. 2009;138:476–488. doi: 10.1016/j.cell.2009.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu MK, Boylan MO, Cohen DE. Cloning and gene structure of rat phosphatidylcholine transfer protein, Pctp. Gene. 1999;235:111–120. doi: 10.1016/s0378-1119(99)00204-8. [DOI] [PubMed] [Google Scholar]
- 12.Rakhshandehroo M, Sanderson LM, Matilainen M, Stienstra R, Carlberg C, de Groot PJ, Muller M, Kersten S. Comprehensive Analysis of PPARalpha-Dependent Regulation of Hepatic Lipid Metabolism by Expression Profiling. PPAR Res. 2007;2007:26839. doi: 10.1155/2007/26839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chu R, Lim H, Brumfield L, Liu H, Herring C, Ulintz P, Reddy JK, Davison M. Protein profiling of mouse livers with peroxisome proliferator-activated receptor alpha activation. Mol. Cell. Biol. 2004;24:6288–6297. doi: 10.1128/MCB.24.14.6288-6297.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Brouwer AP. Functional Studies on the Phosphatidylcholine Transfer Protein. Utrecht, The Netherlands: Utrecht University; 2002. pp. 135–148. [Google Scholar]
- 15.van Helvoort A, de Brouwer A, Ottenhoff R, Brouwers JF, Wijnholds J, Beijnen JH, Rijneveld A, van der Poll T, van der Valk MA, Majoor D, Voorhout W, Wirtz KW, Elferink RP, Borst P. Mice without phosphatidylcholine transfer protein have no defects in the secretion of phosphatidylcholine into bile or into lung airspaces. Proc. Natl. Acad. Sci. U.S.A. 1999;96:11501–11506. doi: 10.1073/pnas.96.20.11501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu MK, Hyogo H, Yadav S, Novikoff PM, Cohen DE. Impaired response of biliary lipid secretion to a lithogenic diet in phosphatidylcholine transfer protein-deficient mice. J. Lipid Res. 2005;46:422–431. doi: 10.1194/jlr.M400387-JLR200. [DOI] [PubMed] [Google Scholar]
- 17.Hyogo H, Roy S, Paigen B, Cohen DE. Leptin promotes biliary cholesterol elimination during weight loss in ob/ob mice by regulating the enterohepatic circulation of bile salts. J. Biol. Chem. 2002;277:34117–34124. doi: 10.1074/jbc.M203912200. [DOI] [PubMed] [Google Scholar]
- 18.Scapa EF, Kanno K, Wang W, Cohen DE. A key regulatory role for phosphatidylcholine transfer protein (PC-TP) in hepatic triglyceride metabolism: Implications for the pathogenesis of non-alcoholic fatty liver disease (abstract) Hepatology. 2005;42:508A–509A. [Google Scholar]
- 19.Kanno K, Wu MK, Agate DA, Fanelli BK, Wagle N, Scapa EF, Ukomadu C, Cohen DE. Interacting proteins dictate function of the minimal START domain phosphatidylcholine transfer protein/StarD2. J. Biol. Chem. 2007;282:30728–30736. doi: 10.1074/jbc.M703745200. [DOI] [PubMed] [Google Scholar]
- 20.Li C, Wong WH. Model-based analysis of oligonucleotide arrays: expression index computation and outlier detection. Proc. Natl. Acad. Sci. U.S.A. 2001;98:31–36. doi: 10.1073/pnas.011404098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim DM, Ramoni MF, Nevins M, Fiorellini JP. The gene expression profile in refractory periodontitis patients. J. Periodontol. 2006;77:1043–1050. doi: 10.1902/jop.2006.050254. [DOI] [PubMed] [Google Scholar]
- 22.Salomonis N, Hanspers K, Zambon AC, Vranizan K, Lawlor SC, Dahlquist KD, Doniger SW, Stuart J, Conklin BR, Pico AR. GenMAPP 2: new features and resources for pathway analysis. BMC Bioinformatics. 2007;8:217. doi: 10.1186/1471-2105-8-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pan M, Maitin V, Parathath S, Andreo U, Lin SX, St Germain C, Yao Z, Maxfield FR, Williams KJ, Fisher EA. Presecretory oxidation, aggregation, and autophagic destruction of apoprotein-B: a pathway for late-stage quality control. Proc. Natl. Acad. Sci. U.S.A. 2008;105:5862–5867. doi: 10.1073/pnas.0707460104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Horton JD, Goldstein JL, Brown MS. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J. Clin. Invest. 2002;109:1125–1131. doi: 10.1172/JCI15593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reue K, Zhang P. The lipin protein family: dual roles in lipid biosynthesis and gene expression. FEBS Lett. 2008;582:90–96. doi: 10.1016/j.febslet.2007.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sampath H, Ntambi JM. Polyunsaturated fatty acid regulation of gene expression. Nutr. Rev. 2004;62:333–339. doi: 10.1111/j.1753-4887.2004.tb00058.x. [DOI] [PubMed] [Google Scholar]
- 27.Oosterveer MH, Grefhorst A, van Dijk TH, Havinga R, Staels B, Kuipers F, Groen AK, Reijngoud DJ. Fenofibrate simultaneously induces hepatic fatty acid oxidation, synthesis and elongation in mice. J Biol Chem. 2009 doi: 10.1074/jbc.M109.051052. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dalen KT, Ulven SM, Arntsen BM, Solaas K, Nebb HI. PPARalpha activators and fasting induce the expression of adipose differentiation-related protein in liver. J Lipid Res. 2006;47:931–943. doi: 10.1194/jlr.M500459-JLR200. [DOI] [PubMed] [Google Scholar]
- 29.Lin J, Tarr PT, Yang R, Rhee J, Puigserver P, Newgard CB, Spiegelman BM. PGC-1beta in the regulation of hepatic glucose and energy metabolism. J. Biol. Chem. 2003;278:30843–30848. doi: 10.1074/jbc.M303643200. [DOI] [PubMed] [Google Scholar]
- 30.Badman MK, Pissios P, Kennedy AR, Koukos G, Flier JS, Maratos-Flier E. Hepatic fibroblast growth factor 21 is regulated by PPARalpha and is a key mediator of hepatic lipid metabolism in ketotic states. Cell Metab. 2007;5:426–437. doi: 10.1016/j.cmet.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 31.Tall AR, Yvan-Charvet L, Terasaka N, Pagler T, Wang N. HDL, ABC transporters, and cholesterol efflux: implications for the treatment of atherosclerosis. Cell Metab. 2008;7:365–375. doi: 10.1016/j.cmet.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 32.Vance JE, Vance DE. Phospholipid biosynthesis in mammalian cells. Biochem. Cell Biol. 2004;82:113–128. doi: 10.1139/o03-073. [DOI] [PubMed] [Google Scholar]
- 33.Radhakrishnan A, Goldstein JL, McDonald JG, Brown MS. Switch-like control of SREBP-2 transport triggered by small changes in ER cholesterol: a delicate balance. Cell Metab. 2008;8:512–521. doi: 10.1016/j.cmet.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yu L, Hammer RE, Li-Hawkins J, Von Bergmann K, Lutjohann D, Cohen JC, Hobbs HH. Disruption of Abcg5 and Abcg8 in mice reveals their crucial role in biliary cholesterol secretion. Proc. Natl. Acad. Sci. U.S.A. 2002;99:16237–16242. doi: 10.1073/pnas.252582399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chiang JY. Regulation of bile acid synthesis: pathways, nuclear receptors, and mechanisms. J Hepatol. 2004;40:539–551. doi: 10.1016/j.jhep.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 36.Lin J, Handschin C, Spiegelman BM. Metabolic control through the PGC-1 family of transcription coactivators. Cell Metab. 2005;1:361–370. doi: 10.1016/j.cmet.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 37.Gross DN, van den Heuvel AP, Birnbaum MJ. The role of FoxO in the regulation of metabolism. Oncogene. 2008;27:2320–2336. doi: 10.1038/onc.2008.25. [DOI] [PubMed] [Google Scholar]
- 38.Iynedjian PB. Molecular physiology of mammalian glucokinase. Cell Mol. Life Sci. 2009;66:27–42. doi: 10.1007/s00018-008-8322-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Haeusler RA, Accili D. The double life of Irs. Cell Metab. 2008;8:7–9. doi: 10.1016/j.cmet.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.